UV-A Radiation: Safe Human Exposure and Antibacterial Activity
Abstract
1. Introduction
2. Results
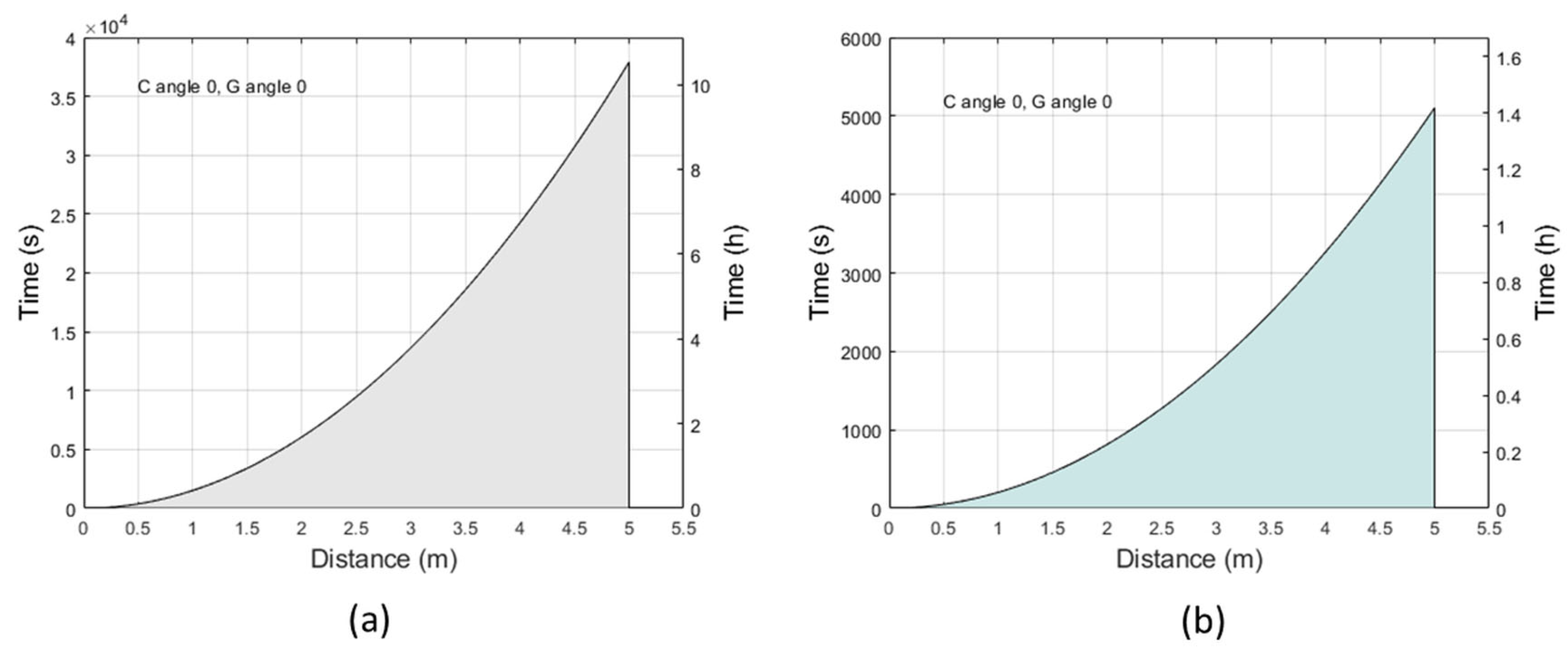
2.1. Exposure Limits at Maximum Irradiance
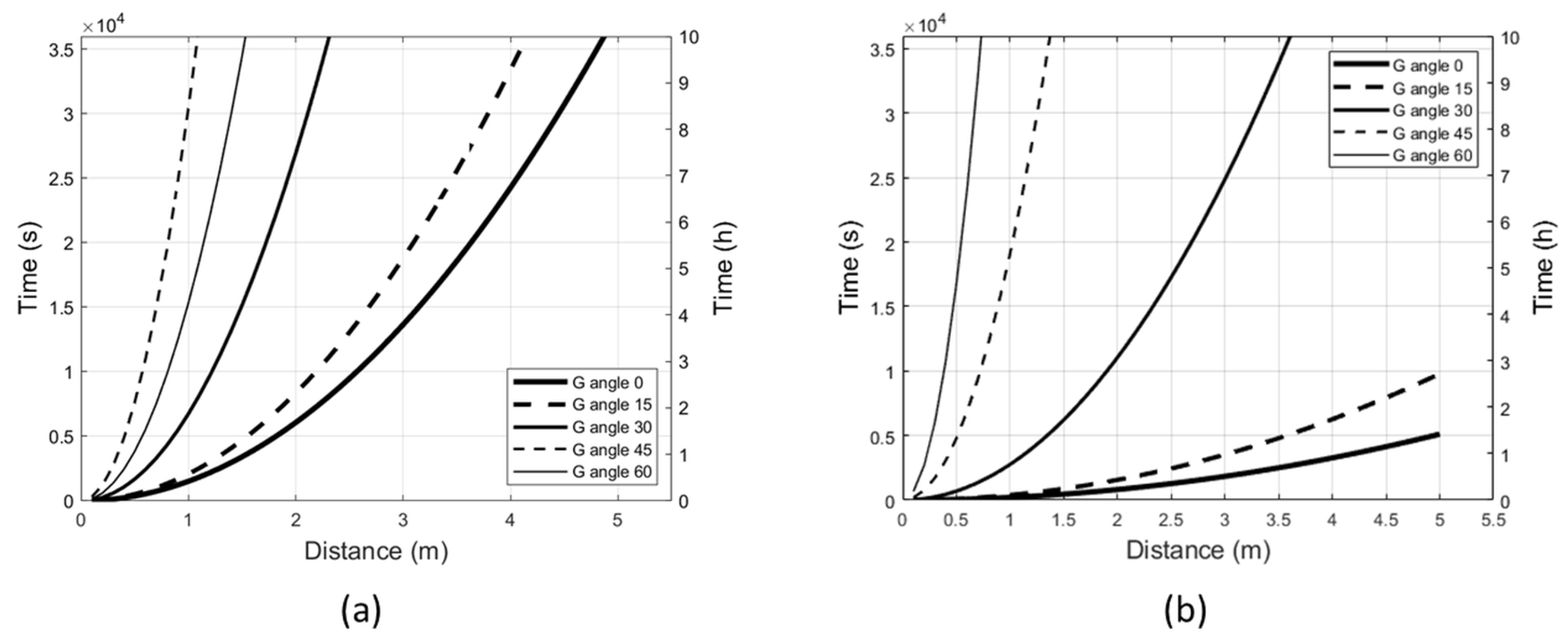
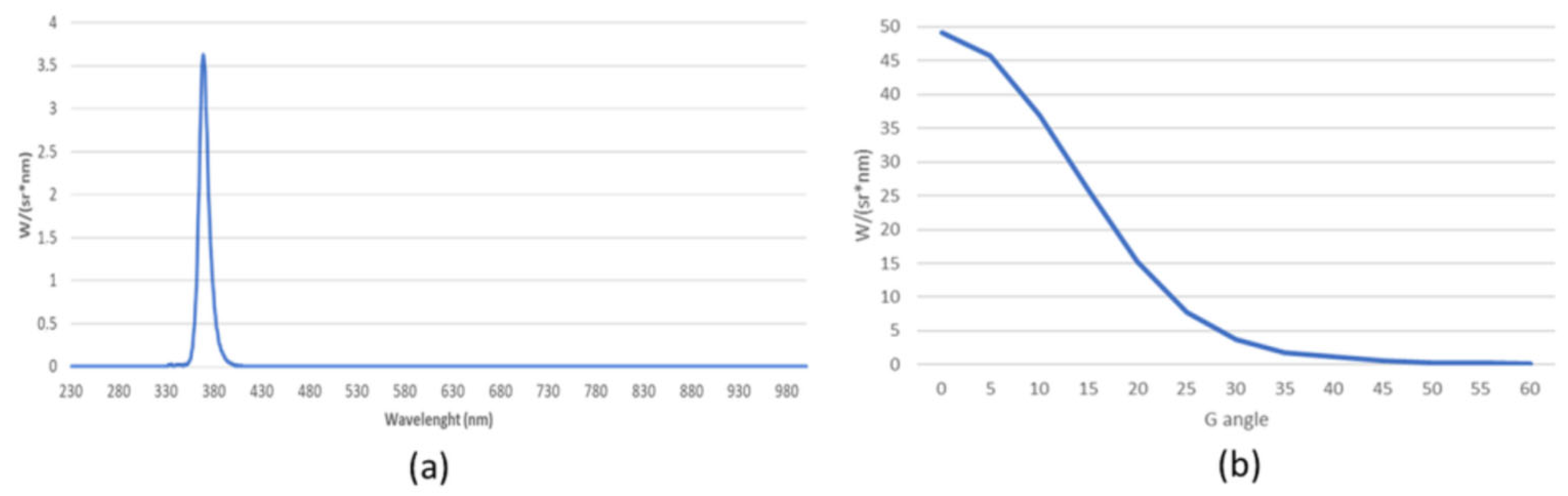
2.2. Radiant Exposure Limits and Irradiation Angles
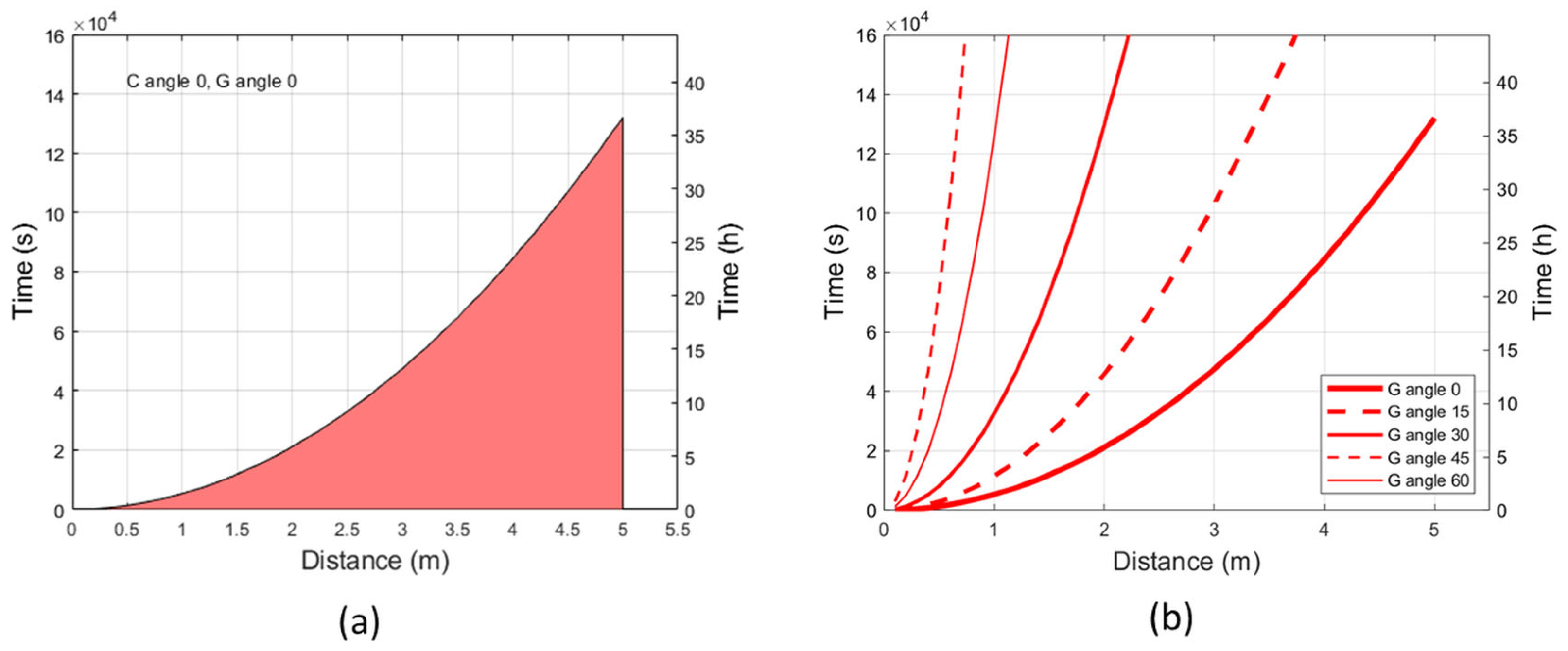
2.3. Erythemal Exposure Limits at Maximum Irradiance and Varying Irradiation Angles
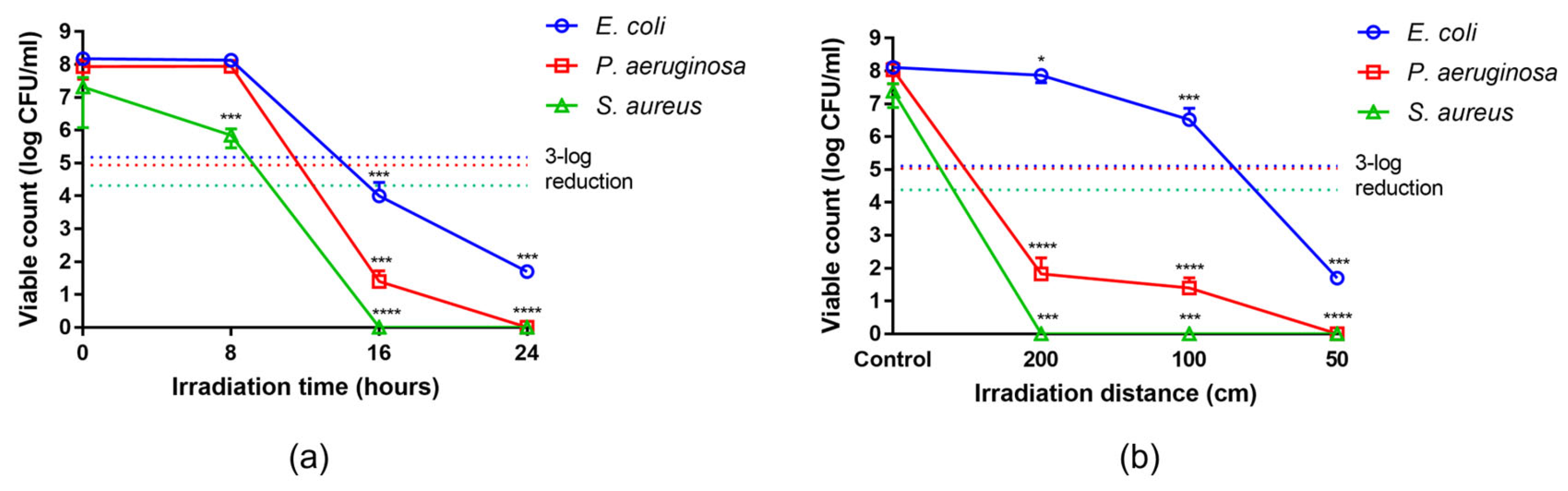
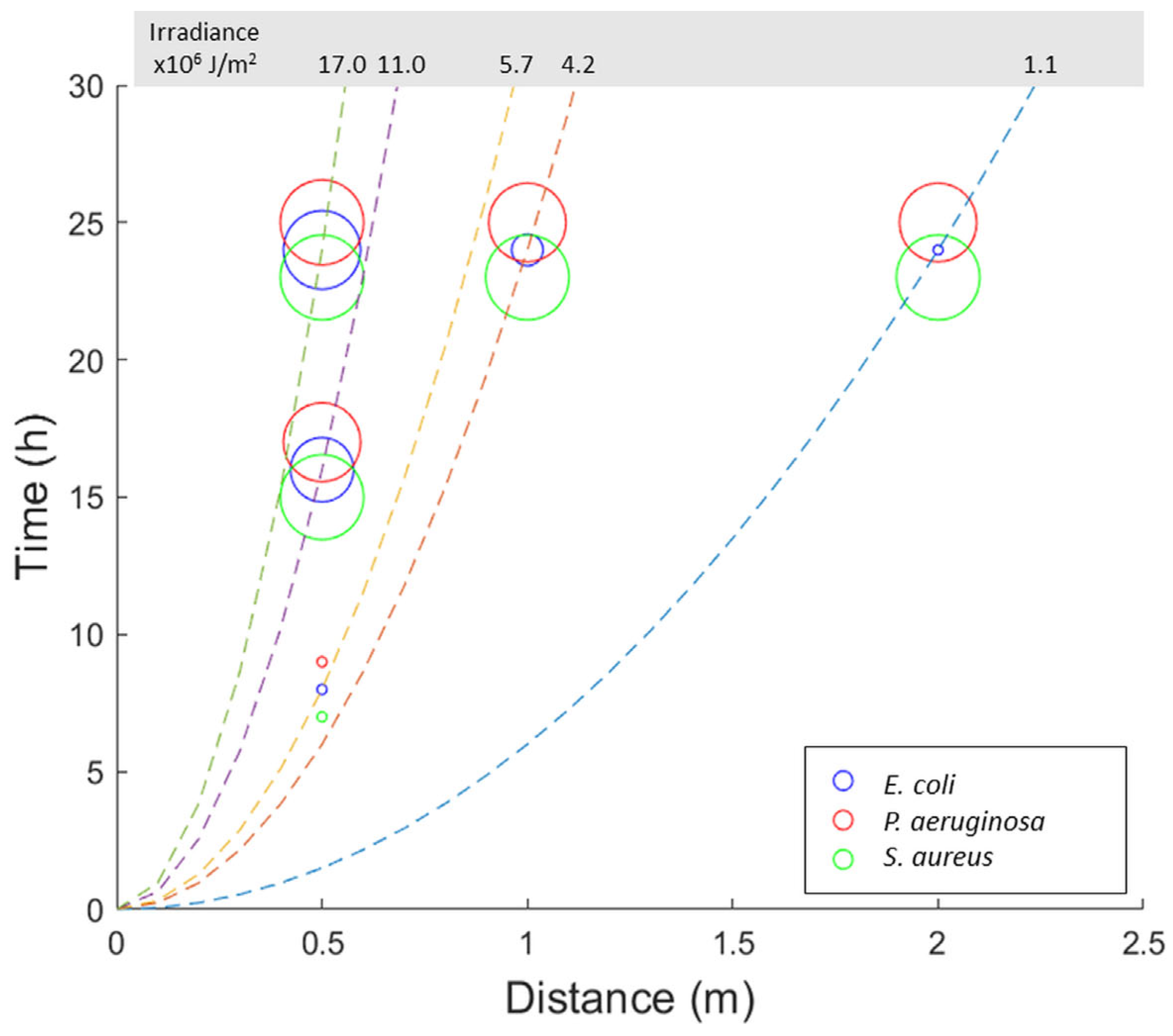
2.4. Antibacterial Activity over Exposure Time and Irradiation Distance
2.5. Bactericidal Activity in Relation to the Irradiance of the Source
3. Discussion
4. Materials and Methods
4.1. UV-A Source
4.2. Exposure Limits
4.3. Antibacterial Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bank, H.L.; John, J.; Schmehl, M.K.; Dratch, R.J. Bactericidal effectiveness of modulated UV light. Appl. Environ. Microbiol. 1990, 56, 3888–3889. [Google Scholar] [CrossRef] [PubMed]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent advances on the application of UV-LED technology for microbial inactivation: Progress and mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef] [PubMed]
- Zemke, V.; Podgorsek, L.; Schoenen, D. Ultraviolet disinfection of drinking water. 1. Communication: Inactivation of E. coli and coliform bacteria. Zentralbl. Hyg. Umweltmed. 1990, 190, 51–61. [Google Scholar] [PubMed]
- Warriner, K.; Rysstad, G.; Murden, A.; Rumsby, P.; Thomas, D.; Waites, W.M. Inactivation of Bacillus subtilis spores on aluminum and polyethylene preformed cartons by UV-excimer laser irradiation. J. Food Prot. 2000, 63, 753–757. [Google Scholar] [CrossRef]
- Favier, G.L.; Escudero, M.E.; de Guzman, A.M. Effect of chlorine, sodium chloride, trisodium phosphate, and ultraviolet radiation on the reduction of Yersinia enterocolitica and mesophilic aerobic bacteria from eggshell surface. J. Food Prot. 2001, 64, 1621–1623. [Google Scholar] [CrossRef]
- Katara, G.; Hemvani, N.; Chitnis, S.; Chitnis, V.; Chitnis, D.S. Surface disinfection by exposure to germicidal UV light. Indian J. Med. Microbiol. 2008, 26, 241–242. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Braz, M.; Costa, P.; Duarte, J.; Pereira, C.; Almeida, A. Evaluation of UV-C Radiation Efficiency in the Decontamination of Inanimate Surfaces and Personal Protective Equipment Contaminated with Phage varphi6. Microorganisms 2022, 10, 593. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Johnson, G.J. The environment and the eye. Eye 2004, 18, 1235–1250. [Google Scholar] [CrossRef]
- Friedberg, E.C. A history of the DNA repair and mutagenesis field: I. The discovery of enzymatic photoreactivation. DNA Repair 2015, 33, 35–42. [Google Scholar] [CrossRef]
- Hemminki, K.; Xu, G.; Le Curieux, F. Ultraviolet radiation-induced photoproducts in human skin DNA as biomarkers of damage and its repair. IARC Sci. Publ. 2001, 154, 69–79. [Google Scholar]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- European Commission D-GfHaFS. Opinion on Biological Effects of UV-C Radiation Relevant to Health with Particular Reference to UV-C Lamps; European Commission: Luxembourg, 2018. [Google Scholar]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Rezaie, A.; Leite, G.G.S.; Melmed, G.Y.; Mathur, R.; Villanueva-Millan, M.J.; Parodi, G.; Sin, J.; Germano, J.F.; Morales, W.; Weitsman, S.; et al. Ultraviolet A light effectively reduces bacteria and viruses including coronavirus. PLoS ONE 2020, 15, e0236199. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation, P. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation). Health Phys. 2004, 87, 171–186. [Google Scholar]
- ISO/CIE 17166:2019; International Commission on Illumination. Erythemal Reference Action Spectrum and Standard Erythemal Dose. ISO: Rome, Italy, 2019.
- Fujiyoshi, S.; Tanaka, D.; Maruyama, F. Transmission of Airborne Bacteria across Built Environments and Its Measurement Standards: A Review. Front. Microbiol. 2017, 8, 2336. [Google Scholar] [CrossRef]
- Boone, S.A.; Gerba, C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007, 73, 1687–1696. [Google Scholar] [CrossRef]
- Li, S.; Eisenberg, J.N.; Spicknall, I.H.; Koopman, J.S. Dynamics and control of infections transmitted from person to person through the environment. Am. J. Epidemiol. 2009, 170, 257–265. [Google Scholar] [CrossRef]
- Wilson, A.M.; Weir, M.H.; Bloomfield, S.F.; Scott, E.A.; Reynolds, K.A. Modeling COVID-19 infection risks for a single hand-to-fomite scenario and potential risk reductions offered by surface disinfection. Am. J. Infect. Control. 2021, 49, 846–848. [Google Scholar] [CrossRef]
- Mori, M.; Hamamoto, A.; Nakano, M.; Akutagawa, M.; Takahashi, A.; Ikehara, T.; Kinouchi, Y. Effects of Ultraviolet LED on Bacteria. In World Congress on Medical Physics and Biomedical Engineering 2006, Proceedings of the IFMBE Proceedings, Seoul, Korea, 27 August–1 September 2007; Magjarevic, R., Nagel, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 14. [Google Scholar]
- Palma, F.; Baldelli, G.; Schiavano, G.F.; Amagliani, G.; Aliano, M.P.; Brandi, G. Use of Eco-Friendly UV-C LEDs for Indoor Environment Sanitization: A Narrative Review. Atmosphere 2022, 13, 1411. [Google Scholar] [CrossRef]
- Unluturk, S.; Atilgan, M.R.; Handan Baysal, A.; Tari, K. Use of UV-C radiation as a non-thermal process for liquid egg products (LEP). J. Food Eng. 2008, 85, 561–568. [Google Scholar] [CrossRef]
- Khan, M.; McDonald, M.; Mundada, K.; Willcox, M. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Malik, S.A.; Swee, T.T.; Malek, N.A.N.N.; Yahya, A.; Emoto, T.; Akutagawa, M.; Meng, L.K.; Hou, T.J.; Iskandar, T.A.; Alang, T.; et al. Effectiveness of visible and ultraviolet light emitting diodes for inactivation of Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli: A comparative study. Malays. J. Fundam. Appl. Sci. 2019, 15, 572–576. [Google Scholar] [CrossRef]
- Marasini, S.; Dean, S.J.; Swift, S.; Perera, J.; Rupenthal, I.D.; Wang, T.; Read, H.; Craig, J.P. Preclinical confirmation of UV-C efficacy in treating infectious keratitis. Ocul. Surf. 2022, 25, 76–86. [Google Scholar] [CrossRef]
- Bosshard, F.; Bucheli, M.; Meur, Y.; Egli, T. The respiratory chain is the cell’s Achilles’ heel during UV-A inactivation in Escherichia coli. Microbiology 2010, 156, 2006–2015. [Google Scholar] [CrossRef]
- Liang, J.Y.; Cheng, C.W.; Yu, C.H.; Chen, L.Y. Investigations of blue light-induced reactive oxygen species from flavin mononucleotide on inactivation of E. coli. J. Photochem. Photobiol. B 2015, 143, 82–88. [Google Scholar] [CrossRef]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.; Almeida, A.; Correia, A.; Cunha, A. Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef]
- Caldeira de Araujo, A.; Favre, A. Near ultraviolet DNA damage induces the SOS responses in Escherichia coli. EMBO J. 1986, 5, 175–179. [Google Scholar] [CrossRef]
- Fernandez, R.O.; Pizarro, R.A. Lethal effect induced in Pseudomonas aeruginosa Exposed to Ultraviolet-A radiation. Photochem. Photobiol. 1996, 64, 334–339. [Google Scholar] [CrossRef]
- Li, T.; Zhou, C.; Jiang, M. UV absorption spectra of polystyrene. Polym. Bull. 1991, 25, 211–216. [Google Scholar] [CrossRef]
| Skin Phototype | Sunburn Susceptibility | Minimal Erythemal Radiation (SED) | Erythemal Exposure Limit (min) |
|---|---|---|---|
| I | Very high | 2 | 90 |
| II | High | 3 | 135 |
| III | Moderate | 4 | 180 |
| IV | Low | 5 | 225 |
| V | Very low | 7 | 315 |
| VI | Extremely low | ≥9 | ≥405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandri, A.; Tessari, A.; Giannetti, D.; Cetti, A.; Lleo, M.M.; Boschi, F. UV-A Radiation: Safe Human Exposure and Antibacterial Activity. Int. J. Mol. Sci. 2023, 24, 8331. https://doi.org/10.3390/ijms24098331
Sandri A, Tessari A, Giannetti D, Cetti A, Lleo MM, Boschi F. UV-A Radiation: Safe Human Exposure and Antibacterial Activity. International Journal of Molecular Sciences. 2023; 24(9):8331. https://doi.org/10.3390/ijms24098331
Chicago/Turabian StyleSandri, Angela, Aldo Tessari, Danilo Giannetti, Alberto Cetti, Maria M. Lleo, and Federico Boschi. 2023. "UV-A Radiation: Safe Human Exposure and Antibacterial Activity" International Journal of Molecular Sciences 24, no. 9: 8331. https://doi.org/10.3390/ijms24098331
APA StyleSandri, A., Tessari, A., Giannetti, D., Cetti, A., Lleo, M. M., & Boschi, F. (2023). UV-A Radiation: Safe Human Exposure and Antibacterial Activity. International Journal of Molecular Sciences, 24(9), 8331. https://doi.org/10.3390/ijms24098331









