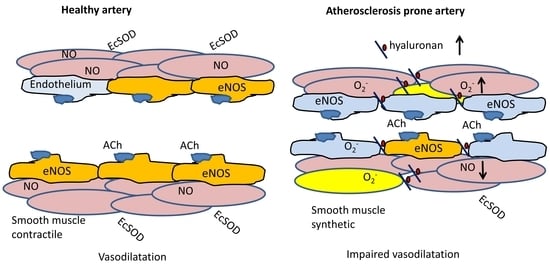
Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media
Abstract
1. Introduction
2. Results
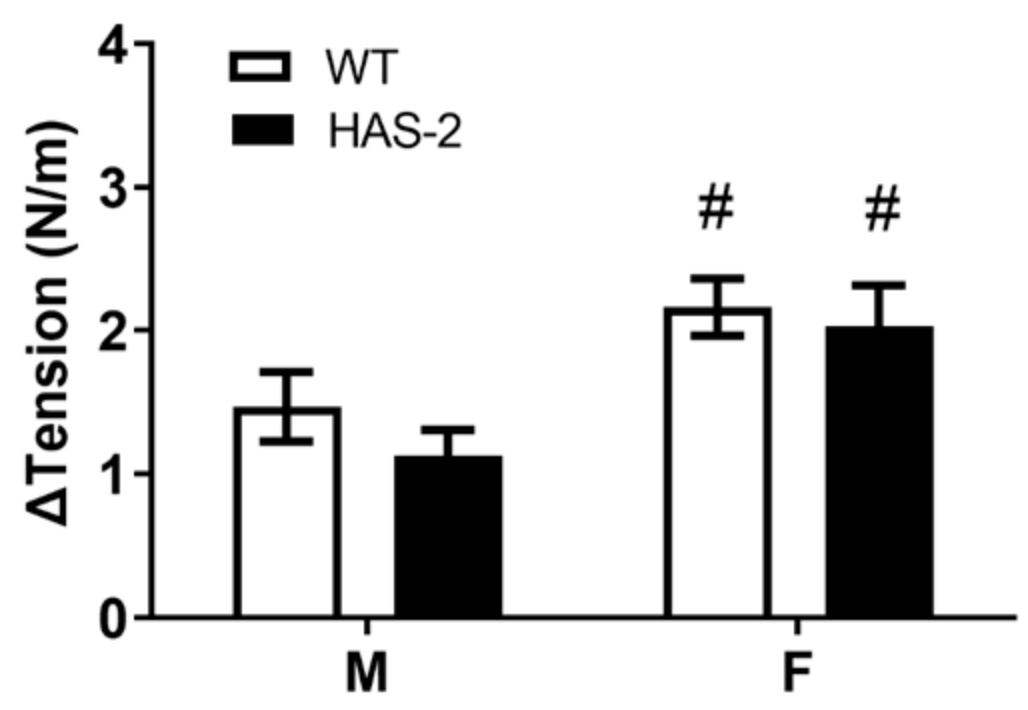
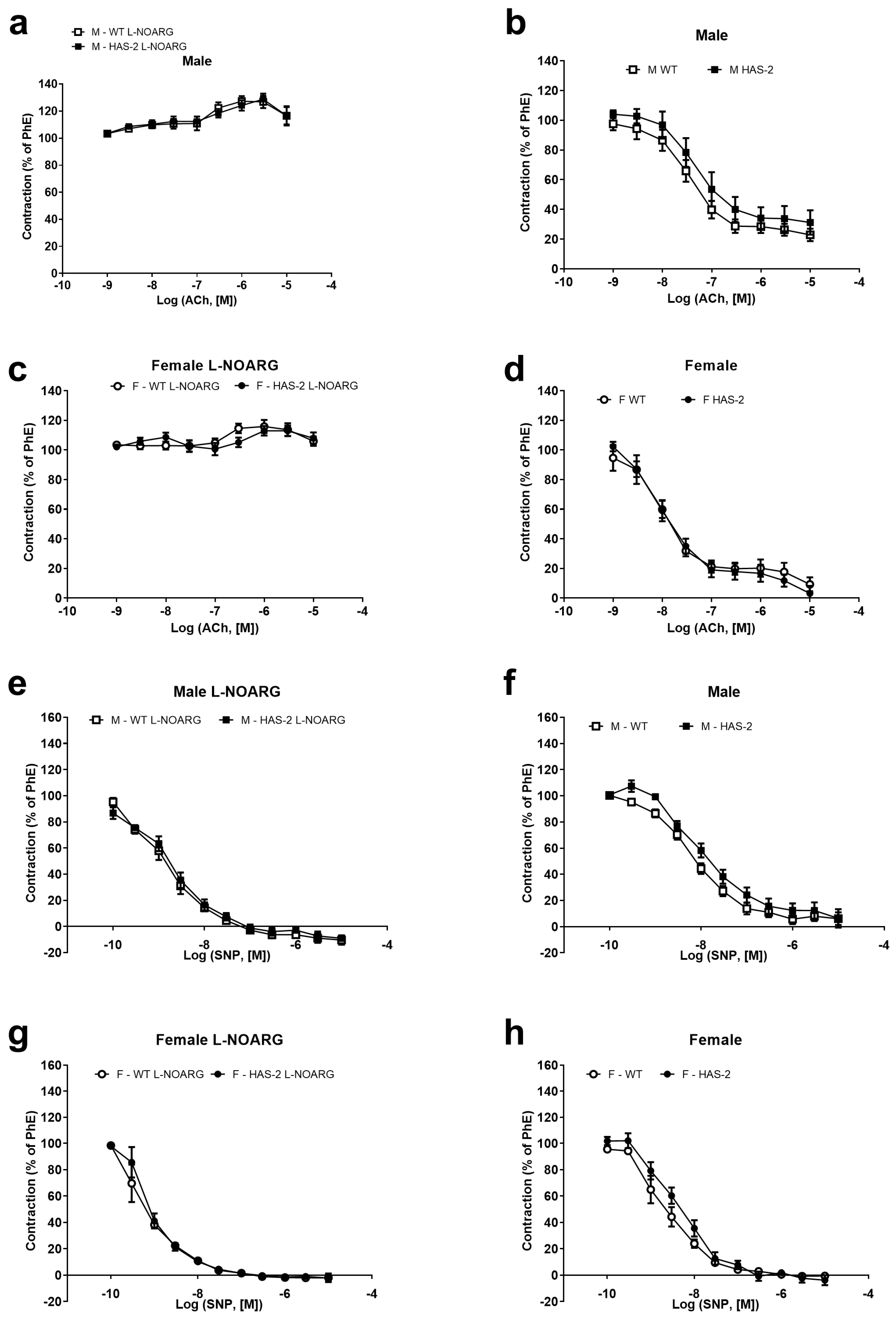
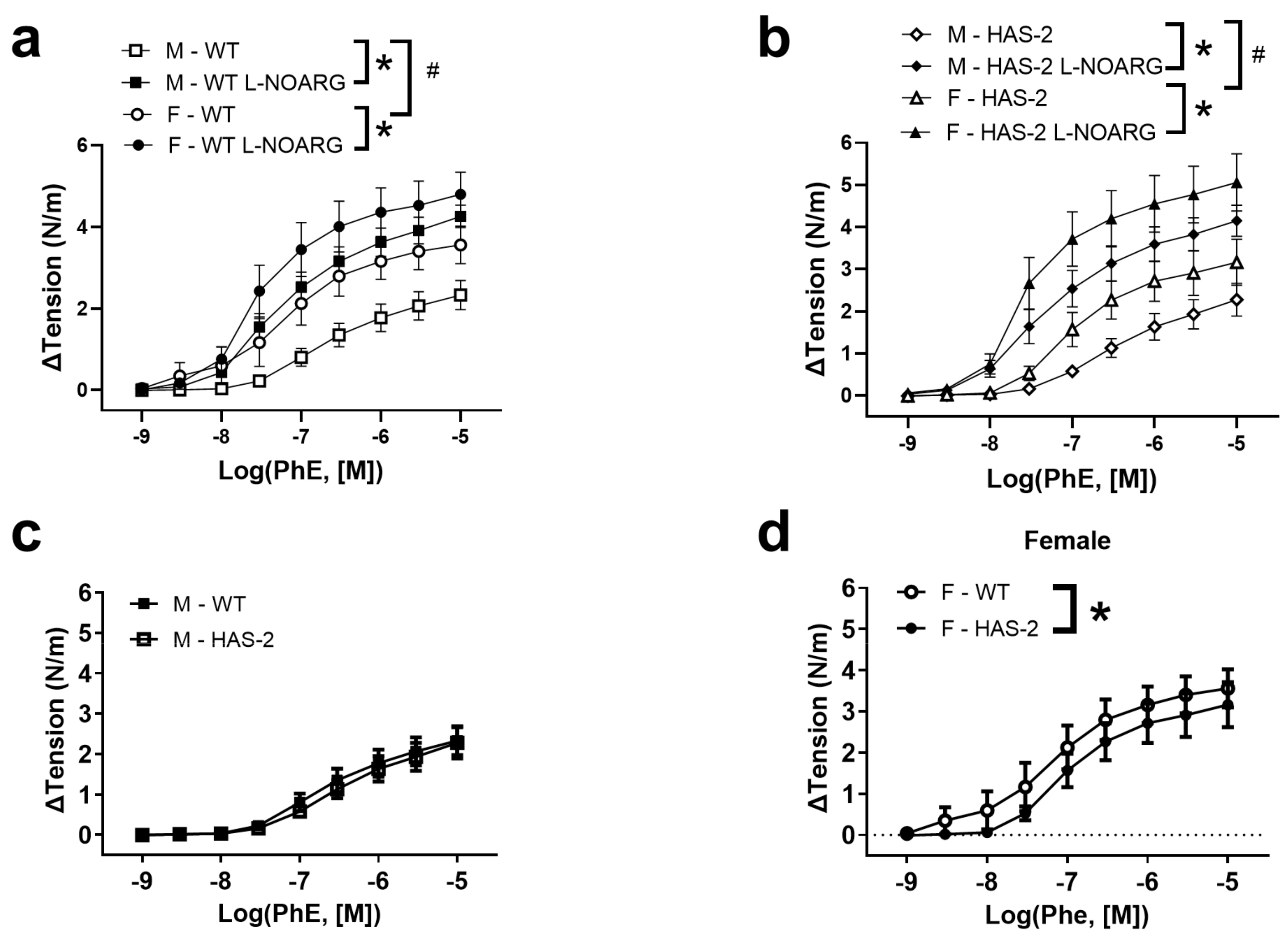
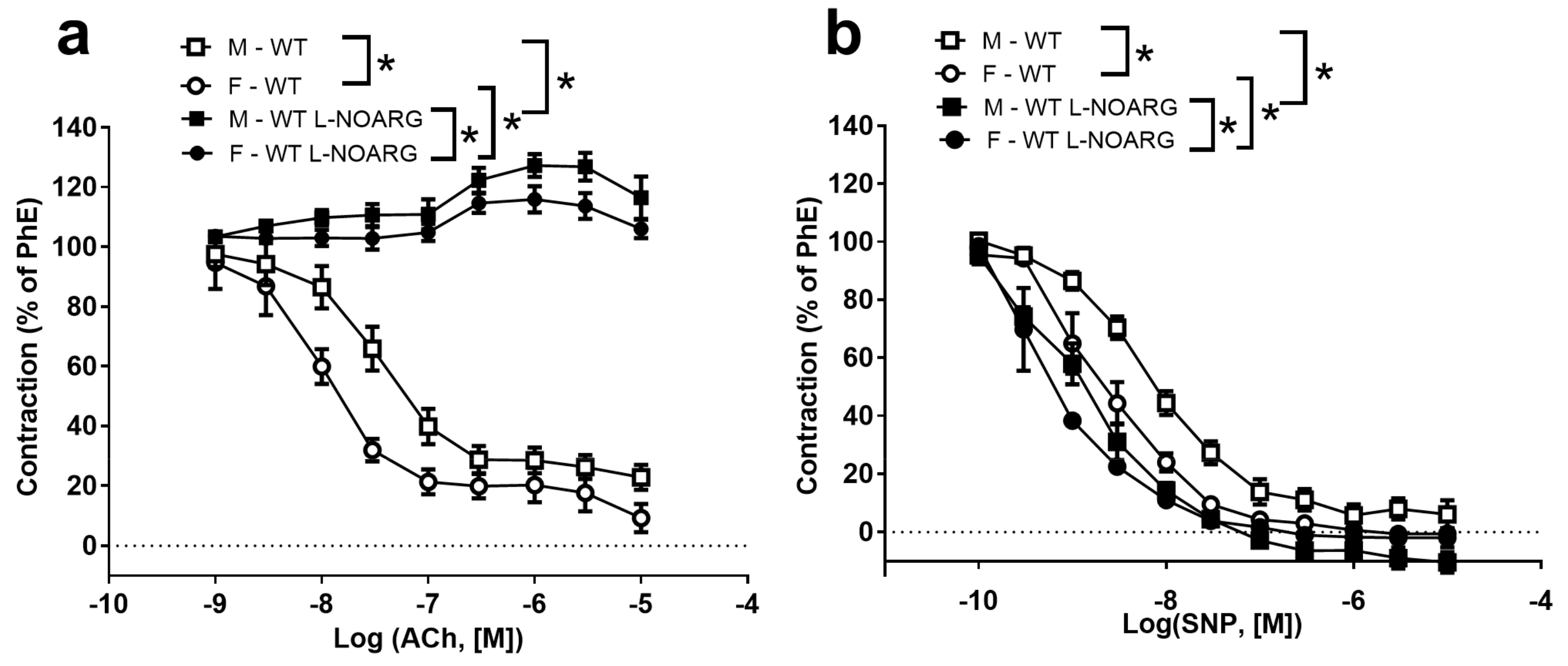
2.1. Functional Studies and NO Measurements
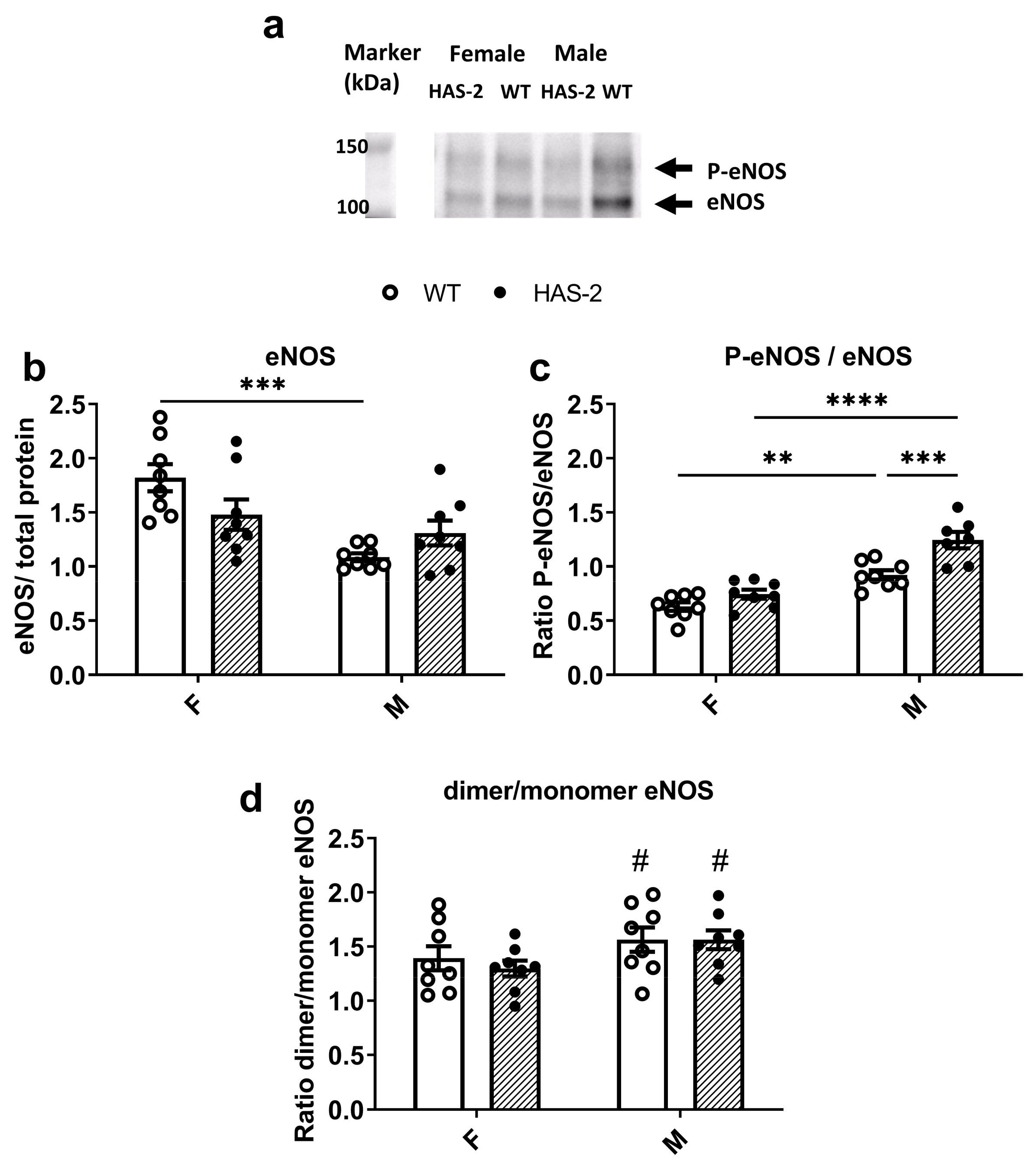
2.2. eNOS Expression and Phosphorylation
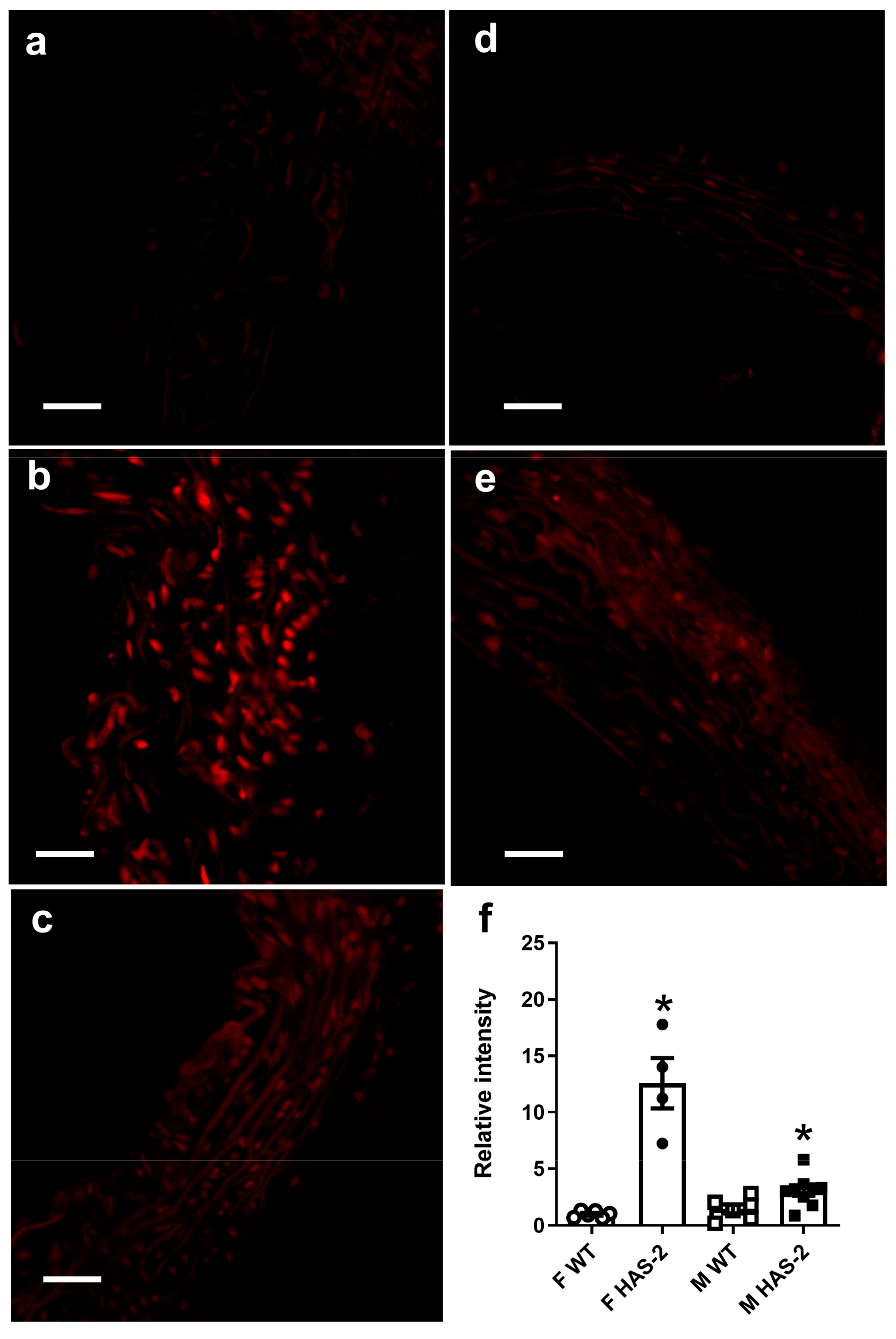
2.3. Assessment of Vascular O2- and O2- Scavenging
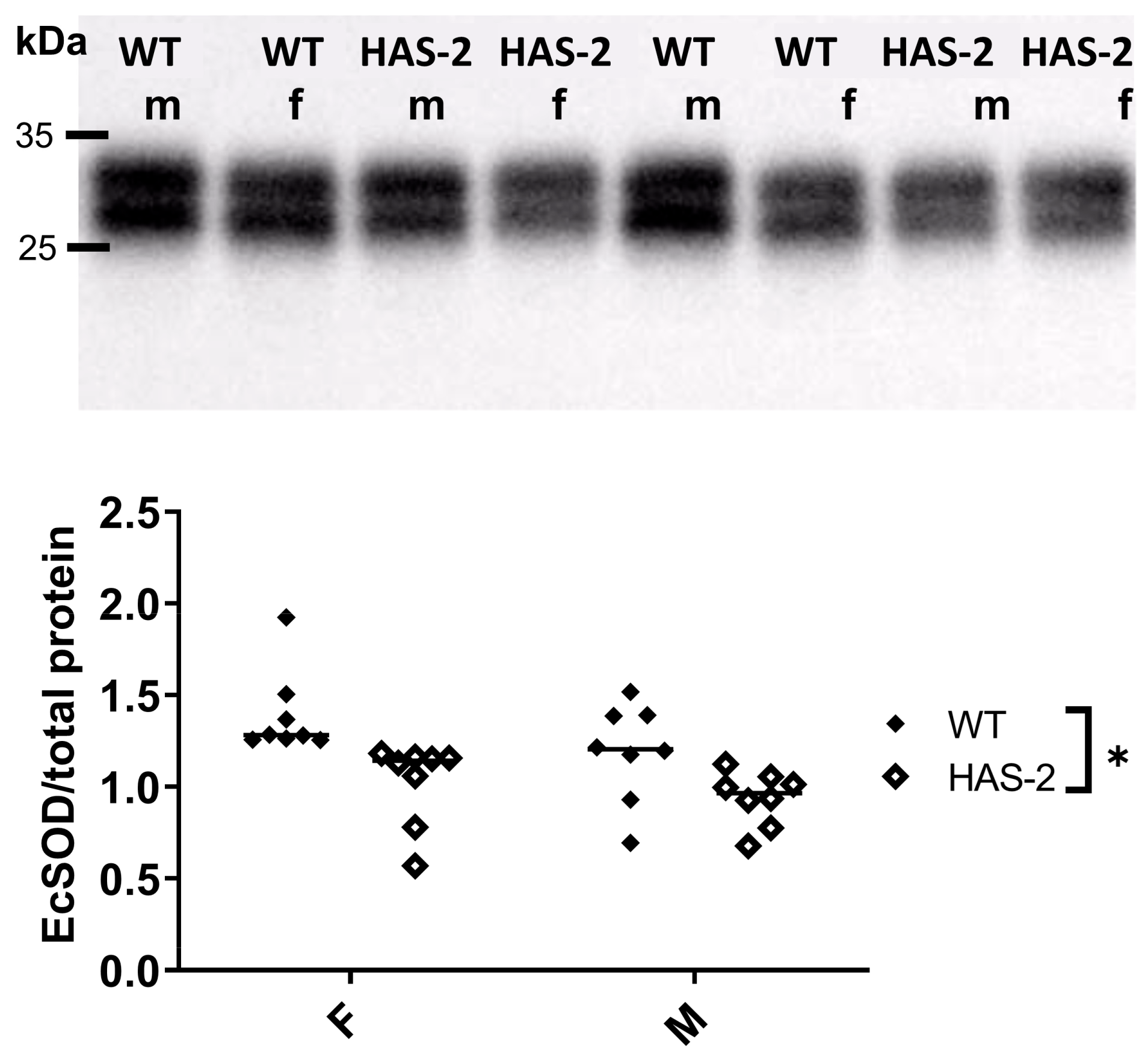
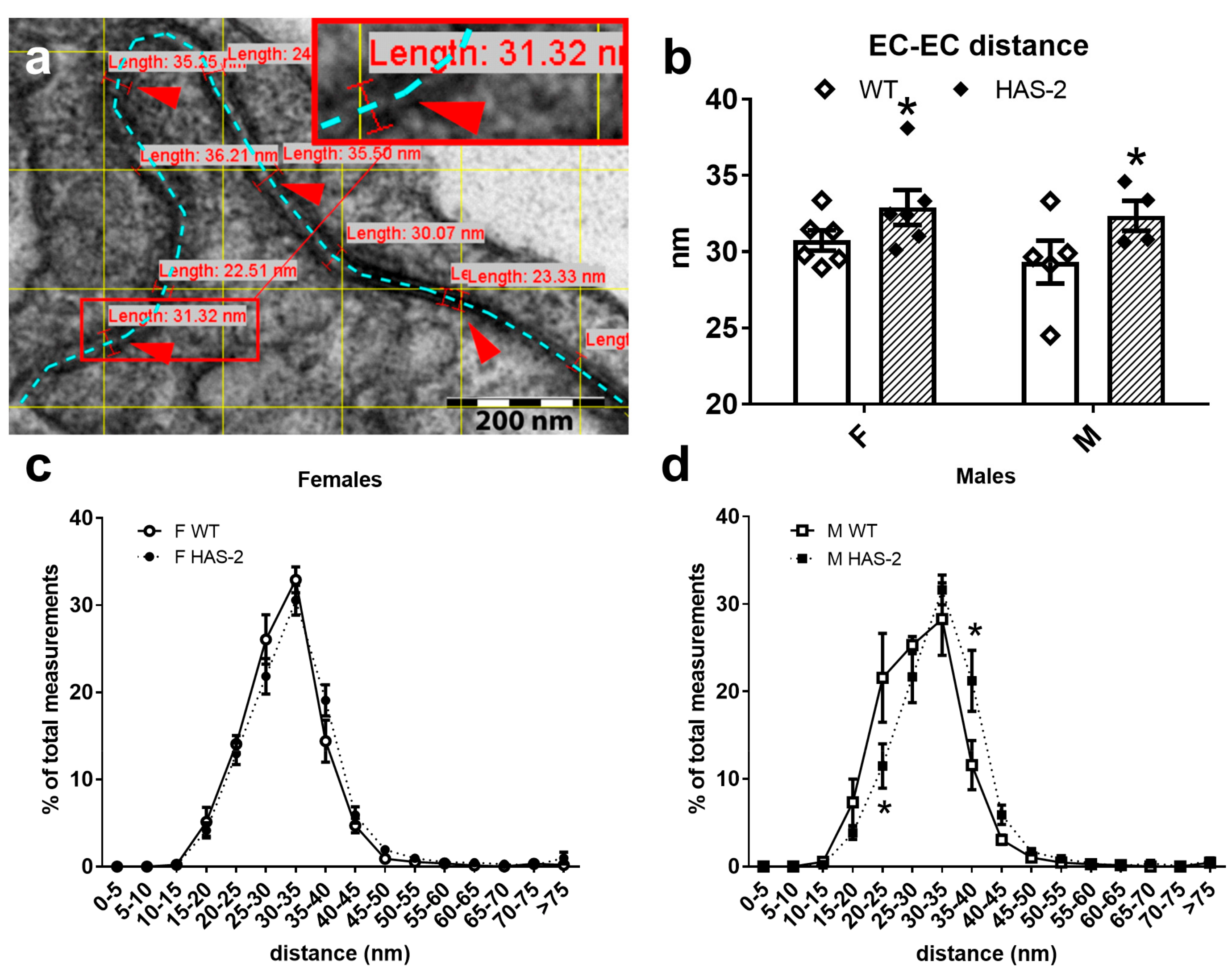
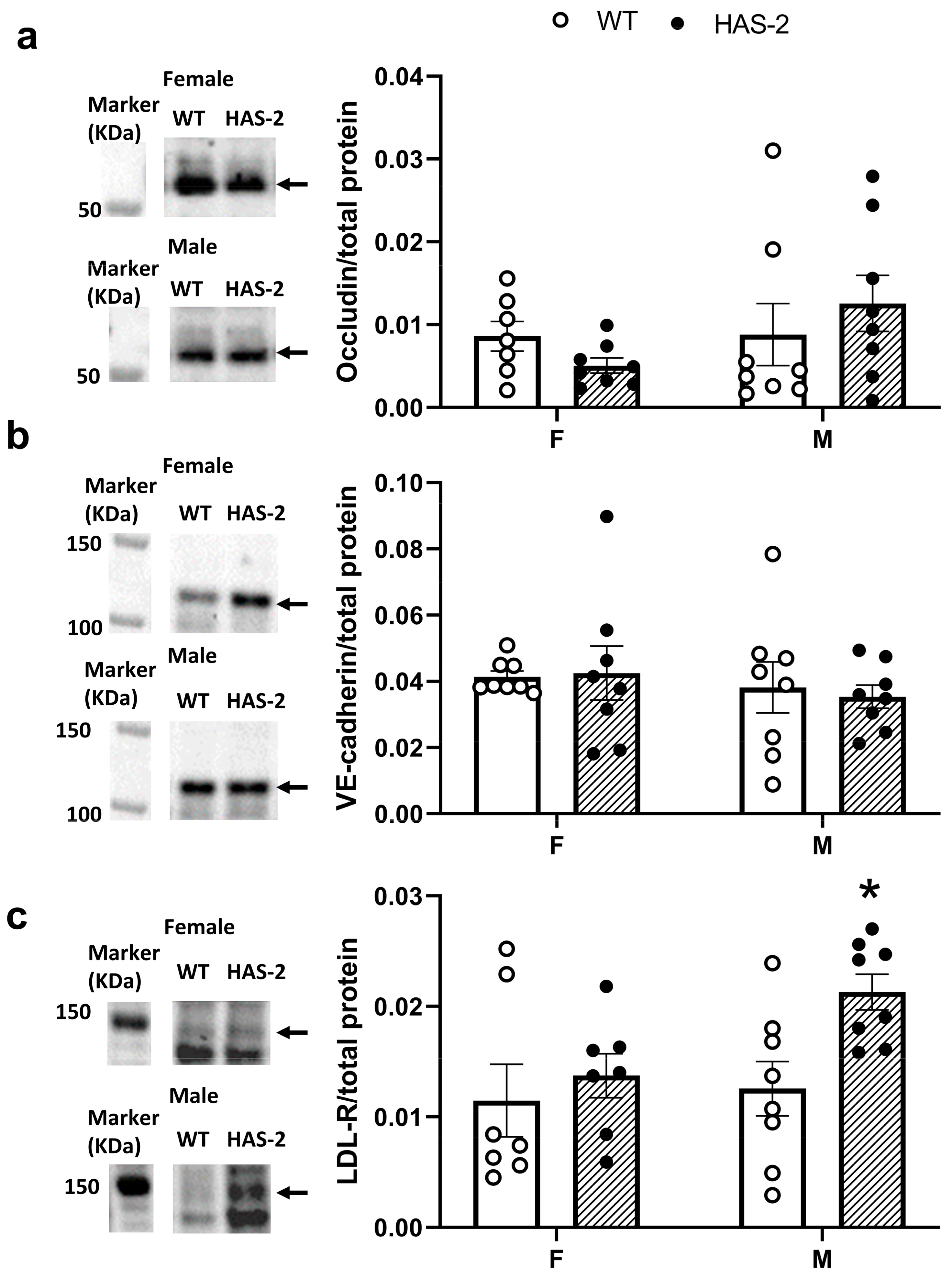
2.4. EC–EC Distance and Expression of Occludin, Vascular Endothelial Cadherin, and the LDL Receptor
3. Discussion
3.1. Sex-Dependent Differences in Endothelial Dysfunction
3.2. Effect of Hyaluronan Overexpression on Endothelial Cell Function
3.3. Pro-Atherogenic Effects of Hyaluronan Overexpression
3.4. Limitations
4. Materials and Methods
4.1. Animals
4.2. Contractility
4.3. Diaminofluorescence
4.4. Detection of Vascular O2−
4.5. Immunoblotting
4.6. EC–EC Distance
4.7. Data and Statistical Analyses
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Wasty, F.; Alavi, M.Z.; Moore, S. Distribution of Glycosaminoglycans in the Intima of Human Aortas: Changes in Atherosclerosis and Diabetes Mellitus. Diabetologia 1993, 36, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Prenner, S.B.; Chirinos, J.A. Arterial Stiffness in Diabetes Mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Yamashita, T.; Takaya, T.; Shinohara, M.; Shiraki, R.; Takeda, M.; Emoto, N.; Fukatsu, A.; Hayashi, T.; Ikemoto, K.; et al. Augmentation of Vascular Remodeling by Uncoupled Endothelial Nitric Oxide Synthase in a Mouse Model of Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Ladeia, A.M. Prognostic Value of Endothelial Dysfunction in Type 1 Diabetes Mellitus. World J. Diabetes 2014, 5, 601. [Google Scholar] [CrossRef]
- Seike, M.; Ikeda, M.; Matsumoto, M.; Hamada, R.; Takeya, M.; Kodama, H. Hyaluronan Forms Complexes with Low Density Lipoprotein While Also Inducing Foam Cell Infiltration in the Dermis. J. Dermatol. Sci. 2006, 41, 197–204. [Google Scholar] [CrossRef]
- Heickendorff, L.; Ledet, T.; Rasmussen, L.M. Glycosaminoglycans in the Human Aorta in Diabetes Mellitus: A Study of Tunica Media from Areas with and without Atherosclerotic Plaque. Diabetologia 1994, 37, 286–292. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 Interactions as Potential Targets for Cancer Therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Williams, K.J.; Tabas, I. The Response-to-Retention Hypothesis of Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–562. [Google Scholar] [CrossRef]
- Nakashima, Y.; Wight, T.N.; Sueishi, K. Early Atherosclerosis in Humans: Role of Diffuse Intimal Thickening and Extracellular Matrix Proteoglycans. Cardiovasc. Res. 2008, 79, 14–23. [Google Scholar] [CrossRef]
- Chai, S.; Chai, Q.; Danielsen, C.C.; Hjorth, P.; Nyengaard, J.R.; Ledet, T.; Yamaguchi, Y.; Rasmussen, L.M.; Wogensen, L. Overexpression of Hyaluronan in the Tunica Media Promotes the Development of Atherosclerosis. Circ. Res. 2005, 96, 583–591. [Google Scholar] [CrossRef]
- Lorentzen, K.A.; Chai, S.; Chen, H.; Danielsen, C.C.; Simonsen, U.; Wogensen, L. Mechanisms Involved in Extracellular Matrix Remodeling and Arterial Stiffness Induced by Hyaluronan Accumulation. Atherosclerosis 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A. Long-Term Follow-up of Patients with Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Nitenberg, A.; Chemla, D.; Antony, I.; Verdier, C.J. Long-Term Follow-up of Hypertensive Patients with Angiographically Normal Coronary Arteries: Prognostic Value of Epicardial Coronary Endothelial Dysfunction. J. Am. Coll. Cardiol. 2003, 41, 282. [Google Scholar] [CrossRef]
- Nitenberg, A.; Pham, I.; Antony, I.; Valensi, P.; Attali, J.R.; Chemla, D. Cardiovascular Outcome of Patients with Abnormal Coronary Vasomotion and Normal Coronary Arteriography Is Worse in Type 2 Diabetes Mellitus than in Arterial Hypertension: A 10 Year Follow-up Study. Atherosclerosis 2005, 183, 113–120. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.; Warnholtz, A.; et al. Mechanisms Underlying Endothelial Dysfunction in Diabetes Mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Lund, D.D.; Chu, Y.; Miller, J.D.; Heistad, D.D. Protective Effect of Extracellular Superoxide Dismutase on Endothelial Function during Aging. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H1920–H1925. [Google Scholar] [CrossRef]
- Sima, A.V.; Stancu, C.S.; Simionescu, M. Vascular Endothelium in Atherosclerosis. Cell Tissue Res. 2009, 335, 191–203. [Google Scholar] [CrossRef]
- Jung, O.; Marklund, S.L.; Geiger, H.; Pedrazzini, T.; Busse, R.; Brandes, R.P. Extracellular Superoxide Dismutase Is a Major Determinant of Nitric Oxide Bioavailability: In Vivo and Ex Vivo Evidence from EcSOD-Deficient Mice. Circ. Res. 2003, 93, 622–629. [Google Scholar] [CrossRef]
- Gao, F.; Koenitzer, J.R.; Tobolewski, J.M.; Jiang, D.; Liang, J.; Noble, P.W.; Oury, T.D. Extracellular Superoxide Dismutase Inhibits Inflammation by Preventing Oxidative Fragmentation of Hyaluronan. J. Biol. Chem. 2008, 283, 6058–6066. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Siegfried, M.R.; Ushio-Fukai, M.; Cheng, Y.; Kojda, G.; Harrison, D.G. Regulation of the Vascular Extracellular Superoxide Dismutase by Nitric Oxide and Exercise Training. J. Clin. Investig. 2000, 105, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell. Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Nishimura, N.; Rana, K.; Peloquin, J.M.; Califano, J.P.; Montague, C.R.; King, M.R.; Schaffer, C.B.; Reinhart-King, C.A. Age-Related Intimal Stiffening Enhances Endothelial Permeability and Leukocyte Transmigration. Sci. Transl. Med. 2011, 3, 12ra122. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Sowers, J.R. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. CardioRenal Med. 2014, 4, 60–71. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M.D.; Østergaard, L.; Andersen, M.R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic Acid Induces Relaxation and Calcium-Independent Release of Endothelium-Derived Nitric Oxide. Br. J. Pharmacol. 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Matlung, H.L.; Neele, A.E.; Groen, H.C.; van Gaalen, K.; Tuna, B.G.; van Weert, A.; de Vos, J.; Wentzel, J.J.; Hoogenboezem, M.; van Buul, J.D.; et al. Transglutaminase Activity Regulates Atherosclerotic Plaque Composition at Locations Exposed to Oscillatory Shear Stress. Atherosclerosis 2012, 224, 355–362. [Google Scholar] [CrossRef]
- Van Herck, J.L.; Schrijvers, D.M.; De Meyer, G.R.Y.; Martinet, W.; Van Hove, C.E.; Bult, H.; Vrints, C.J.; Herman, A.G. Transglutaminase 2 Deficiency Decreases Plaque Fibrosis and Increases Plaque Inflammation in Apolipoprotein-E-Deficient Mice. J. Vasc. Res. 2010, 47, 231–240. [Google Scholar] [CrossRef]
- Wellman, G.C.; Bonev, A.D.; Nelson, M.T.; Brayden, J.E. Gender Differences in Coronary Artery Diameter Involve Estrogen, Nitric Oxide, and Ca2+-Dependent K+ Channels. Circ. Res. 1996, 79, 1024–1030. [Google Scholar] [CrossRef]
- Takenouchi, Y.; Kobayashi, T.; Taguchi, K.; Matsumoto, T.; Kamata, K. Gender Differences in Vascular Reactivity of Aortas from Streptozotocin-Induced Diabetic Mice. Biol. Pharm. Bull. 2010, 33, 1692–1697. [Google Scholar] [CrossRef]
- Taguchi, K.; Matsumoto, T.; Kamata, K.; Kobayashi, T. Akt/ENOS Pathway Activation in Endothelium-Dependent Relaxation Is Preserved in Aortas from Female, but Not from Male, Type 2 Diabetic Mice. Pharmacol. Res. 2012, 65, 56–65. [Google Scholar] [CrossRef]
- Chen, H.; Simonsen, U.; Aalkjaer, C. A Sex-Specific, COX-Derived/Thromboxane Receptor Activator Causes Depolarization and Vasoconstriction in Male Mice Mesenteric Resistance Arteries. Basic Clin. Pharmacol. Toxicol. 2020, 127, 152–159. [Google Scholar] [CrossRef]
- Kauser, K.; Rubanyi, G.M. Gender Difference in Endothelial Dysfunction in the Aorta of Spontaneously Hypertensive Rats. Hypertension 1995, 25, 517–523. [Google Scholar] [CrossRef]
- Carnethon, M.R.; Biggs, M.L.; Barzilay, J.; Kuller, L.H.; Mozaffarian, D.; Mukamal, K.; Smith, N.L.; Siscovick, D. Diabetes and Coronary Heart Disease as Risk Factors for Mortality in Older Adults. Am. J. Med. 2010, 123, 556.e1. [Google Scholar] [CrossRef]
- Pabbidi, M.R.; Kuppusamy, M.; Didion, S.P.; Sanapureddy, P.; Reed, J.T.; Sontakke, S.P. Sex Differences in the Vascular Function and Related Mechanisms: Role of 17β-Estradiol. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1499–H1518. [Google Scholar] [CrossRef]
- Beck, L.; Su, J.; Comerma-Steffensen, S.; Pinilla, E.; Carlsson, R.; Hernanz, R.; Sheykhzade, M.; Danielsen, C.C.; Simonsen, U. Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic Db/Db Mice. Front. Physiol. 2020, 11, 667. [Google Scholar] [CrossRef]
- Comerma-Steffensen, S.; Prat-Duran, J.; Mogensen, S.; Fais, R.; Pinilla, E.; Simonsen, U. Erectile Dysfunction and Altered Contribution of KCa1.1 and KCa2.3 Channels in the Penile Tissue of Type-2 Diabetic Db/Db Mice. J. Sex. Med. 2022, 19, 697–710. [Google Scholar] [CrossRef]
- Chen, H.; Kold-Petersen, H.; Laher, I.; Simonsen, U.; Aalkjaer, C. Impaired Endothelial Calcium Signaling Is Responsible for the Defective Dilation of Mesenteric Resistance Arteries from Db/Db Mice to Acetylcholine. Eur. J. Pharmacol. 2015, 767, 17–23. [Google Scholar] [CrossRef]
- Boittin, F.X.; Alonso, F.; Le Gal, L.; Allagnat, F.; Bény, J.L.; Haefliger, J.A. Connexins and M3 Muscarinic Receptors Contribute to Heterogeneous Ca Signaling in Mouse Aortic Endothelium. Cell. Physiol. Biochem. 2013, 31, 166–178. [Google Scholar] [CrossRef]
- Tangirala, R.K.; Rubin, E.M.; Palinski, W. Quantitation of Atherosclerosis in Murine Models: Correlation between Lesions in the Aortic Origin and in the Entire Aorta, and Differences in the Extent of Lesions between Sexes in LDL Receptor-Deficient and Apolipoprotein E-Deficient Mice. J. Lipid Res. 1995, 36, 2320–2328. [Google Scholar] [CrossRef]
- Russell, R. Mechanisms of Disease: Atherosclerosis An Inflammatory Disease. N. Engl. J. Med. 1999, 1, 115–126. [Google Scholar]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement Fromthe European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, E.; Comerma-Steffensen, S.; Prat-Duran, J.; Rivera, L.; Matchkov, V.V.; Buus, N.H.; Simonsen, U. Transglutaminase 2 Inhibitor LDN 27219 Age-Dependently Lowers Blood Pressure and Improves Endothelium-Dependent Vasodilation in Resistance Arteries. Hypertension 2020, 77, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Swoap, S.J.; Gutilla, M.J. Cardiovascular Changes during Daily Torpor in the Laboratory Mouse. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R769–R774. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard Operating Procedures for Describing and Performing Metabolic Tests of Glucose Homeostasis in Mice. DMM Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Buus, N.H.; Hansson, N.C.; Rodriguez-Rodriguez, R.; Stankevicius, E.; Andersen, M.R.; Simonsen, U. Antiatherogenic Effects of Oleanolic Acid in Apolipoprotein e Knockout Mice. Eur. J. Pharmacol. 2011, 670, 519–526. [Google Scholar] [CrossRef]
- Bangshaab, M.; Gutierrez, A.; Huynh, K.D.; Knudsen, J.S.; Arcanjo, D.D.R.; Petersen, A.G.; Rungby, J.; Gejl, M.; Simonsen, U. Different Mechanisms Involved in Liraglutide and Glucagon-like Peptide-1 Vasodilatation in Rat Mesenteric Small Arteries. Br. J. Pharmacol. 2019, 176, 386–399. [Google Scholar] [CrossRef]
- Gilda, J.E.; Gomes, A.V. Stain-Free Total Protein Staining Is a Superior Loading Control to b-Actin for Western Blots. Anal. Biochem. 2013, 440, 186–188. [Google Scholar] [CrossRef]


| Male WT | Male HAS-2 | Female WT | Female HAS-2 | |
|---|---|---|---|---|
| PhE −log EC50 | 6.49 ± 0.11 # (13) | 6.35 ± 0.11 # (13) | 7.19 ± 0.24 (5) | 6.92 ± 0.08 (5) |
| ACh −log EC50 | 7.45 ± 0.20 # (11) | 7.26 ± 0.23 # (11) | 8.13 ± 0.25 (5) | 7.92 ± 0.11 (5) |
| SNP −log EC50 | 8.05 ± 0.05 # (11) | 7.70 ± 0.07 #* (119) | 8.62 ± 0.05 # (5) | 8.31 ± 0.05 #* (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorentzen, K.A.; Hernanz, R.; Pinilla, E.; Nyengaard, J.R.; Wogensen, L.; Simonsen, U. Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media. Int. J. Mol. Sci. 2023, 24, 8436. https://doi.org/10.3390/ijms24098436
Lorentzen KA, Hernanz R, Pinilla E, Nyengaard JR, Wogensen L, Simonsen U. Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media. International Journal of Molecular Sciences. 2023; 24(9):8436. https://doi.org/10.3390/ijms24098436
Chicago/Turabian StyleLorentzen, Karen Axelgaard, Raquel Hernanz, Estéfano Pinilla, Jens Randel Nyengaard, Lise Wogensen, and Ulf Simonsen. 2023. "Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media" International Journal of Molecular Sciences 24, no. 9: 8436. https://doi.org/10.3390/ijms24098436
APA StyleLorentzen, K. A., Hernanz, R., Pinilla, E., Nyengaard, J. R., Wogensen, L., & Simonsen, U. (2023). Sex-Dependent Impairment of Endothelium-Dependent Relaxation in Aorta of Mice with Overexpression of Hyaluronan in Tunica Media. International Journal of Molecular Sciences, 24(9), 8436. https://doi.org/10.3390/ijms24098436