Chronic Administration of Diethylnitrosamine and 2-Acetylaminofluorene Induces Hepatocellular Carcinoma in Wistar Rats
Abstract
1. Introduction
2. Results
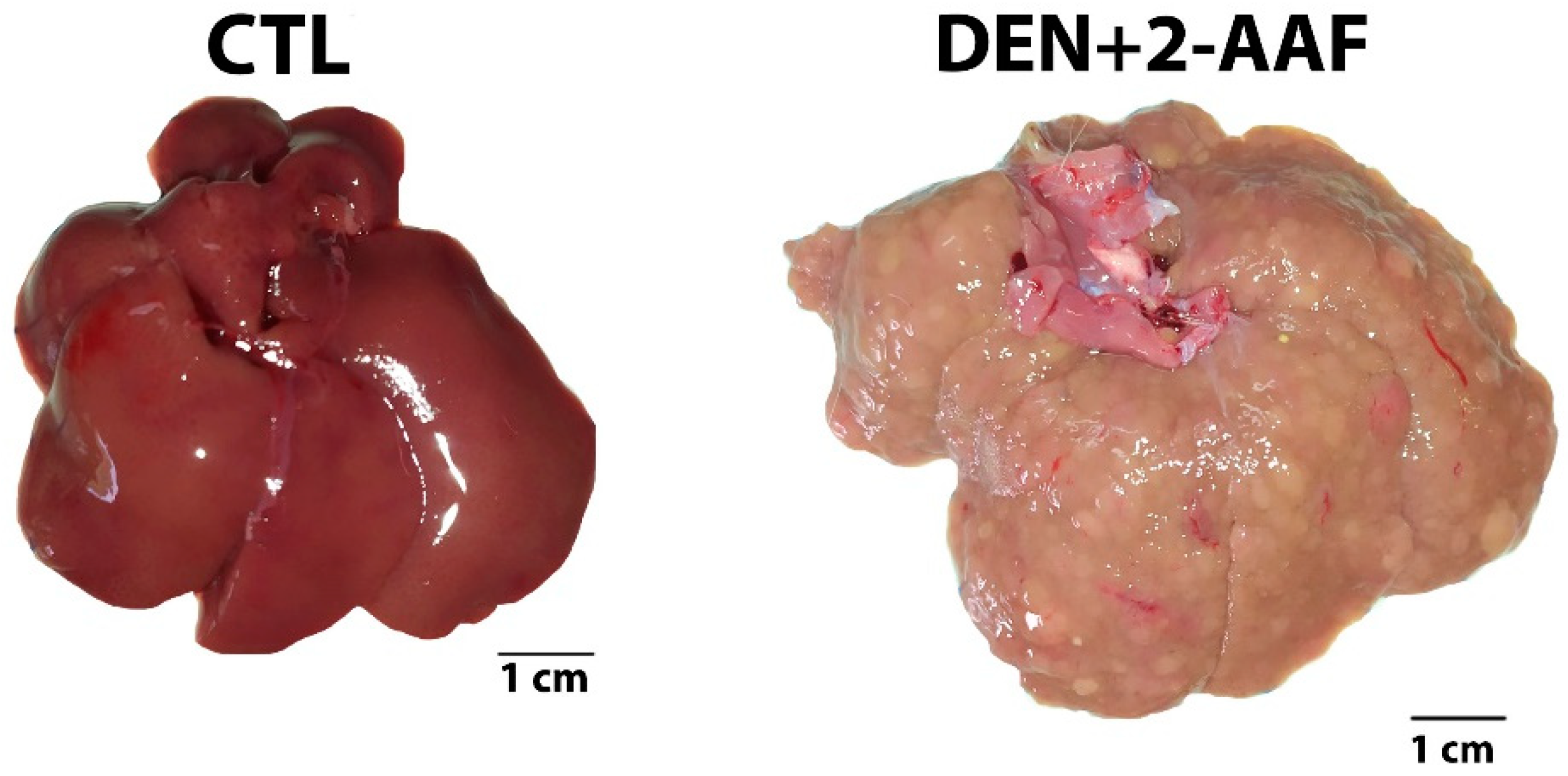
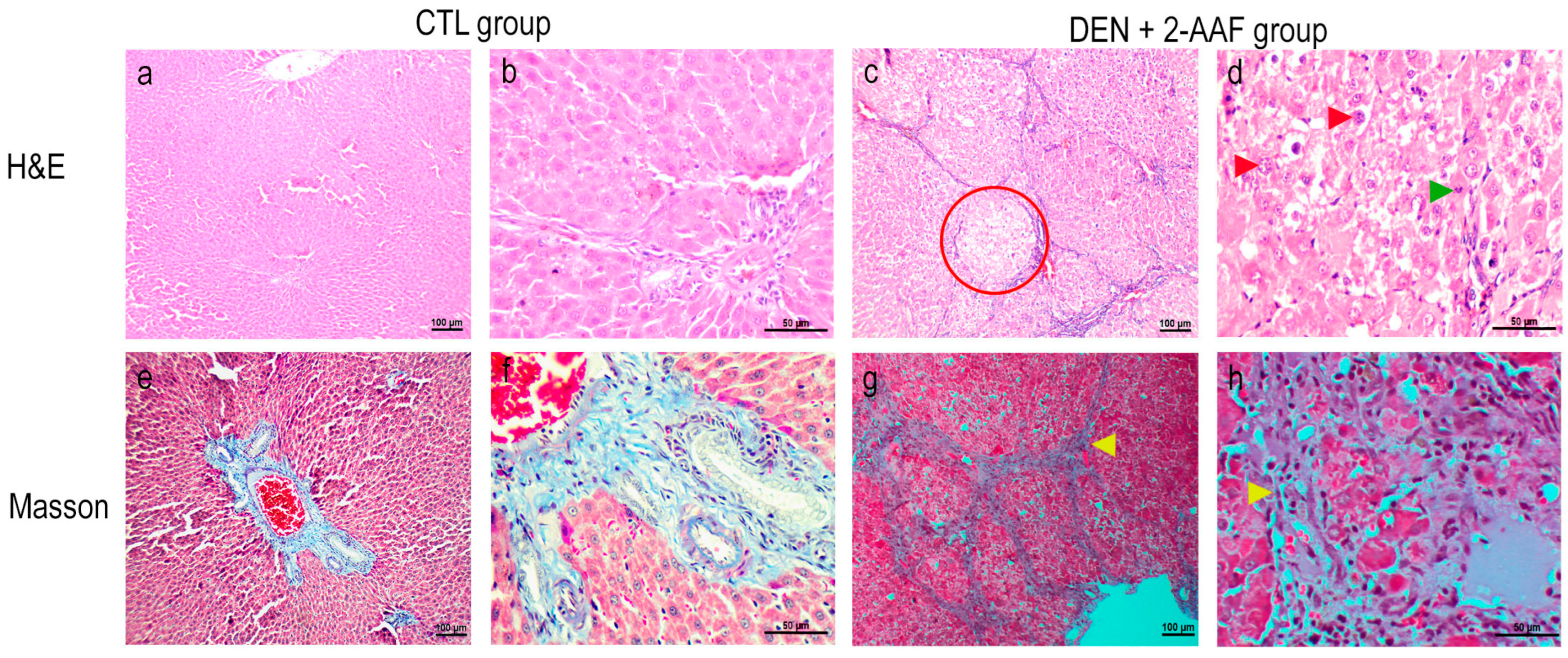
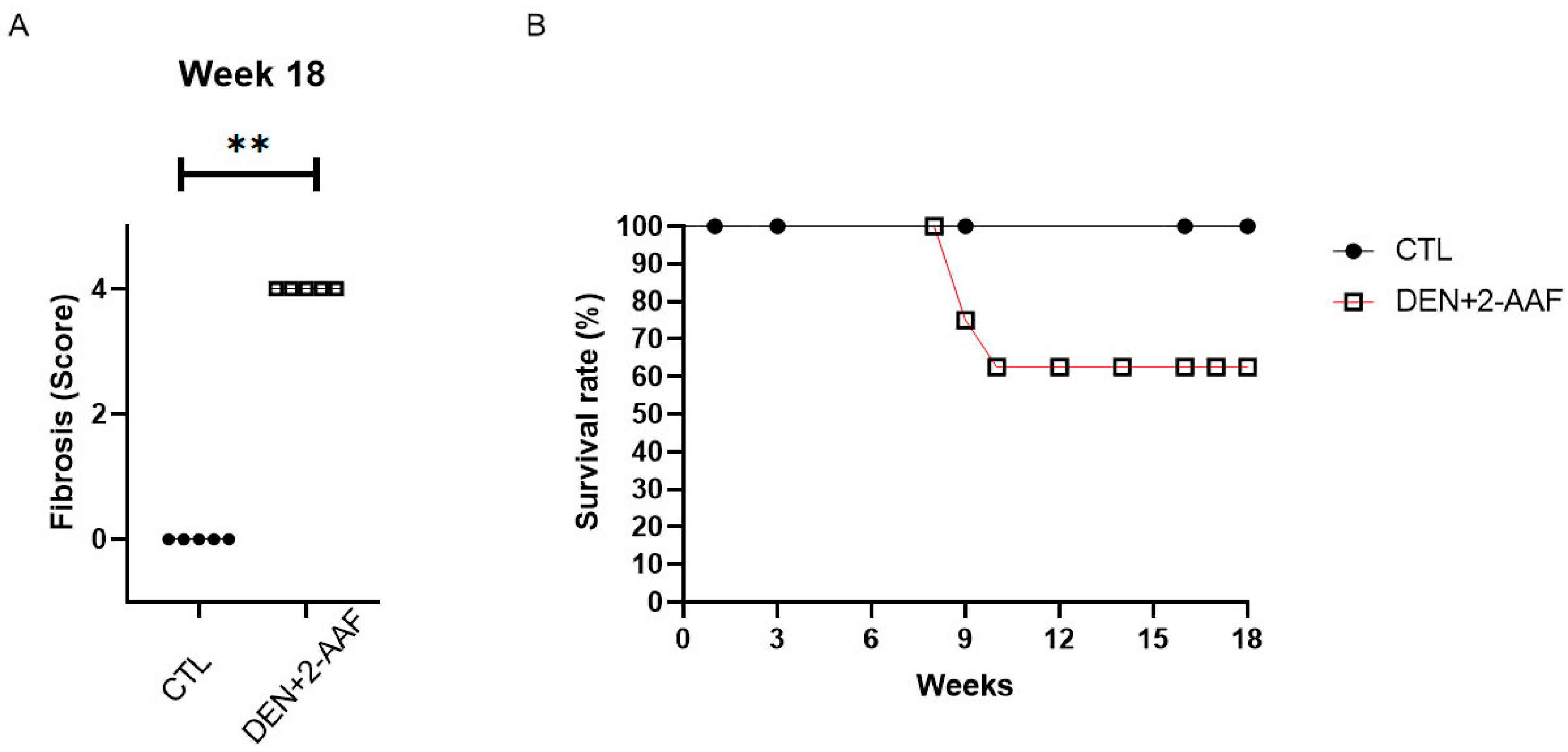
2.1. Macroscopic and Histological Findings
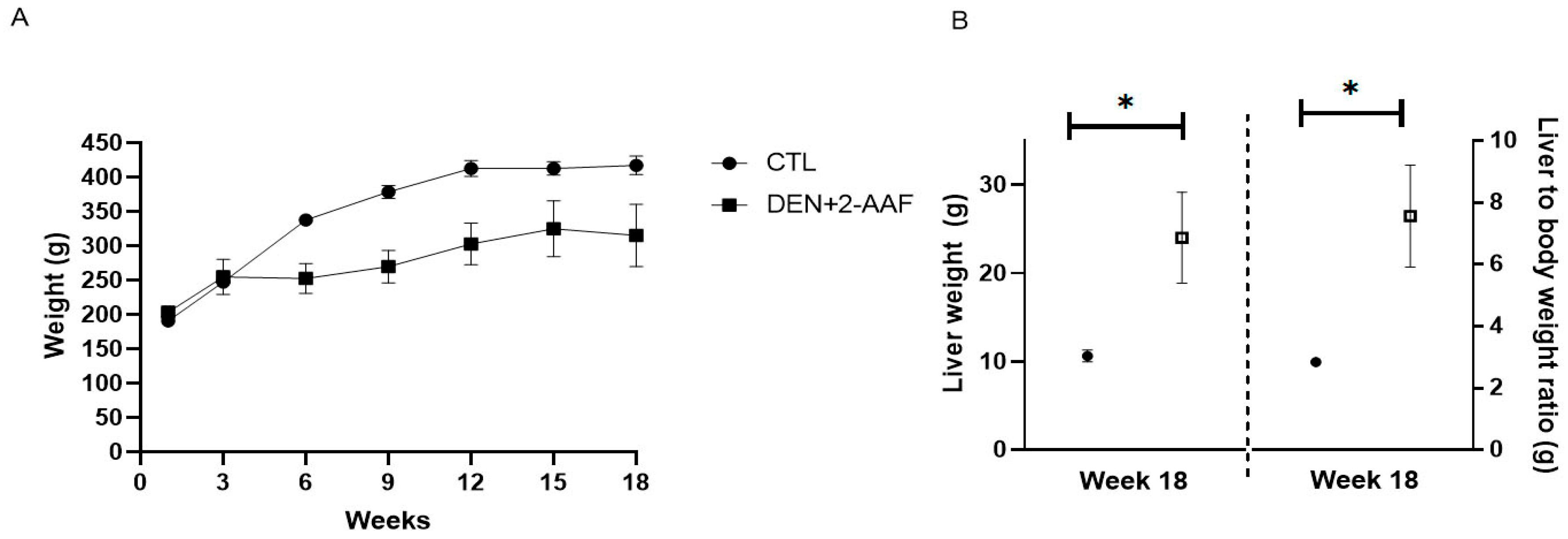
2.2. Serum Biochemistry Assays
2.3. Changes in mRNA Expression Induced by DEN+2-AAF Treatment
3. Discussion
4. Materials and Methods
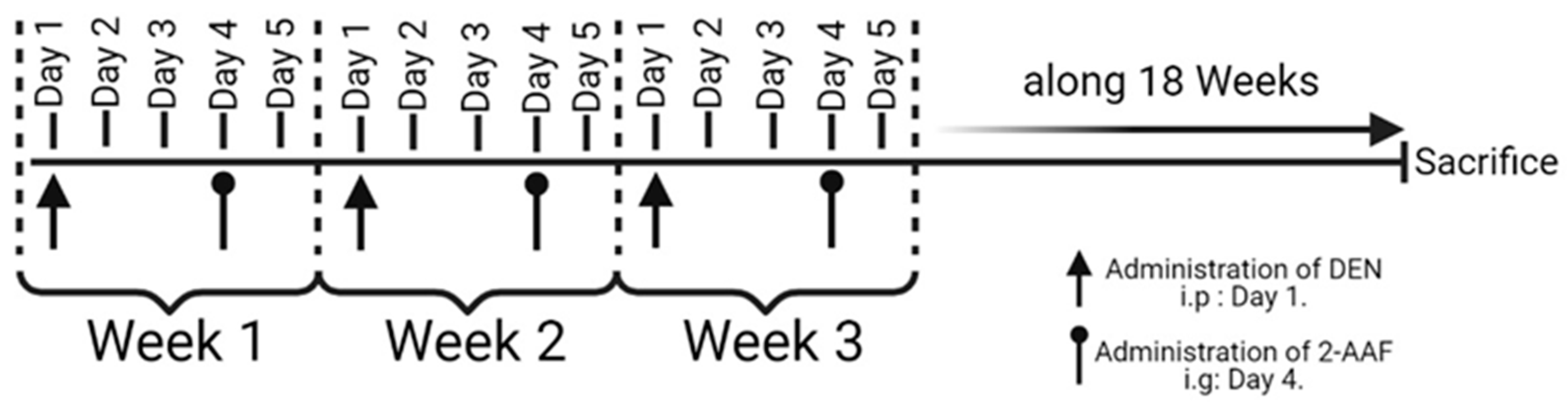
4.1. Hepatocellular Carcinoma Model Chemical Induction
4.2. Clinical Biochemistry
4.3. Histological Analyses
4.4. Total RNA Extraction and cDNA Synthesis
4.5. Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tunissiolli, N.M.; Castanhole-Nunes, M.M.U.; Biselli-Chicote, P.M.; Pavarino, E.C.; da Silva, R.F.; da Silva, R.C.; Goloni-Bertollo, E.M. Hepatocellular Carcinoma: A Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. Asian Pac. J. Cancer Prev. 2017, 18, 863–872. [Google Scholar] [PubMed]
- Koulouris, A.; Tsagkaris, C.; Spyrou, V.; Pappa, E.; Troullinou, A.; Nikolaou, M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J. Hepatocell. Carcinoma 2021, 8, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Monroy-Ramirez, H.C.; Campos-Valdez, M.; Sanchez-Meza, J.; Sanchez-Orozco, L.; Armendariz-Borunda, J. Hepatocellular carcinoma and hepatitis C virus infection in Latin America: Epidemiology, diagnosis and treatment. Hepatoma Res. 2020, 6, 20. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Z.Y.; Hou, J.X. Hepatocellular carcinoma: Insight from animal models. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Jilkova, Z.; Kurma, K.; Decaens, T. Animal Models of Hepatocellular Carcinoma: The Role of Immune System and Tumor Microenvironment. Cancers 2019, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- IARC/WHO. GLOBOCAN 2021: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Cancer Today Data Base, Population Fact Sheets. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 20 March 2021).
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Ioannou, G.N. Epidemiology and risk-stratification of NAFLD-associated HCC. J. Hepatol. 2021, 75, 1476–1484. [Google Scholar] [CrossRef]
- Verna, L.; Whysner, J.; Williams, G.M. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996, 71, 57–81. [Google Scholar] [CrossRef]
- Schulien, I.; Hasselblatt, P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods Cell Biol. 2021, 163, 137–152. [Google Scholar]
- Heflich, R.H.; Neft, R.E. Genetic toxicity of 2-acetylaminofluorene, 2-aminofluorene and some of their metabolites and model metabolites. Mutat. Res. 1994, 318, 73–114. [Google Scholar] [CrossRef]
- Hasanin, A.H.; Habib, E.K.; El-Gayar, N.; Matboli, M. Promotive action of 2-acetylaminofluorene on hepatic precancerous lesions initiated by diethylnitrosamine in rats: Molecular study. World J. Hepatol. 2021, 13, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Kriek, E. Fifty years of research on N-acetyl-2-aminofluorene, one of the most versatile compounds in experimental cancer research. J. Cancer Res. Clin. Oncol. 1992, 118, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.P.; Colaço, A.A.; Oliveira, P.A. Animal models as a tool in hepatocellular carcinoma research: A Review. Tumour Biol. 2017, 39, 1010428317695923. [Google Scholar] [CrossRef] [PubMed]
- Tolba, R.; Kraus, T.; Liedtke, C.; Schwarz, M.; Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 2015, 49 (Suppl. S1), 59–69. [Google Scholar] [CrossRef]
- Ghufran, H.; Azam, M.; Mehmood, A.; Butt, H.; Riazuddin, S. Standardization of diethylnitrosamine-induced hepatocellular carcinoma rat model with time based molecular assessment. Exp. Mol. Pathol. 2021, 123, 104715. [Google Scholar] [CrossRef]
- Zhang, H.E.; Henderson, J.M.; Gorrell, M.D. Animal models for hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 993–1002. [Google Scholar] [CrossRef]
- Castro-Gil, M.P.; Sánchez-Rodríguez, R.; Torres-Mena, J.E.; López-Torres, C.D.; Quintanar-Jurado, V.; Gabiño-López, N.B.; Villa-Treviño, S.; Del-Pozo-Jauner, L.; Arellanes-Robledo, J.; Pérez-Carreón, J.I. Enrichment of progenitor cells by 2-acetylaminofluorene accelerates liver carcinogenesis induced by diethylnitrosamine in vivo. Mol. Carcinog. 2021, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Leroy, K.; Costa, C.J.S.; Prata, G.B.; Vanderborght, B.; da Silva, T.C.; Barbisan, L.F.; Andraus, W.; Devisscher, L.; Câmara, N.O.S.; et al. In Vivo and In Vitro Models of Hepatocellular Carcinoma: Current Strategies for Translational Modeling. Cancers 2021, 13, 5583. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Bai, X.L.; Liang, T.B. Intratumoral heterogeneity of hepatocellular carcinoma: From single-cell to population-based studies. World J. Gastroenterol. 2020, 26, 3720–3736. [Google Scholar] [CrossRef]
- Hoshida, Y.; Nijman, S.M.; Kobayashi, M.; Chan, J.A.; Brunet, J.P.; Chiang, D.Y.; Villanueva, A.; Newell, P.; Ikeda, K.; Hashimoto, M.; et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009, 69, 7385–7392. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Dow, M.; Pyke, R.M.; Tsui, B.Y.; Alexandrov, L.B.; Nakagawa, H.; Taniguchi, K.; Seki, E.; Harismendy, O.; Shalapour, S.; Karin, M.; et al. Integrative genomic analysis of mouse and human hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2018, 115, E9879–E9888. [Google Scholar] [CrossRef]
- De Minicis, S.; Kisseleva, T.; Francis, H.; Baroni, G.S.; Benedetti, A.; Brenner, D.; Alvaro, D.; Alpini, G.; Marzioni, M. Liver carcinogenesis: Rodent models of hepatocarcinoma and cholangiocarcinoma. Dig. Liver Dis. 2013, 45, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Nakayama, S.M.; Watanabe, K.P.; Kawai, Y.K.; Ohno, M.; Ikenaka, Y.; Ishizuka, M. Strain differences in cytochrome P450 mRNA and protein expression, and enzymatic activity among Sprague Dawley, Wistar, Brown Norway and Dark Agouti rats. J. Vet. Med. Sci. 2016, 78, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.A.; Korkola, J.E.; Archer, M.C. Tissue-specific resistance to cancer development in the rat: Phenotypes of tumor-modifier genes. Carcinogenesis 2002, 23, 1–9. [Google Scholar] [CrossRef]
- Newton, J.F.; Bailie, M.B.; Hook, J.B. Acetaminophen nephrotoxicity in the rat. Renal metabolic activation in vitro. Toxicol. Appl. Pharmacol. 1983, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Han, J.; Xing, H.; Zhang, H.; Li, Z.; Liang, L.; Li, C.; Dai, S.; Wu, M.; Shen, F.; et al. Dysregulated fatty acid metabolism in hepatocellular carcinoma. Hepat. Oncol. 2016, 3, 241–251. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Goliasch, G.; Wurm, R.; Strunk, G.; Cho, A.; Novak, J.F.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Subclinical involvement of the liver is associated with prognosis in treatment naïve cancer patients. Oncotarget 2017, 8, 81250–81260. [Google Scholar] [CrossRef]
- Hann, H.W.; Wan, S.; Myers, R.E.; Hann, R.S.; Xing, J.; Chen, B.; Yang, H. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PLoS ONE 2012, 7, e47687. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Mohamed, Y.; El-Shetry, E.S.; Elsayed, S.A.; Abo Gazia, M.; Abdel-Aleem, G.A.; Shafik, N.M.; Abdo, W.S.; El-Desouki, N.I.; Basyony, M.A. Melatonin maximizes the therapeutic potential of non-preconditioned MSCs in a DEN-induced rat model of HCC. Biomed. Pharmacother. 2019, 114, 108732. [Google Scholar] [CrossRef]
- You, Y.; Zhu, F.; Li, Z.; Zhang, L.; Xie, Y.; Chinnathambi, A.; Alahmadi, T.A.; Lu, B. Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomed. Pharmacother. 2021, 137, 111335. [Google Scholar] [CrossRef] [PubMed]
- Yassin, N.Y.S.; AbouZid, S.F.; El-Kalaawy, A.M.; Ali, T.M.; Almehmadi, M.M.; Ahmed, O.M. Silybum marianum total extract, silymarin and silibinin abate hepatocarcinogenesis and hepatocellular carcinoma growth via modulation of the HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2022, 145, 112409. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.F.; Wu, Z.H.; Wei, Y.J.; Shu, L.; Peng, Y.R. Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine. J. Cancer Res. Clin. Oncol. 2017, 143, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, S.; Shokri, S.; Taherkhani, R.; Farshadpour, F. Hepatitis C virus core protein modulates several signaling pathways involved in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Huang, W.; Huang, C.; Luo, Z.; Yan, X. Contextual Regulation of TGF-β Signaling in Liver Cancer. Cells 2019, 8, 1235. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, S.; Wang, N.; Guan, L.; Shao, C.; Lin, Y.; Liu, J.; Li, Y. High Expression of TGF-β1 Contributes to Hepatocellular Carcinoma Prognosis via Regulating Tumor Immunity. Front. Oncol. 2022, 12, 861601. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Fowell, A.J.; Collins, J.E.; Duncombe, D.R.; Pickering, J.A.; Rosenberg, W.M.; Benyon, R.C. Silencing tissue inhibitors of metalloproteinases (TIMPs) with short interfering RNA reveals a role for TIMP-1 in hepatic stellate cell proliferation. Biochem. Biophys. Res. Commun. 2011, 407, 277–282. [Google Scholar] [CrossRef]
- Sánchez-Pérez, Y.; Carrasco-Legleu, C.; García-Cuellar, C.; Pérez-Carreón, J.; Hernández-García, S.; Salcido-Neyoy, M.; Alemán-Lazarini, L.; Villa-Treviño, S. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005, 217, 25–32. [Google Scholar] [CrossRef]
- Solt, D.B.; Medline, A.; Farber, E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am. J. Pathol. 1977, 88, 595–618. [Google Scholar]
- Sivalingam, K.; Amirthalingam, V.; Ganasan, K.; Huang, C.Y.; Viswanadha, V.P. Neferine suppresses diethylnitrosamine-induced lung carcinogenesis in Wistar rats. Food Chem. Toxicol. 2019, 123, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hu, Y.; Ingelman-Sundberg, M. Mechanisms of down-regulation of CYP2E1 expression by inflammatory cytokines in rat hepatoma cells. J. Pharmacol. Exp. Ther. 2003, 304, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, X.; Feng, J.; Mao, J.; Zhang, Q.; He, M.; Mi, Y.; Mei, Y.; Jin, G.; Zhang, H. CYP2E1 plays a suppressive role in hepatocellular carcinoma by regulating Wnt/Dvl2/β-catenin signaling. J. Transl. Med. 2022, 20, 194. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Fahim, S.A.; Tadros, S.A.; Badary, O.A. Suppressive effects of thymoquinone on the initiation stage of diethylnitrosamine hepatocarcinogenesis in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23078. [Google Scholar] [CrossRef]
- Wang, R.; Yin, C.; Li, X.X.; Yang, X.Z.; Yang, Y.; Zhang, M.Y.; Wang, H.Y.; Zheng, X.F. Reduced SOD2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging 2016, 8, 1184–1200. [Google Scholar] [CrossRef]
- Min, J.Y.; Lim, S.O.; Jung, G. Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett. 2010, 584, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, N.G.; Mohamed, W.R.; Mahmoud, H.S.; Alqarni, M.A.; Naguib, I.A.; Fahmy, A.M.; Ahmed, O.M. Isatin Counteracts Diethylnitrosamine/2-Acetylaminofluorene-Induced Hepatocarcinogenesis in Male Wistar Rats by Upregulating Anti-Inflammatory, Antioxidant, and Detoxification Pathways. Antioxidants 2022, 11, 699. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ahmed, A.A.; Fahim, H.I.; Zaky, M.Y. Quercetin and naringenin abate diethylnitrosamine/acetylaminofluorene-induced hepatocarcinogenesis in Wistar rats: The roles of oxidative stress, inflammation and cell apoptosis. Drug Chem. Toxicol. 2022, 45, 262–273. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Tsung, A.; Huang, H.; Du, Q.; Yang, M.; Deng, M.; Xiong, S.; Wang, X.; Zhang, L.; et al. iNOS promotes CD24+CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc. Natl. Acad. Sci. USA 2018, 115, E10127–E10136. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Althobaiti, F.; Omran, A.F.; Eldomany, E.B.; El-Shazly, S.A.; Alharthi, F.; Elkattawy, A.M.; Kahilo, K.A.A.; Dorghamm, D.A. The chemoprevention of spirulina platensis and garlic against diethylnitrosamine induced liver cancer in rats via amelioration of inflammatory cytokines expression and oxidative stress. Toxicol. Res. 2021, 11, 22–31. [Google Scholar] [CrossRef]
- Wiest, R.; Groszmann, R.J. The paradox of nitric oxide in cirrhosis and portal hypertension: Too much, not enough. Hepatology 2002, 35, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; El-Beih, N.M.; Swellam, M.; El-Hussieny, E.A. Pomegranate juice and punicalagin-mediated chemoprevention of hepatocellular carcinogenesis via regulating miR-21 and NF-κB-p65 in a rat model. Cancer Cell Int. 2022, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- Pocasap, P.; Weerapreeyakul, N.; Wongpoomchai, R. Chemopreventive Effect of Cratoxylum formosum (Jack) ssp. pruniflorum on Initial Stage Hepatocarcinogenesis in Rats. Molecules 2021, 26, 4235. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.F.; Huang, Y.L.; Xie, Y.K.; Tan, Y.H.; Chen, B.C.; Zhou, M.T.; Shi, H.Q.; Yu, Z.P.; Song, Q.T.; Zhang, Q.Y. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med. Oncol. 2011, 28, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, C.; Li, B.; Chen, J.; Peng, J.; Cao, Y.; Yue, Y.; Mai, X.; Yu, D. Combined clinical features and MRI parameters for the prediction of VEGFR2 in hepatocellular carcinoma patients. Front. Oncol. 2022, 12, 961530. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Cui, A.; Yu, K.; Huang, C.; Zhu, M.; Chen, M. Development of a Genetic and Clinical Data-Based (GC) Risk Score for Predicting Survival of Hepatocellular Carcinoma Patients After Tumor Resection. Cell. Physiol. Biochem. 2018, 48, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Long, X.; Zhang, J.; Huang, X.; Aa, J.; Yang, H.; Chen, Z.; Xing, J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J. Hepatol. 2015, 63, 1378–1389. [Google Scholar] [CrossRef]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef]
- Carr, B.I.; Akkiz, H.; Üsküdar, O.; Yalçın, K.; Guerra, V.; Kuran, S.; Karaoğullarından, Ü.; Altıntaş, E.; Özakyol, A.; Tokmak, S.; et al. HCC with low- and normal-serum alpha-fetoprotein levels. Clin. Pract. 2018, 15, 453–464. [Google Scholar]
- Bağırsakçı, E.; Şahin, E.; Atabey, N.; Erdal, E.; Guerra, V.; Carr, B.I. Role of Albumin in Growth Inhibition in Hepatocellular Carcinoma. Oncology 2017, 93, 136–142. [Google Scholar] [CrossRef]
- Wu, G.X.; Lin, Y.M.; Zhou, T.H.; Gao, H.; Pei, G. Significant down-regulation of alpha-albumin in human hepatoma and its implication. Cancer Lett. 2000, 160, 229–236. [Google Scholar] [CrossRef] [PubMed]



| Parameter | CTL Group | DEN+2-AAF Group |
|---|---|---|
| Glucose (mg/dL) | 153.5 ± 14.34 | 165.8 ± 13.5 |
| Urea (mg/dL) | 44.98 ± 1.88 | 39.24 ± 6.9 |
| Creatinine (mg/dL) | 0.53 ± 0.02 | 0.58 ± 0.05 |
| Cholesterol (mg/dL) | 48 ± 2.62 | 98.4 ± 13.8 * |
| Triglycerides (mg/dL) | 51.83 ± 7.60 | 62 ± 8.34 |
| HDL-C (mg/dL) | 27.5 ± 2.14 | 57.8 ± 7.39 * |
| VLDL (mg/dL) | 10.5 ± 1.47 | 12.2 ± 1.68 |
| Total Protein (g/dL) | 6.22 ± 0.19 | 6.82 ± 0.1 * |
| AST (U/L) | 141.83 ± 11.89 | 263.8 ± 45.4 * |
| ALKP (U/L) | 171.17 ± 21.39 | 429.4 ± 30.37 * |
| GGT (U/L) | 5.17 ± 0.16 | 71.8 ± 14.11 * |
| ALT (U/L) | 35.83 ± 2.31 | 130.2 ± 22.4 * |
| Name | Forward (F) Sequence | Reverse (R) Sequence |
|---|---|---|
| P38 | F: 5’- AGCAACCTCGCTGTGAATGA | R: 5’- TCCCCGTCAGACGCATTATC |
| AFP | F: 5’- CTTGGTGAAGCAAAAGCCTGAA | R: 5’- GGACCCTCTTCTGTGAAACAGACT |
| ALB | F: 5’- CCCGATTACTCCGTGT | R: 5’- TGGCGTTTTGGAATCCATA |
| BAX ** | F: 5′- TGGAGATGAACTGGACAATA | R: 5′- CAAAGTAGAAGAGGGCAAC |
| BCL2 ** | F: 5′- CGACTTTGCAGAGATGTCC | R: 5′- ATGCCGGTTCAGGTACTCAG |
| CAT | F: 5’- GGAGGCGGGAACCCAATAG | R: 5′- GTGTGCCATCTCGTCAGTGAA |
| Col1α1 ** | S: 5′- CAAGATGGTGGCCGTTACTAC | R: 5′- AGTACTCTCCGCTCTTCCAG |
| Col3α1 * | S: 5′- TGGACAGATGCTGGTGCTGAG | R: 5′- GAAGGCCAGCTGTACATCAAGG |
| Cyp2e1 * | S: 5′- CTTTCCCTCTTCCCATCCTT | R: 5′- CCCGTCCAGAAAACTCATTC |
| CPT1A | F: 5’- GGTCGGAAGCCCATGTTGTA | R: 5’- TTTGGGTCCGAGGTTGACAG |
| ERK-2 | F: 5’- GGAACACTGCATCTTTGAGTGAG | R: 5’- GCACACAGTGCAGGAACAAAA |
| HGF * | F: 5′- ATGAGAGAGGCGAGGAGAAAC | R: 5′- GTAGCCCCAGCCGTAAATACT |
| HIF-1α * | F: 5′- CCCATCCATGTGACCATGAG | R: 5′- AATCAGCACCAAGCACGTCA |
| IL-1β ** | F: 5′- CCAAGCACCTTCTTTTCCTTC | R: 5′- GTCAGACAGCACGAGGCATT |
| IL-6 ** | F: 5′- CCACCCACAACAGACCAGTA | R: 5′- CTCCAGAAGACCAGAGCAGAT |
| iNOS ** | F: 5′- GCCCCTTCAATGGTTGGTAC | R: 5′- AGGCCAGTGTGTGGGTCTC |
| PCNA ** | F: 5′- GTGAACCTCACCAGCATGTC | R: 5′- GTTGCTCAACGTCTAAGTCC |
| PPARα | F: 5′- GTGGTCCCTAATCAGGCCTATATC | R: 5′- ACAATACTACCTGACCACCACT |
| RPL41 | F: 5′- GGCAGAGGTCCAAGTAAACCA | R: 5′- ATCTCGGCGAGGTGACATTC |
| SOD | F: 5′- AATGTGTCCATTGAAGATCGTGTGA | R: 5′- GCTTCCAGCATTTCCAGTCTTTGTA |
| TGFβ1 | F: 5’- TGCTAATGGTGGACCGCAA | R: 5’- CACTGCTTCCCGAATGTCTGA |
| TIMP-1 * | F: 5′- TCCCCAGAAATCATCGAGAC | R: 5′- TCAGATTATGCCAGGGAACC |
| TNF-α ** | F: 5′- TGCCTCAGCCTCTTCTCATT | R: 5′- GCTTGGTGGTTTGCTACGAC |
| VEGF-R2 * | F: 5′- AGATGACAGCCAGACAGACAG | R: 5′- CCACAGACTCCCTGCTTTTAC |
| Number of Cycles | Step | ALB, BCL2, CAT, COL1α1, COL3α1, CYP2E1, ERK2, HGF, HIF-1α, IL-1β, IL-6, iNOS, PCNA, RPL41, TGFβ1, TIMP-1, TNF-α, and VEGF-R2 | rt-qPCR Program CPT1A, P38, and SOD | AFP, BAX, and PPARα |
|---|---|---|---|---|
| 1 | Initial Denaturation | 95 °C–10 min | 95 °C–10 min | 95 °C–10 min |
| 40 | Denaturation | 95 °C–15 s | 95 °C–15 s | 95 °C–15 s |
| Annealing | 60 °C–30 s | 59 °C–30 s | 58 °C–30 s | |
| Extension | 72 °C–25 s | 72 °C–25 s | 72 °C–25 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Meza, J.; Campos-Valdez, M.; Domínguez-Rosales, J.A.; Godínez-Rubí, J.M.; Rodríguez-Reyes, S.C.; Martínez-López, E.; Zúñiga-González, G.M.; Sánchez-Orozco, L.V. Chronic Administration of Diethylnitrosamine and 2-Acetylaminofluorene Induces Hepatocellular Carcinoma in Wistar Rats. Int. J. Mol. Sci. 2023, 24, 8387. https://doi.org/10.3390/ijms24098387
Sánchez-Meza J, Campos-Valdez M, Domínguez-Rosales JA, Godínez-Rubí JM, Rodríguez-Reyes SC, Martínez-López E, Zúñiga-González GM, Sánchez-Orozco LV. Chronic Administration of Diethylnitrosamine and 2-Acetylaminofluorene Induces Hepatocellular Carcinoma in Wistar Rats. International Journal of Molecular Sciences. 2023; 24(9):8387. https://doi.org/10.3390/ijms24098387
Chicago/Turabian StyleSánchez-Meza, Jaime, Marina Campos-Valdez, José Alfredo Domínguez-Rosales, Juliana Marisol Godínez-Rubí, Sarai Citlalic Rodríguez-Reyes, Erika Martínez-López, Guillermo M. Zúñiga-González, and Laura Verónica Sánchez-Orozco. 2023. "Chronic Administration of Diethylnitrosamine and 2-Acetylaminofluorene Induces Hepatocellular Carcinoma in Wistar Rats" International Journal of Molecular Sciences 24, no. 9: 8387. https://doi.org/10.3390/ijms24098387
APA StyleSánchez-Meza, J., Campos-Valdez, M., Domínguez-Rosales, J. A., Godínez-Rubí, J. M., Rodríguez-Reyes, S. C., Martínez-López, E., Zúñiga-González, G. M., & Sánchez-Orozco, L. V. (2023). Chronic Administration of Diethylnitrosamine and 2-Acetylaminofluorene Induces Hepatocellular Carcinoma in Wistar Rats. International Journal of Molecular Sciences, 24(9), 8387. https://doi.org/10.3390/ijms24098387







