1. Introduction
In 2019, a new infectious disease, COVID-19, caused by SARS-CoV-2, spread rapidly around the world, posing major challenges for healthcare systems, economy and society. The highly contagious pathogen is mainly transmitted via respiratory droplets and aerosols during direct person-to-person contact [
1] and, due to its high stability, via contaminated surfaces [
2]. Approaches to combat the pandemic comprise reducing exposure and the use of vaccines, as well as the development and testing of newly developed or already approved drugs that reduce the infectivity of the virus. Mechanisms to interact with the virus include blocking of host cell receptors, inhibiting viral binding to and fusion with cell membranes of host cells, blocking receptor-mediated endocytosis, inhibiting viral enzymes, inhibiting viral nucleic acid synthesis, and inhibiting viral assembly and release [
3]. To reduce infections, not only the use of antiviral drugs but also the decontamination of surfaces and personal protective equipment (PPE) is crucial. Surface and PPE disinfection have been reported using ultraviolet radiation, vaporized hydrogen peroxide, heat and liquid chemicals [
4]. Most of the described methods damage materials or do not reliably disinfect all parts of the surface, as with ultraviolet radiation that is ineffective on areas shielded from light. Alternatives are the use of more material-friendly methods, such as ozone [
5] or nanomaterial coatings.
The field of nanotechnology has grown very fast over the past decade, and nanomaterials are used in a variety of applications, such as electronics, cosmetics, water treatment, textile manufacturing and pharmaceuticals, due to their various physiochemical properties [
6,
7]. Nanoparticles are characterized by a large surface area compared to their particle size of 1–100 nm [
8], which enhances reactivity and interaction with viruses or other agents. They exhibit a broad spectrum of antiviral activities, such as the inhibiting receptor binding of virus particles to host cell surface structures, degrading viral particles through the generation of reactive oxygen species (ROS) or interacting with the viral envelope and degrading its structure [
9,
10,
11]. These properties make them highly interesting candidates for virus neutralization with therapeutics or surface coatings [
10].
Antiviral nanomaterials can be classified into the following groups: metal nanomaterials, metal oxide-based nano-photocatalysts and nonmetallic nanomaterials [
12]. Metallic nanomaterials like Ag, Cu, Au and Fe exhibit broad-spectrum antiviral activities which were described in different studies [
13,
14,
15,
16]. They can inhibit viral entry into the host cells, inactivate the viruses before cellular entry or can enter cells and block viral replication by inactivating viral nucleic acids [
11,
13,
16,
17].
Metal oxide-based nano-photocatalysts, such as ZnO or SnO
2, are often semiconductors and have an energetic band gap between the valence band (VB) and the conduction band (CB). When the photocatalyst is illuminated at a suitable wavelength, electrons (e
−) can be excited from the VB into the CB if the energy is greater than the band gap. Thereby, a positively charged hole (h
+) in which oxidation can take place is left in the VB of the photocatalyst. Hydroxyl radicals (
•OH) can be formed by a reaction with water. The electrons in the CB induce reductions of absorbed oxygen atoms. During the photocatalytic process, superoxide radicals (
•O
2−) and other reactive oxygen species (ROS) are formed [
18]. The metal oxide-based nanostructures can thus cause physical damage to the virus structure [
12,
19,
20]. The antiviral activity of materials such as titanium dioxide (TiO
2), tungsten trioxide (WO
3), copper (II) oxide (CuO), zinc oxide (ZnO) and tin (IV) oxide (SnO
2) against enveloped and non-enveloped viruses, including SARS-CoV-2, has been described in previous studies [
21].
The advantage of nonmetallic nanomaterials is their low toxicity compared to most metallic materials, which are heavy metals that may be harmful to human health and the environment. Their antiviral properties are based on electrostatic adsorption, nanometer size effect [
22] and photocatalytic oxidation, similar to metal nanoparticles. Materials used include carbon nanotubes, graphene-based materials and graphitic carbon nitrides [
12]. ZnO is generally recognized as safe by the FDA [
23] and was described as a promising versatile inorganic material with a broad range of applications [
8]. The antiviral activity of ZnO was already described for many different viruses, such as rhinovirus [
24], human immunodeficiency virus (HIV) [
25], hepatitis E and hepatitis C [
26], influenza [
27], herpes viruses [
28] and, recently, SARS-CoV-2 [
29]. In addition to their antiviral activities, ZnO nanoparticles (ZnO-NPs) have antimicrobial and UV-blocking properties and are used in many products, such as cosmetics, food, textiles, electronics and also in PPE [
30].
Compared to current knowledge on the antibacterial activity of ZnO-NPs [
8], data on their antiviral activity are limited. Therefore, the aim of the present study was to investigate the antiviral activity of two ZnO-NPs, ZnO-NP-45 and ZnO-NP-76, against SARS-CoV-2 including relevant variants in cell culture-based virus neutralization assays. The nanoparticles tested were prepared using an environmentally friendly, highly efficient method. The synthesis uses salts (nitrates, chlorates and sulfates) as a starting material and whey as a chelating agent instead of conventional analytical reagents [
31]. The results of this research may form the basis for the development of an antiviral coating for PPE and filters to improve protection and reduce the transmission of pathogens such as SARS-CoV-2.
3. Discussion
In this study, we examined the antiviral activity of two novel ZnO-NPs (ZnO-NP-45 and ZnO-NP-76) against the Delta and Omicron variants of SARS-CoV-2 in several independent cell-culture-based experimental series.
These nanomaterials are of great interest due to their physical and chemical properties, low cytotoxicity and sustainable manufacturing process, which allows an environmentally friendly “green” synthesis using salts (nitrates, chlorates and sulfates) as a starting material and whey as a chelating agent instead of conventional analytical reagents. Additionally, the manufacturing process allows doping of the ZnO with other antiviral materials, such as Ag or Cu, which could provide additional antiviral activities for future research and applications. In doped NPs, the atoms of the dopant are located inside the ZnO lattice and not on its surface. To our knowledge, such combinations have not yet been tested.
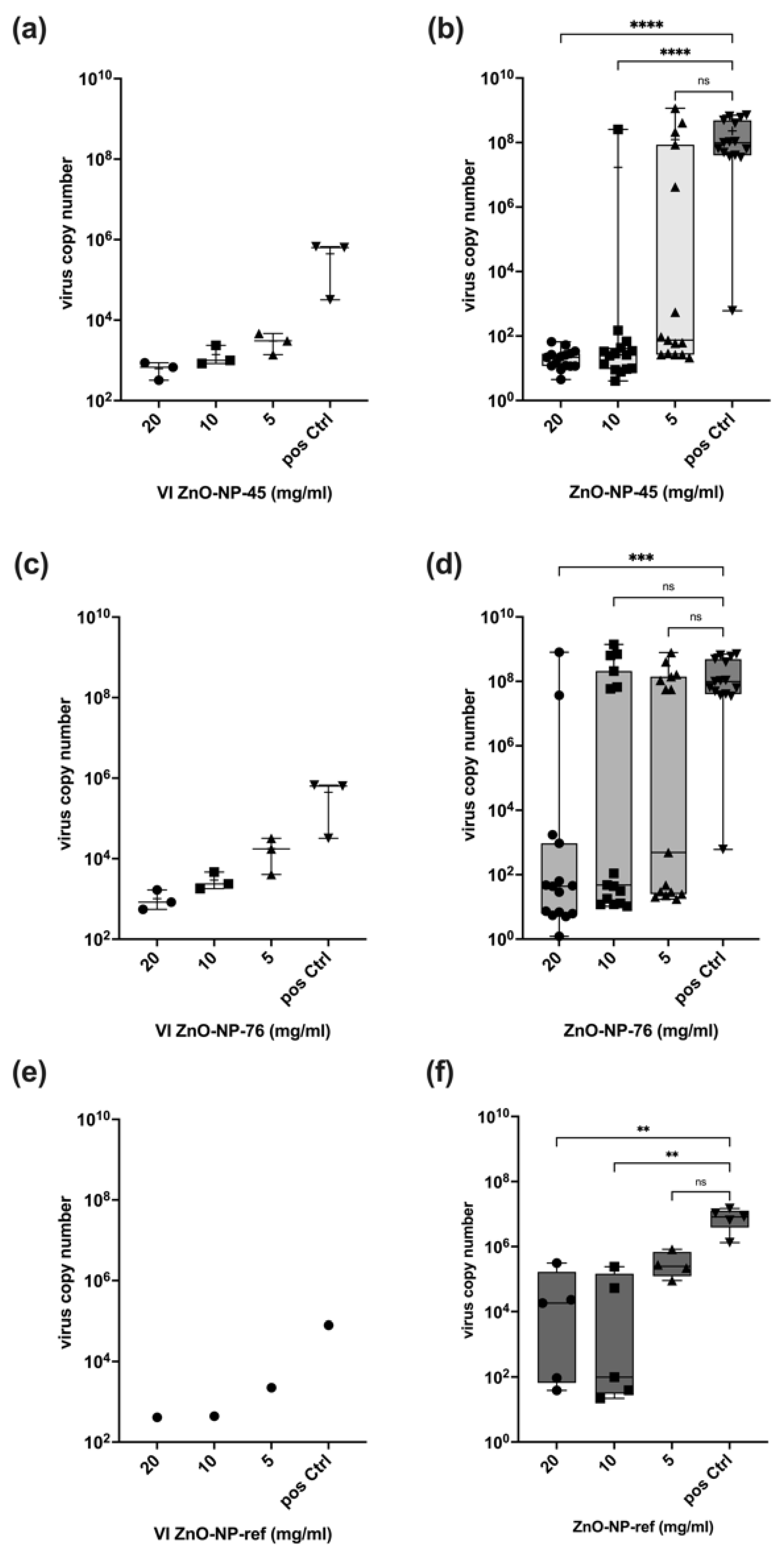
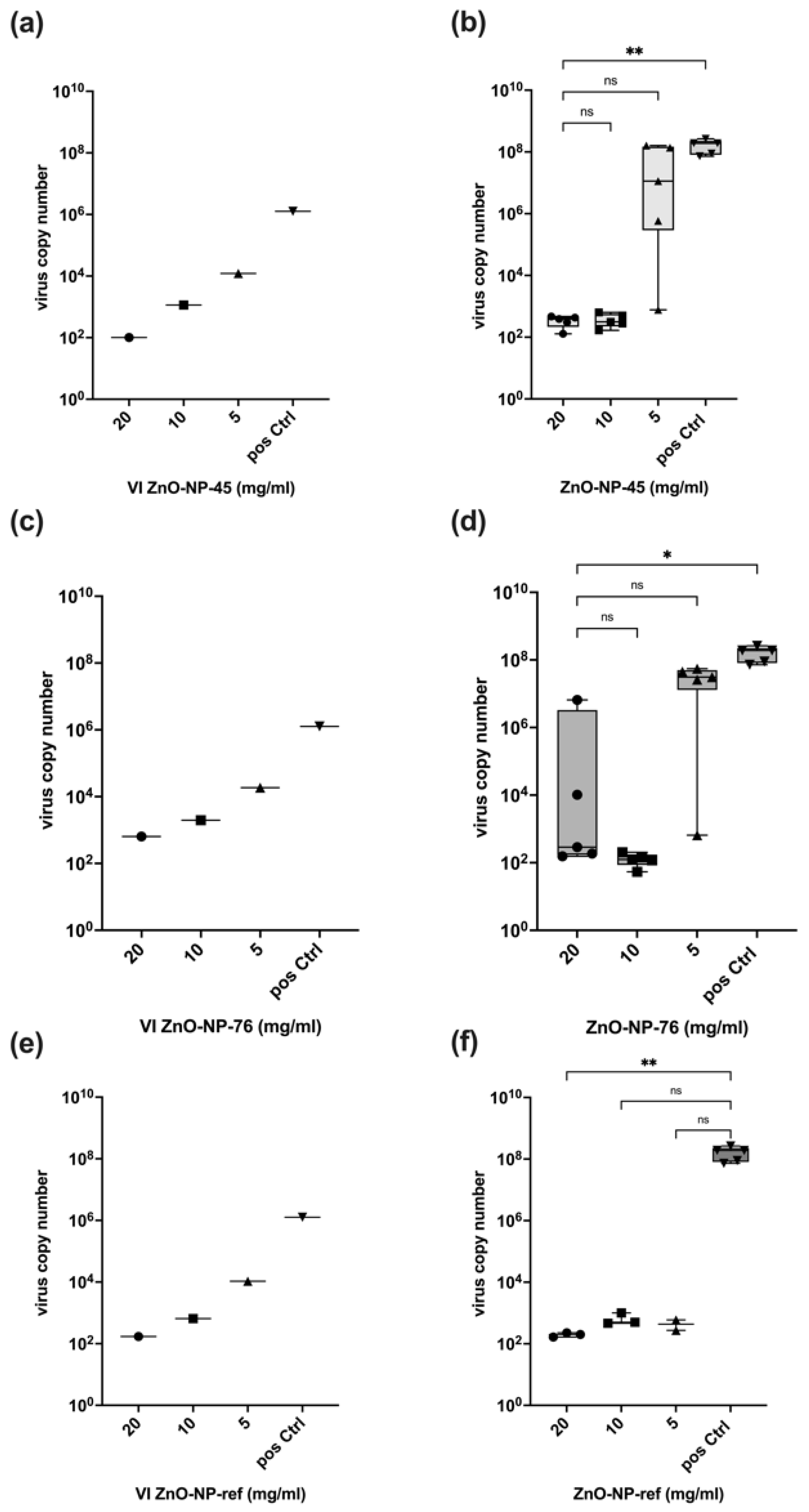
Our results show that ZnO-NP-45 inhibits replication of SARS-CoV-2 by a factor of up to 10
6 when incubated at a concentration of 20 mg/mL for 1 h prior to cell infection. This high factor of virus inactivation even exceeds the requirements for disinfectants since, according to the Robert Koch Institute (RKI) and the guideline of the German Association for the Control of Virus Diseases (DVV), a reduction of at least 10
4 is required for virus disinfection [
36]. Lower concentrations of ZnO-NP-45 or ZnO-NP-76 also reduced virus replication, but not in all samples tested. In particular, after treatment with ZnO-NP-76, large differences in virus inactivation were observed at all concentrations tested, ranging from a strong reduction of infectious virus to high replication. This heterogeneity could be explained by incomplete virus inactivation after nanoparticle pre-treatment as well as by secondary infections occurring during the virus neutralization assay. This may be the case because within the 48 h of incubation of infected cells, even a few virus-replicating cells produce sufficient virus particles that may lead to secondary infections of most cells resulting in very high virus copy numbers as determined by RT-qPCR and also seen in IHC.
The reference nanoparticles ZnO-NP-ref also reduced the number of infectious virus particles but seemed to exhibit a stronger antiviral effect on the Omicron variant, as shown in
Figure 5. Inactivation of infectious virus was observed for all concentrations and all replicates. With SARS-CoV-2 Delta, only a small number of replicates was inactivated at all NP concentrations. NP-ZnO-ref and NP-ZnO-76 are comparable in their inconsistent antiviral effect on the Delta variant, while 20 mg/mL ZnO-NP-45 resulted in an inactivation of infectious particles by a factor of 10
6 in all replicates. For the Omicron variant, a constant inactivation using ZnO-NP-45 was observed at 20 mg/mL and also at 10 mg/mL. Therefore, inactivation with 20 mg/mL ZnO-NP-45 seems to have the greatest effect on both SARS-CoV-2 variants tested.
We also noticed poor dispersion of the nanoparticles in the medium, leading to unstable suspensions, which made it difficult to standardize the experimental conditions, which may also contribute to some variations. To reduce this problem, each nanoparticle suspension was mixed immediately before use for each working step. During pre-treatment, the samples were shaken at 300 min−1 to keep as many particles as possible suspended and to minimize precipitation before centrifugation.
Pre-treatment of the virus with nanoparticles led to binding and co-precipitation of the virus and nanoparticles after centrifugation. This interaction was dose-dependent and reduced the VI used for the infection assay performed with the supernatant after pre-treatment and centrifugation. Although the pre-treatment and centrifugation reduced virus copy numbers by a factor of up to 104, the reduction of virus replication was up to a factor of 106, which indicates that in addition to adsorption to its surface, the nanoparticles tested reduced virus infectivity by further inactivation. This appears to be a chemical antiviral effect on the virus that could inhibit infectivity when incubated together for one hour. The reference NPs (ZnO-NP-ref) also demonstrated an antiviral effect, as the reduction in viral replication cannot be explained only by particle loss in the VI after pre-incubation and centrifugation. Whether nanoparticles that were not precipitated by centrifugation exert some additional effect on the cells used for the neutralization assay cannot be excluded. However, the metabolic interference assay performed excluded major interference with cell function at concentrations of up to 20 mg/mL nanoparticles.
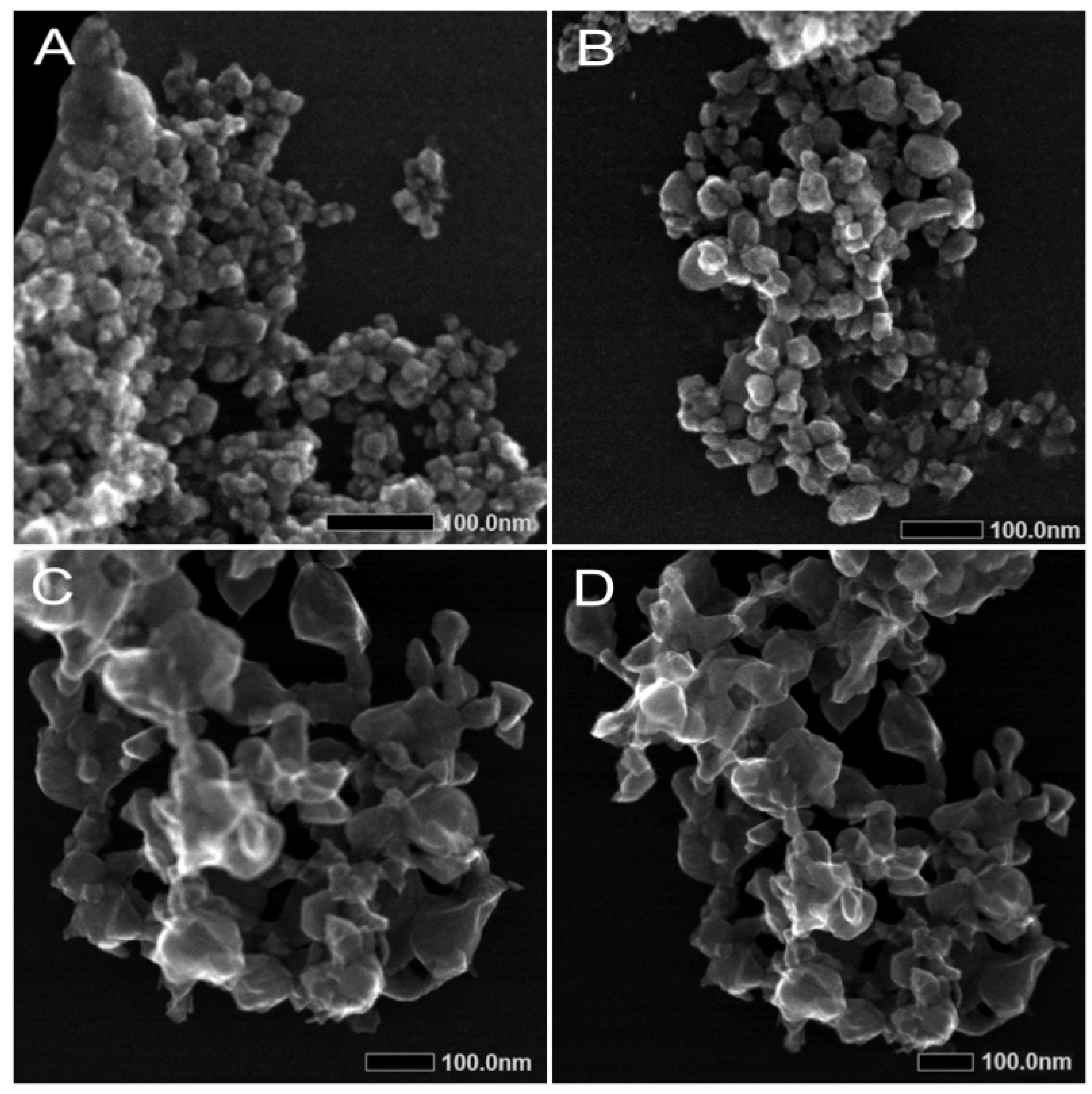
The different antiviral effects of the two nanoparticle preparations might be explained by their differences in size distribution. As revealed by TEM and DLS, ZnO-NP-45 is composed of particles with sizes from approximately 20 nm to 80 nm, whereas ZnO-NP-76 is monodispersed and contains particles of about 80 nm. The enhanced virus binding and neutralization activity of ZnO-NP-45 might, therefore, be a consequence of the greater total reactive surface than that of ZnO-NP-76.
Previous studies on ZnO-NP have primarily focused on their inhibitory effect on various bacteria. For this reason, data on the effect of ZnO-NP on viruses are less common in the literature and are not yet available for SARS-CoV-2 variants. One possible antiviral effect could be hydrolyzation and inactivation of SARS-CoV-2 bound to ZnO-NPs by the alkaline zinc oxide. Another observation was made for the influenza virus H1N1. ZnO-NPs, both uncoated and PEGylated, were found to inhibit the intracellular replication of the H1N1 virus after cell infection by a mechanism possibly related to the release of Zn
2+ ions [
27]. No comparable observations were made for SARS-CoV-2, but a similar mechanism can be expected and needs further research. The antiviral activity of ZnO-NPs on SARS-CoV-2 was already shown with VeroE6 cells, reporting severe damage to the viral envelope by free radicals causing oxidative stress to SARS-CoV-2 [
29]. Reactive oxygen species (ROS) generated through photocatalysis can destroy proteins, lipids, carbohydrates and nucleic acids, ultimately leading to viral inactivation [
37].
All of the mechanisms described here could be responsible for inactivating the virus, but there is no specific evidence of which chemical mechanism was responsible for the virus inactivation observed in our experiments.
In conclusion, the findings of this study clearly demonstrate a high antiviral activity of ZnO nanoparticles against the two SARS-CoV-2 variants Delta and Omicron, which is based on adsorption and an additional not yet defined antiviral effect. Particularly the ZnO-NP-45 nanomaterial, which is more active than the ZnO-NP-76, could be a promising material for future usage as a surface coating for antiviral PPE, especially coverall suits, face masks, and antiviral filters that could be used for air-conditioning and room ventilation systems.
4. Materials and Methods
4.1. Cell Culture
Calu-3 cells (Biomedica, Vienna, Austria) were cultured in Minimal Essential Medium (MEM) (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal calf serum (FCS) (Thermo Fisher Scientific, Waltham, MA, USA), 2% L-glutamine (Merck KGaA, Darmstadt, Germany) and 1% Penicillin–Streptomycin (PenStrep) (Thermo Fisher Scientific, Waltham, MA, USA). VeroE6 cells (Biomedica, Vienna, Austria) were cultured in Minimal Essential Medium (MEM) (Thermo Fisher Scientific, Waltham, MA, USA) containing 5% FCS (Thermo Fisher Scientific, Waltham, MA, USA), 2% L-glutamine (Merck KGaA, Darmstadt, Germany) and 1% PenStrep (Thermo Fisher Scientific, Waltham, MA, USA). All cells were cultured at 37 °C and 5% CO2.
4.2. Synthesis and Characterization of Zinc Oxide Nanoparticles
The two zinc oxide nanoparticle types, ZnO-NP-45 and ZnO-NP-76, were synthesized using a modified green sol-gel method, as described by Soares et al. in 2020 [
31]. In brief, ZnO nanoparticles (NPs) were successfully synthesized by a whey-assisted sol-gel method. The composition of the gel and the subsequent calcination temperature showed efficiency in controlling the growth of nanocrystals. The sustainable method proved to be highly efficient for the synthesis of crystalline ZnO-NPs. As reference, commercially available ZnO-NPs <100 nm particle size (Cat# 544906, Sigma-Aldrich, Saint Louis, MO, USA) were used in separate neutralization assays.
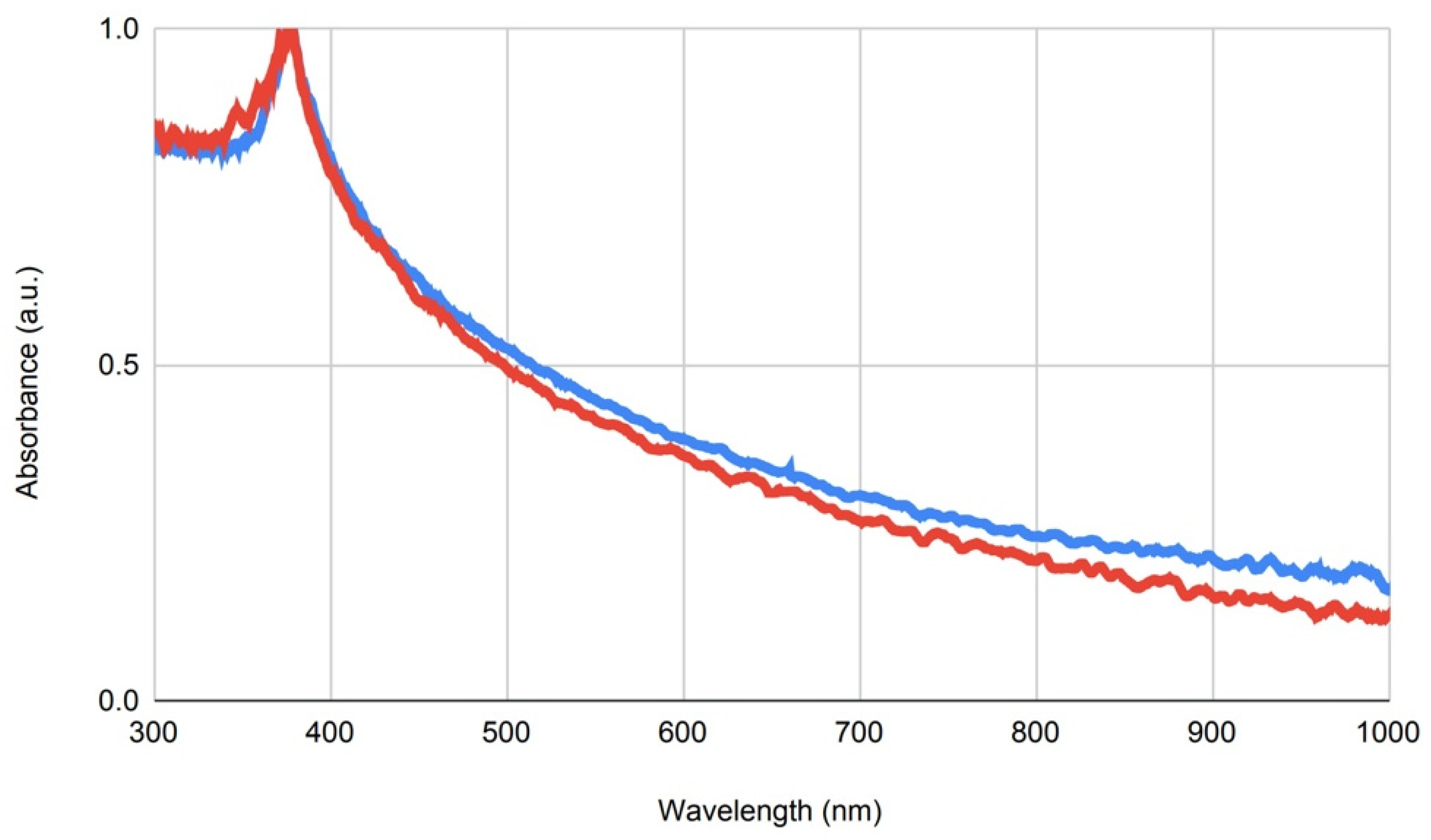
The hydrodynamic sizes and zeta potentials of the ZnO-NPs were evaluated using dynamic light scattering (DLS). A concentration of 100 µg/mL of NPs was prepared in Millipore water, and the measurements were performed using the ZetaSizerNano ZS (Malvern Panalytical, Malvern, UK) and the ZetaSizer Software (Malvern, 7.03, Malvern Panalytical, Malvern, UK), modulating the settings for refractive index of the NP composition and dispersant (2.0 for zinc oxide). The primary particle size and elemental composition of the ZnO-NPs were further determined by scanning transmission electron microscopy (STEM) and energy-dispersive X-ray spectroscopy (XPS). For measurement, 2 µL of a 10 µg/mL NP dispersion was dried overnight on a lacy carbon-coated copper TEM grid and imaged using the JEM F200 (JEOL, Freising, Germany) electron microscope in STEM mode operated at 200 kV. Primary particle size was determined by calculating the mean ± SD of minimum 10 particles via image processing with the ImageJ software (NIH, Bethesda, MD, USA) and manual measuring. EDX intensity maps were acquired with a beam current of 0.1 nA and a beam diameter of 0.16 nm.
4.3. Dispersion of Zinc Oxide Nanoparticles
NPs were washed with 70% EtOH and air dried. The required amount of each NP preparation was weighed into 5 mL Eppendorf tubes and suspended in MEM medium, initially without FCS, to avoid unspecific adsorption of the NPs to BSA and other proteins present in the serum. The tubes were mixed for 30 s. using a vortex mixer to minimize agglomeration. It was observed that the nanoparticles precipitate within a short time. Changes in pH or suspension in other solvents did not improve the stability of the NP suspensions. The tubes were mixed before each working step to keep the NPs in suspension during the experiments. During pre-incubation of NPs and SARS-CoV-2, tubes were gently shaken at 300 min−1.
4.4. Metabolic Interference Assay
The possible metabolic interference of ZnO-NP on Calu-3 (human lung epithelial cells) cells was evaluated using the resazurin reduction assay. Resazurin is a non-toxic dye that is reduced in metabolically active cells to fluorescent resorufin. Its concentration is measured fluorometrically at an excitation wavelength of 530 nm and an emission wavelength of 590 nm [
32,
33,
34]. The optimal excitation wavelength for this assay in our experiments was found to be 485 nm. A stock solution of 1 mM resazurin (Sigma Aldrich R7017-1G, Merck KGaA, Darmstadt, Germany) was prepared in phosphate-buffered saline (PBS) (Thermo Fisher Scientific, Waltham, MA, USA), sterile filtered using 0.2 µm syringe filters (Thermo Fisher Scientific, Waltham, MA, USA) and stored at 4 °C protected from light. To determine the possible metabolic interference, the resazurin assay was performed following the same conditions as the neutralization assays but without the virus (
Figure S1, Supplementary Files). Calu-3 cells were seeded into 48-well microtiter plates (Corning Incorporated, Kennebunk, ME, USA) at a density of 30,000 cells per well and incubated at 37 °C and 5% CO
2 48 h before the assay. Suspensions of decreasing ZnO-NP concentrations (100, 80, 40, 20, 10, 5 and 1 mg/mL) were prepared in MEM medium containing 2% L-glutamine and 1% Penicillin–Streptomycin. ZnO-NP were incubated in medium for 1 h at 37 °C and 300 min
−1 and subsequently centrifuged at 13,000×
g for 5 min. The supernatant was collected and added to Calu-3 cells at 37 °C and 5% CO
2 for 1 h. Then, the cells were washed with MEM and fresh medium containing 10% FCS, 2% L-glutamine and 1% Penicillin–Streptomycin, was added. Cells without ZnO-NP treatment were used as references. The plate was incubated at 37 °C and 5% CO
2 for 48 h. To measure the metabolic activity of the cells, resazurin was added to each well at a final concentration of 10 µM. The fluorescent signal was recorded at a wavelength of 485/590 nm over a time period of 2 h at 37 °C using a microplate reader (BioTek Synergy 4, Szabo-Scandic HandelsgmbH, Vienna, Austria). The cell metabolic activity was measured 48 h after ZnO-NP treatment for each concentration. Linear regression equations of the recorded signals were generated, and the resulting slopes were normalized to the reference and plotted in a graph.
4.5. Preparation of SARS-CoV-2 Virus Stocks
All work with infectious SARS-CoV-2 was performed under biosafety level (BSL)-3 conditions [
38]. The experimental series was performed using a SARS-CoV-2 Delta virus patient isolate (isolated at the Diagnostic and Research Institute of Pathology, Graz, Austria, GISAID accession number EPI_ISL_4847176 delta-like variant) and SARS-CoV-2 Omicron (Strain HCOV-19/Netherlands/NH-EMC-1720/2021, Omicron variant, Lineage B.1.1.529—Calu 3 culture, Erasmus-MC). For propagation of the SARS-CoV-2 viruses, VeroE6 cells were infected with the different isolates and incubated at 37 °C and 5% CO
2 for 72 h. To release intracellular viral particles from adherent cells in the cell culture flasks, cells were lysed by a freeze and thaw cycle, followed by a centrifugation step (10 min at 3000×
g) to remove cell debris and sterile filtered with 0.2 µm syringe filters (Thermo Fisher Scientific, Waltham, MA, USA). Virus stocks were stored at −80 °C until use. The titer of the virus stock was determined using a focus-forming assay. Briefly, VeroE6 cells were seeded in 48-well cell culture plates at a density of 30,000 cells per well and infected with 200 µL of the 10-fold serially diluted virus for 1 h at 37 °C and 5% CO
2. Each dilution was tested in triplicate. After infection, the cells were washed with MEM, and 500 µL of overlay medium consisting of 1.5% carboxymethylcellulose sodium salt (Merck KGaA, Darmstadt, Germany) in MEM containing 2% FCS and 1% PenStrep was added to each well. After 72 h of incubation at 37 °C and 5% CO
2, the cells were fixed with 4% neutral-buffered formalin (SAV Liquid Production GmbH, Flintsbach am Inn, Germany) for 30 min followed by antibody staining according to the immunohistochemistry protocol below to determine the number of infected cells.
4.6. Immunohistochemistry (IHC)
For immunohistochemical staining, SARS-CoV-2 infected and control cells were fixed for at least 30 min with 4% neutral-buffered formalin (SAV Liquid Production GmbH, Flintsbach am Inn, Germany) and washed 3 times with PBS (Gatt-Koller GmbH, Absam, Austria). The cells were then incubated with 0.1% Triton X-100 (Merck KGaA, Darmstadt, Germany) for 10 min, washed 3 times with PBS and incubated for 30 min in 3% H2O2 (Merck KGaA, Darmstadt, Germany) dissolved in methanol (Merck KGaA, Darmstadt, Germany) and washed again with PBS. The primary antibody, SARS-CoV-2 nucleocapsid antibody (Sino Biological, Bejing, China, Cat# 40143-V08B), diluted at 1:1000, was added to the cells and incubated at RT for 1 h. The cells were washed 3 times with PBS, and the ready-to-use detection system reagent EnVision Detection Systems, Peroxidase/DAB, Rabbit/Mouse (Agilent Dako, Glostrup, Denmark, Cat# K5007) was added for 30 min, followed by 3 washing steps with PBS. DAB + Chromogen X50 (Agilent Dako, Cat# K5007) was applied to each well to visualize bound secondary antibodies. The reaction was stopped by adding PBS. Cells were washed again with PBS to remove reagent, and fresh PBS was added to keep them humid. Images were taken by light microscope (Nikon, Eclipse, TS100; Nikon Europe BV, Amsterdam, The Netherlands) equipped with a JENOPTIK GRYPHAX® camera (Breitschopf, Innsbruck, Austria). SARS-CoV-2 infected cells appear red after antibody staining.
4.7. SARS-CoV-2 Neutralization Assays
To determine the neutralizing effect of ZnO-NP on SARS-CoV-2, Calu-3 cells were seeded into 48-well cell culture plates (30,000 cells/well) two days prior to the assay.
Based on the cell viability measurement, the following ZnO-NP concentrations were chosen: 20, 10 and 5 mg/mL (in MEM, without FCS). SARS-CoV-2 virus at an MOI (multiplicity of infection) of 0.002 was added to the prepared NP solutions and incubated for 1 h at 37 °C and 300 min−1. Afterward, the samples were centrifuged for 5 min at 13,000× g, and supernatants were transferred to fresh tubes. To determine the virus input (VI) for cell infection, 140 µL of each supernatant was collected for RNA isolation and RT-qPCR (reverse transcription quantitative polymerase chain reaction). Five wells per supernatant tube were infected for 1 h at 37 °C and 5% CO2 to test each NP concentration in five replicates. Virus without NP pre-treatment was used as positive control, and non-infected cells served as negative control for the assay.
After infection, the cells were washed with MEM (no FCS), and fresh MEM (10% FCS) was added to the cells. After 48 h incubation at 37 °C and 5% CO
2, supernatant of each well was collected to determine virus concentrations at t48 (timepoint 48 h after infection) via RNA isolation and RT-qPCR. A schematic workflow of the neutralization assay is shown in
Figure S2 (Supplementary Files).
4.8. RNA Isolation and Reverse Transcription Quantitative PCR (RT-qPCR)
SARS-CoV-2 viral RNA from cell culture supernatant was extracted using the QIAamp
® Viral RNA Mini Kit (Qiagen, GmbH, Hilden, Germany) according to the manufacturer’s recommendations. RNA samples were eluted with 40 µL Milli-Q water and stored at −80 °C. Viral replication was detected via RT-qPCR using a Rotor-Gene Q thermal cycler (Qiagen) and the QuantiTect
®Probe PCR Kit (Qiagen). Forward primer 2019-nCoV_N2-F (5′-TTA CAA ACA TTG GCC GCA AA-3′), reverse primer 2019-nCoV-N2-R (5′-GCG CGA CAT TCC GAA GAA-3′) and the N2 probe 2019-nCoV_N2-P (FAM-ACA ATT TGC CCC CAG CGC TTC AG-BHQ1) were obtained from Eurofins Genomics (Ebersberg, Germany). The primer and probe sequences were used as recommended by the Centers for Disease Control and Prevention (CDC) in February 2020 [
39]. The total volume of each RT-qPCR reaction was 25 µL. The thermal profile was 50 °C for 30 min, followed by 95 °C for 15 min and 45 cycles of 95 °C for 3 s and 55 °C for 30 s.
4.9. Data Analysis
Data analysis, copy number calculations, statistics and graphical presentations were performed with Dotmatics GraphPad Prism 9 (Boston, Massachusetts). Statistical differences between groups were determined using Kruskal–Wallis test corrected for multiple comparisons. Symbol meaning are: ns =
p > 0.05, * =
p ≤ 0.05, ** =
p ≤ 0.01, *** =
p ≤ 0.001, **** =
p ≤ 0.0001. The virus copy numbers were calculated using a calibration curve based on a certified RNA standard (VR-1986D
TM from 2019 Novel Coronavirus, Lot: 70035624, ATCC, Glasgow, UK). This commercially available standard, containing 4.73 × 10
3 genome copy numbers per 1 µL, was serially diluted and analyzed by RT-qPCR. The resulting cq-values were plotted against ln[copy numbers], and the equation obtained from a simple linear regression analysis was used to calculate the copy numbers from the cq-values. The standard curve to calculate between cq-values and virus copy numbers is the equation: