Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata
Abstract
1. Introduction
2. Results and Discussion
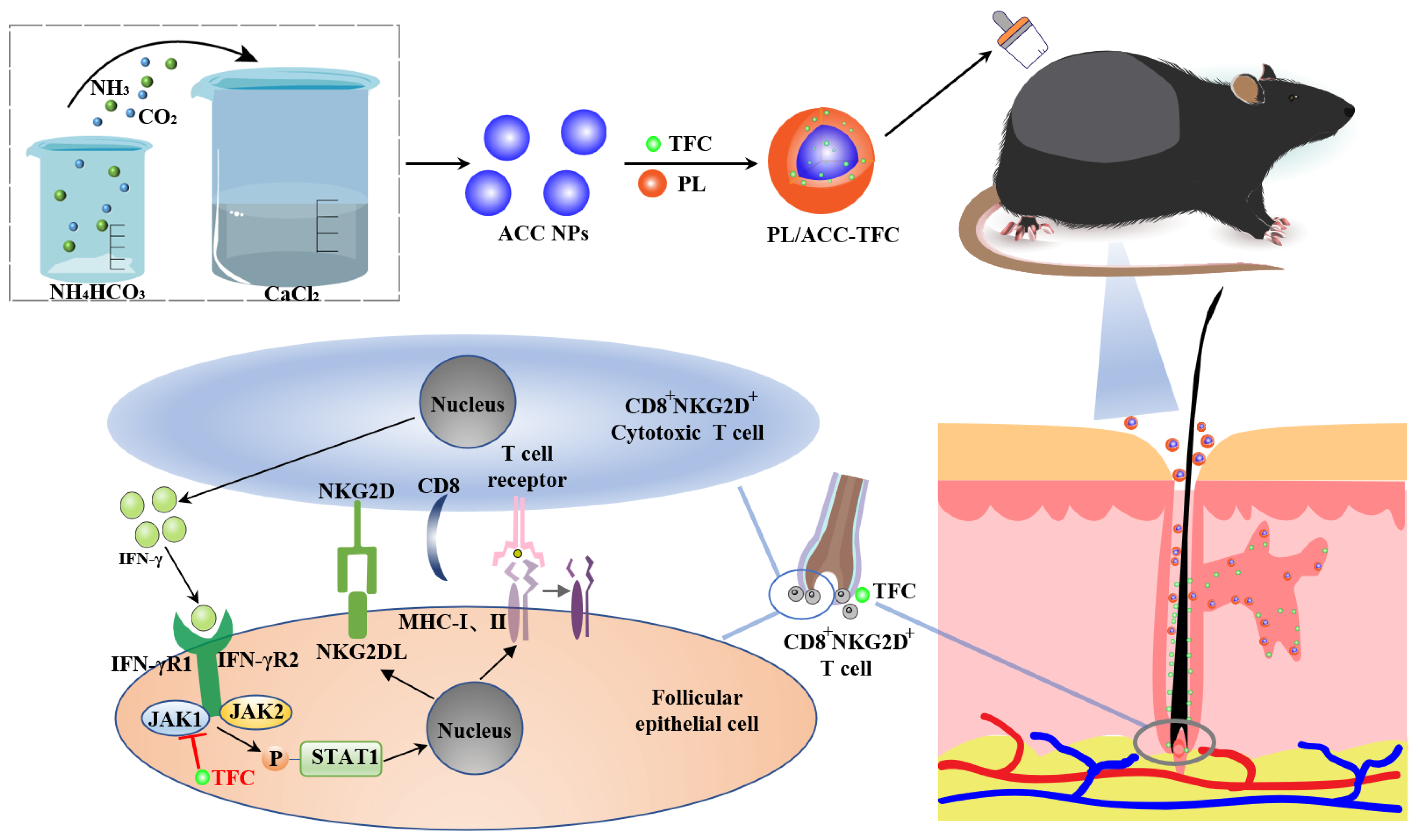
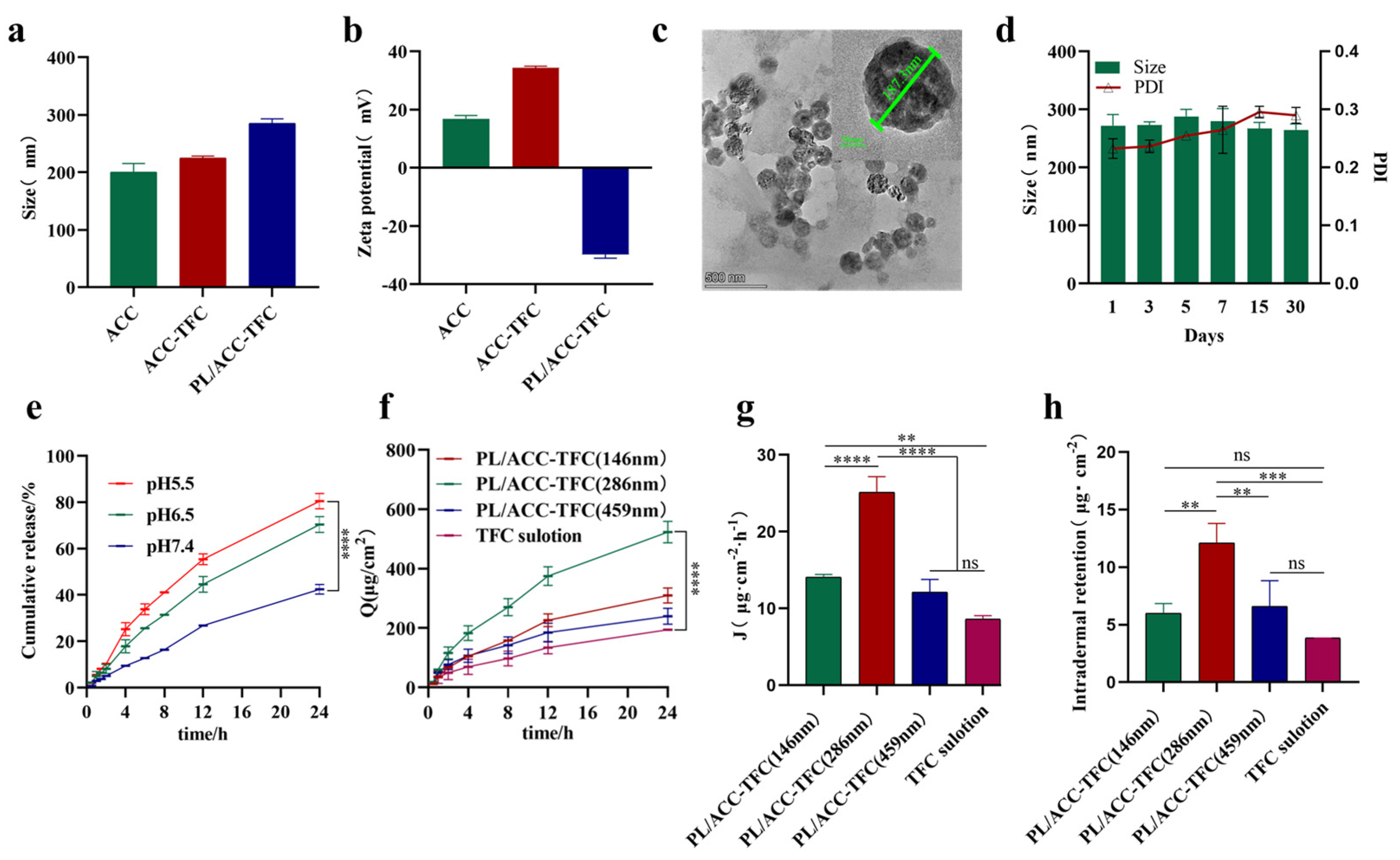
2.1. Preparation and Characterization of PL/ACC-TFC
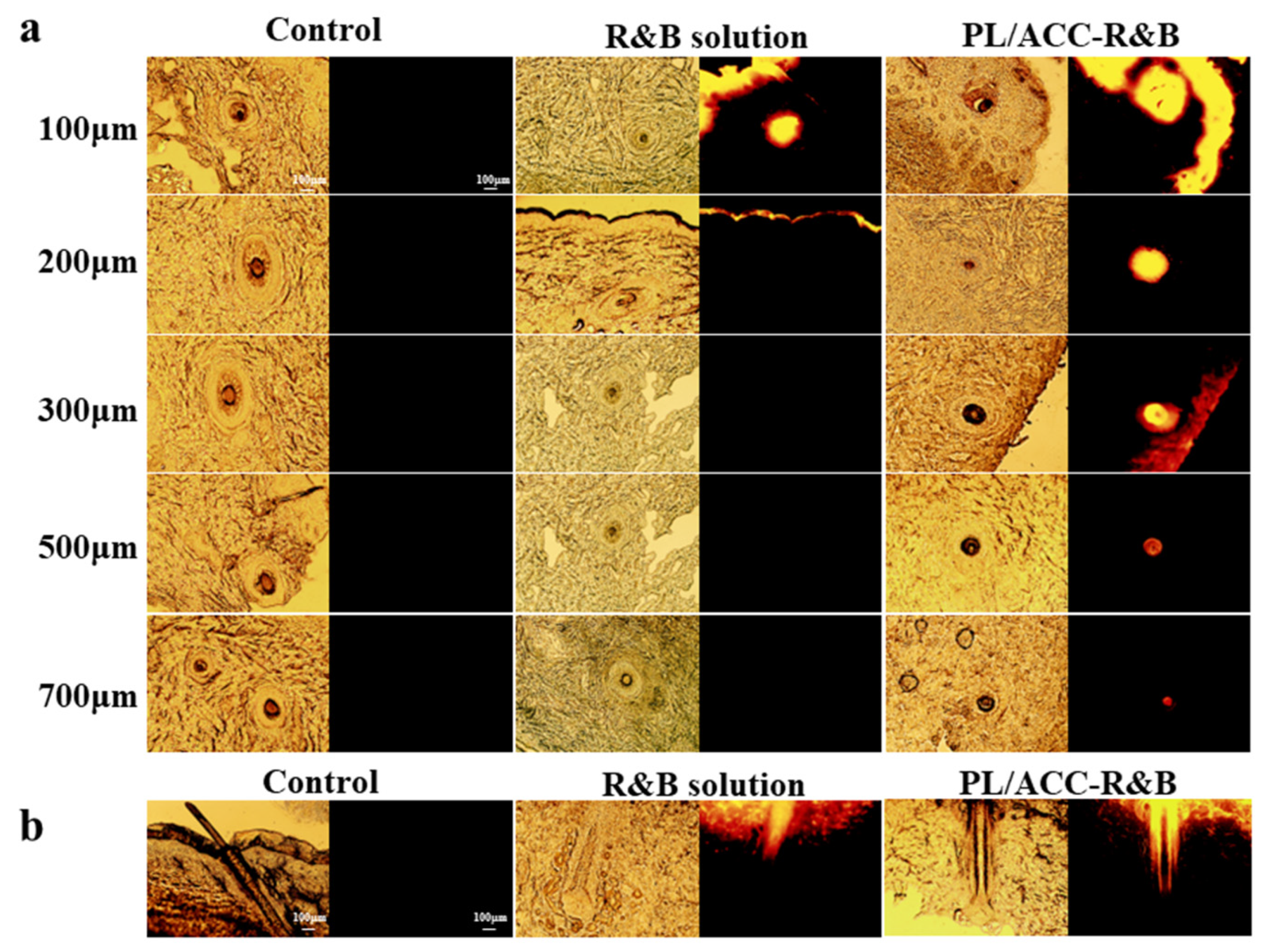
2.2. Determination of Passive Dermal and Follicular Penetration
2.3. Dermal Penetration of PL/ACC NPs In Vitro
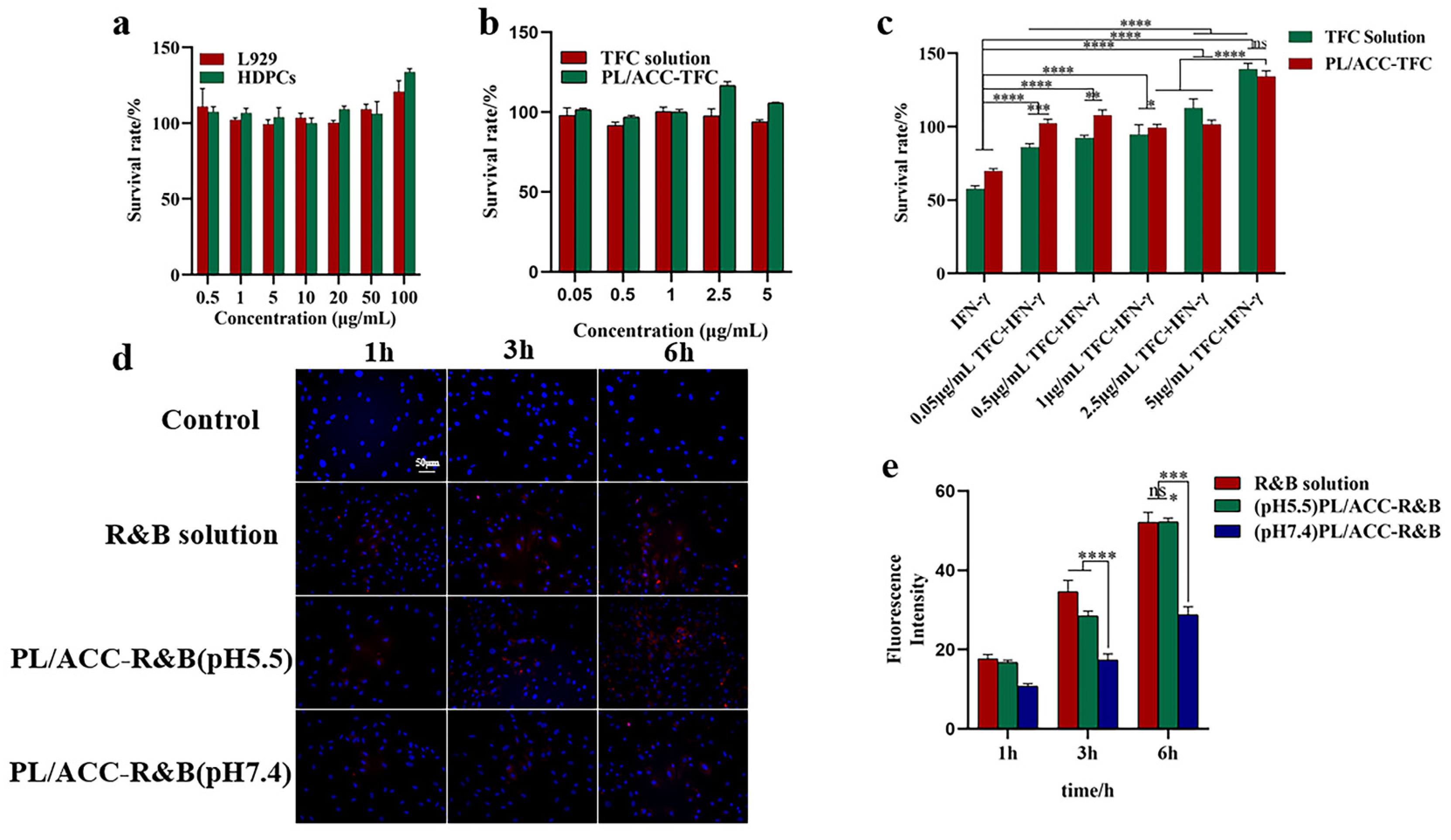
2.4. Viability of Cells and Cellular Uptake
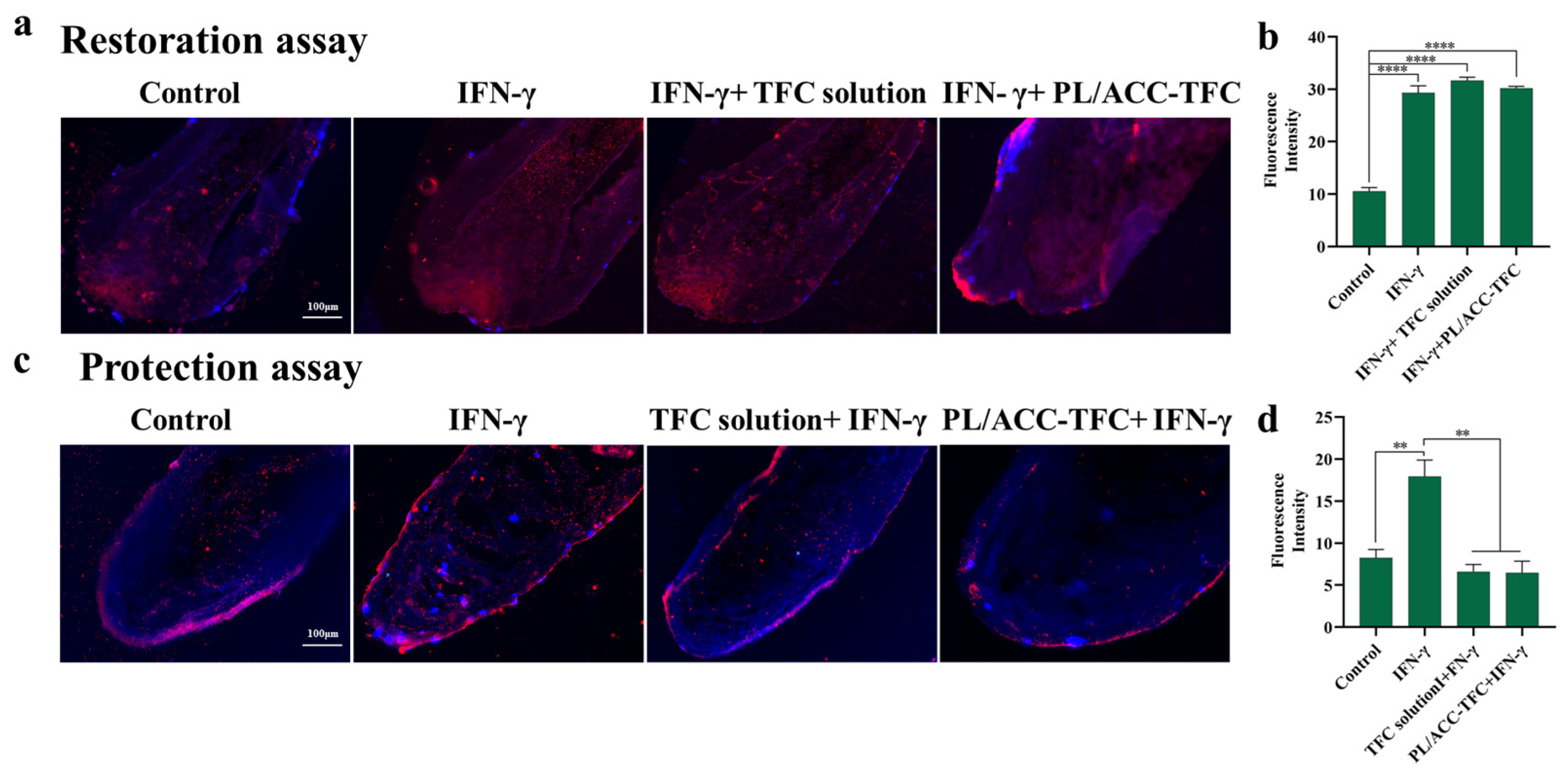
2.5. Hair Follicle Immune Privilege (HF-IP) Restoration/Protection Assay
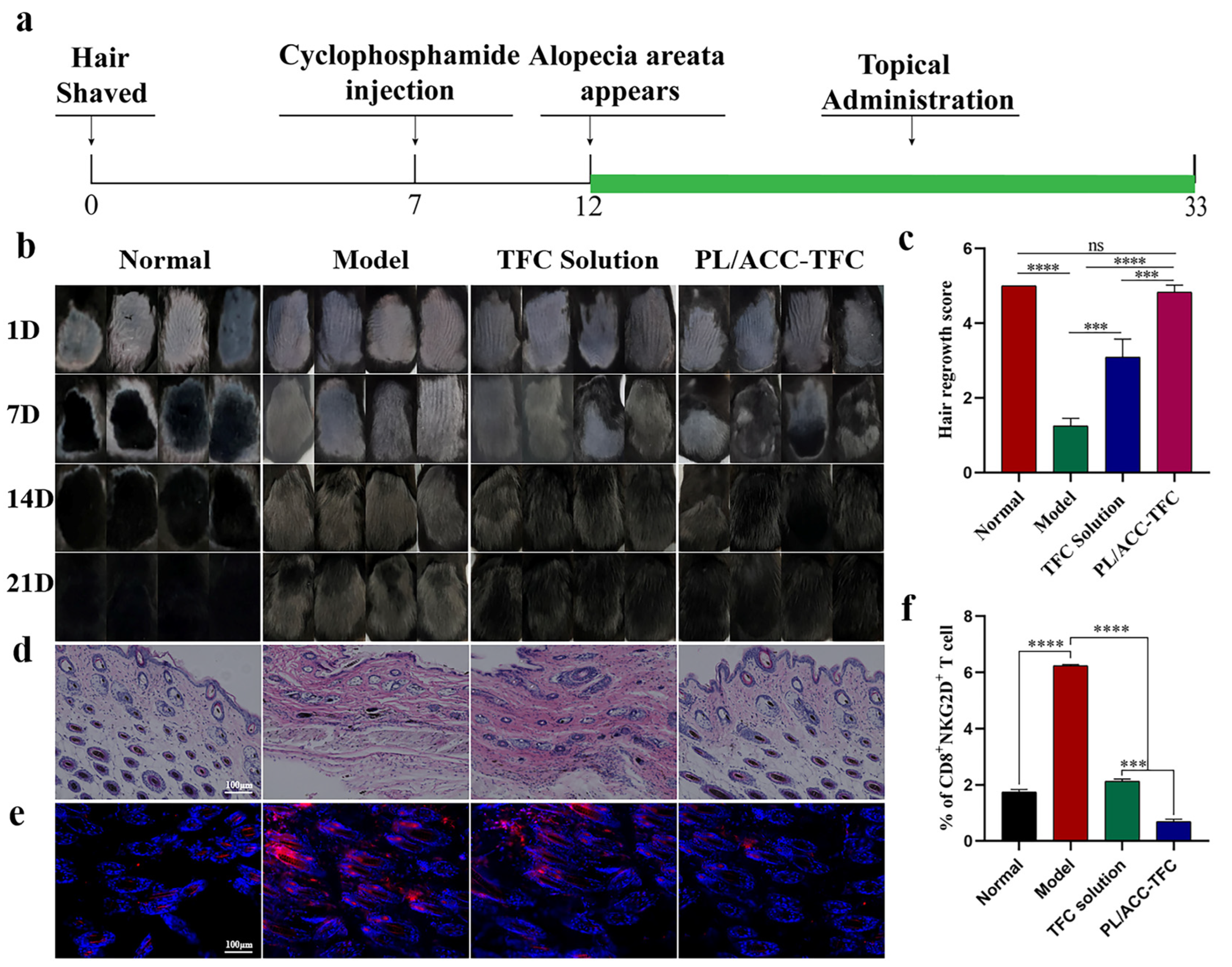
2.6. PL/ACC-TFC NPs Controls Alopecia Areata
3. Materials and Methods
3.1. Materials
3.2. Cell Line
3.3. Preparation of Lipid-Coated Calcium Carbonate Nanocarrier (PL/ACC-TFC)
3.4. Characterization of PL/ACC-TFC NPs
3.5. Evaluation of PL/ACC-TFC NPs Delivery In Vitro
3.5.1. Ex Vivo Model of Dermal and Follicular Penetration
3.5.2. Investigation of TFC Delivery to Skin In Vitro
3.6. Cell Viability and Cellular Uptake Test
3.7. Hair Follicle Immune Privilege (HF-IP) Restoration/Protection Assay
3.7.1. Hair Follicle (HF) Organ Culture Assay
3.7.2. Immunohistochemistry Staining
3.8. Application for Cyclophosphamide (CYP)-Induced Alopecia Areata
3.8.1. Hair Regrowth Assays
3.8.2. Histological Staining of Skin Samples
3.9. Flow Cytometry
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Prim. 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 2018, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Bhoyrul, B.; Asfour, L.; Lutz, G.; Mitchell, L.; Jerjen, R.; Sinclair, R.D.; Holmes, S.; Chaudhry, I.H.; Harries, M.J. Clinicopathologic Characteristics and Response to Treatment of Persistent Chemotherapy-Induced Alopecia in Breast Cancer Survivors. JAMA Dermatol. 2021, 157, 1335–1342. [Google Scholar] [CrossRef]
- Pourang, A.; Mesinkovska, N.A. New and Emerging Therapies for Alopecia Areata. Drugs 2020, 80, 635–646. [Google Scholar] [CrossRef]
- Shen, X.F.; Ru, L.X.; Yao, X.B. Efficacy of scalp cooling for prevention of chemotherapy induced alopecia: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5090–5103. [Google Scholar]
- Almutairi, N.; Nour, T.M.; Hussain, N.H. Janus Kinase Inhibitors for the Treatment of Severe Alopecia Areata: An Open-Label Comparative Study. Dermatology 2019, 235, 130–136. [Google Scholar] [CrossRef]
- Guo, L.; Feng, S.; Sun, B.; Jiang, X.; Liu, Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: A systemic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 192–201. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J. Recent advances in follicular drug delivery of nanoparticles. Expert. Opin. Drug. Deliv. 2020, 17, 49–60. [Google Scholar] [CrossRef]
- Christmann, R.; Thomas, C.; Jager, N.; Raber, A.S.; Loretz, B.; Schaefer, U.F.; Tschernig, T.; Vogt, T.; Lehr, C.M. Nanoparticle Targeting to Scalp Hair Follicles: New Perspectives for a Topical Therapy for Alopecia Areata. J. Investig. Dermatol. 2020, 140, 243–246.e245. [Google Scholar] [CrossRef]
- Salim, S.; Kamalasanan, K. Controlled drug delivery for alopecia: A review. J. Control. Release 2020, 325, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.; Ho, D.K.; Wilzopolski, J.; Lee, S.; Koch, M.; Loretz, B.; Vogt, T.; Baumer, W.; Schaefer, U.F.; Lehr, C.M. Tofacitinib Loaded Squalenyl Nanoparticles for Targeted Follicular Delivery in Inflammatory Skin Diseases. Pharmaceutics 2020, 12, 1131. [Google Scholar] [CrossRef]
- Dimde, M.; Sahle, F.F.; Wycisk, V.; Steinhilber, D.; Camacho, L.C.; Licha, K.; Lademann, J.; Haag, R. Synthesis and Validation of Functional Nanogels as pH-Sensors in the Hair Follicle. Macromol. Biosci. 2017, 17, 1600505. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.H.; Zhao, Y. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem. Int. Ed. Engl. 2015, 54, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, C.; Xu, L.; Wang, D.; Liu, W.; Zhang, K.; Zhang, Z.; Shi, J. Nanoenabled Intracellular Calcium Bursting for Safe and Efficient Reversal of Drug Resistance in Tumor Cells. Nano Lett. 2020, 20, 8102–8111. [Google Scholar] [CrossRef]
- Santos, G.A.; Angelo, T.; Andrade, L.M.; Silva, S.M.M.; Magalhaes, P.O.; Cunha-Filho, M.; Gelfuso, G.M.; Taveira, S.F.; Gratieri, T. The role of formulation and follicular pathway in voriconazole cutaneous delivery from liposomes and nanostructured lipid carriers. Colloids Surf. B. Biointerfaces 2018, 170, 341–346. [Google Scholar] [CrossRef]
- Kaden, D.; Dahne, L.; Knorr, F.; Richter, H.; Lademann, J.; Meinke, M.C.; Patzelt, A.; Darvin, M.E.; Jung, S. Determination of the pH Gradient in Hair Follicles of Human Volunteers Using pH-Sensitive Melamine Formaldehyde-Pyranine Nile Blue Microparticles. Sensors 2020, 20, 5243. [Google Scholar] [CrossRef]
- Younis, U.S.; Vallorz, E.; Addison, K.J.; Ledford, J.G.; Myrdal, P.B. Preformulation and Evaluation of Tofacitinib as a Therapeutic Treatment for Asthma. AAPS PharmSciTech 2019, 20, 167. [Google Scholar] [CrossRef]
- Carcamo-Martinez, A.; Mallon, B.; Anjani, Q.K.; Dominguez-Robles, J.; Utomo, E.; Vora, L.K.; Tekko, I.A.; Larraneta, E.; Donnelly, R.F. Enhancing intradermal delivery of tofacitinib citrate: Comparison between powder-loaded hollow microneedle arrays and dissolving microneedle arrays. Int. J. Pharm. 2021, 593, 120152. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2021, 329, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.C.; Patzelt, A.; Richter, H.; Renneberg, R.; Lai, K.K.; Ruhl, E.; Sterry, W.; Lademann, J. Triggering of drug release of particles in hair follicles. J. Control. Release 2012, 160, 509–514. [Google Scholar] [CrossRef]
- Kathuria, H.; Nguyen, D.T.P.; Handral, H.K.; Cai, J.; Cao, T.; Kang, L. Proposome for transdermal delivery of tofacitinib. Int. J. Pharm. 2020, 585, 119558. [Google Scholar] [CrossRef] [PubMed]
- Kandekar, S.G.; Del Rio-Sancho, S.; Lapteva, M.; Kalia, Y.N. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale 2018, 10, 1099–1110. [Google Scholar] [CrossRef]
- Sagawa, N.; Oshima, Y.; Hiratsuka, T.; Kono, Y.; Etoh, T.; Inomata, M. Role of increased vascular permeability in chemotherapy-induced alopecia: In vivo imaging of the hair follicular microenvironment in mice. Cancer Sci. 2020, 111, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Aljuffali, I.A.; Sung, C.T.; Shen, F.M.; Huang, C.T.; Fang, J.Y. Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells. AAPS J. 2014, 16, 140–150. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, Y.J.; Park, H.R.; Lee, D.G.; Jeong, K.H.; Kang, H. The Effect of JAK Inhibitor on the Survival, Anagen Re-Entry, and Hair Follicle Immune Privilege Restoration in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 5137. [Google Scholar] [CrossRef]
- Kim, J.E.; Oh, J.H.; Woo, Y.J.; Jung, J.H.; Jeong, K.H.; Kang, H. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models. Exp. Dermatol. 2020, 29, 265–272. [Google Scholar] [CrossRef]
- Lim, Y.C.; Kim, H.; Lim, S.M.; Kim, J.S. Genetic analysis of a novel antioxidant multi-target iron chelator, M30 protecting against chemotherapy-induced alopecia in mice. BMC Cancer 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, K.; Zhao, J.; Maloney, N.J.; Atassi, G.; Beestrum, M.; Lio, P.A. Off-label studies on tofacitinib in dermatology: A review. J. Dermatol. Treat. 2021, 32, 399–409. [Google Scholar] [CrossRef]
- Hendrix, S.; Handjiski, B.; Peters, E.M.; Paus, R. A Guide to Assessing Damage Response Pathways of the Hair Follicle: Lessons From Cyclophosphamide-Induced Alopecia in Mice. J. Invest. Dermatol. 2005, 125, 43–53. [Google Scholar] [CrossRef]
- Kim, M.J.; Seong, K.Y.; Kim, D.S.; Jeong, J.S.; Kim, S.Y.; Lee, S.; Yang, S.Y.; An, B.S. Minoxidil-loaded hyaluronic acid dissolving microneedles to alleviate hair loss in an alopecia animal model. Acta Biomater. 2022, 143, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Chen, J.; Chang, Y.; Christiano, A.M. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight 2021, 6, e142205. [Google Scholar] [CrossRef]
- Qin, T.; Dai, Z.; Xu, X.D.; Zhang, Z.L.; You, X.Y.; Sun, H.M.; Liu, M.X.; Zhu, H.D. Nanosuspension as an Efficient Carrier for Improved Ocular Permeation of Voriconazole. Curr. Pharm. Biotechnol. 2021, 22, 245–253. [Google Scholar] [CrossRef]
- Kinori, M.; Bertolini, M.; Funk, W.; Samuelov, L.; Meyer, K.C.; Emelianov, V.U.; Hasse, S.; Paus, R. Calcitonin gene-related peptide (CGRP) may award relative protection from interferon-gamma-induced collapse of human hair follicle immune privilege. Exp. Dermatol. 2012, 21, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Fujii, M.; Imaoka, A.; Kobayashi, R.; Hayashi, R.; Yoshida, Y.; Kohno, T.; Tsuji, T. Preventive effect of edaravone ointment on cyclophosphamide-chemotherapy induced alopecia. Support. Care Cancer 2021, 29, 6127–6134. [Google Scholar] [CrossRef]
- Chen, S.S.; Zhang, Y.; Lu, Q.L.; Lin, Z.; Zhao, Y. Preventive effects of cedrol against alopecia in cyclophosphamide-treated mice. Environ. Toxicol. Pharmacol. 2016, 46, 270–276. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.W.; Yin, X.Q.; Lao, Z.Z.; Zhang, Z.; Wu, Q.G.; Yu, L.W.; Lai, X.P.; Wan, Y.H.; Li, G. Inhibitory activities of some traditional Chinese herbs against testosterone 5alpha-reductase and effects of Cacumen platycladi on hair re-growth in testosterone-treated mice. J. Ethnopharmacol. 2016, 177, 1–9. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Q.; Miao, L.; Musetti, S.; Huo, M.; Huang, L. Remodeling the fibrotic tumor microenvironment of desmoplastic melanoma to facilitate vaccine immunotherapy. Nanoscale 2020, 12, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Lv, Y.; Huang, W.; Fang, Z.; Qi, J.; Chen, Z.; Zhao, W.; Wu, W.; Lu, Y. Enhanced transdermal delivery of curcumin nanosuspensions: A mechanistic study based on co-localization of particle and drug signals. Int. J. Pharm. 2020, 588, 119737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Yan, A.; Qiang, W.; Ruan, R.; Yang, C.; Ma, K.; Sun, H.; Liu, M.; Zhu, H. Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata. Int. J. Mol. Sci. 2023, 24, 8427. https://doi.org/10.3390/ijms24098427
Guan Y, Yan A, Qiang W, Ruan R, Yang C, Ma K, Sun H, Liu M, Zhu H. Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata. International Journal of Molecular Sciences. 2023; 24(9):8427. https://doi.org/10.3390/ijms24098427
Chicago/Turabian StyleGuan, Yeneng, Aqin Yan, Wei Qiang, Rui Ruan, Chaobo Yang, Kai Ma, Hongmei Sun, Mingxing Liu, and Hongda Zhu. 2023. "Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata" International Journal of Molecular Sciences 24, no. 9: 8427. https://doi.org/10.3390/ijms24098427
APA StyleGuan, Y., Yan, A., Qiang, W., Ruan, R., Yang, C., Ma, K., Sun, H., Liu, M., & Zhu, H. (2023). Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata. International Journal of Molecular Sciences, 24(9), 8427. https://doi.org/10.3390/ijms24098427






