Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons
Abstract
:1. Common Features of Dopaminergic Neurons
1.1. Dopaminergic Neurons
1.2. Dopaminergic Receptors
1.3. DA Synthesis
1.4. DA Metabolism
1.5. DA Negative Feedback
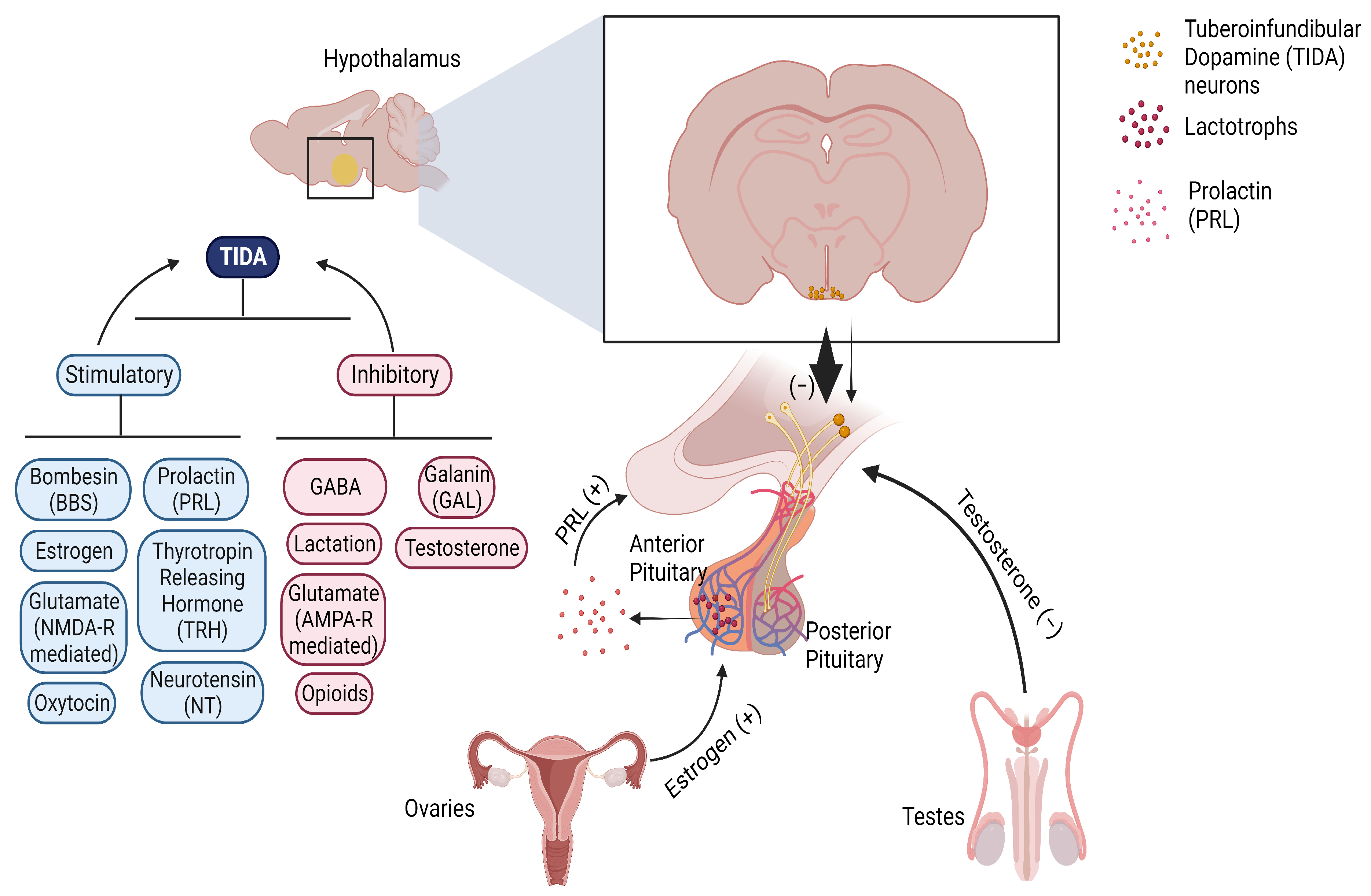
2. TIDA Neurons
- Anatomical Structure
- 2.
- Functions
- 3.
- Inherent Membrane Characteristics
- 4.
- Regulation of TIDA Neurons
2.1. Regulation by Autoreceptors
2.2. Regulation by PRL, Estradiol, and Testosterone
2.3. Regulation by Glutamate
2.4. Regulation by GABA
2.5. Regulation by Neuropeptides
2.5.1. TRH
2.5.2. Opioids
2.5.3. Neurotensin (NT)
2.5.4. Bombesin (BBS)
2.5.5. Galanin (GAL)
2.6. Regulation by Lactation
3. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-X. Electrophysiological Characteristics of Dopamine Neurons: A 35-Year Update. In Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra; Giovanni, G., Di Matteo, V., Esposito, E., Eds.; Springer: Vienna, Austria, 2009; pp. 103–119. [Google Scholar]
- Madadi Asl, M.; Vahabie, A.-H.; Valizadeh, A. Dopaminergic Modulation of Synaptic Plasticity, Its Role in Neuropsychiatric Disorders, and Its Computational Modeling. Basic Clin. Neurosci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 117906951877982. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.E. Hypothalamic dopaminergic neuronal systems. In Psychopharmacology: The Third Generation of Progress; Meltzer, H.Y., Ed.; Raven Press: New York, NY, USA, 1987; pp. 127–139. [Google Scholar]
- Langer, S.Z.; Arbilla, S. Presynaptic receptors and modulation of the release of noradrenaline, dopamine and GABA. Postgrad. Med. J. 1981, 57 (Suppl. S1), 18–29. [Google Scholar] [PubMed]
- Mercuri, N.B.; Saiardi, A.; Bonci, A.; Picetti, R.; Calabresi, P.; Bernardi, G.; Borrelli, E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience 1997, 79, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef]
- Loose, M.; Ronnekleiv, O.; Kelly, M. Membrane properties and response to opioids of identified dopamine neurons in the guinea pig hypothalamus. J. Neurosci. 1990, 10, 3627–3634. [Google Scholar] [CrossRef]
- Lyons, D.J.; Broberger, C. TIDAL WAVES: Network mechanisms in the neuroendocrine control of prolactin release. Front. Neuroendocrinol. 2014, 35, 420–438. [Google Scholar] [CrossRef]
- MacLeod, R.M. Regulation of prolactin secretion. Front. Neuroendocrinol. 1976, 4, 169–194. [Google Scholar]
- Cramer, O.M.; Parker, R.C.; Porter, J.C. Secretion of Dopamine into Hypophysial Portal Blood by Rats Bearing Prolactin-Secreting Tumors or Ectopic Pituitary Glands. Endocrinology 1979, 105, 636–640. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.M.; Lumpkin, M.D.; Mizunuma, H.; Khorram, O.; Ottlecz, A.; Samson, W.K. Peptidergic and dopaminergic control of prolactin release. Trends Neurosci. 1984, 7, 127–131. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Oliver, C.; Weiner, H.J.; Mical, R.S.; Porter, J.C. Dopamine in Hypophysial Portal Plasma of the Rat during the Estrous Cycle and Throughout Pregnancy. Endocrinology 1977, 100, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, L.A.; Voogt, J.L. The responsiveness of tuberoinfundibular dopaminergic neurons to prolactin feedback is diminished between early lactation and midlactation in the rat. Endocrinology 1996, 137, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Berghorn, K.A.; Le, W.W.; Sherman, T.G.; Hoffman, G.E. Suckling stimulus suppresses messenger RNA for tyrosine hydroxylase in arcuate neurons during lactation. J. Comp. Neurol. 2001, 438, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Selmanoff, M.; Wise, P.M. Decreased dopamine turnover in the median eminence in response to suckling in the lactating rat. Brain Res. 1981, 212, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; North, R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, S.; Greenfield, S.A. Sub-populations of pars compacta neurons in the substantia nigra: The significance of qualitatively and quantitatively distinct conductances. Neuroscience 1992, 48, 423–437. [Google Scholar] [CrossRef]
- Stagkourakis, S.; Dunevall, J.; Taleat, Z.; Ewing, A.G.; Broberger, C. Dopamine Release Dynamics in the Tuberoinfundibular Dopamine System. J. Neurosci. 2019, 39, 4009–4022. [Google Scholar] [CrossRef]
- Wolfart, J.; Neuhoff, H.; Franz, O.; Roeper, J. Differential Expression of the small-Conductance, Calcium-Activated Potassium Channel SK3 Is Critical for Pacemaker Control in Dopaminergic Midbrain Neurons. J. Neurosci. 2001, 21, 3443–3456. [Google Scholar] [CrossRef]
- Bosch, M.A.; Kelly, M.J.; Rønnekleiv, O.K. Distribution, Neuronal Colocalization, and 17β-E2 Modulation of Small Conductance Calcium-Activated K+ Channel (SK3) mRNA in the Guinea Pig Brain. Endocrinology 2002, 143, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.J.; Horjales-Araujo, E.; Broberger, C. Synchronized Network Oscillations in Rat Tuberoinfundibular Dopamine Neurons: Switch to Tonic Discharge by Thyrotropin-Releasing Hormone. Neuron 2010, 65, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine Receptors: From Structure to Function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Romanelli, R.J.; Williams, J.T.; Neve, K.A. Dopamine Receptor Signaling: Intracellular Pathways to Behavior. In The Dopamine Receptors; Neve, K.A., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 137–173. [Google Scholar]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Ford, C.P. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 2014, 282, 13–22. [Google Scholar] [CrossRef]
- Demarest, K.T.; Moore, K.E. Comparison of dopamine synthesis regulation in the terminals of nigrostriatal, mesolimbic, tuberoinfundibular and tuberohypophyseal neurons. J. Neural Transm. 1979, 46, 263–277. [Google Scholar] [CrossRef]
- Anden, N.E.; Grabowska-Anden, M.; Liljenberg, B. On the presence of autoreceptors on dopamine neurons in different brain regions. J. Neural Transm. 1983, 57, 129–137. [Google Scholar] [CrossRef]
- Anden, N.E.; Grabowska-Anden, M.; Liljenberg, B. Demonstration of autoreceptors on dopamine neurons in different brain regions of rats treated with gammabutyrolactone. J. Neural Transm. 1983, 58, 143–152. [Google Scholar] [CrossRef]
- Timmerman, W.; Deinum, M.E.; Westerink, B.H.C.; Schuiling, G.A. Lack of evidence for dopamine autoreceptors in the mediobasal hypothalamus: A microdialysis study in awake rats. Neurosci. Lett. 1995, 195, 113–116. [Google Scholar] [CrossRef]
- Stagkourakis, S.; Kim, H.; Lyons, D.J.; Broberger, C. Dopamine Autoreceptor Regulation of a Hypothalamic Dopaminergic Network. Cell Rep. 2016, 15, 735–747. [Google Scholar] [CrossRef]
- Gonzalez, H.A.; Porter, J.C. Mass and in situ activity of tyrosine hydroxylase in the median eminence: Effect of hyperprolactinemia. Endocrinology 1988, 122, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.J.; Hellysaz, A.; Broberger, C. Prolactin Regulates Tuberoinfundibular Dopamine Neuron Discharge Pattern: Novel Feedback Control Mechanisms in the Lactotrophic Axis. J. Neurosci. 2012, 32, 8074–8083. [Google Scholar] [CrossRef] [PubMed]
- Shull, J.D.; Gorski, J. Estrogen Regulation of Prolactin Gene Transcription in Vivo: Paradoxical Effects of 17β-Estradiol Dose. Endocrinology 1989, 124, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Shull, J.D.; Gorski, J. Estrogen Stimulates Prolactin Gene Transcription by a Mechanism Independent of Pituitary Protein Synthesis. Endocrinology 1984, 114, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Toney, T.W.; Pawsat, D.E.; Fleckenstein, A.E.; Lookingland, K.J.; Moore, K.E. Evidence That Prolactin Mediates the Stimulatory Effects of Estrogen on Tuberoinfundibular Dopamine Neurons in Female Rats. Neuroendocrinology 1992, 55, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-T.; Kow, L.-M.; Pfaff, D.W. Single-Unit Activity of Hypothalamic Arcuate Neurons in Brain Tissue Slices. Neuroendocrinology 1986, 43, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.J.; Moore, K.E.; Lookingland, K.J. Sexual differences in receptor-mediated regulation of tuberoinfundibular dopaminergic neurons in the rat. Brain Res. 1993, 611, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Wagner, E.J.; LaVigne, S.D.; Lookingland, K.J.; Moore, K.E. Sexual Differences in Kappa Opioid Receptor-Mediated Regulation of Tuberoinfundibular Dopaminergic Neurons. Neuroendocrinology 1992, 55, 301–307. [Google Scholar] [CrossRef]
- Toney, T.W.; Manzanares, J.; Moore, K.E.; Lookingland, K.J. Sexual differences in the stimulatory effects of bombesin on tuberoinfundibular dopaminergic neurons. Brain Res. 1992, 598, 279–285. [Google Scholar] [CrossRef]
- Toney, T.W.; Lookingland, K.J.; Moore, K.E. Role of testosterone in the regulation of tuberoinfundibular dopaminergic neurons in the male rat. Neuroendocrinology 1991, 54, 23–29. [Google Scholar] [CrossRef]
- Lookingland, K.J.; Gunnet, J.W.; Toney, T.W.; Moore, K.E. Comparison of the effects of ether and restraint stress on the activity of tuberoinfundibular dopaminergic neurons in female and male rats. Neuroendocrinology 1990, 52, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, B.M.; Davis, M.; Pankevich, D.E. (Eds.) Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System: Workshop Summary; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Pohl, C.R.; Smith, M.S.; Plant, T.M. Studies of the role of the N-methyl-D-aspartate (NMDA) receptor in the hypothalamic control of prolactin secretion. Life Sci. 1992, 50, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.J.; Moore, K.E.; Lookingland, K.J. Non-NMDA receptor-mediated regulation of hypothalamic dopaminergic neurons in the rat. Eur. J. Pharmacol. 1994, 254, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.J.; Moore, K.E.; Lookingland, K.J. Neurochemical evidence that AMPA receptor-mediated tonic inhibition of hypothalamic dopaminergic neurons occurs via activation of inhibitory interneurons. Brain Res. 1994, 660, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Kantamneni, S. Cross-talk and regulation between glutamate and GABAB receptors. Front. Cell. Neurosci. 2015, 9, 135. [Google Scholar] [CrossRef]
- Schimchowitsch, S.; Vuillez, P.; Tappaz, M.L.; Klein, M.J.; Stoeckel, M.E. Systematic presence of GABA-immunoreactivity in the tubero-infundibular and tubero-hypophyseal dopaminergic axonal systems: An ultrastructural immunogold study on several mammals. Exp. Brain Res. 1991, 83, 575–586. [Google Scholar] [CrossRef]
- Zhang, X.; Van Den Pol, A.N. Dopamine/Tyrosine Hydroxylase Neurons of the Hypothalamic Arcuate Nucleus Release GABA, Communicate with Dopaminergic and Other Arcuate Neurons, and Respond to Dynorphin, Met-Enkephalin, and Oxytocin. J. Neurosci. 2015, 35, 14966–14982. [Google Scholar] [CrossRef]
- Anderson, R.; Mitchell, R. Effects of GABA receptor agonists on [3H]dopamine release from median eminence and pituitary neurointermediate lobe. Eur. J. Pharmacol. 1985, 115, 109–112. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Pan, J.-T. Involvement of central GABAergic neurons in basal and diurnal changes of tuberoinfundibular dopaminergic neuronal activity and prolactin secretion. Life Sci. 2001, 68, 1965–1975. [Google Scholar] [CrossRef]
- Wagner, E.J.; Goudreau, J.L.; Moore, K.E.; Lookingland, K.J. GABAergic regulation of tuberoinfundibular dopaminergic neurons in the male rat. Brain Res. 1994, 659, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ammari, R.; Broberger, C. Pre- and post-synaptic modulation by GABA(B) receptors of rat neuroendocrine dopamine neurones. J. Neuroendocr. 2020, 32, e12881. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.G.; Convey, E.M. TRH-Stimulation of Prolactin Release from Bovine Pituitary Cells. Exp. Biol. Med. 1975, 149, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Manaker, S.; Winokur, A.; Rostene, W.; Rainbow, T. Autoradiographic localization of thyrotropin-releasing hormone receptors in the rat central nervous system. J. Neurosci. 1985, 5, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Schafer, M.K.H.; O’Donnell, D.; Walker, P.; Bauer, K. Expression of thyrotropin-releasing hormone receptor 2 (TRH-R2) in the central nervous system of rats. J. Comp. Neurol. 2000, 428, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Dudas, B.; Merchenthaler, I. Thyrotropin-releasing hormone axonal varicosities appear to innervate dopaminergic neurons in the human hypothalamus. Brain Struct. Funct. 2020, 225, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Ikegami, H.; Jikihara, H.; Koike, K.; Masumoto, N.; Kasahara, K.; Tasaka, K.; Hirota, K.; Miyake, A.; Tanizawa, O. Effects of thyrotropin-releasing hormone and phorbol ester on dopamine release from dispersed rat tuberoinfundibular dopaminergic neurons. Peptides 1993, 14, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, H.; Jikihara, H.; Koike, K.; Morishige, K.; Kurachi, H.; Yamamoto, N.; Hirota, K.; Miyake, A.; Tanizawa, O. Intraventricular administration of thyrotrophin-releasing hormone (TRH) suppresses prolactin secretion and synthesis: A possible involvement of dopamine release by TRH from rat hypothalamus. J. Endocrinol. 1992, 133, 59–66. [Google Scholar] [CrossRef]
- Yuan, Z.F.; Yang, S.-C.; Pan, J.-T. Effects of prolactin-releasing peptide on tuberoinfundibular dopaminergic neuronal activity and prolactin secretion in estrogen-treated female rats. J. Biomed Sci. 2002, 9, 112–118. [Google Scholar] [CrossRef]
- Meites, J.; Bruni, J.F.; Van Vugt, D.A.; Smith, A.F. Relation of endogenous opioid peptides and morphine to neuroendocrine functions. Life Sci. 1979, 24, 1325–1336. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Harms, P.G.; McCann, S.M. Possible Role of Cyclic AMP and Prostaglandin E in the Dopaminergic Control of Prolactin Release. Endocrinology 1974, 95, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Panerai, A.E.; Casanueva, F.; Martini, A.; Mantegazza, P.; Di Giulio, A.M. Opiates act centrally on GH and PRL Release. Endocrinology 1981, 108, 2400–2402. [Google Scholar] [CrossRef] [PubMed]
- Rivier, C.; Vale, W.; Ling, N.; Brown, M.; Guillemin, R. Stimulation in vivo of the secretion of prolactin and growth hormone by β-endorphin. Endocrinology 1977, 100, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, J.B.; Koenig, J.I.; Krulich, L.; Moss, R.L. Site of action for β-endorphin-induced changes in plasma luteinizing hormone and prolactin in the ovariectomized rat. Life Sci. 1984, 34, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, D.A.; Meites, J. Influence of endogenous opiates on anterior pituitary function. Fed. Proc. 1980, 39, 2533–2538. [Google Scholar] [PubMed]
- Deyo, S.N.; Swift, R.M.; Miller, R.J.; Fang, V.S. Development of Tolerance to the Prolactin-Releasing Action of Morphine and Its Modulation by Hypothalamic Dopamine. Endocrinology 1980, 106, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Grandison, L.; Guidotti, A. Regulation of prolactin release by endogenous opiates. Nature 1977, 270, 357–359. [Google Scholar] [CrossRef]
- Gudelsky, G.A.; Porter, J.C. Morphine- and opioid peptide-induced inhibition of the release of dopamine from tuberoinfundibular neurons. Life Sci. 1979, 25, 1697–1702. [Google Scholar] [CrossRef]
- Haskins, J.T.; Gudelsky, G.A.; Moss, R.L.; Porter, J.C. Iontophoresis of Morphine into the Arcuate Nucleus: Effects on Dopamine Concentrations in Hypophysial Portal Plasma and Serum Prolactin Concentrations. Endocrinology 1981, 108, 767–771. [Google Scholar] [CrossRef]
- Manzanares, J.; Wagner, E.J.; Lookingland, K.J.; Moore, K.E. Effects of immunoneutralization of dynorphin1–17 and dynorphin1–18 on the activity of central dopaminergic neurons in the male rat. Brain Res. 1992, 587, 301–305. [Google Scholar] [CrossRef]
- Loose, M.D.; Kelly, M.J. Opioid inhibition of spontaneously active neurons of the rat arcuate nucleus in vitro. Brain Res. Bull. 1989, 22, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Loose, M.D.; Kelly, M.J. Opioids act at μ-receptors to hyperpolarize arcuate neurons via an inwardly rectifying potassium conductance. Brain Res. 1990, 513, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.J.; Rønnekleiv, O.K.; Grandy, D.K.; Kelly, M.J. The Peptide Orphanin FQ Inhibits β-Endorphin Neurons and Neurosecretory Cells in the Hypothalamic Arcuate Nucleus by Activating an Inwardly-Rectifying K+ Conductance. Neuroendocrinology 1998, 67, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Lennard, D.E. The Hypophysiotropic Neurotensin-Immunoreactive Neuronal System of the Rat Brain. Endocrinology 1991, 129, 2875–2880. [Google Scholar] [CrossRef] [PubMed]
- Rostène, W.H.; Alexander, M.J. Neurotensin and Neuroendocrine Regulation. Front. Neuroendocrinol. 1997, 18, 115–173. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-T.; Tian, Y.; Lookingland, K.J.; Moore, K.E. Neurotensin-induced activation of hypothalamic dopaminergic neurons is accompanied by a decrease in pituitary secretion of prolactin and α-melanocyte-stimulating hormone. Life Sci. 1992, 50, 2011–2017. [Google Scholar] [CrossRef]
- Memo, M.; Castelletti, L.; Valerio, A.; Pizzi, M.; Missale, C.; Carruba, M.O.; Spano, P.F. Cellular mechanisms for neurotensin receptor-mediated release of prolactin. Regul. Pept. 1985, 10, 203–208. [Google Scholar] [CrossRef]
- Canonico, P.L.; Sortino, M.A.; Speciale, C.; Scapagnini, U. Neurotensin stimulates polyphosphoinositide breakdown and prolactin release in anterior pituitary cells in culture. Mol. Cell. Endocrinol. 1985, 42, 215–220. [Google Scholar] [CrossRef]
- Ciofi, P.; Crowley, W.R.; Pillez, A.; Schmued, L.L.; Tramu, G.; Mazzuca, M. Plasticity in Expression of Immunoreactivity for Neuropeptide Y, Enkephalins and Neurotensin in the Hypothalamic Tubero-lnfundibular Dopaminergic System during Lactation in Mice. J. Neuroendocrinol. 1993, 5, 599–602. [Google Scholar] [CrossRef]
- McDonald, T.J.; Jörnvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.R.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Anastasi, A.; Erspamer, V.; Bucci, M. Isolation and amino acid sequences of alytesin and bombesin, two analogous active tetradecapeptides from the skin of European discoglossid frogs. Arch. Biochem. Biophys. 1972, 148, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Hakim, E.; El-Azouzi, M.; Black, P.M. The effect of prolactin and bombesin on the growth of meningioma-derived cells in monolayer culture. J. Neuro-Oncol. 1993, 16, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Bjøro, T.; Torjesen, P.A.; Østberg, B.C.; Sand, O.; Iversen, J.G.; Gautvik, K.M.; Haug, E. Bombesin stimulates prolactin secretion from cultured rat pituitary tumour cells (GH4C1) via activation of phospholipase C. Regul. Pept. 1987, 19, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Toney, T.W.; Lookingland, K.J.; Moore, K.E. Activation of tuberoinfundibular and tuberohypophysial dopamine neurons following intracerebroventricular administration of bombesin. Brain Res. 1991, 565, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.F.; Keller, B.K. Galanin Secretion from Anterior Pituitary Cells In Vitro Is Regulated by Dopamine, Somatostatin, and Thyrotropin-Releasing Hormone. Endocrinology 1991, 128, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, C.; Tian, Y.; Moore, K.E.; Lookingland, K.J. Neurochemical Evidence that the Inhibitory Effect of Galanin on Tuberoinfundibular Dopamine Neurons Is Activity Dependent. Neuroendocrinology 1993, 58, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Selmanoff, M.; Gregerson, K.A. Suckling-Induced Prolactin Release is Suppressed by Naloxone and Simulated by β-Endorphin. Neuroendocrinology 1986, 42, 255–259. [Google Scholar] [CrossRef]
- Callahan, P.; Baumann, M.H.; Rabii, J. Inhibition of Tuberoinfundibular Dopaminergic Neural Activity During Suckling: Involvement of μ and κ Opiate Receptor Subtypes. J. Neuroendocrinol. 1996, 8, 771–776. [Google Scholar] [CrossRef]
- Voogt, J.L.; Lee, Y.; Yang, S.; Arbogast, L. Chapter 12 Regulation of prolactin secretion during pregnancy and lactation. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2001; Volume 133, pp. 173–185. [Google Scholar]
- Meza, E.; Waliszewski, S.M.; Caba, M. Circadian nursing induces PER1 protein in neuroendocrine tyrosine hydroxylase neurones in the rabbit doe. J. Neuroendocrinol. 2011, 23, 472–480. [Google Scholar] [CrossRef]
- Lumpkin, M.D.; Samson, W.K.; McCann, S.M. Hypothalamic and Pituitary Sites of Action of Oxytocin to Alter Prolactin Secretion in the Rat. Endocrinology 1983, 112, 1711–1717. [Google Scholar] [CrossRef]
- Samson, W.K.; Lumpkin, M.D.; McCann, S.M. Evidence for a Physiological Role for Oxytocin in the Control of Prolactin Secretion. Endocrinology 1986, 119, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Arey, B.J.; Freeman, M.E. Hypothalamic Factors Involved in the Endogenous Stimulatory Rhythm Regulating Prolactin Secretion. Endocrinology 1989, 124, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Briffaud, V.; Williams, P.; Courty, J.; Broberger, C. Excitation of Tuberoinfundibular Dopamine Neurons by Oxytocin: Crosstalk in the Control of Lactation. J. Neurosci. 2015, 35, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Romanò, N.; Yip, S.H.; Hodson, D.J.; Guillou, A.; Parnaudeau, S.; Kirk, S.; Tronche, F.; Bonnefont, X.; Le Tissier, P.; Bunn, S.J.; et al. Plasticity of Hypothalamic Dopamine Neurons during Lactation Results in Dissociation of Electrical Activity and Release. J. Neurosci. 2013, 33, 4424–4433. [Google Scholar] [CrossRef]
- Zhang, X.; Van Den Pol, A.N. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat. Neurosci. 2016, 19, 1341–1347. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi-Lytle, X.; Sayers, S.; Wagner, E.J. Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons. Int. J. Mol. Sci. 2024, 25, 110. https://doi.org/10.3390/ijms25010110
Qi-Lytle X, Sayers S, Wagner EJ. Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons. International Journal of Molecular Sciences. 2024; 25(1):110. https://doi.org/10.3390/ijms25010110
Chicago/Turabian StyleQi-Lytle, Xiaojun, Sarah Sayers, and Edward J. Wagner. 2024. "Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons" International Journal of Molecular Sciences 25, no. 1: 110. https://doi.org/10.3390/ijms25010110
APA StyleQi-Lytle, X., Sayers, S., & Wagner, E. J. (2024). Current Review of the Function and Regulation of Tuberoinfundibular Dopamine Neurons. International Journal of Molecular Sciences, 25(1), 110. https://doi.org/10.3390/ijms25010110




