Mechanism of ADP-Inhibited ATP Hydrolysis in Single Proton-Pumping FoF1-ATP Synthase Trapped in Solution
Abstract
1. Introduction
2. Results
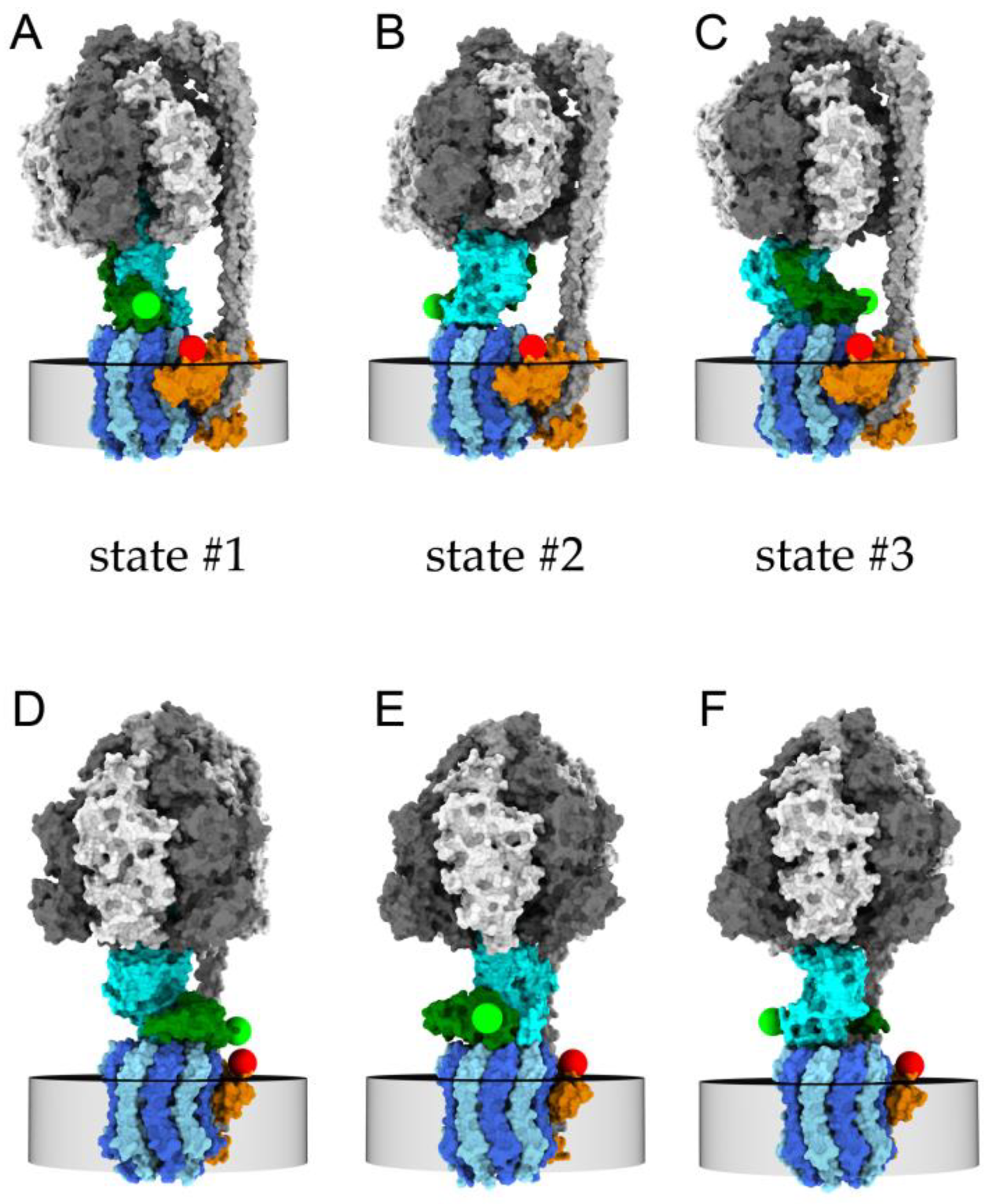
2.1. Monitoring Catalysis-Related ε-Subunit Rotation by Single-Molecule FRET
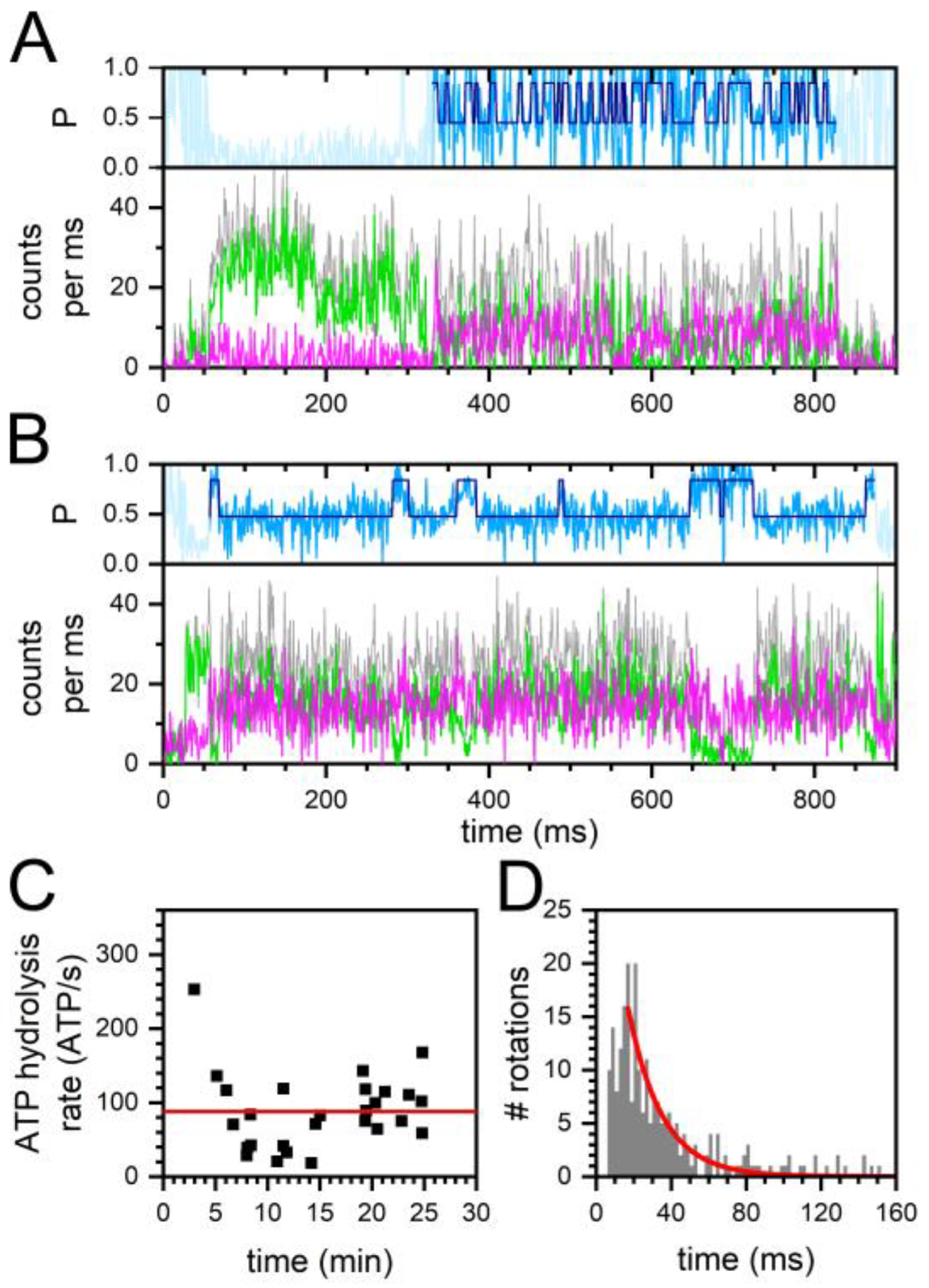
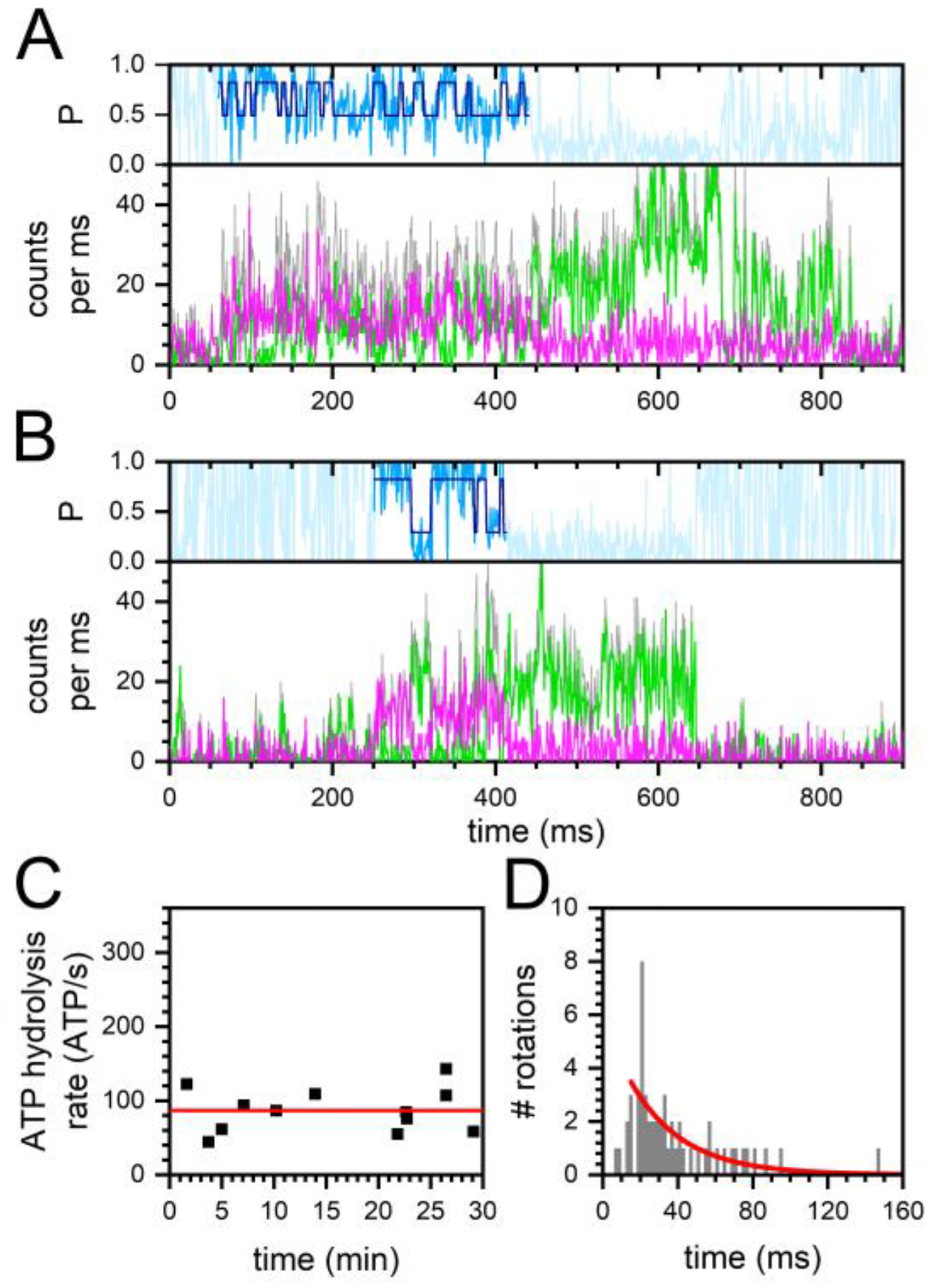
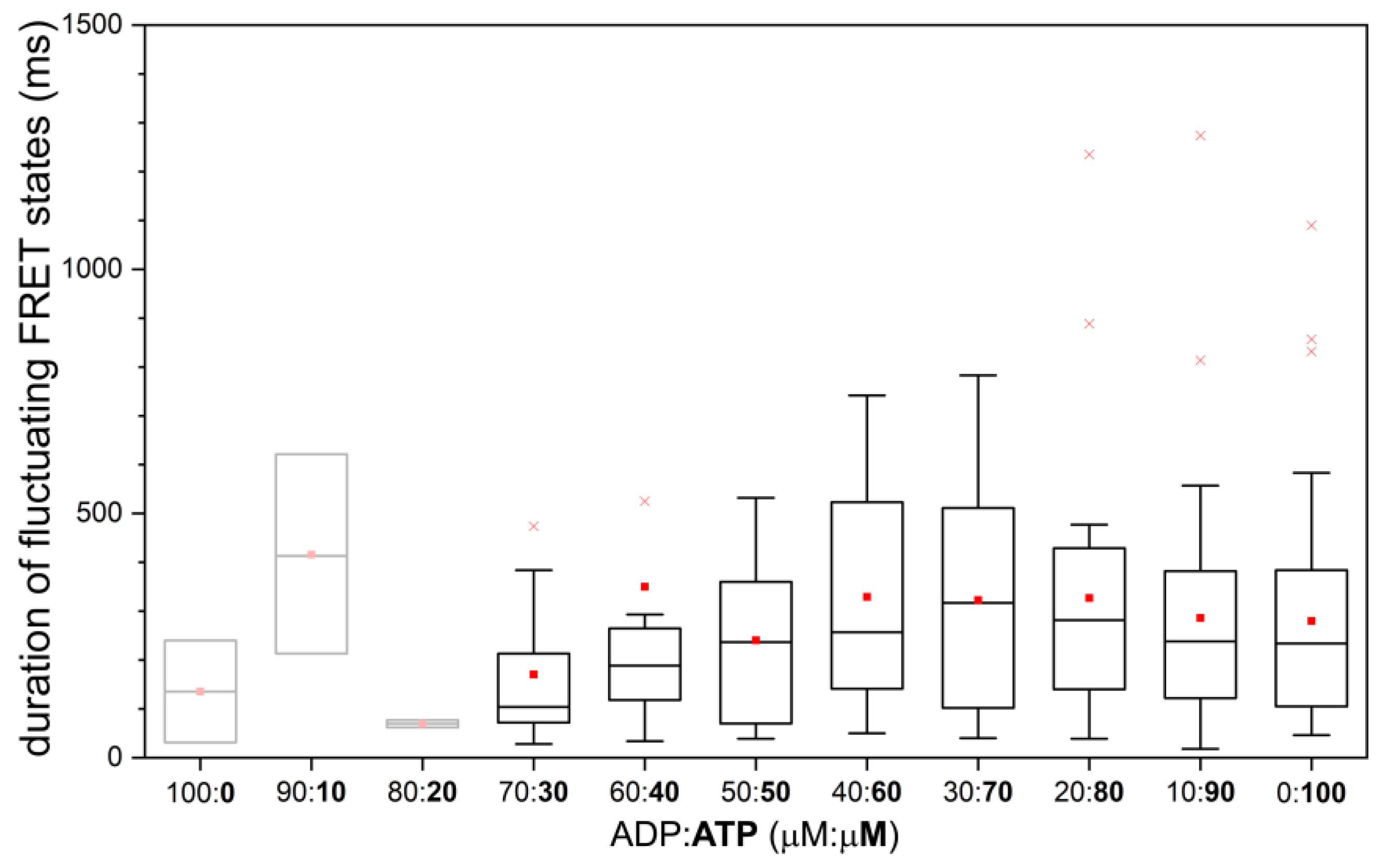
2.2. Constant and Fluctuating FRET States at Different ADP/ATP Ratios
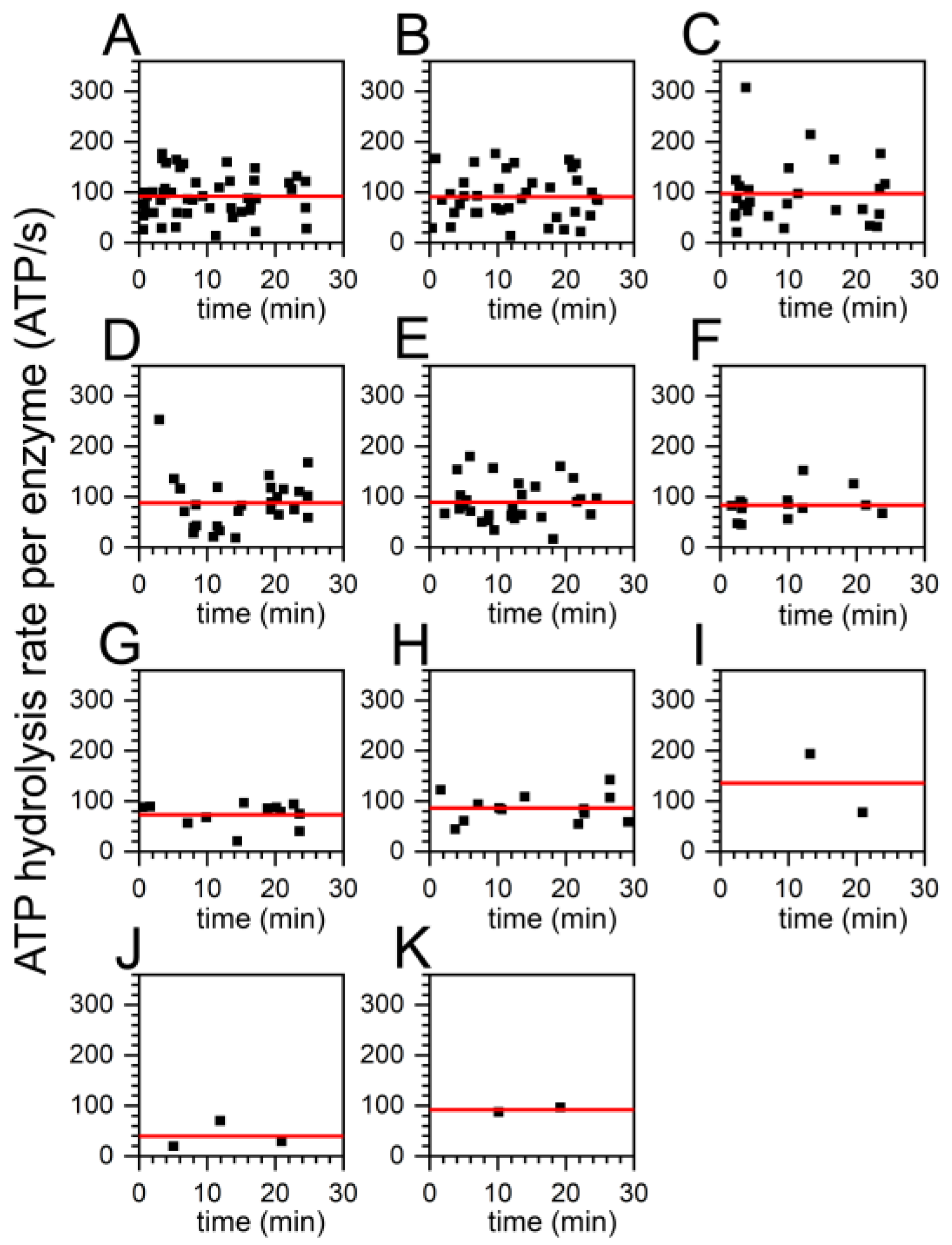
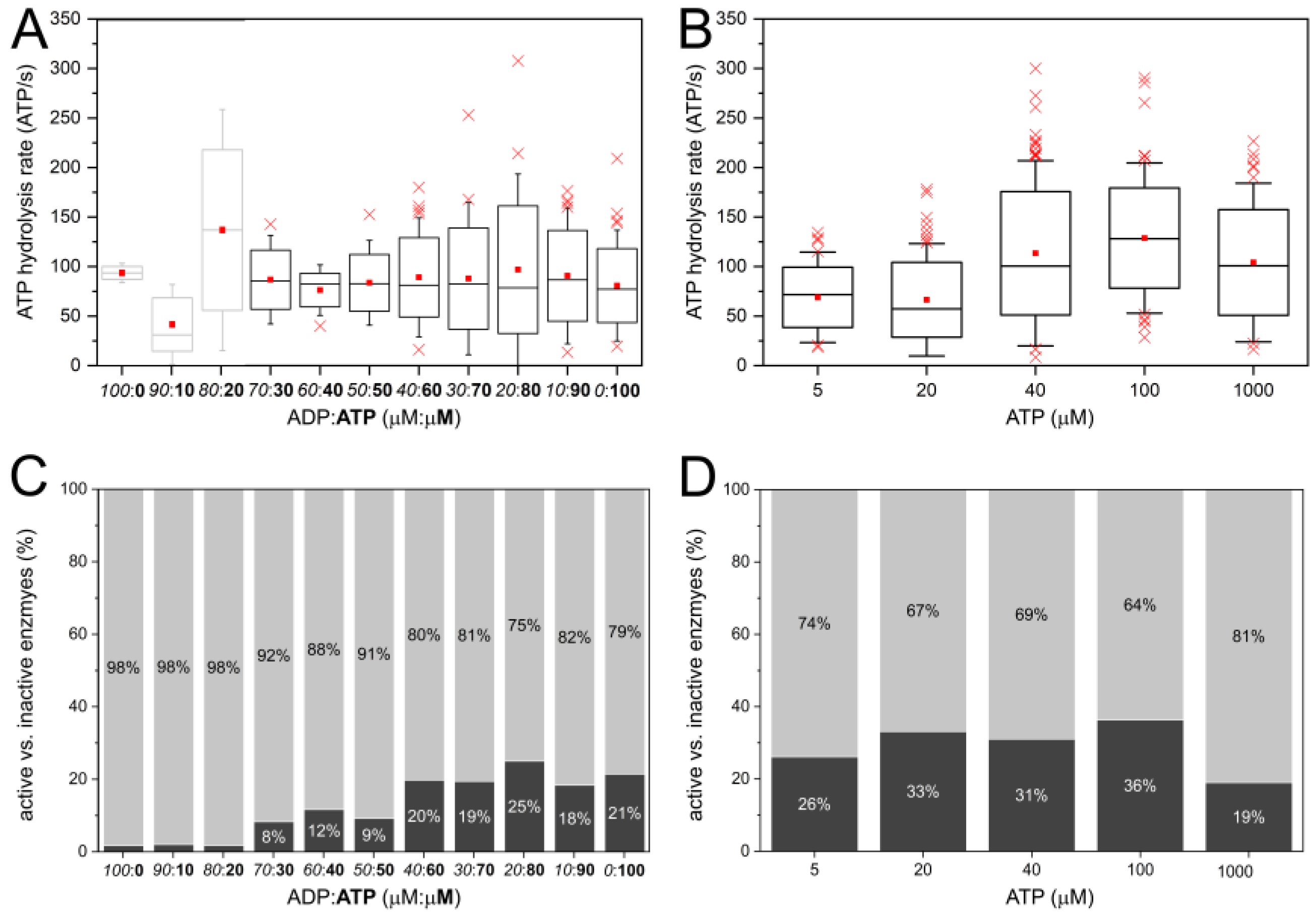
2.3. Similar ATP Turnover Found for All ADP/ATP Ratios
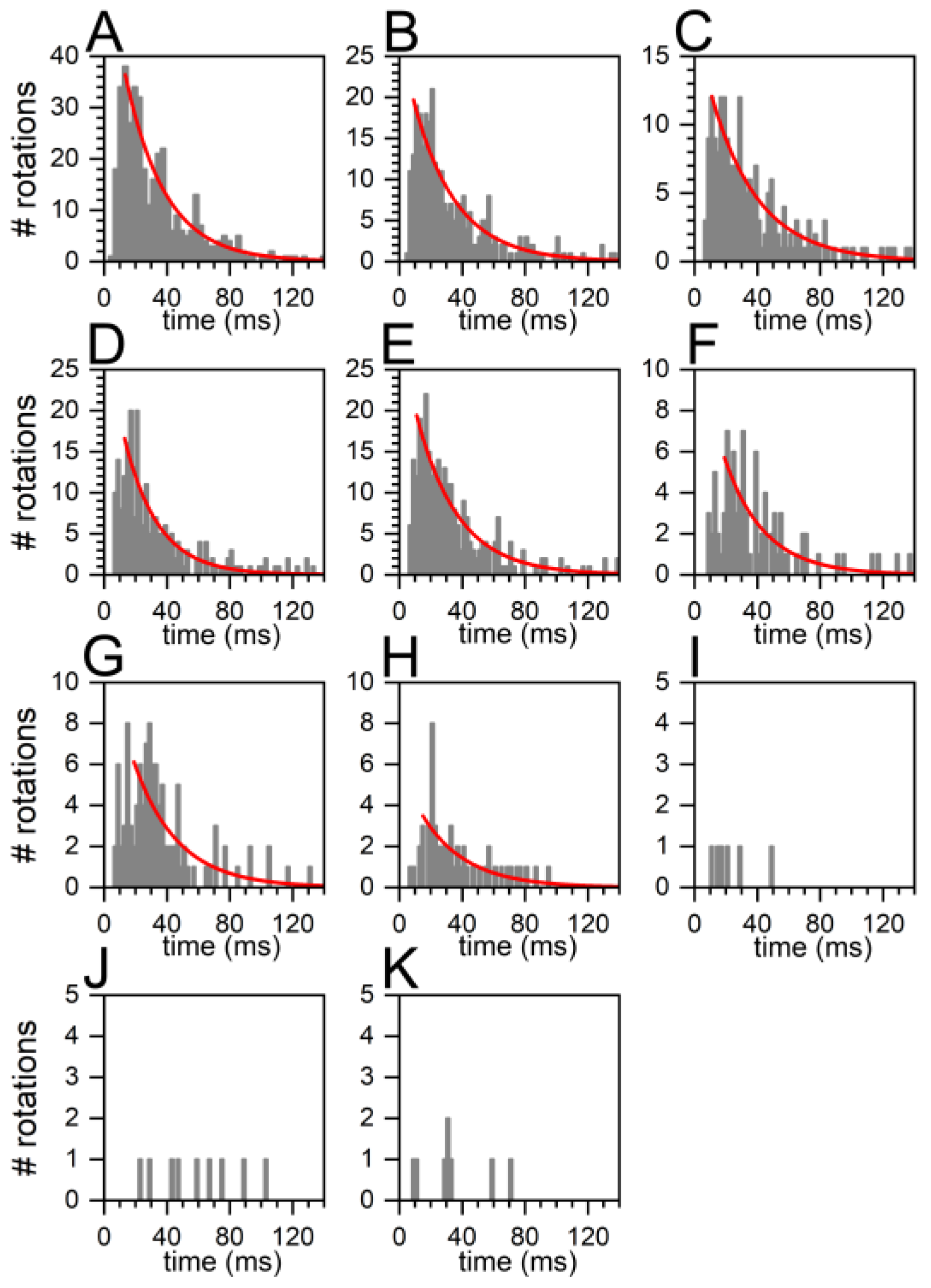
2.4. Similar Distributions of the FRET State Fluctuation Periods for All ADP/ATP Ratios
2.5. Decreasing Percentage of Active FoF1-ATP Synthases with Higher ADP Content
3. Discussion
4. Materials and Methods
4.1. FRET-Labeled FoF1-ATP Synthase from E. coli
4.2. Recording of FRET-Labeled FoF1-ATP Synthase in a Confocal ABEL Trap
4.3. Data Analysis of FRET-Labeled FoF1-ATP Synthase Held by the ABEL Trap
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junge, W.; Nelson, N. ATP Synthase. Annu. Rev. Biochem 2015, 84, 631–657. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Structure and Mechanisms of F-Type ATP Synthases. Annu. Rev. Biochem. 2019, 88, 515–549. [Google Scholar] [CrossRef] [PubMed]
- Boyer, P.D. The ATP synthase—A splendid molecular machine. Annu. Rev. Biochem. 1997, 66, 717–749. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Senior, A.E. ATP synthase: What we know about ATP hydrolysis and what we do not know about ATP synthesis. Biochim. Biophys. Acta 2000, 1458, 300–309. [Google Scholar] [CrossRef]
- Zhou, Y.; Duncan, T.M.; Cross, R.L. Subunit rotation in Escherichia coli FoF1-ATP synthase during oxidative phosphorylation. Proc. Natl. Acad. Sci. USA 1997, 94, 10583–10587. [Google Scholar] [CrossRef]
- Elston, T.; Wang, H.; Oster, G. Energy transduction in ATP synthase. Nature 1998, 391, 510–513. [Google Scholar] [CrossRef]
- Sambongi, Y.; Iko, Y.; Tanabe, M.; Omote, H.; Iwamoto-Kihara, A.; Ueda, I.; Yanagida, T.; Wada, Y.; Futai, M. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): Direct observation. Science 1999, 286, 1722–1724. [Google Scholar] [CrossRef]
- Pänke, O.; Gumbiowski, K.; Junge, W.; Engelbrecht, S. F-ATPase: Specific observation of the rotating c subunit oligomer of EFoEF1. FEBS Lett. 2000, 472, 34–38. [Google Scholar] [CrossRef]
- Steigmiller, S.; Turina, P.; Gräber, P. The thermodynamic H+/ATP ratios of the H+-ATP synthases from chloroplasts and Escherichia coli. Proc. Natl. Acad. Sci. USA 2008, 105, 3745–3750. [Google Scholar] [CrossRef]
- Noji, H.; Yasuda, R.; Yoshida, M.; Kinosita, K., Jr. Direct observation of the rotation of F1-ATPase. Nature 1997, 386, 299–302. [Google Scholar] [CrossRef]
- Abrahams, J.P.; Leslie, A.G.; Lutter, R.; Walker, J.E. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 1994, 370, 621–628. [Google Scholar] [CrossRef]
- Duncan, T.M.; Bulygin, V.V.; Zhou, Y.; Hutcheon, M.L.; Cross, R.L. Rotation of subunits during catalysis by Escherichia coli F1-ATPase. Proc. Natl. Acad. Sci. USA 1995, 92, 10964–10968. [Google Scholar] [CrossRef]
- Sabbert, D.; Engelbrecht, S.; Junge, W. Intersubunit rotation in active F-ATPase. Nature 1996, 381, 623–625. [Google Scholar] [CrossRef]
- Yoshida, M.; Muneyuki, E.; Hisabori, T. ATP synthase—A marvellous rotary engine of the cell. Nat. Rev. Mol. Cell Biol. 2001, 2, 669–677. [Google Scholar] [CrossRef]
- Noji, H.; Ueno, H. How Does F1-ATPase Generate Torque?: Analysis From Cryo-Electron Microscopy and Rotational Catalysis of Thermophilic F1. Front. Microbiol. 2022, 13, 904084. [Google Scholar] [CrossRef]
- Frasch, W.D.; Bukhari, Z.A.; Yanagisawa, S. F1FO ATP synthase molecular motor mechanisms. Front. Microbiol. 2022, 13, 965620. [Google Scholar] [CrossRef]
- Nakanishi-Matsui, M.; Sekiya, M.; Futai, M. ATP synthase from Escherichia coli: Mechanism of rotational catalysis, and inhibition with the ε subunit and phytopolyphenols. Biochim. Biophys. Acta 2016, 1857, 129–140. [Google Scholar] [CrossRef]
- Börsch, M.; Diez, M.; Zimmermann, B.; Reuter, R.; Gräber, P. Stepwise rotation of the γ-subunit of EF0F1-ATP synthase observed by intramolecular single-molecule fluorescence resonance energy transfer. FEBS Lett. 2002, 527, 147–152. [Google Scholar] [CrossRef]
- Diez, M.; Zimmermann, B.; Börsch, M.; König, M.; Schweinberger, E.; Steigmiller, S.; Reuter, R.; Felekyan, S.; Kudryavtsev, V.; Seidel, C.A.; et al. Proton-powered subunit rotation in single membrane-bound FoF1-ATP synthase. Nat. Struct. Mol. Biol. 2004, 11, 135–141. [Google Scholar] [CrossRef]
- Zimmermann, B.; Diez, M.; Zarrabi, N.; Gräber, P.; Börsch, M. Movements of the ε-subunit during catalysis and activation in single membrane-bound H+-ATP synthase. EMBO J. 2005, 24, 2053–2063. [Google Scholar] [CrossRef]
- Düser, M.G.; Zarrabi, N.; Cipriano, D.J.; Ernst, S.; Glick, G.D.; Dunn, S.D.; Börsch, M. 36 degrees step size of proton-driven c-ring rotation in FoF1-ATP synthase. EMBO J. 2009, 28, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Düser, M.G.; Zarrabi, N.; Börsch, M. Three-color Förster resonance energy transfer within single FoF1-ATP synthases: Monitoring elastic deformations of the rotary double motor in real time. J. Biomed. Opt. 2012, 17, 011004. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.E.; Moerner, W.E. The anti-Brownian electrophoretic trap (ABEL trap): Fabrication and software. Proc. SPIE 2005, 5699, 296–305. [Google Scholar]
- Cohen, A.E.; Moerner, W.E. Method for trapping and manipulating nanoscale objects in solution. Appl. Phys. Lett. 2005, 86, 093109. [Google Scholar] [CrossRef]
- Wilson, H.; Wang, Q. ABEL-FRET: Tether-free single-molecule FRET with hydrodynamic profiling. Nat. Methods 2021, 18, 816–820. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Q.; Cohen, A.E.; Douglas, N.; Frydman, J.; Moerner, W.E. Hardware-based anti-Brownian electrokinetic trap (ABEL trap) for single molecules: Control loop simulations and application to ATP binding stoichiometry in multi-subunit enzymes. Proc. SPIE 2008, 7038, 703807. [Google Scholar]
- Fields, A.P.; Cohen, A.E. Electrokinetic trapping at the one nanometer limit. Proc. Natl. Acad. Sci. USA 2011, 108, 8937–8942. [Google Scholar] [CrossRef]
- Wang, Q.; Moerner, W.E. Optimal strategy for trapping single fluorescent molecules in solution using the ABEL trap. Appl. Phys. B 2010, 99, 23–30. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shaw, A.; Wilson, H.; Woringer, M.; Darzacq, X.; Marqusee, S.; Wang, Q.; Bustamante, C. Single-molecule diffusometry reveals no catalysis-induced diffusion enhancement of alkaline phosphatase as proposed by FCS experiments. Proc. Natl. Acad. Sci. USA 2020, 117, 21328–21335. [Google Scholar] [CrossRef]
- Wang, Q.; Serban, A.J.; Wachter, R.M.; Moerner, W.E. Single-molecule diffusometry reveals the nucleotide-dependent oligomerization pathways of Nicotiana tabacum Rubisco activase. J. Chem. Phys. 2018, 148, 123319. [Google Scholar] [CrossRef]
- Wilson, H.; Lee, M.; Wang, Q. Probing DNA-protein interactions using single-molecule diffusivity contrast. Biophys. Rep. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Heitkamp, T.; Börsch, M. Fast ATP-Dependent Subunit Rotation in Reconstituted FoF1-ATP Synthase Trapped in Solution. J. Phys. Chem. B. 2021, 125, 7638–7650. [Google Scholar] [CrossRef]
- Duncan, T.M.; Düser, M.G.; Heitkamp, T.; McMillan, D.G.; Börsch, M. Regulatory conformational changes of the ε subunit in single FRET-labeled FoF1-ATP synthase. Proc. SPIE 2014, 8948, 89481J. [Google Scholar]
- Sobti, M.; Smits, C.; Wong, A.S.W.; Ishmukhametov, R.; Stock, D.; Sandin, S.; Stewart, A.G. Cryo-EM structures of the autoinhibited E. coli ATP synthase in three rotational states. eLife 2016, 5, e21598. [Google Scholar] [CrossRef]
- Sobti, M.; Walshe, J.L.; Wu, D.; Ishmukhametov, R.; Zeng, Y.C.; Robinson, C.V.; Berry, R.M.; Stewart, A.G. Cryo-EM structures provide insight into how E. coli F1Fo ATP synthase accommodates symmetry mismatch. Nat. Commun. 2020, 11, 2615. [Google Scholar] [CrossRef]
- Sobti, M.; Zeng, Y.C.; Walshe, J.L.; Brown, S.H.J.; Ishmukhametov, R.; Stewart, A.G. Changes within the central stalk of E. coli F1Fo ATP synthase observed after addition of ATP. Commun. Biol. 2023, 6, 26. [Google Scholar] [CrossRef]
- White, D.S.; Goldschen-Ohm, M.P.; Goldsmith, R.H.; Chanda, B. Top-down machine learning approach for high-throughput single-molecule analysis. eLife 2020, 9, e53357. [Google Scholar] [CrossRef]
- Xie, X.S.; Lu, H.P. Single-molecule enzymology. J. Biol. Chem. 1999, 274, 15967–15970. [Google Scholar] [CrossRef]
- Kobayashi, H.; Anraku, Y. Membrane-Bound Adenosine Triphosphatase of Escherichia coli: 1. Partial Purification and Properties. J. Biochem. 1972, 71, 387–399. [Google Scholar]
- Weber, J.; Dunn, S.D.; Senior, A.E. Effect of the ε-subunit on nucleotide binding to Escherichia coli F1-ATPase catalytic sites. J. Biol. Chem. 1999, 274, 19124–19128. [Google Scholar] [CrossRef]
- Roisin, M.P.; Kepes, A. Membrane ATPase of Escherichia coli. 1. Ion Dependence and ATP-ADP Exchange-Reaction. Biochim. Biophys. Acta 1972, 275, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Lapashina, A.S.; Shugaeva, T.E.; Berezina, K.M.; Kholina, T.D.; Feniouk, B.A. Amino Acid Residues beta139, beta189, and beta319 Modulate ADP-Inhibition in Escherichia coli H+-FOF1-ATP Synthase. Biochemistry Biokhimiia 2019, 84, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Lapashina, A.S.; Prikhodko, A.S.; Shugaeva, T.E.; Feniouk, B.A. Residue 249 in subunit beta regulates ADP inhibition and its phosphate modulation in Escherichia coli ATP synthase. Biochim. Biophys. Acta-Bioenerg. 2019, 1860, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Gräber, P.; Turina, P. The activity of the ATP synthase from Escherichia coli is regulated by the transmembrane proton motive force. J. Biol. Chem. 2000, 275, 30157–30162. [Google Scholar] [CrossRef] [PubMed]
- Noji, H.; Häsler, K.; Junge, W.; Kinosita, K., Jr.; Yoshida, M.; Engelbrecht, S. Rotation of Escherichia coli F1-ATPase. Biochem. Biophys. Res. Commun. 1999, 260, 597–599. [Google Scholar] [CrossRef]
- Sekiya, M.; Hosokawa, H.; Nakanishi-Matsui, M.; Al-Shawi, M.K.; Nakamoto, R.K.; Futai, M. Single molecule behavior of inhibited and active states of Escherichia coli ATP synthase F1 rotation. J. Biol. Chem. 2010, 285, 42058–42067. [Google Scholar] [CrossRef]
- Bilyard, T.; Nakanishi-Matsui, M.; Steel, B.C.; Pilizota, T.; Nord, A.L.; Hosokawa, H.; Futai, M.; Berry, R.M. High-resolution single-molecule characterization of the enzymatic states in Escherichia coli F1-ATPase. Philos. Trans. B R. Soc. Lond. 2013, 368, 20120023. [Google Scholar] [CrossRef]
- Cohen, A.E.; Moerner, W.E. Controlling Brownian motion of single protein molecules and single fluorophores in aqueous buffer. Opt. Express 2008, 16, 6941–6956. [Google Scholar] [CrossRef]
- Dunn, S.D.; Tozer, R.G.; Zadorozny, V.D. Activation of Escherichia coli F1-ATPase by lauryldimethylamine oxide and ethylene glycol: Relationship of ATPase activity to the interaction of the ε and β subunits. Biochemistry 1990, 29, 4335–4340. [Google Scholar] [CrossRef]
- Hyndman, D.J.; Milgrom, Y.M.; Bramhall, E.A.; Cross, R.L. Nucleotide-binding sites on Escherichia coli F1-ATPase. Specificity of noncatalytic sites and inhibition at catalytic sites by MgADP. J. Biol. Chem. 1994, 269, 28871–28877. [Google Scholar] [CrossRef]
- Milgrom, Y.M.; Duncan, T.M. F-ATP-ase of Escherichia coli membranes: The ubiquitous MgADP-inhibited state and the inhibited state induced by the ε-subunit’s C-terminal domain are mutually exclusive. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148189. [Google Scholar] [CrossRef]
- Shah, N.B.; Duncan, T.M. Aerobic growth of Escherichia coli is reduced and ATP synthesis is selectively inhibited when five C-terminal residues are deleted from the ε subunit of ATP synthase. J. Biol. Chem. 2015, 290, 21032–21041. [Google Scholar] [CrossRef]
- Shah, N.B.; Hutcheon, M.L.; Haarer, B.K.; Duncan, T.M. F1-ATPase of Escherichia coli: The ε-inhibited state forms after ATP hydrolysis, is distinct from the ADP-inhibited state, and responds dynamically to catalytic site ligands. J. Biol. Chem. 2013, 288, 9383–9395. [Google Scholar] [CrossRef]
- Cingolani, G.; Duncan, T.M. Structure of the ATP synthase catalytic complex F1 from Escherichia coli in an autoinhibited conformation. Nat. Struct. Mol. Biol. 2011, 18, 701–707. [Google Scholar] [CrossRef]
- Fischer, S.; Gräber, P. Comparison of ΔpH- and Δψ-driven ATP synthesis catalyzed by the H+-ATPases from Escherichia coli or chloroplasts reconstituted into liposomes. FEBS Lett. 1999, 457, 327–332. [Google Scholar] [CrossRef]
- Su, B.; Düser, M.G.; Zarrabi, N.; Heitkamp, T.; Starke, I.; Börsch, M. Observing conformations of single FoF1-ATP synthases in a fast anti-Brownian electrokinetic trap. Proc. SPIE 2015, 9329, 93290A. [Google Scholar]
- Dathe, A.; Heitkamp, T.; Pérez, I.; Sielaff, H.; Westphal, A.; Reuter, S.; Mrowka, R.; Börsch, M. Observing monomer—dimer transitions of neurotensin receptors 1 in single SMALPs by homoFRET and in an ABELtrap. Proc. SPIE 2019, 10884, 108840N. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, I.; Heitkamp, T.; Börsch, M. Mechanism of ADP-Inhibited ATP Hydrolysis in Single Proton-Pumping FoF1-ATP Synthase Trapped in Solution. Int. J. Mol. Sci. 2023, 24, 8442. https://doi.org/10.3390/ijms24098442
Pérez I, Heitkamp T, Börsch M. Mechanism of ADP-Inhibited ATP Hydrolysis in Single Proton-Pumping FoF1-ATP Synthase Trapped in Solution. International Journal of Molecular Sciences. 2023; 24(9):8442. https://doi.org/10.3390/ijms24098442
Chicago/Turabian StylePérez, Iván, Thomas Heitkamp, and Michael Börsch. 2023. "Mechanism of ADP-Inhibited ATP Hydrolysis in Single Proton-Pumping FoF1-ATP Synthase Trapped in Solution" International Journal of Molecular Sciences 24, no. 9: 8442. https://doi.org/10.3390/ijms24098442
APA StylePérez, I., Heitkamp, T., & Börsch, M. (2023). Mechanism of ADP-Inhibited ATP Hydrolysis in Single Proton-Pumping FoF1-ATP Synthase Trapped in Solution. International Journal of Molecular Sciences, 24(9), 8442. https://doi.org/10.3390/ijms24098442




