Abstract
The intramolecular Heck reaction is a well-established strategy for natural product total synthesis. When constructing large rings, this reaction is also referred to as Heck macrocyclization, which has proved a viable avenue to access diverse naturally occurring macrocycles. Less noticed but likewise valuable, it has created novel macrocycles of non-natural origin that neither serve as nor derive from natural products. This review presents a systematic account of the title reaction in forging this non-natural subset of large rings, thereby addressing a topic rarely covered in the literature. Walking through two complementary sections, namely (1) drug discovery research and (2) synthetic methodology development, it demonstrates that beyond the well-known domain of natural product synthesis, Heck macrocyclization also plays a remarkable role in forming synthetic macrocycles, in particular macrocyclic drugs.
1. Introduction
Heck cross-coupling, alternatively named the Mizoroki–Heck reaction [1], is a time-tested synthetic methodology that has transformed organic chemistry [2,3]. Intramolecularly, the Heck reaction effects cyclic structures ranging from small (n = 3–7) through medium (n = 8–11) to large (n ≥ 12) rings. In contrast to the ample literature addressing Heck-type ring closure for small and medium rings [4,5,6,7,8,9,10], there are only a few works covering the intramolecular Heck reaction for large ring formation [11,12,13], which are unexceptionally dedicated to natural product total synthesis. From time to time, however, this long-neglected transformation, more often termed Heck macrocyclization, has been exploited to prepare synthetic macrocycles including macrocyclic drugs. Having unmatched architecture and functional group disposition, macrocycles constitute a cutting edge of modern drug discovery well poised to engage challenging pharmaceutical targets [14,15,16]. To our surprise, though sporadically mentioned [17,18], the title reaction has hitherto not been scrutinized in the context of making non-natural macrocycles. Accordingly, the present review aims to conduct a systematic survey of this reaction forging diverse synthetic macrocycles beyond natural products. Covering the literature from 1995 to 2022, this review consists of two sections: (1) drug discovery research and (2) synthetic methodology developmentogural products. The first section showcases Heck macrocyclization as employed to build biologically relevant (drug-like) peptidomimetic as well as non-peptidic macrocycles. Of utmost interest is the latest manufacturing route to lorlatinib, a CNS penetrable ALK inhibitor approved for the treatment of lung cancer, which hinges upon a highly efficient intramolecular Heck arylation to close its rigid 13-membered ring. In the second section, attention is paid to the capacity of the Heck reaction to yield an array of unprecedented large rings by virtue of (1) novel allene-containing precursors, (2) sequential multifold couplings, or (3) supramolecular catalysts. Though not immediately translatable to medicinal chemistry, the interesting results compiled in the second part serve to advance our appreciation of the intramolecular Heck reaction and as such will foster its future application in drug synthesis.
2. Drug Discovery Research
2.1. Peptidomimetic Macrocycles
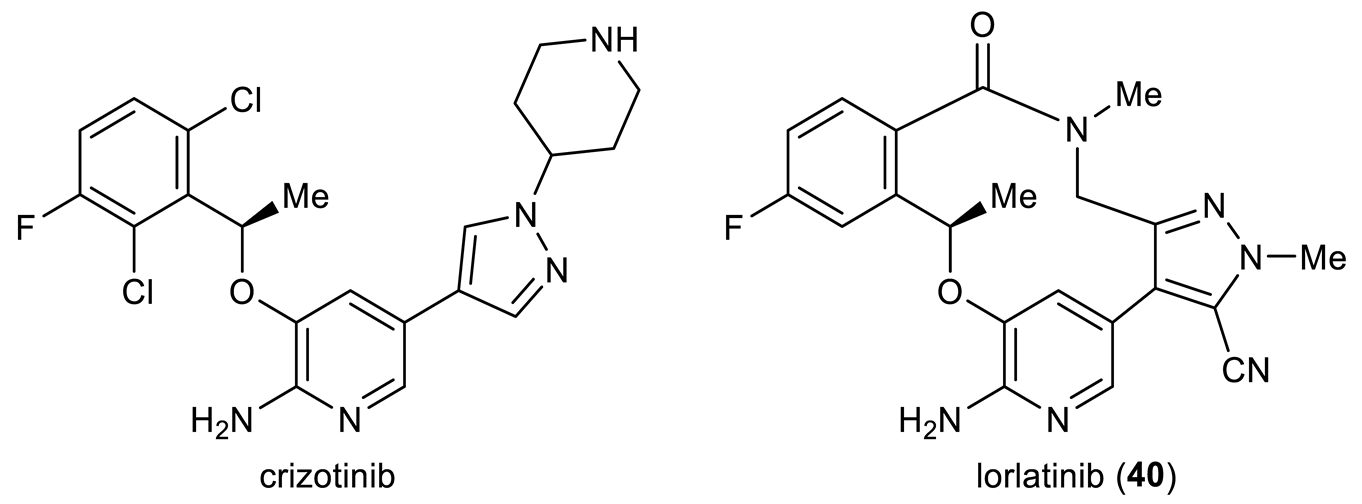
Solid-phase synthesis represents a breakthrough technology in organic chemistry, easing product isolation and enabling automated multistep preparation in a combinatorial fashion [19]. Though originally invented for constructing biopolymers, its scope was later broadened to include carbon–carbon bond forming reactions. Hauske et al. depicted the first Pd-mediated macrocyclization in 1995 by means of such a strategy (Figure 1A) [20]. In this pioneering work, a combinatorial library of 15 bifunctional molecules encompassing different amino acid building blocks at R1 and R2 were grafted to Tenta Gel PHB resin. These supported reactants 1 were treated with Pd(OAc)2, PPh3, and Bu4NCl in a mixed solvent of DMF/H2O/Et3N at room temperature overnight, followed by TFA-assisted cleavage from the supporting resin for structural analysis. Remarkably, the products 3, with a variety of ring sizes (20–24 membered) occurring predominantly as E-isomers, were recovered in 75–85% overall yields. Two notable features of this system, namely mild cyclization conditions and high yields, may be attributed to the pseudodilution effect [21], a phenomenon referring to the immobilization-induced separation of reactive sites in favor of intramolecular reactions. However, no further biological evaluation of these compounds has been disclosed since.
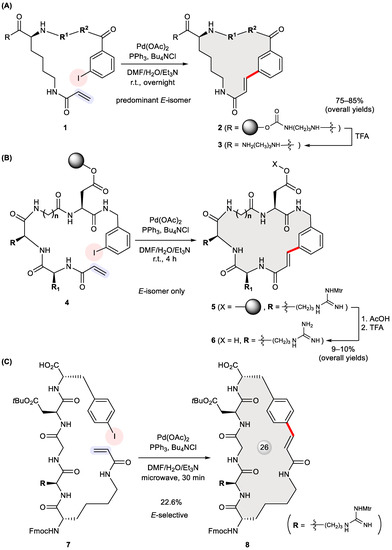
Figure 1.
Heck macrocyclization for solid-phase synthesis of cyclic peptidomimetics 3 (A), 6 (B), and 8 (C). (Mtr = 4-methoxy-2,3,6-trimethylbenzenesulfonyl).
Solid-phase Heck macrocyclization was also explored in the context of building cyclic tetrapeptides bearing a signature sequence of arginine–glycine–aspartic acid (RGD) [22]. Following an initial proof of concept study [23], Akaji et al. applied the split and mix approach to prepare multicomponent cyclic peptidomimetic libraries 6 varied with ring size (n) and substitution at R1 via supported Heck macrocyclization of 4 (Figure 1B) [24]. Two combinatorial libraries, each containing 15 compounds, were isolated in 9–10% overall yields after detachment from the resin and deprotection. According to NMR spectroscopy, only E-configuration was observed in the nascent alkene. Notably, this heterogeneous cyclization occurred more rapidly than did a corresponding substrate in solution. A preliminary assay found that one purified cyclic RGD derivative (R1 = H, n = 1 in 6) from this library selectively inhibited fibrinogen binding to immobilized GPIIb/IIIa with an IC50 value of 2 × 10−5 M but without inhibitory activity against the vitronectin receptor [25], another member of the integrin family of receptors. In 2006, Byk et al. demonstrated microwave-assisted Heck macrocyclization both on resin and in solution [26]. As an illustration of their approach, an RGD-containing seco precursor 7 prepared via solid-phase synthesis underwent cyclization within 30 min to give biologically relevant macrocycle 8 in a 22.6% yield as an E-isomer (Figure 1C). This work shows the potential of microwave-assisted Heck cyclization for preparing conformationally restrained peptidomimetics.
The helix–turn–helix (HTH) motif is instrumental to many DNA-binding proteins such as transcription factors that are capable of recognizing a particular DNA sequence for regulatory purposes [27]. To develop chemical probes gauging DNA–protein interactions, Iqbal et al. designed cyclic peptides bridged by a meta benzene ring such as 10 (Figure 2A) [28]. As the final step of their synthesis, acyclic substrate 9 was treated with Pd(OAc)2, tri(o-tolyl)phosphine, and diisopropylethylamine in refluxing acetonitrile for 36 h. The product 10 was isolated solely as an E-isomer in a 39% yield. Since NMR spectroscopy detected the presence of hydrogen bonds (marked as dashed lines in Figure 2A) in both the linear precursor and its corresponding product, conformational preorganization through such intramolecular hydrogen bonding was believed to promote cyclization.
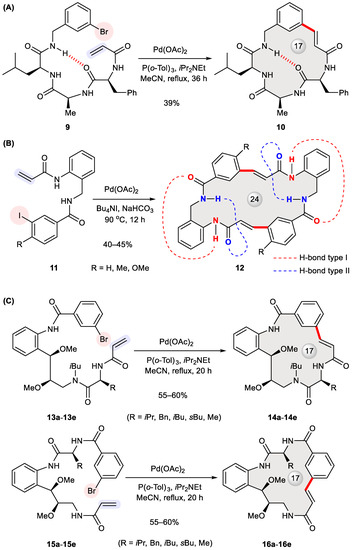
Figure 2.
Synthesis of cyclic peptides 10 (A), 12 (B), 14a–14e and 16a–16e (C) via Heck cyclization.
More recently, Banerji et al. synthesized symmetrical pseudo-turn mimics 12 via double Heck cross-coupling (Figure 2B) [29]. Initially intending to build 12-membered rings, they were unable to obtain any monomeric cyclization product, even under high dilution conditions; rather, dimeric 24-membered macrocycles were produced in 40–45% yields with exclusive E-geometry at the newly formed alkenes. NMR and FT-IR spectroscopy proved the presence of intramolecular hydrogen bonds in the precursors 11 as well as the cyclized products 12 (marked in Figure 2B). This stabilizing force was deemed conducive to forming turn-like structures that help with binding to DNA minor grooves [30]. The binding of 11a (R = H) to DNA minor grooves was confirmed through a variety of experiments including a DAPI displacement assay, mobility shift DNA-binding assay, and melting temperature assay. Based on the fluorescence emission spectra of 5a at 380 nm in the presence of varying concentrations of CT-DNA, its DNA-binding constant (KA) was calculated to be 7.89 × 104 M−1.
The abundance of bioactive 17-membered natural macrocycles motivated Arya et al. to design two sets of natural product-like compounds 14a–14e and 16a–16e (Figure 2C) [31]. These analogues were prepared in 55–60% yields via Heck macrocyclization of 13a–13e and 15a–15e using Pd(OAc)2, P(o-tolyl)3, and diisopropylethylamine in refluxing acetonitrile. Screening these compounds in various zebrafish assays identified 16a (R = isopropyl) as a potent antiangiogenic agent with complete inhibition of angiogenesis at 2.5 μM. The fact that its acyclic precursor 15a was inactive substantiated the importance of a macrocycle-constrained framework.
Macrocyclization is a popular strategy to create conformationally restrained hepatitis C virus (HCV) non-structural (NS)3/4a protease inhibitors [32], which belong to the group of direct-acting antiviral agents against HCV infection [33]. With a view to enhancing binding affinity and pharmacokinetic properties, Chen et al. designed peptidomimetics 19a–19c and 21a–21c bearing a P2–P3 macrocycle (Figure 3A) [34]. These HCV NS3 protease inhibitors were synthesized via the Heck cyclization of 17 into 18 in a 37% yield as a Z/E-isomeric mixture. The stapled dipeptide 18 and its hydrogenated intermediate 20 were elaborated at their C-termini to give rise to 19a–19c and 21a–21c, respectively. A bioassay indicated that the presence or absence of an olefinic bond in the macrocyclic tether has a marginal effect on inhibitory activity, while two carboxylic acids 19b and 21b were the most potent (Table 1). The conformation of 21b bound to HCV NS3 protease was further elucidated with X-ray crystallography.
Figure 3.
Synthesis of HCV protease inhibitors 19a–19c, 21a–21c (A) and vaniprevir (25, B) via Heck macrocyclization.

Table 1.
Inhibitory activity of macrocyclic peptidomimetics 19a–19c and 21a–21c against HCV NS3 protease.
Another pertinent example appeared in 2011 when process chemists at Merck disclosed their first-generation scale-up route to vaniprevir (25, MK-7009, shown in Figure 3B) [35], a 20-membered P2–P4 macrocyclic inhibitor of HCV NS3/4a protease [36]. To support clinical development, its practical synthesis was worked out to optimize macrocycle formation. A variety of ring-closing methods were evaluated in terms of robustness and cost-effectiveness, including ring-closing metathesis, Pd-catalyzed macrocyclization, and macrolactamization. Among three Pd-catalyzed cross-couplings tested, the Heck reaction utilizing a hindered ferrocene-based palladacycle 23 stood out with the highest yield (47%) on a 60 mg scale. By contrast, the Suzuki and Sonogashira reactions with their corresponding substrates offered 5% and 35% yields, respectively. Notwithstanding a mixture of configurational and positional isomers, the ring-closed product 24 was hydrogenated and then elaborated to 25. However, the vaniprevir ring was finally closed via more efficient macrolactamization at a >10 g scale, prior to which intermolecular Heck cross-coupling took place to form the same linkage as created by the previous intramolecular Heck reaction.
Very recently, peptide stapling through macrocyclization reactions [37,38,39] was explored by Spring et al. with a view to discovering a pan-KRAS inhibitor based on the pharmacophore model of a 13-mer peptide binding to KRASG12V. Having identified key interacting residues, they designed a library of smaller peptides whose KRAS-binding conformation as well as drug-likeness is reinforced by means of a one-component stapling strategy [40]. These linear 5- and 6-mer peptides were efficiently assembled via solid-phase peptide synthesis (SPPS) and further subjected to diverse macrocyclization reactions including azide–alkyne cycloaddition, Glaser coupling, ene–yne metathesis, cross-alkene metathesis, Heck cross-coupling, and Sonogashira cross-coupling. While Sonogashira cyclization failed to bring about any observable product, Heck cyclization (unoptimized) smoothly converted pentapeptide 26 into stapled peptide 27 in a modest yield, thus supplying adequate amount of the macrocyclic sample for in vitro biological characterization (Figure 4) [40]. Unfortunately, initial screening for KRAS-binding potency determined its IC50 value to be over 100 μM, while more active low-micromolar macrocyclic binders of KRAS were prepared alternatively via Ru-catalyzed azide–alkyne cycloaddition.
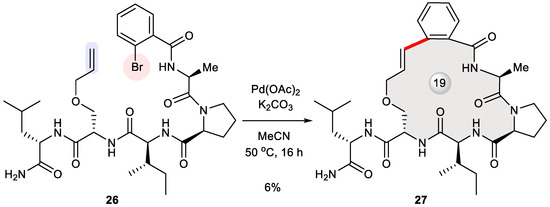
Figure 4.
Intramolecular Heck coupling for the synthesis of a stapled pentapeptide 27 as a potential KRAS binder.
2.2. Non-Peptidic Macrocycles
FK506 is a natural immunosuppressant featuring a 23-membered macrolide, which can be functionally dissected into two domains: one for engaging the FK506 binding protein (FKBP) and the other for downstream signal transduction [41]. To design FK506 mimics with the dual domain concept [42], Stocks et al. reserved a simplified binding domain of the parent macrocycle while replacing its effector domain with hydrocarbon tethers of varying length [43]. For example, macrocycles 29, 31, and 33 were prepared from their corresponding acyclic substrates 28, 30, and 32 through Heck macrocyclization (Figure 5) using a protocol developed by Gaudin [44]. In the case of 32, the exclusive formation of E-alkenes was observed. Dating back to 1995, this work is among the earliest examples of Heck macrocyclization.
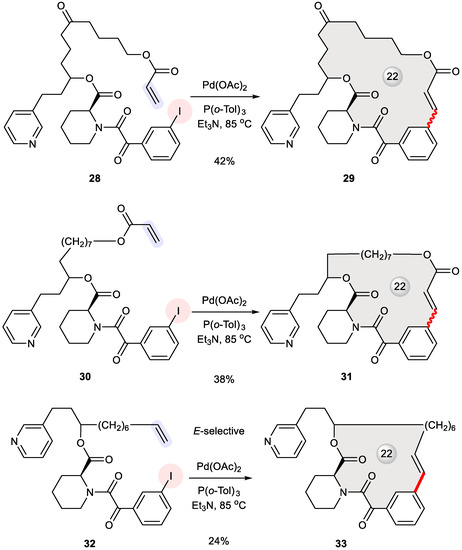
Figure 5.
Synthesis of FK506 mimics 29, 31, and 33 via Heck macrocyclization.
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase implicated in several types of cancer [45]. ALK inhibitors have proved an efficacious modality for targeted cancer therapy [46] since the launch of crizotinib in 2011 for the clinical treatment of ALK-positive non-small cell lung cancer (NSCLC) [47]. Inspired by the inverted U-shaped conformation of a 2,4-diaminopyrimidine (DAP) derivative revealed through X-ray crystallography, Breslin et al. adopted a macrocyclization strategy by designing an array of macrocyclic DAPs 36 capable of enforcing such an active conformation [48]. To access them, acyclic substrates 34 were subjected to microwave-assisted Heck cyclization. The products 35 were obtained in 32–94% yields and further converted to 36 (Figure 6A). Systematic structure–activity relationship (SAR) investigation of thus prepared macrocycles found that sp2 hybridization at the restraining two-carbon linchpin (35) tends to undermine activity relative to the saturated counterparts (36). Among them, 36c (R = 4-Me-piperazinyl, R1 = –OMe, R2 = –N(Me)SO2Me) exhibited the highest in vitro activity at both enzymatic and cellular levels (IC50 = 0.5 nM and 10 nM, respectively), with a desirable kinase selectivity (173-fold) for ALK over the closely homologous insulin receptor (IR) kinase (Table 2). Recently, the same ring scaffolds modified with different phosphine oxides at ring C were disclosed in a patent as potent inhibitors of normal and mutated ALKs [49]. Again, microwave-assisted Heck macrocyclization was invoked to build a heteroaryl tethered 13-membered ring.
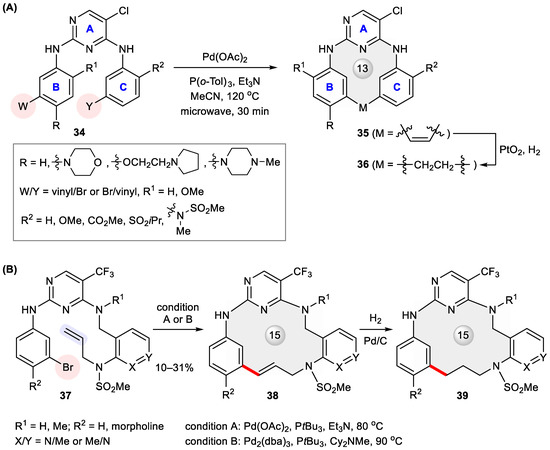
Figure 6.
Heck macrocyclization for the preparation of (A) ALK inhibitors 35–36 and (B) Pyk2-selective inhibitors 38–39.

Table 2.
SAR of DAP-containing macrocyclic drugs 35a–35c and 36a–36c.
The macrocyclization strategy was also applied by Gilead scientists to develop selective inhibitors of proline-rich tyrosine kinase 2 (Pyk2) [50], a potential target for the treatment of invasive cancers [51]. Because Pyk2 shares a similar catalytic domain with focal adhesion kinase (FAK) [52], a challenge is to find a Pyk2-specific inhibitor with low off-target binding to FAK. Starting from PF-562271, a first-in-class dual inhibitor of FAK and Pyk2 [53], ring closure by amidation gave rise to first-generation macrocyclic inhibitors with improved Pyk2 selectivity but unsatisfactory metabolic stability [54]. Introducing a three-carbon linker via Heck macrocyclization led to 38 and 39 (Figure 6B), with not only better stability but also dramatically enhanced Pyk2-binding potency and selectivity compared with their corresponding acyclic precursor 37 (Table 3) [54]. Among these analogues, 38c displayed the highest stability in the human microsomal stability assay, with a half-life (t1/2) of 263 min.

Table 3.
SAR of Pyk2-targeting macrocyclic inhibitors 38a–38c and 39a–39b and their acyclic precursors 37a–37c (structures shown in Figure 6B).
Though efficacious against ALK-positive cancers, crizotinib is incapable of blocking mutated ALK, a problem relentlessly haunting the first and second generations of ALK inhibitors. Meanwhile, hardly permeable across the blood–brain barrier (BBB), it cannot control ALK-driven brain metastases that stem from peripheral tumors. To tackle these drawbacks, Pfizer scientists conducted a structure-based drug design that focused on two crucial factors, namely lipophilic efficiency (lipE) and molecular weight (MW) during structural optimization [55]. The former, expressed as pIC50 minus logD (octanol/buffer distribution coefficient), is a measure of the binding effectiveness of a drug molecule per unit of lipophilicity, helpful to guide the improvement of potency and ADME properties in parallel [56]. Having its root in Lipinski’s rule of five, the latter is negatively correlated with permeability so that a smaller or more compact size is generally preferred. In addition, a substantial challenge is working out a structure efficient at penetrating the BBB to enhance central nervous system (CNS) availability. To this end, an in vitro transwell assay was utilized throughout the drug discovery phase to monitor P-glycoprotein (P-gp) efflux liability in terms of the MDR BA/AB ratio [57]. A high MDR BA/AB ratio (for example, >2.5) indicates significant P-gp efflux and accordingly lack of CNS activity. Through intensive efforts, this drug discovery campaign eventually led to macrocyclic inhibitor lorlatinib (40), a third-generation ALK inhibitor and, significantly, the first macrocyclic kinase inhibitor. As shown in Table 4, 40 is highly potent against both wild-type ALK and all known ALK mutants including the gatekeeper L1196M mutant with excellent CNS penetration, which was approved in 2018 for the treatment of NSCLC [58].

Table 4.
Potency and key physicochemical properties of crizotinib and lorlatinib (40).
The medicinal chemistry route culminating in 40 required the preparation of diverse 12- to 14-membered macrocycles holding one stereogenic center and three (hetero)aromatic rings. These rigidifying elements make their synthesis a nontrivial task, particularly in view of their close resemblance to synthetically more demanding medium-sized (8- to 11-membered) rings compared with larger (≥15 membered) macrocycles [59]. Intramolecular Heck arylation [6,60], as testified through the successful assembly of numerous 5- and 6-membered rings [61,62,63,64,65,66,67,68,69,70,71], turned out to be indispensable to access macrocyclic (R)-42, 44, 46, and 40 during the initial drug discovery campaign (Figure 7A) [55]. Subsequently, this synthetic approach was implemented to produce radiolabeled isotopologues for positron emission tomography (PET) imaging [72]. Crucial to this ring-closing transformation is the addition of di-1-adamantyl-n-butylphosphine (cataCXium®A) [73] to promote Pd-catalyzed arylation. In preclinical studies, an exploratory scale-up route was initially reported, relying on amidation to close the macrolactam ring [74]. However, safety concerns over the large-scale use of high-energy condensation reagent HATU prompted Pfizer chemists to work out a second-generation process synthesis through the intermediates 48 and 49 (Figure 7B), in this way delivering 10–20 kg batches of the drug for clinical investigation [75]. To avoid the use of proprietary cataCXium®A, an alternative ligand di(n-butyl)-t-butylphosphine was employed in the form of an air-stable HBF4 salt. The endgame of the commercial route features an efficient intramolecular Heck arylation of crystalline 48 t-amyl alcohol to yield another crystalline solvate, 49 acetonitrile, in 65–70% yields, followed by acidic deprotection so as to manufacture the API at a >120 kg scale for the time being (Figure 7C) [75]. It is worth noting that intramolecular Suzuki coupling had been evaluated in parallel throughout the process optimization study, but poor yields were achieved even after extensive screening of multiple reaction parameters and, accordingly, its applicability was ruled out.
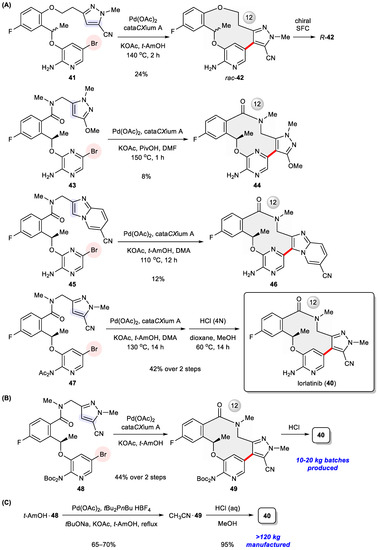
Figure 7.
(A) Initial medicinal chemistry synthesis of lorlatinib (40) and its analogues 42, 44, 46 via intramolecular Heck arylation. (B) Second-generation and (C) commercial-scale synthesis of lorlatinib via intramolecular Heck arylation.
2.3. Natural Product Analogues
In a broad sense, natural product analogues should contain those initially targeted for total synthesis but only found later to be misassigned structures, a serendipitous twist alluding to the charm of natural product research. Pertaining to the title reaction, the synthesized nominal structures of diazonamide A [76], kulkenon [77], the aglycone of mandelalide A [78], and maltepolide C [79] belong to this category. More often though, analogues of structurally validated natural products are prepared deliberately, rather than unexpectedly, in order to explore biologically relevant chemical space, wherein Heck macrocyclization again plays a substantial role. Earlier examples include conformationally restricted taxoids [80], side chain derivatives of mandelalide A [81], and stereodivergent solomonamides [82]. Recently, our total synthesis of highly antiproliferative nannocystin A through Heck macrocyclization [83,84] secured subsequent SAR investigations by facilely preparing dozens of non-natural analogues including 50–54 that deviate from the natural lead either stereochemically, along the macrocycle backbone, or at the peripheral substituent (Figure 8A) [85,86,87,88]. Aiming at site-directed late-stage diversification for quickly exploring chemical space around the nannocystin framework [89], we next remodeled its macrocycle composition in which a homochiral serine (highlighted in the structure) has been substituted for the innate D-serine to give the macrocyclic alcohol 57. To our satisfaction, the precursor 55 underwent smooth ring closure in a 70% yield under Heck coupling conditions, TBS deprotection then furnishing 57 ready for divergent post-macrocyclization esterification (Figure 8B) [90]. Although nannocystin A was shown to be a specific inhibitor of eukaryotic elongation factor 1A (eEF1A) [91], its exact anticancer mode of action awaits further elucidation [92]. Informed by thus obtained SAR and pursuing a nannocystin-based fluorescent probe [93], we designed and synthesized a coumarin conjugate 58 with good cell permeability. It was observed by means of confocal fluorescent microscopy that this probe is localized predominantly to the endoplasmic reticulum (Figure 8C), most likely acting upon its target eEF1A at the ER-bound ribosome [90]. Interestingly, our result is in good agreement with a recent work that visualized eEF1A associated with the ribosome on the ER membrane at molecular resolution by the use of cryo-electron tomography [94], thereby shedding light on the intracellular mode of action of nannocystins.
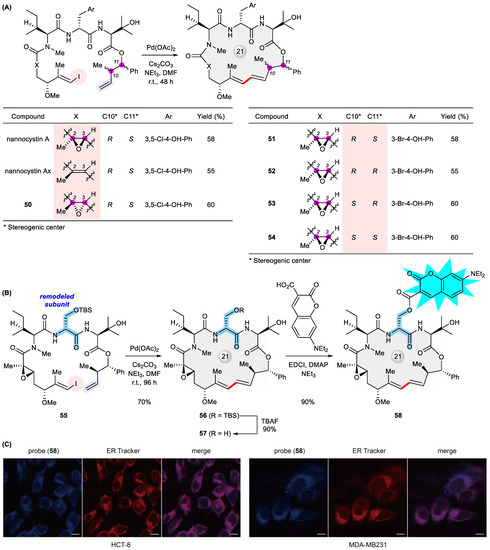
Figure 8.
(A) Synthesis of nannocystin A and analogues 50–54 permutated stereochemically along the macrocyclic backbone via Heck macrocyclization. (B) Synthesis of a diversity-conferring nannocystin intermediate 57 via Heck macrocyclization and elaboration into a macrocyclic fluorescent probe 58. (C) Confocal fluorescence images of cancer cells co-stained with ER-tracker and 58 (scale bars, 10 μm). Reproduced from ref. [90] with permission from the Royal Society of Chemistry.
3. Synthetic Methodology Development
3.1. Allenic Precursors
An interesting Heck-type cyclic carbopalladation was reported by Ma and Negishi in 1995, employing allenes as the alkenyl coupling partner, thereby producing carbocycles of varying sizes including macrocycles [95]. This work was based on their earlier discovery of facile access to 7- and 8-membered rings from allene-containing organohalides via Pd catalysis [96]. Substrates pertaining to the subject of this review are given in Figure 9, including ω-iodoallenes 59a–59d and ω-iodoalkenes 61a–61c highlighted at their alkenyl functionalities. Aside from catalytic amount (5 mol%) of Pd(PPh3)2Cl2 and five equivalents of K2CO3, critical to their ring closure are (1) the addition of the phase transfer agent Bu4NCl as pioneered by Jeffery [97] and (2) executing the reaction at reduced concentrations. Despite three possible pathways for carbon–carbon bond formation, the actual cyclization took place invariably at the central allenic carbon with exclusive formation of an exo alkene. The 12- and 20-membered rings 60a–60d were prepared from their allenic precursors 59a–59d in higher yields than the 13- and 21-membered endo macrocycles from their corresponding ω-iodoalkenes (59a vs. 61a, 59c vs. 61b, 59d vs. 61c, Figure 9). Such a superior performance may originate from the cumulated double bonds of allenes, which have gained increasing popularity in organic synthesis [98,99,100,101,102].
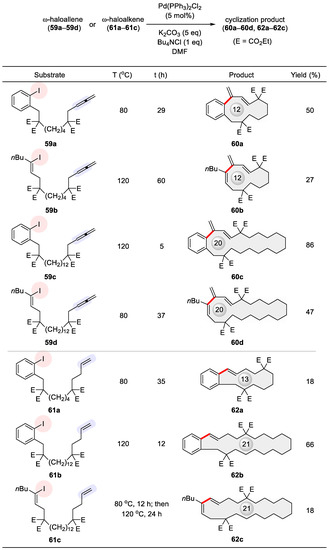
Figure 9.
Heck macrocyclization using allene 59a–59d or alkene 61a–61c as the alkenyl coupling partner.
More recently, this methodology was upgraded to embrace intermolecular cyclization between organoiodides and allenes (Figure 10 top), where the allene coupling partner A was equipped distally with an extra nucleophile X so as to self-trap the transient allylpalladium species B generated from initial addition of Ar-Pd-I, resulting in C with high regio- and E/Z stereoselectivity, finally yielding the saturated ring D via Pd/C hydrogenation [103,104]. A variety of unprecedented 9–20-membered rings were prepared via this strategy, as showcased in Figure 10 (bottom).
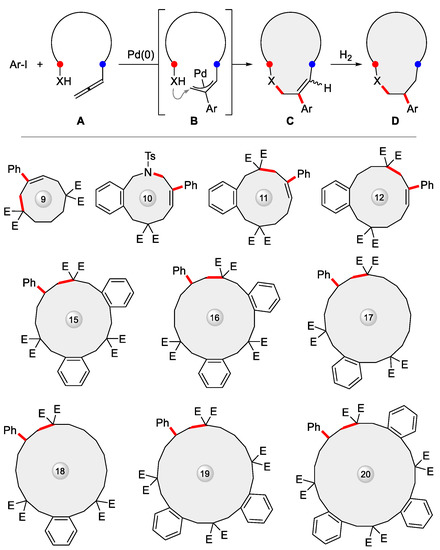
Figure 10.
Pd-catalyzed intermolecular cyclization between Ar-I and allenes carrying a remote tethered nucleophile X brought about diverse medium and large rings (E = CO2Et). A: nucleophile-tethered allene; B: reaction intermediate; C: cyclized product; D: further hydrogenated product.
3.2. Single, Double, or Multifold Heck Reactions
In exploring novel analogues of bisbenzylisoquinoline alkaloids [105], Pyne et al. synthesized laudanosine dimers bound with carbon tethers [106]. Since laudanosine is an active metabolite of the neuromuscular-blocking drugs atracurium and cisatracurium [107], its dimer may have interesting properties. One compound they obtained is macrocyclic 64, deriving from the intramolecular Heck reaction of the acyclic substrate 63. Conventional Heck reaction conditions using Pd(OAc)2, PPh3, and Et3N at 110 °C delivered 64 in a 15% yield, whereas the optimal conditions for the intermolecular Heck reaction of other laudanosine analogues utilizing Pd(OAc)2, NaOAc, N,N-dimethylglycine, and NMP at 130 °C paradoxically resulted in a complex mixture (Figure 11).
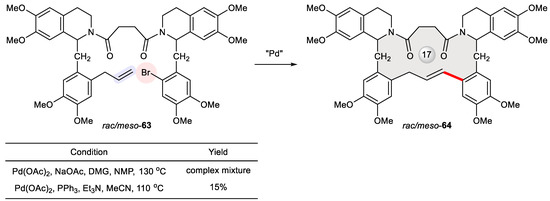
Figure 11.
Synthesis of macrocyclic laudanosine dimer 64 via Heck cyclization of rac/meso-63.
Due to the reversibility of β-hydride elimination, the Heck cross-coupling of allylic and homoallylic alcohols renders carbonyl products through double bond migration [108]. Coupled with other transformations, this reaction can initiate domino processes that give annulated ring systems. Pursuing this goal [109,110,111,112,113], Dyker et al. devised a double transannular cyclization strategy to access the tetracyclic steroid skeleton in the form of isomeric cis/trans-68 and cis/trans-69, which relied on the intramolecular Heck cyclization of allylic alcohol 65 to form the precursor macrocycle 66 (Figure 12) [114]. In addition to 66, a 26-membered macrocycle 67 was isolated in an unneglectable yield of 17%. Intrigued by this finding, the authors performed a follow-up study to show the opportunity of creating C2v-symmetric macrocycles 71 and 72 through a fourfold Heck reaction with p-diiodobenzene and m-diiodobenzene, respectively [115].
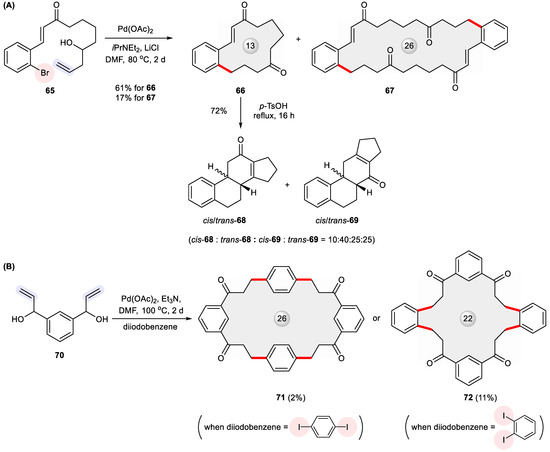
Figure 12.
Heck macrocyclization for constructing (A) steroid frameworks 68–69 from homoallylic alcohol 65, as well as (B) ketonic macrocycles 71 and 72 from bisallylic alcohol 70.
The double Heck cyclization approach was likewise investigated by Harrowven et al. for macrocycle synthesis [116]. Because the formation of a biphenyl-embedded 13-membered ring from 73 is likely to experience significant strain, as encountered in an independent study before [117], the authors envisioned that such an energetically disfavored process would be surpassed by the closure of a relaxed dimeric 26-membered ring (Figure 13). Nevertheless, the initial precursor 73 failed to cyclize under Pd(0) catalysis; a mixture of polar by-products were detected instead, likely as a result of competitive polymerization. The impasse was overcome by oxidizing its allylic alcohol (highlighted in Figure 13) with Dess–Martin periodinane (DMP). The resulting α,β-unsaturated ketone 74 gratifyingly boosted the anticipated intermolecular–intramolecular Heck couplings, affording 75 in a 54% yield. Echoing the preference for dimer formation, recently, an unexpected 26-membered macrocyclic dimer was observed via RCM in an attempted total synthesis of myricanol wherein no mono-cyclization occurred to give a strained 13-membered ring [118].
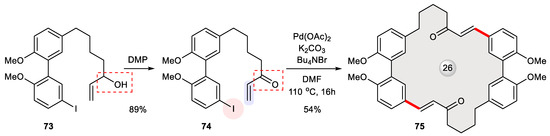
Figure 13.
Double Heck cross-coupling of 74 leading to 26-membered cyclic dimer 75.
Pondaplin (76) is a strained 13-membered macrocycle because of its rigid 1,4-benzenoid linkage and two built-in Z-alkenes [119]. In an effort to synthesize 76, Joullie et al. explored the intramolecular Heck reaction of the seco precursor 77 but without yielding the target molecule under various conditions (Figure 14) [120]. A serendipitous result from their trials was the formation of the pondaplin dimer 78 in a 38% yield under high dilution conditions (0.001 M). Clearly, high strain energy accrued along the self cross-coupling pathway and as such defied ring closure. As a result, the sequential intermolecular–intramolecular process came into play and generated the dimeric macrocycle. At a 10-fold increased concentration, that is, 0.01 M of 77, a head-to-tail cyclized trimer 79 (7% yield) was isolated along with 78 (7% yield).
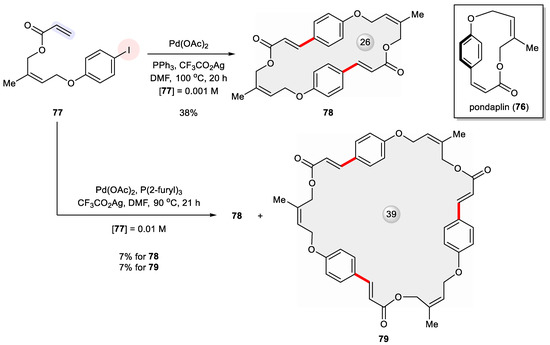
Figure 14.
Attempted total synthesis of pondaplin (76) via Heck cyclization of 77 unexpectedly led to its dimeric and trimeric macrocycles 78 and 79.
The versatility of multifold Heck-type cross-coupling [121] was demonstrated by Gibson et al. for rapidly preparing a collection of dimeric and trimeric macrocycles from simple starting materials. Both achiral [122,123] and chiral [124,125,126] macrocycles were produced via this strategy. As shown in Figure 15A, achiral cyclophanes possessing two (Z)-dehydrophenylalanine subunits such as 81a–81d arise from the Heck-type head-to-tail dimerization of the corresponding dehydroalanine derivatives 80a–80d, to which p-iodobenzene is attached via a hydrocarbon spacer of different lengths [123]. On the other hand, a variety of bifunctional ω-iodo-1-alkenes 82–87 derived from (S)-valinol (for 82–84) or (S)-prolinol (for 85–87) underwent double and/or triple Heck cross-coupling, giving rise to chiral macrocycles of varying ring sizes, as depicted in Figure 15B [124,125,126].
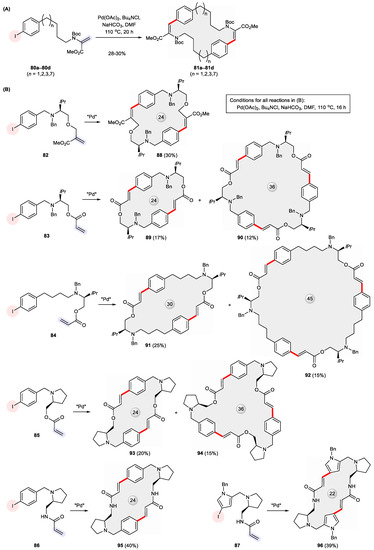
Figure 15.
Formation of (A) benzene-bridged cyclophanes 81a–81d bearing (Z)-dehydrophenylalanine substructures via double Heck cross-coupling and (B) chiral dimeric and/or trimeric macrocycles 88–96 via multifold Heck reaction.
3.3. Supramolecular Catalysts
An intriguing case was made by the use of a dinuclear Pd precatalyst 98 mounted onto a rotaxane platform (Figure 16) [127]. This mechanically interlocked supramolecular catalyst was derived from a bidentate N,N ligand 97 containing a crown ether motif so that two Pd-complexed macrocycles could be threaded through a confining α,ω-bisferrocenyl shaft. Its catalytic performance was compared with the standard Pd(OAc)2/PPh3 system in the Heck cross-coupling of two pairs of bifunctional substrates, namely (1) 99a, 100a and (2) 99b, 100b. While both reactions suffered from competing oligomerization, the rotaxane-based catalyst 98 produced higher yields of the macrocycles 101a and 101b relative to oligomers than the discrete Pd species.
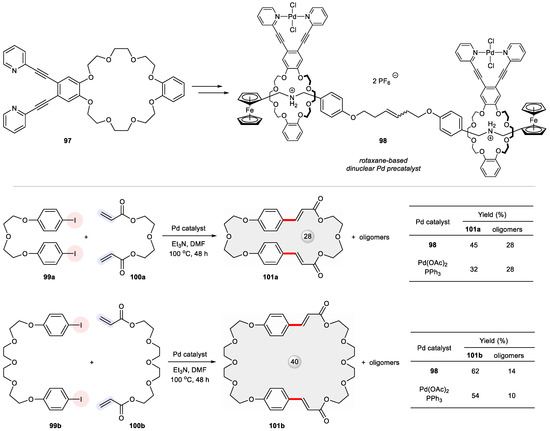
Figure 16.
Rotaxane-based dinuclear Pd precatalyst 98 for Heck-type macrocyclization between 99 and 100.
4. Conclusions and Future Perspectives
In contrast to alternative Pd-catalyzed macrocyclization reactions [11], an appealing facet of the Heck reaction lies in the fact that there is no prerequisite for a heteroatom-functionalized alkene coupling partner. Hence, it is an apt embodiment of the KISS (keep it simple …) principle with regard to atom economy. The absence of a directing/activating group, such as a boronic acid moiety, which defines the Suzuki reaction, is prone to engendering ambiguous regio- and stereoselectivity, an issue often challenging intermolecular Heck cross-coupling. Fortunately, such a complication is less seen in intramolecular Heck cyclization. In truth, its relative lack of certainty or predictability compared with other well-practiced cross-coupling processes happens to call for a creative mindset in synthetic design, as elegantly exemplified by the recent total synthesis of polycyclic natural products such as lyconadin A [128], dysiherbol A [129], clionastatins A [130], octanorcucurbitacin B [131], himalensine A [132], and shearilicine [133] (Figure 17A). When coupled to carbonylation with the one-carbon feedstock CO, the intramolecular Heck reaction offers the opportunity to generate two consecutive rings in a one-pot cascade [134]. As illustrated in Figure 17B, Pd-catalyzed carbonylative Heck-type macrolactonization, C-H functionalization, lactonization, and lactamization have inspiringly led to the total synthesis of spinosyn A [135], cephanolides B [136], perseanol [137], and α-schizozygol [138], respectively. With regard to large rings, shortly after a 2021 review [12], more progress was made in the total synthesis of isoriccardin C [139], pulvomycin D (Figure 17C) [140], and the (2E) isomer of macrolactin 3 [141].
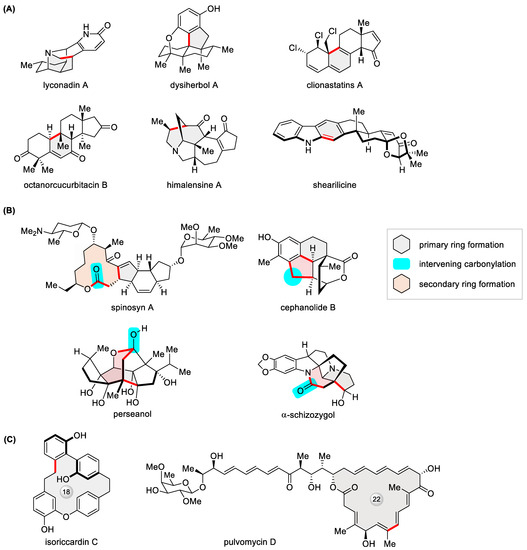
Figure 17.
Recent examples of polycyclic natural products constructed via (A) intramolecular Heck reaction and (B) Heck-type carbonylative tandem cyclization, as well as (C) macrocyclic natural products built via Heck macrocyclization.
The theme of the present review, on the other hand, is to raise awareness of the fact that this reaction is likewise useful in forging synthetic macrocycles, especially macrocyclic drugs. Although first reported in the early 1980s [142], it was only more than 10 years later, that is, in 1995, that three independent studies led by Ma and Negishi [95], Hauske [20], and Stocks [43] attested the utility of the intramolecular Heck reaction in generating innovative synthetic macrocycles. These achievements, in turn, could have inspired broader exploration of Heck macrocyclization in natural product synthesis, as initiated by Harran’s landmark synthesis of the originally proposed structure of diazonamide A [76,143,144]. Aside from being investigated as a synthetic method, this reaction has made prominent contributions to macrocyclic drugs including stapled peptides as well as non-peptidic kinase inhibitors, as evidenced by the lab-scale and commercial synthesis of lorlatinib, an approved latest-generation broad-spectrum ALK inhibitor bearing a rigid 13-membered biheteroaryl macrolactam ring. In this regard, Heck cross-coupling proves a viable option in the arsenal of available macrocyclization reactions to enable macrocycle-based drug design, a frontier of modern drug discovery [145,146,147,148,149,150,151,152].
Thus far, both phosphine-assisted and phosphine-free catalytic systems have found wide applications in Heck macrocyclization (Table 5). Of note, the former proceeds at elevated temperatures typical of a routine inter- or intramolecular Heck reaction, often adopted to prepare non-natural macrocycles, whereas the latter involves a much milder condition amenable to the total synthesis of natural macrocycles and their analogues. These contrasting reaction settings betoken fundamentally different emphases in making natural products and designer macrocycles. To access a non-natural macrocycle, particularly a macrocyclic drug under development, efficiency is a pivotal factor, so drastic conditions are required to drive the completion of large ring formation. When it comes to constructing a natural macrocycle, however, caution must be exercised to not spoil its rich functional groups. Therefore, it is reasonable to effect large ring closure at or close to room temperature to reduce side reactions. It is evident that no matter which method is used, the yield of Heck macrocyclization remains suboptimal, ranging from moderate to good yet seldom reaching up to 90% (Table 5). Consistent with this observation, Knapp and Hanke et al. recently assessed the efficiency of various macrocyclization reactions yielding macrocyclic kinase inhibitors reported over the past 15 years [59] in terms of the Emac index [153]. Clearly, there is much room to improve the productivity of Heck macrocyclization when compared with conventional macrocyclization reactions such as macrolactonization and macrolactamization. Looking forward, this would likely be accomplished through not only extensive optimization of reaction parameters (ligand, additive, solvent, temperature, etc.) during scale-up, but more decisively, in the long run, the advent of more capable catalytic systems. Moreover, it is the existence of stable, long-acting Pd species that could sustain efficient large ring formation in a highly diluted medium. As the macrocyclization strategy extends outside the sphere of kinase inhibitors to harness mechanistically diverse therapeutic agents, Heck macrocyclization will reveal its value in due course.

Table 5.
Two types of Pd-based catalytic systems for Heck macrocyclization and application examples thereof.
Author Contributions
Conceptualization, W.Z.; investigation, W.Z., J.C. and B.S.; data curation, J.C. and B.S.; validation: S.Y. and H.Z.; writing—original draft preparation, W.Z.; writing—review and editing, W.Z., J.C., B.S., S.Y. and H.Z.; funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is generously supported by the National Natural Science Foundation of China (NNSFC) (NO. 21772101) and the Natural Science Foundation of Tianjin City (Grant No. 17JCYBJC28400).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oestreich, M. (Ed.) The Mizoroki-Heck Reaction; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Seechurn, C.C.J.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.E.; Middleton, R.J. The intramolecular Heck reaction. Contemp. Org. Synth. 1996, 3, 447–471. [Google Scholar] [CrossRef]
- Dounay, A.B.; Overman, L.E. The asymmetric intramolecular Heck reaction in natural product total synthesis. Chem. Rev. 2003, 103, 2945–2964. [Google Scholar] [CrossRef] [PubMed]
- Link, J.T. The intramolecular Heck reaction. Org. React. 2004, 60, 157–561. [Google Scholar] [CrossRef]
- Race, N.J.; Hazelden, I.R.; Faulkner, A.; Bower, J.F. Recent developments in the use of aza-Heck cyclizations for the synthesis of chiral N-heterocycles. Chem. Sci. 2017, 8, 5248–5260. [Google Scholar] [CrossRef]
- Ghosh, T. Reductive Heck Reaction: An Emerging Alternative in Natural Product Synthesis. ChemistrySelect 2019, 4, 4747–4755. [Google Scholar] [CrossRef]
- Reznikov, A.N.; Ashatkina, M.A.; Klimochkin, Y.N. Recent developments in asymmetric Heck type cyclization reactions for constructions of complex molecules. Org. Biomol. Chem. 2021, 19, 5673–5701. [Google Scholar] [CrossRef]
- Liang, R.-X.; Jia, Y.-X. Aromatic π-Components for Enantioselective Heck Reactions and Heck/Anion-Capture Domino Sequences. Acc. Chem. Res. 2022, 55, 734–745. [Google Scholar] [CrossRef]
- Ronson, T.O.; Taylor, R.J.; Fairlamb, I.J. Palladium-catalysed macrocyclisations in the total synthesis of natural products. Tetrahedron 2015, 7, 989–1009. [Google Scholar] [CrossRef]
- Zhang, W. Heck macrocyclization in natural product total synthesis. Nat. Prod. Rep. 2021, 38, 1109–1135. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Das, S.; Saha, S.; Sharma, H.; Goswami, R.K. Intramolecular Heck Reaction in Total Synthesis of Natural Products: An Update. Eur. J. Org. Chem. 2021, 2057–2076. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Marsault, E.; Peterson, M.L. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef] [PubMed]
- Giordanetto, F.; Kihlberg, J. Macrocyclic drugs and clinical candidates: What can medicinal chemists learn from their properties? J. Med. Chem. 2014, 57, 278–295. [Google Scholar] [CrossRef]
- Peterson, M.L. Chapter 11 The Synthesis of Macrocycles for Drug Discovery. In Macrocycles in Drug Discovery; Levin, J.I., Ed.; Royal Society of Chemistry: London, UK, 2015; Volume 40, pp. 398–486. [Google Scholar]
- Ronson, T.O.; Unsworth, W.P.; Fairlamb, I.J. Palladium-Catalyzed Synthesis of Macrocycles. In Practical Medicinal Chemistry with Macrocycles: Design, Synthesis, and Case Studies; Marsault, E., Peterson, M.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 281–305. [Google Scholar]
- Toy, P.H.; Lam, Y. (Eds.) Solid-Phase Organic Synthesis: Concepts, Strategies, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Hiroshige, M.; Hauske, J.R.; Zhou, P. Palladium-Mediated Macrocyclization on Solid Support and Its Applications to Combinatorial Synthesis. J. Am. Chem. Soc. 1995, 117, 11590–11591. [Google Scholar] [CrossRef]
- Mazur, S.; Jayalekshmy, P. Chemistry of polymer-bound o-benzyne. Frequency of encounter between substituents on crosslinked polystyrenes. J. Am. Chem. Soc. 1979, 101, 677–683. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Shen, Y.; Wang, A.; Wang, S.; Xie, T. The functions and applications of RGD in tumor therapy and tissue engineering. Int. J. Mol. Sci. 2013, 14, 13447–13462. [Google Scholar] [CrossRef]
- Akaji, K.; Kiso, Y. Macrocyclization on solid support using Heck reaction. Tetrahedron Lett. 1997, 38, 5185–5188. [Google Scholar] [CrossRef]
- Akaji, K.; Teruya, K.; Akaji, M.; Aimoto, S. Synthesis of cyclic RGD derivatives via solid phase macrocyclization using the Heck reaction. Tetrahedron 2001, 57, 2293–2303. [Google Scholar] [CrossRef]
- Ruzha, Y.; Ni, J.; Quan, Z.; Li, H.; Qing, H. Role of Vitronectin and Its Receptors in Neuronal Function and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 12387. [Google Scholar] [CrossRef] [PubMed]
- Byk, G.; Cohen-Ohana, M.; Raichman, D. Fast and versatile microwave-assisted intramolecular Heck reaction in peptide macrocyclization using microwave energy. Biopolymers 2006, 84, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bartas, M.; Cerven, J.; Guziurova, S.; Slychko, K.; Pecinka, P. Amino acid composition in various types of nucleic acid-binding proteins. Int. J. Mol. Sci. 2021, 22, 922. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Balraju, V.; Madhavan, G.R.; Banerji, B.; Iqbal, J. Synthesis of small cyclic peptides via intramolecular Heck reactions. Tetrahedron Lett. 2003, 44, 353–356. [Google Scholar] [CrossRef]
- Banerji, B.; Chatterjee, S.; Killi, S.K.; Srinivas, D.; Prodhan, C.; Katarkar, A.; Chaudhuri, K. Synthesis and DNA-Binding Studies of A New Cyclic Dimeric Symmetrical Pseudo-Turn Mimetic. Chemistryselect 2018, 3, 2103–2107. [Google Scholar] [CrossRef]
- Neidle, S. DNA minor-groove recognition by small molecules. Nat. Prod. Rep. 2001, 18, 291–309. [Google Scholar] [CrossRef]
- Aeluri, M.; Gaddam, J.; Trinath, D.V.K.S.; Chandrasekar, G.; Kitambi, S.S.; Arya, P. An Intramolecular Heck Approach To Obtain 17-Membered Macrocyclic Diversity and the Identification of an Antiangiogenesis Agent from a Zebrafish Assay. Eur. J. Org. Chem. 2013, 3955–3958. [Google Scholar] [CrossRef]
- McCauley, J.A.; Rudd, M.T. Hepatitis C virus NS3/4a protease inhibitors. Curr. Opin. Pharmacol. 2016, 30, 84–92. [Google Scholar] [CrossRef]
- Gotte, M.; Feld, J.J. Direct-acting antiviral agents for hepatitis C: Structural and mechanistic insights. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 338–351. [Google Scholar] [CrossRef]
- Chen, K.X.; Njoroge, F.G.; Prongay, A.; Pichardo, J.; Madison, V.; Girijavallabhan, V. Synthesis and biological activity of macrocyclic inhibitors of hepatitis C virus (HCV) NS3 protease. Bioorg. Med. Chem. Lett. 2005, 15, 4475–4478. [Google Scholar] [CrossRef]
- Song, Z.J.; Tellers, D.M.; Journet, M.; Kuethe, J.T.; Lieberman, D.; Humphrey, G.; Zhang, F.; Peng, Z.; Waters, M.S.; Zewge, D.; et al. Synthesis of Vaniprevir (MK-7009): Lactamization To Prepare a 20-Membered Macrocycle. J. Org. Chem. 2011, 76, 7804–7815. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.A.; McIntyre, C.J.; Rudd, M.T.; Nguyen, K.T.; Romano, J.J.; Butcher, J.W.; Gilbert, K.F.; Bush, K.J.; Holloway, M.K.; Swestock, J.; et al. Discovery of Vaniprevir (MK-7009), a Macrocyclic Hepatitis C Virus NS3/4a Protease Inhibitor. J. Med. Chem. 2010, 53, 2443–2463. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.H.; De Andrade, P.; Wu, Y.; Spring, D.R. Peptide stapling techniques based on different macrocyclisation chemistries. Chem. Soc. Rev. 2015, 44, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bechtler, C.; Lamers, C. Macrocyclization strategies for cyclic peptides and peptidomimetics. RSC Med. Chem. 2021, 12, 1325–1351. [Google Scholar] [CrossRef]
- Bozovicar, K.; Bratkovic, T. Small and Simple, yet Sturdy: Conformationally Constrained Peptides with Remarkable Properties. Int. J. Mol. Sci. 2021, 22, 1611. [Google Scholar] [CrossRef]
- Fumagalli, G.; Carbajo, R.J.; Nissink, J.W.M.; Tart, J.; Dou, R.; Thomas, A.P.; Spring, D.R. Targeting a Novel KRAS Binding Site: Application of One-Component Stapling of Small (5–6-mer) Peptides. J. Med. Chem. 2021, 64, 17287–17303. [Google Scholar] [CrossRef]
- Bierer, B.E.; Somers, P.K.; Wandless, T.J.; Burakoff, S.J.; Schreiber, S.L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science 1990, 250, 556–559. [Google Scholar] [CrossRef]
- Holt, D.A.; Luengo, J.I.; Yamashita, D.S.; Oh, H.J.; Konialian, A.L.; Yen, H.K.; Rozamus, L.W.; Brandt, M.; Bossard, M.J. Design, synthesis, and kinetic evaluation of high-affinity FKBP ligands and the X-ray crystal structures of their complexes with FKBP12. J. Am. Chem. Soc. 1993, 115, 9925–9938. [Google Scholar] [CrossRef]
- Stocks, M.J.; Harrison, R.P.; Teague, S.J. Macrocyclic ring closures employing the Intramolecular Heck reaction. Tetrahedron Lett. 1995, 36, 6555–6558. [Google Scholar] [CrossRef]
- Gaudin, J.M. Intramolecular Heck reaction with substrates possessing an allylic alcohol moiety. Tetrahedron Lett. 1991, 32, 6113–6116. [Google Scholar] [CrossRef]
- Holla, V.R.; Elamin, Y.Y.; Bailey, A.M.; Johnson, A.M.; Litzenburger, B.C.; Khotskaya, Y.B.; Sanchez, N.S.; Zeng, J.; Abu Shufean, M.; Shaw, K.R.; et al. ALK: A tyrosine kinase target for cancer therapy. Cold Spring Harbor Mol. Case Stud. 2017, 3, a001115. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Gray, N.S. Small molecule inhibitors of ALK. Top. Med. Chem. 2018, 28, 435–467. [Google Scholar]
- Cui, J.J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P.-P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure Based Drug Design of Crizotinib (PF-02341066), a Potent and Selective Dual Inhibitor of Mesenchymal–Epithelial Transition Factor (c-MET) Kinase and Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363. [Google Scholar] [CrossRef] [PubMed]
- Breslin, H.J.; Lane, B.M.; Ott, G.R.; Ghose, A.K.; Angeles, T.S.; Albom, M.S.; Cheng, M.; Wan, W.; Haltiwanger, R.C.; Wells-Knecht, K.J.; et al. Design, Synthesis, and Anaplastic Lymphoma Kinase (ALK) Inhibitory Activity for a Novel Series of 2,4,8,22-Tetraazatetracyclo[14.3.1.13,7.19,13]docosa-1(20),3(22),4,6,9(21),10,12,16,18-nonaene Macrocycles. J. Med. Chem. 2012, 55, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, S. Phosphine-Containing Macrocyclic Compound, Its Preparation Method, and Application in Inhibiting ALK Gene Mutation, ALK Gene Rearrangement and/or ALK Gene Amplification. Patent CN113549113A, 26 October 2021. [Google Scholar]
- Lipinski, C.A.; Loftus, J.C. Targeting Pyk2 for therapeutic intervention. Expert Opin. Ther. Tar. 2010, 14, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hong, J.-H. Activated PyK2 and Its Associated Molecules Transduce Cellular Signaling from the Cancerous Milieu for Cancer Metastasis. Int. J. Mol. Sci. 2022, 23, 15475. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Zhen, Y.-Y.; Tsai, Y.-C.; Chuang, C.-H.; Hsiao, M.; Huang, M.-S.; Yang, C.-J. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Roberts, W.G.; Ung, E.; Whalen, P.; Cooper, B.; Hulford, C.; Autry, C.; Richter, D.; Emerson, E.; Lin, J.; Kath, J.; et al. Antitumor Activity and Pharmacology of a Selective Focal Adhesion Kinase Inhibitor, PF-562,271. Cancer Res. 2008, 68, 1935–1944. [Google Scholar] [CrossRef]
- Farand, J.; Mai, N.; Chandrasekhar, J.; Newby, Z.E.; Van Veldhuizen, J.; Loyer-Drew, J.; Venkataramani, C.; Guerrero, J.; Kwok, A.; Li, N.; et al. Selectivity switch between FAK and Pyk2: Macrocyclization of FAK inhibitors improves Pyk2 potency. Bioorg. Med. Chem. Lett. 2016, 26, 5926–5930. [Google Scholar] [CrossRef]
- Johnson, T.W.; Richardson, P.F.; Bailey, S.; Brooun, A.; Burke, B.J.; Collins, M.R.; Cui, J.J.; Deal, J.G.; Deng, Y.-L.; Dinh, D.; et al. Discovery of (10R)-7-Amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a Macrocyclic Inhibitor of Anaplastic Lymphoma Kinase (ALK) and c-ros Oncogene 1 (ROS1) with Preclinical Brain Exposure and Broad-Spectrum Potency against ALK-Resistant Mutations. J. Med. Chem. 2014, 57, 4720–4744. [Google Scholar] [PubMed]
- Freeman-Cook, K.D.; Hoffman, R.L.; Johnson, T.W. Lipophilic efficiency: The most important efficiency metric in medicinal chemistry. Future Med. Chem. 2013, 5, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Mills, J.B.; Davidson, R.E.; Mireles, R.J.; Janiszewski, J.S.; Troutman, M.D.; de Morais, S.M. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab. Dispos. 2008, 36, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Lorlatinib: First Global Approval. Drugs 2019, 79, 93–98. [Google Scholar] [CrossRef]
- Amrhein, J.A.; Knapp, S.; Hanke, T. Synthetic Opportunities and Challenges for Macrocyclic Kinase Inhibitors. J. Med. Chem. 2021, 64, 7991–8009. [Google Scholar] [CrossRef]
- Prashad, M. Palladium-catalyzed heck arylations in the synthesis of active pharmaceutical ingredients. Topics Organomet. Chem. 2004, 6, 181–203. [Google Scholar]
- Comins, D.L.; Baevsky, M.F.; Hong, H. A 10-step, asymmetric synthesis of (S)-camptothecin. J. Am. Chem. Soc. 1992, 114, 10971–10972. [Google Scholar] [CrossRef]
- Heim, A.; Terpin, A.; Steglich, W. Biomimetic Synthesis of Lamellarin G. Trimethyl Ether. Angew. Chem. Int. Ed. 1997, 36, 155–156. [Google Scholar] [CrossRef]
- Yue, W.S.; Li, J.J. A concise synthesis of all four possible benzo [4, 5] furopyridines via palladium-mediated reactions. Org. Lett. 2002, 4, 2201–2203. [Google Scholar] [CrossRef]
- Bringmann, G.; Menche, D.; Kraus, J.; Mühlbacher, J.; Peters, K.; Peters, E.-M.; Brun, R.; Bezabih, M.; Abegaz, B.M. Atropo-enantioselective total synthesis of knipholone and related antiplasmodial phenylanthraquinones. J. Org. Chem. 2002, 67, 5595–5610. [Google Scholar] [CrossRef]
- Singh, S.K.; Ruchelman, A.L.; Li, T.-K.; Liu, A.; Liu, L.F.; LaVoie, E.J. Nitro and Amino Substitution in the D-Ring of 5-(2-Dimethylaminoethyl)-2,3-methylenedioxy-5H-dibenzo [c,h][1,6] naphthyridin-6-ones: Effect on Topoisomerase-I Targeting Activity and Cytotoxicity. J. Med. Chem. 2003, 46, 2254–2257. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.; Chattopadhyay, B.; Nath, S. New Heck coupling strategies for the arylation of secondary and tertiary amides via palladium-catalyzed intramolecular cyclization. Tetrahedron Lett. 2008, 49, 1609–1612. [Google Scholar] [CrossRef]
- Cappoen, D.; Jacobs, J.; Tuyen, N.V.; Claessens, S.; Diels, G.; Anthonissen, R.; Einarsdottir, T.; Fauville, M.; Verschaeve, L.; Huygen, K.; et al. Straightforward palladium-mediated synthesis and biological evaluation of benzo[j]phenanthridine-7,12-diones as anti-tuberculosis agents. Eur. J. Med. Chem. 2012, 48, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ng, K.; Panesar, H.; Moon, S.-J.; Paredes, M.; Ishida, K.; Hertweck, C.; Minehan, T.G. Total Synthesis of the Antitumor Natural Product Polycarcin V and Evaluation of Its DNA Binding Profile. Org. Lett. 2014, 16, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Saravanan, V.; Pavunkumar, V.; Mohanakrishnan, A.K. Pd(0)-Catalyzed Intramolecular Heck reaction of 2/3-Aryl(amino)methyl-3/2-bromoindoles: Syntheses of 3,4-Benzo[c]-β-carbolines, Benzo[4,5]isothiazolo[2,3-a]indole 5,5-Dioxides, and 1,2-Benzo[a]-γ-carbolines. J. Org. Chem. 2021, 86, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-B.; Zhang, H.-Y.; Ma, Y.; Zhao, J.; Han, Y.-P.; Zhang, Y.; Liang, Y.-M. Palladium-Catalyzed Intramolecular Heck Dearomative Alkenylation of Indoles with N-Tosylhydrazones. J. Org. Chem. 2022, 87, 10917–10927. [Google Scholar] [CrossRef]
- Shi, W.; Yang, X.; Li, X.; Meng, L.; Zhang, D.; Zhu, Z.; Xiao, X.; Zhao, D. Syntheses of Anthracene-Centered Large PAH Diimides and Conjugated Polymers. Chem. Eur. J. 2022, 28, e202104598. [Google Scholar] [CrossRef]
- Collier, T.L.; Normandin, M.D.; Stephenson, N.A.; Livni, E.; Liang, S.H.; Wooten, D.W.; Esfahani, S.A.; Stabin, M.G.; Mahmood, U.; Chen, J.; et al. Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat. Commun. 2017, 8, 15761. [Google Scholar] [CrossRef]
- Klaus, S.; Neumann, H.; Zapf, A.; Strübing, D.; Hübner, S.; Almena, J.; Riermeier, T.; Groß, P.; Sarich, M.; Krahnert, W.-R.; et al. A General and Efficient Method for the Formylation of Aryl and Heteroaryl Bromides. Angew. Chem. Int. Ed. 2006, 45, 154–158. [Google Scholar] [CrossRef]
- Li, B.; Barnhart, R.W.; Hoffman, J.E.; Nematalla, A.; Raggon, J.; Richardson, P.; Sach, N.; Weaver, J. Exploratory Process Development of Lorlatinib. Org. Process. Res. Dev. 2018, 22, 1289–1293. [Google Scholar] [CrossRef]
- Dugger, R.; Li, B.; Richardson, P. Discovery and Development of Lorlatinib: A Macrocyclic Inhibitor of EML4-ALK for the Treatment of NSCLC. In Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry Volume 2; American Chemical Society: Washington, DC, USA, 2019; Volume 1332, pp. 27–59. [Google Scholar]
- Li, J.; Jeong, S.; Esser, L.; Harran, P.G. Total synthesis of nominal Diazonamides—Part 1: Convergent preparation of the structure proposed for (−)-Diazonamide A. Angew. Chem. Int. Ed. 2001, 40, 4765–4769. [Google Scholar] [CrossRef]
- Symkenberg, G.; Kalesse, M. Structure Elucidation and Total Synthesis of Kulkenon. Angew. Chem. Int. Ed. 2014, 53, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Yamini, V.; Singarapu, K.K.; Ghosh, S. Synthesis of Proposed Aglycone of Mandelalide A. Org. Lett. 2014, 16, 2658–2660. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Kanakaraju, M.; Kunwar, A.C.; Ghosh, S. Total Synthesis of the Proposed Structure of Maltepolide C. Org. Lett. 2016, 18, 4092–4095. [Google Scholar] [CrossRef]
- Geng, X.; Miller, M.L.; Lin, S.; Ojima, I. Synthesis of Novel C2−C3‘N-Linked Macrocyclic Taxoids by Means of Highly Regioselective Heck Macrocyclization. Org. Lett. 2003, 5, 3733–3736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Imanishi, M.; Kurogi, T.; Wan, X.; Ishmael, J.E.; McPhail, K.L.; Smith, A.B. Synthetic Access to the Mandelalide Family of Macrolides: Development of an Anion Relay Chemistry Strategy. J. Org. Chem. 2018, 83, 4287–4306. [Google Scholar] [CrossRef]
- Jachak, G.R.; Athawale, P.R.; Choudhury, R.; Kashinath, K.; Reddy, D.S. Access to a Stereoisomer Library of Solomonamide Macrocycles. Chem. Asian J. 2019, 14, 4572–4576. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Yang, C.H.; Tian, Y.; Chen, X.; Lian, L.; Pan, W.; Su, X.; Zhang, W.; Chen, Y. Total Synthesis of Nannocystin A. Org. Lett. 2016, 18, 5768–5770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. From Target-Oriented to Motif-Oriented: A Case Study on Nannocystin Total Synthesis. Molecules 2020, 25, 5327. [Google Scholar] [CrossRef]
- Tian, Y.; Ding, Y.; Xu, X.; Bai, Y.; Tang, Y.; Hao, X.; Zhang, W.; Chen, Y. Total synthesis and biological evaluation of nannocystin analogues modified at the polyketide phenyl moiety. Tetrahedron Lett. 2018, 59, 3206–3209. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, X.; Ding, Y.; Hao, X.; Bai, Y.; Tang, Y.; Zhang, X.; Li, Q.; Yang, Z.; Zhang, W.; et al. Synthesis and biological evaluation of nannocystin analogues toward understanding the binding role of the (2R,3S)-Epoxide in nannocystin A. Eur. J. Med. Chem. 2018, 150, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, J.; Liu, W.; Yuan, X.; Tang, Y.; Li, J.; Chen, Y.; Zhang, W. Stereodivergent total synthesis of Br-nannocystins underpinning the polyketide (10R,11S) configuration as a key determinant of potency. J. Mol. Struct. 2019, 1181, 568–578. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, X.; Ji, J.; Zhang, S.-L.; He, Y. Novel nannocystin A analogues as anticancer therapeutics: Synthesis, biological evaluations and structure-activity relationship studies. Eur. J. Med. Chem. 2019, 170, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Karageorgis, G. Natural product-informed exploration of chemical space to enable bioactive molecular discovery. RSC Med. Chem. 2021, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, Y.; Yuan, X.; Xie, F.; Yu, S.; Cai, J.; Sun, B.; Shan, C.; Zhang, W. Site-directed Late-Stage Diversification of Macrocyclic Nannocystins Facilitating Anticancer SAR and Mode of Action Studies. RSC Med. Chem. 2023, 14, 299–312. [Google Scholar] [CrossRef]
- Krastel, P.; Roggo, S.; Schirle, M.; Ross, N.T.; Perruccio, F.; Aspesi, P., Jr.; Aust, T.; Buntin, K.; Estoppey, D.; Liechty, B.; et al. Nannocystin A: An Elongation Factor 1 Inhibitor from Myxobacteria with Differential Anti-Cancer Properties. Angew. Chem. Int. Ed. 2015, 54, 10149–10154. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Yu, S.; Sun, B.; Zhang, W. Anticancer Small-Molecule Agents Targeting Eukaryotic Elongation Factor 1A: State of the Art. Int. J. Mol. Sci. 2023, 24, 5184. [Google Scholar] [CrossRef]
- Sung, D.-B.; Lee, J.S. Natural-product-based fluorescent probes: Recent advances and applications. RSC Med. Chem. 2023, 14, 412–432. [Google Scholar] [CrossRef]
- Gemmer, M.; Chaillet, M.L.; van Loenhout, J.; Cuevas Arenas, R.; Vismpas, D.; Gröllers-Mulderij, M.; Koh, F.A.; Albanese, P.; Scheltema, R.A.; Howes, S.C.; et al. Visualization of translation and protein biogenesis at the ER membrane. Nature 2023, 614, 160–167. [Google Scholar] [CrossRef]
- Ma, S.; Negishi, E.-I. Palladium-Catalyzed Cyclization of ω-Haloallenes. A New General Route to Common, Medium, and Large Ring Compounds via Cyclic Carbopalladation. J. Am. Chem. Soc. 1995, 117, 6345–6357. [Google Scholar] [CrossRef]
- Ma, S.; Negishi, E.-I. Facile Formation of Seven- and Eight-Membered Cycloalkenes via Catalytic and Cyclic Carbopalladation of Allenes. J. Org. Chem. 1994, 59, 4730–4732. [Google Scholar] [CrossRef]
- Jeffery, T. On the Efficiency of Tetraalkylammonium Salts in Heck Type Reactions. Tetrahedron 1996, 52, 10113–10130. [Google Scholar] [CrossRef]
- Zimmer, R.; Dinesh, C.U.; Nandanan, E.; Khan, F.A. Palladium-Catalyzed Reactions of Allenes. Chem. Rev. 2000, 100, 3067–3125. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Some typical advances in the synthetic applications of allenes. Chem. Rev. 2005, 105, 2829–2872. [Google Scholar] [CrossRef]
- Yu, S.; Ma, S. Allenes in Catalytic Asymmetric Synthesis and Natural Product Syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112. [Google Scholar] [CrossRef]
- Mascarenas, J.L.; Varela, I.; Lopez, F. Allenes and Derivatives in Gold(I)- and Platinum(II)-Catalyzed Formal Cycloadditions. Acc. Chem. Res. 2019, 52, 465–479. [Google Scholar] [CrossRef]
- Blieck, R.; Taillefer, M.; Monnier, F. Metal-catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C-N, C-C and C-O Bonds. Chem. Rev. 2020, 120, 13545–13598. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, X.; Fu, C.; Ma, S. Palladium(0)-Catalyzed Regioselective Synthesis of Macrocycles from Allenes with a Nucleophilic Functionality and Organic Iodides. Adv. Synth. Catal. 2013, 355, 3295–3303. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Q.; Yu, Y.; Fu, C.; Ma, S. Highly Regio- and Stereoselective Synthesis of Nine- to Twelve-Membered Cyclic Compounds by a Pd0-Catalyzed Cyclization Reaction between Allenes with a Nucleophilic Functionality and Organic Halides. Chem. Eur. J. 2009, 15, 7283–7286. [Google Scholar] [CrossRef]
- Weber, C.; Opatz, T. Bisbenzylisoquinoline alkaloids. Alkaloids 2019, 81, 1–114. [Google Scholar]
- Batenburg-Nguyen, U.; Ung, A.T.; Pyne, S.G. The synthesis of carbon linked bis-benzylisoquinolines using Mizoroki-Heck and Sonagashira coupling reactions. Tetrahedron 2009, 65, 318–327. [Google Scholar] [CrossRef]
- Fodale, V.; Santamaria, L.B. Laudanosine, an atracurium and cisatracurium metabolite. Eur. J. Anaesthesiol. 2002, 19, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Dyker, G. Heck-type reactions with a migrating double bond. In Handbook of C-H Transformations; Dyker, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; Volume 2, pp. 427–430, 490–491. [Google Scholar]
- Dyker, G.; Grundt, P. Annulated ring-system by domino-Heck-aldol-condensation and domino-Heck-Michael-addition processes. Tetrahedron Lett. 1996, 37, 619–622. [Google Scholar] [CrossRef]
- Dyker, G.; Grundt, P.; Markwitz, H.; Henkel, G. Heck Reaction and Robinson-Type Annulation: A Versatile Combination. J. Org. Chem. 1998, 63, 6043–6047. [Google Scholar] [CrossRef]
- Dyker, G.; Grundt, P. Synthesis of Functionalized 4a-Methyl-1,2,3,4,4a,9,10,10a-octahydrophenanthrenes. Helv. Chim. Acta 1999, 82, 588–596. [Google Scholar] [CrossRef]
- Dyker, G.; Thöne, A. Palladium catalyzed coupling reactions of diiodoarenes with allylic and homoallylic alcohols. J. Prakt. Chem. 1999, 341, 138–141. [Google Scholar] [CrossRef]
- Dyker, G.; Markwitz, H.; Henkel, G. A putatively unfeasible Heck reaction—From cyclopentenones to annulated ring systems. Eur. J. Org. Chem. 2001, 2415–2423. [Google Scholar] [CrossRef]
- Dyker, G.; Grundt, P. Construction of the Steroid Framework via a Functionalized Macrocyclic Compound. Eur. J. Org. Chem. 1999, 323–327. [Google Scholar] [CrossRef]
- Dyker, G.; Kadzimirsz, D.; Henkel, G. Macrocycles from simple building blocks by a multifold Heck-type coupling reaction. Tetrahedron Lett. 2003, 44, 7905–7907. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Woodcock, T.; Howes, P.D. A tandem Heck reaction leading to a 26-membered carbocycle. Tetrahedron Lett. 2002, 43, 9327–9329. [Google Scholar] [CrossRef]
- Dansou, B.; Pichon, C.; Dhal, R.; Brown, E.; Mille, S. Isolation of macrocyclic metacyclophanes from the attempted synthesis of [7.0]metacyclophanes of the myricanone series by Thorpe-Ziegler intramolecular cyclization of diaryls substituted by ω-cyanoalkyl chains. Eur. J. Org. Chem. 2000, 1527–1533. [Google Scholar]
- Masse, P.; Choppin, S.; Chiummiento, L.; Hanquet, G.; Colobert, F. Unintended Formation of a 26-Membered Cycle in the Course of a Novel Approach to Myricanol, a Strained [7,0]-Metacyclophane. Synlett 2020, 31, 559–564. [Google Scholar] [CrossRef]
- Liu, X.-X.; Pilarinou, E.; McLaughlin, J.L. Pondaplin: A novel cyclic prenylated phenylpropanoid from Annona glabra. Tetrahedron Lett. 1999, 40, 399–402. [Google Scholar] [CrossRef]
- Leonard, M.S.; Carroll, P.J.; Joullié, M.M. Synthesis of a Pondaplin Dimer and Trimer. Aromatic Interactions in Novel Macrocycles. J. Org. Chem. 2004, 69, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- de Meijere, A.; Kurahashi, T. 11.09 Sequential Formation of More than One C–C and Other Bonds by Multiple Heck-type Reactions. In Comprehensive Organometallic Chemistry III; Michael, D., Mingos, P., Crabtree, R.H., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; Volume 11, pp. 311–334. [Google Scholar]
- Gibson, S.E.; Jones, J.O.; Kalindjian, S.B.; Knight, J.D.; Steed, J.W.; Tozer, M.J. Synthesis and structural analysis of dehydrophenylalanine cyclophanes. Chem. Commun. 2002, 1938–1939. [Google Scholar] [CrossRef]
- Gibson, S.E.; Jones, J.O.; Kalindjian, S.B.; Knight, J.D.; Mainolfi, N.; Rudd, M.; Steed, J.W.; Tozer, M.J.; Wright, P.T. Synthesis of meta- and paracyclophanes containing unsaturated amino acid residues. Tetrahedron 2004, 60, 6945–6958. [Google Scholar] [CrossRef]
- Gibson, S.E.; Mainolfi, N.; Barret Kalindjian, S.; Wright, P.T. A versatile approach to chiral macrocycles. Chem. Commun. 2003, 1568–1569. [Google Scholar] [CrossRef]
- Gibson, S.E.; Mainolfi, N.; Kalindjian, S.B.; Wright, P.T.; White, A.J.P. A New Class of Non-Racemic Chiral Macrocycles: A Conformational and Synthetic Study. Chem. Eur. J. 2005, 11, 69–80. [Google Scholar] [CrossRef]
- Gibson, S.E.; Lecci, C.; White, A.J.P. Application of the Heck reaction in the synthesis of macrocycles derived from amino alcohols. Synlett 2006, 2929–2934. [Google Scholar] [CrossRef]
- Suzaki, Y.; Shimada, K.; Chihara, E.; Saito, T.; Tsuchido, Y.; Osakada, K. [3]Rotaxane-Based Dinuclear Palladium Catalysts for Ring-closure Mizoroki-Heck Reaction. Org. Lett. 2011, 13, 3774–3777. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Y.; Hu, R.; Li, T.; Bai, W.-J.; Yang, Y. Enantioselective Total Syntheses of Lyconadins A-E through a Palladium-Catalyzed Heck-Type Reaction. Angew. Chem. Int. Ed. 2020, 59, 2860–2866. [Google Scholar] [CrossRef]
- Chong, C.; Zhang, Q.; Ke, J.; Zhang, H.; Yang, X.; Wang, B.; Ding, W.; Lu, Z. Total Synthesis of Anti-Cancer Meroterpenoids Dysideanone B and Dysiherbol A and Structural Reassignment of Dysiherbol A. Angew. Chem. Int. Ed. 2021, 60, 13807–13813. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Wang, X.; Tian, H.; Gui, J. Asymmetric Total Synthesis of Clionastatins A and B. J. Am. Chem. Soc. 2021, 143, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Bucknam, A.R.; Micalizio, G.C. Asymmetric De Novo Synthesis of a Cucurbitane Triterpenoid: Total Synthesis of Octanorcucurbitacin B. J. Am. Chem. Soc. 2022, 144, 8493–8497. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Ellis, S.R.; Yamazaki, K.; Hayward Cooke, J.; Chekshin, N.; Christensen, K.E.; Hamlin, T.A.; Dixon, D.J. Enantioselective Total Synthesis of (-)-Himalensine A via a Palladium and 4-Hydroxyproline Co-catalyzed Desymmetrization of Vinyl-bromide-tethered Cyclohexanones. J. Am. Chem. Soc. 2023, 145, 5422–5430. [Google Scholar] [CrossRef]
- Kim, D.E.; Zhu, Y.; Harada, S.; Aguilar, I.; Cuomo, A.E.; Wang, M.; Newhouse, T.R. Total Synthesis of (+)-Shearilicine. J. Am. Chem. Soc. 2023, 145, 4394–4399. [Google Scholar] [CrossRef]
- Sims, H.S.; Dai, M. Palladium-Catalyzed Carbonylations: Application in Complex Natural Product Total Synthesis and Recent Developments. J. Org. Chem. 2023, 88, 4925–4941. [Google Scholar] [CrossRef]
- Bai, Y.; Shen, X.; Li, Y.; Dai, M. Total Synthesis of (−)-Spinosyn A via Carbonylative Macrolactonization. J. Am. Chem. Soc. 2016, 138, 10838–10841. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Gao, Z.; Zhao, Y.-M. Total Synthesis of (±)-Cephanolides B and C via a Palladium-Catalyzed Cascade Cyclization and Late-Stage sp3 C-H Bond Oxidation. J. Am. Chem. Soc. 2018, 140, 5653–5658. [Google Scholar] [CrossRef]
- Han, A.; Tao, Y.; Reisman, S.E. A 16-step synthesis of the isoryanodane diterpene (+)-perseanol. Nature 2019, 573, 563–567. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, T.; Tian, M.; Jiang, Y.; Yang, J.; Lei, S.; Wang, Q.; Zhang, C.; Qiu, H.; He, L.; et al. Asymmetric Total Syntheses of Schizozygane Alkaloids. J. Am. Chem. Soc. 2021, 143, 19975–19982. [Google Scholar] [CrossRef]
- Marx, L.; Lamberty, D.; Choppin, S.; Colobert, F.; Speicher, A. Atroposelective Synthesis of Isoriccardin C through a C-H Activated Heck Type Macrocyclization. Eur. J. Org. Chem. 2021, 2021, 1351–1354. [Google Scholar] [CrossRef]
- Fritz, L.; Wienhold, S.; Hackl, S.; Bach, T. Total Synthesis of Pulvomycin D. Chem. Eur. J. 2023, 28, e202104064. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.V.; Choudhury, U.M.; Sarma, A.V.S.; Mohapatra, D.K. Asymmetric Total Synthesis of (2E)-Macrolactin 3. Synlett 2023, 34, 67–72. [Google Scholar]
- Ziegler, F.E.; Chakraborty, U.R.; Weisenfeld, R.B. A palladium-catalyzed carbon-carbon bond formation of conjugated dienones. A macrocyclic dienone lactone model for the carbomycins. Tetrahedron 1981, 37, 4035–4040. [Google Scholar] [CrossRef]
- Jeong, S.; Chen, X.; Harran, P.G. Macrocyclic Triarylethylenes via Heck Endocyclization: A System Relevant to Diazonamide Synthesis. J. Org. Chem. 1998, 63, 8640–8641. [Google Scholar] [CrossRef]
- Chen, X.; Esser, L.; Harran, P.G. Stereocontrol in Pinacol Ring-Contraction of Cyclopeptidyl Glycols: The Diazonamide C10 Problem. Angew. Chem. Int. Ed. 2000, 112, 937–940. [Google Scholar] [CrossRef]
- Collins, S.; Bartlett, S.; Nie, F.; Sore, H.F.; Spring, D.R. Diversity-Oriented Synthesis of Macrocycle Libraries for Drug Discovery and Chemical Biology. Synthesis 2016, 48, 1457–1473. [Google Scholar]
- Ermert, P. Design, properties and recent application of macrocycles in medicinal chemistry. Chimia 2017, 71, 678–702. [Google Scholar] [CrossRef]
- Vinogradov, A.A.; Yin, Y.; Suga, H. Macrocyclic peptides as drug candidates: Recent progress and remaining challenges. J. Am. Chem. Soc. 2019, 141, 4167–4181. [Google Scholar] [CrossRef]
- Cummings, M.D.; Sekharan, S. Structure-based macrocycle design in small-molecule drug discovery and simple metrics to identify opportunities for macrocyclization of small-molecule ligands. J. Med. Chem. 2019, 62, 6843–6853. [Google Scholar] [CrossRef]
- Mortensen, K.T.; Osberger, T.J.; King, T.A.; Sore, H.F.; Spring, D.R. Strategies for the Diversity-Oriented Synthesis of Macrocycles. Chem. Rev. 2019, 119, 10288–10317. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Begnini, F.; Poongavanam, V.; Doak, B.C.; Kihlberg, J. Drug Syntheses Beyond the Rule of 5. Chem. Eur. J. 2020, 26, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fang, R.; Rao, Q. An Insight into the Medicinal Chemistry Perspective of Macrocyclic Derivatives with Antitumor Activity: A Systematic Review. Molecules 2022, 27, 2837. [Google Scholar] [CrossRef] [PubMed]
- Garcia Jimenez, D.; Poongavanam, V.; Kihlberg, J. Macrocycles in Drug Discovery-Learning from the Past for the Future. J. Med. Chem. 2023, 66, 5377–5396. [Google Scholar] [CrossRef]
- Collins, J.C.; James, K. Emac–a comparative index for the assessment of macrocyclization efficiency. MedChemComm 2012, 3, 1489–1495. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Arikan, F.; Ahlbrecht, W.; Dieckmann, M.; Menche, D. Total Synthesis of Etnangien. J. Am. Chem. Soc. 2009, 131, 11678–11679. [Google Scholar] [CrossRef]
- Paul, D.; Das, S.; Goswami, R.K. Total Synthesis of Pestalotioprolide G and Putative Structure of Pestalotioprolide H. J. Org. Chem. 2017, 82, 7437–7445. [Google Scholar] [CrossRef]
- Das, S.; Paul, D.; Goswami, R.K. Stereoselective Total Synthesis of Bioactive Marine Natural Product Biselyngbyolide B. Org. Lett. 2016, 18, 1908–1911. [Google Scholar] [CrossRef] [PubMed]
- Jägel, J.; Maier, M.E. Formal Total Synthesis of Palmerolide A. Synthesis 2009, 2881–2892. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).