ABCA3 Deficiency—Variant-Specific Response to Hydroxychloroquine
Abstract
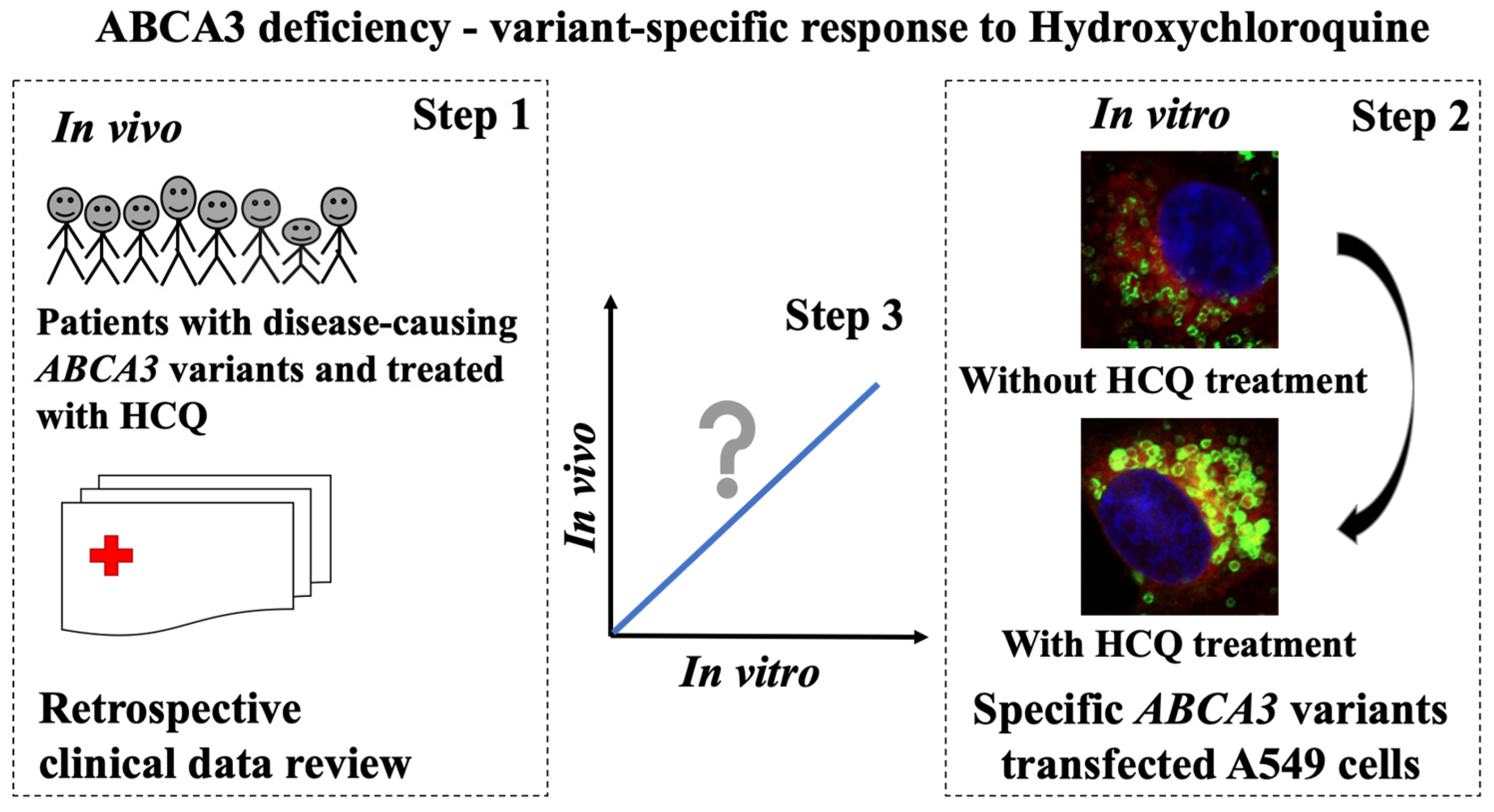
1. Introduction
2. Results
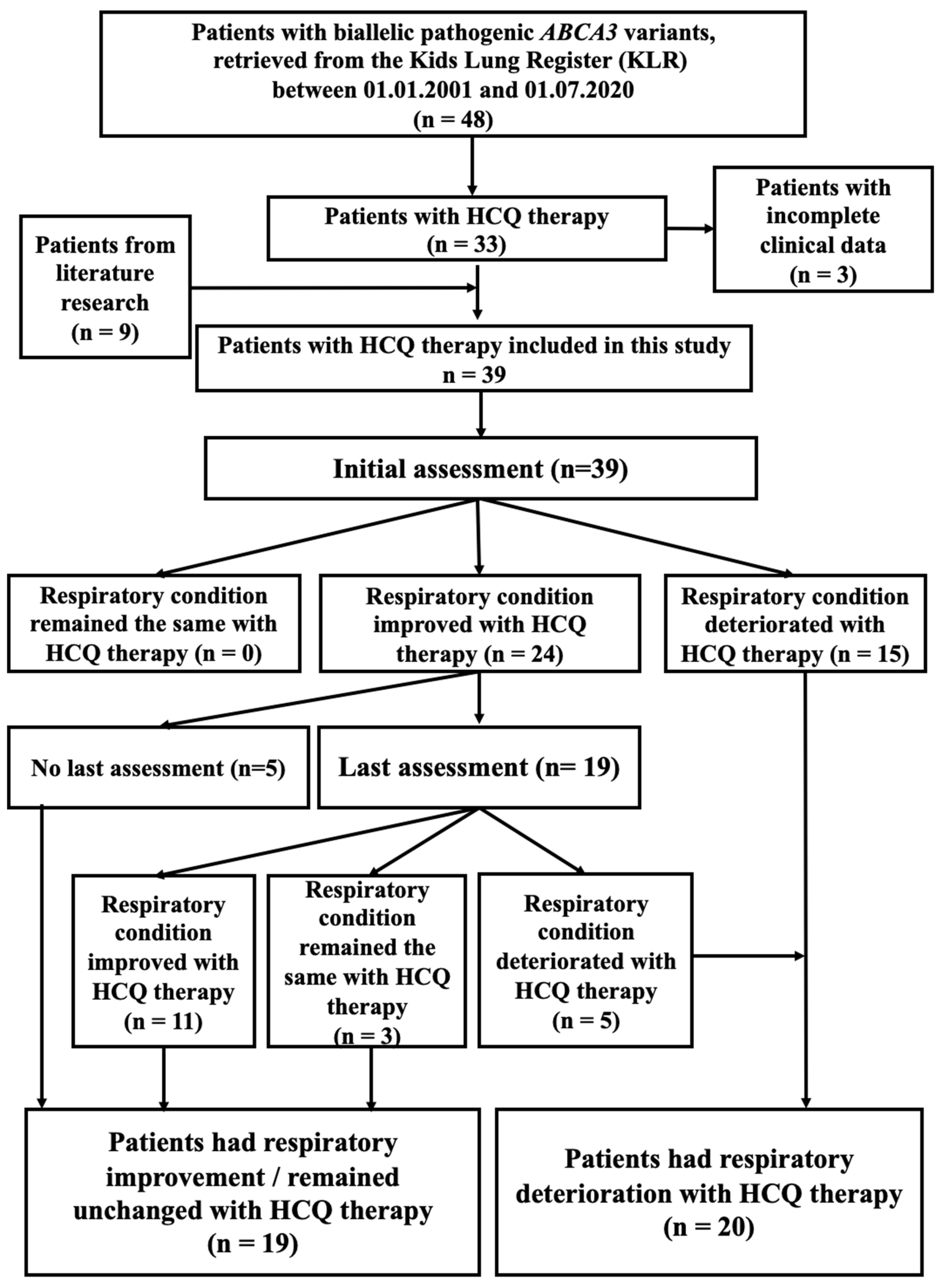
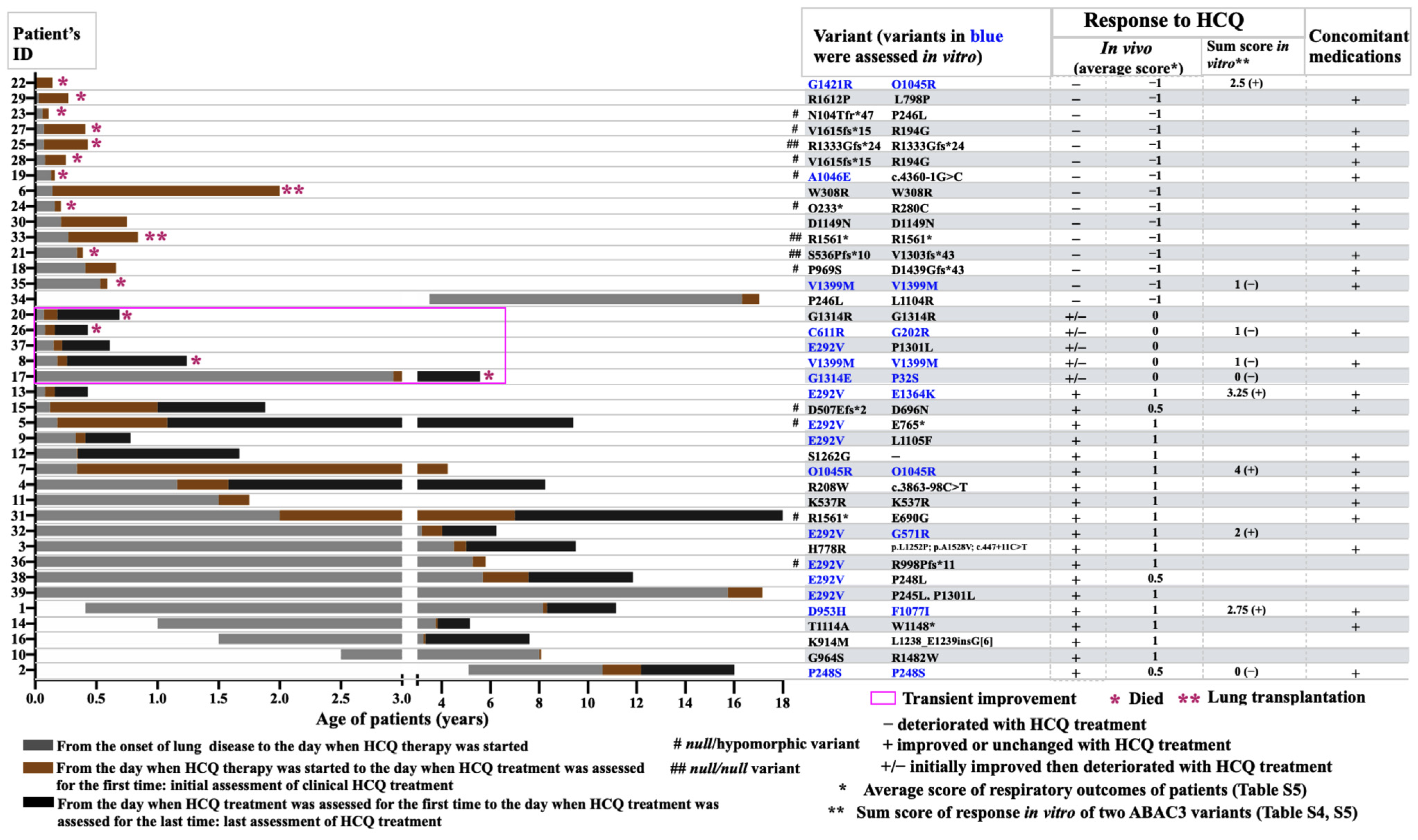
2.1. Outcome of Patients with ABCA3-Related Lung Disease and HCQ Therapy
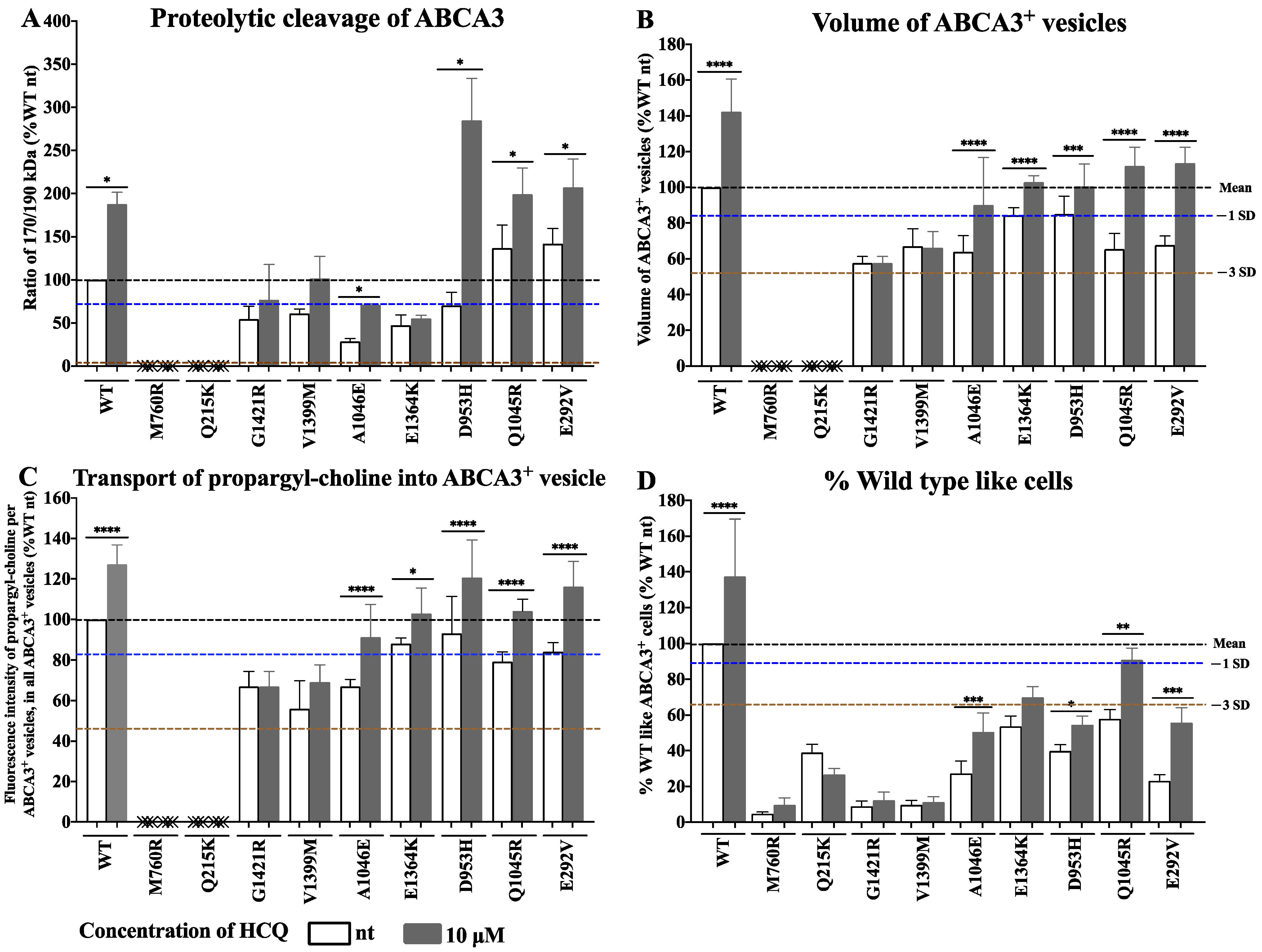
2.2. Variant-Specific and Dose-Dependent Effects of HCQ on ABCA3 In Vitro Using a High-Content Screening (HCS) Assay
2.3. Detailed Evaluation and Validation of HCQ Response in Small-Format Assays (In Vitro)
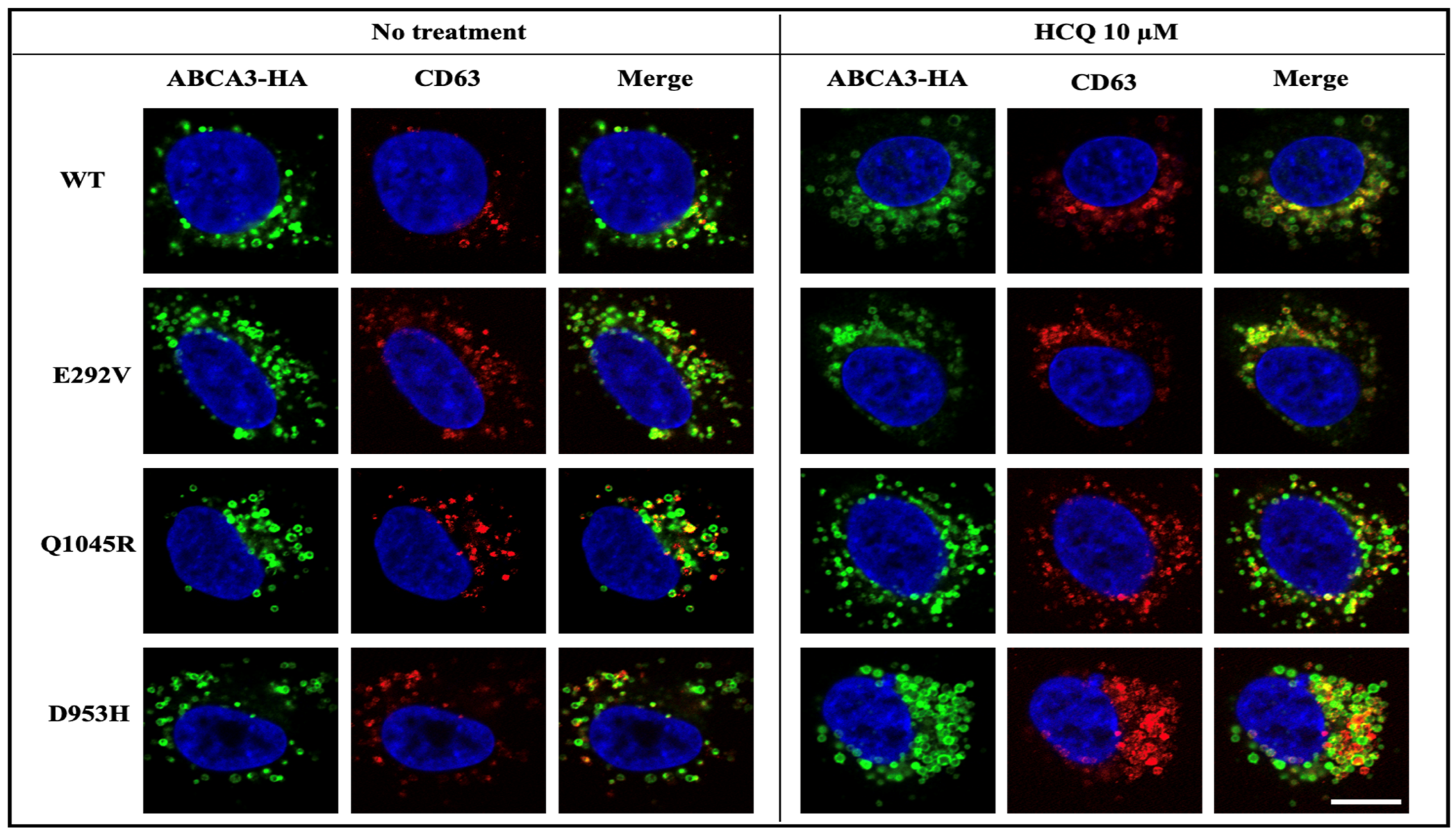
2.4. HCQ Acts on the Lysosome-Related Components in ABCA3 Transfected Cells
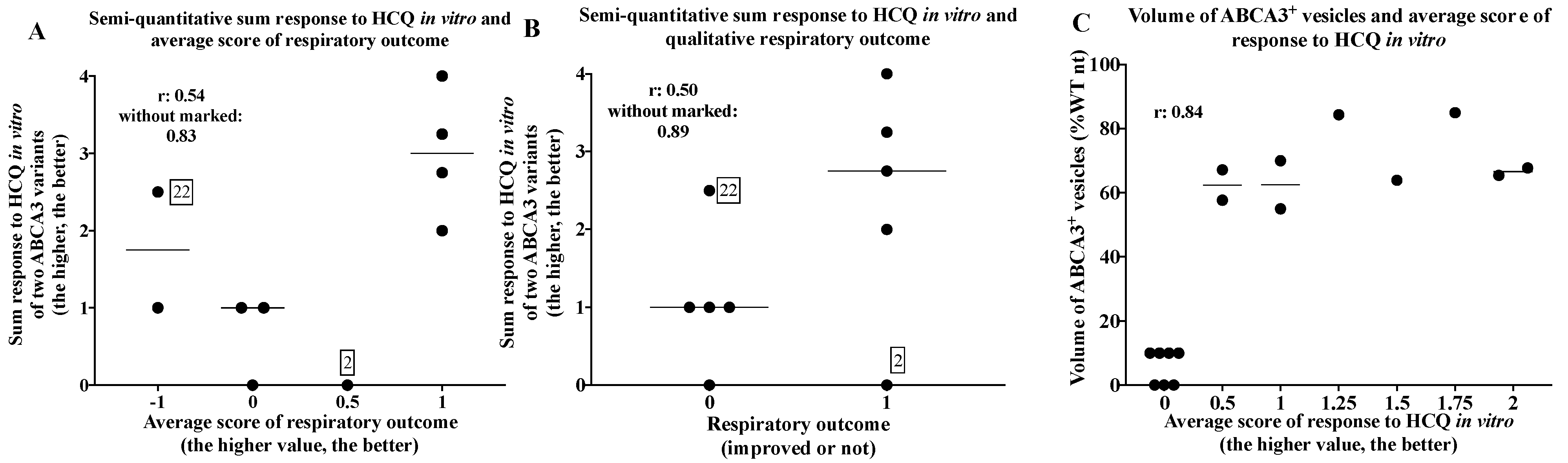
2.5. Comparison of In Vivo and In Vitro Results
3. Discussion
4. Materials and Methods
4.1. Response of Patients with ABCA3 Deficiency to HCQ (In Vivo)
4.2. Response of Cells Expressing ABCA3 Variants to HCQ (In Vitro)
4.2.1. Cell Culture, Viability, Western Blotting, and Immunofluorescence Staining
4.2.2. High-Content Screening Method (HCS)
4.2.3. Expression of Results of the Functional Assays, Criteria for In Vitro Response to HCQ, and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, N.; Matsumura, Y.; Sakai, H.; Takanezawa, Y.; Sasaki, M.; Arai, H.; Inagaki, N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J. Biol. Chem. 2007, 282, 9628–9634. [Google Scholar] [CrossRef]
- Griese, M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur. Respir. J. 1999, 13, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Griese, M. Interstitial lung disease in children—Genetic background and associated phenotypes. Respir. Res. 2005, 6, 32. [Google Scholar] [CrossRef]
- Schindlbeck, U.; Wittmann, T.; Höppner, S.; Kinting, S.; Liebisch, G.; Hegermann, J.; Griese, M. ABCA3 missense mutations causing surfactant dysfunction disorders have distinct cellular phenotypes. Hum. Mutat. 2018, 39, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Wambach, J.A.; Casey, A.M.; Fishman, M.P.; Wegner, D.J.; Wert, S.E.; Cole, F.S.; Hamvas, A.; Nogee, L.M. Genotype–Phenotype Correlations for Infants and Children with ABCA3 Deficiency. Am. J. Respir. Crit. Care Med. 2014, 189, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Kröner, C.; Wittmann, T.; Reu, S.; Teusch, V.; Klemme, M.; Rauch, D.; Hengst, M.; Kappler, M.; Cobanoglu, N.; Sismanlar, T.; et al. Lung disease caused by ABCA3 mutations. Thorax 2017, 72, 213–220. [Google Scholar] [CrossRef]
- Li, Y.; Seidl, E.; Knoflach, K.; Gothe, F.; Forstner, M.E.; Michel, K.; Pawlita, I.; Gesenhues, F.; Sattler, F.; Yang, X.; et al. ABCA3-related interstitial lung disease beyond infancy. Thorax 2023. [Google Scholar] [CrossRef]
- Braun, S.; Ferner, M.; Kronfeld, K.; Griese, M. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr. Pulmonol. 2015, 50, 410–419. [Google Scholar] [CrossRef]
- Griese, M.; Kappler, M.; Stehling, F.; Schulze, J.; Baden, W.; Koerner-Rettberg, C.; Carlens, J.; Prenzel, F.; Nährlich, L.; Thalmeier, A.; et al. Randomized controlled phase 2 trial of hydroxychloroquine in childhood interstitial lung disease. Orphanet J. Rare Dis. 2022, 17, 289. [Google Scholar] [CrossRef]
- Klay, D.; Hoffman, T.W.; Harmsze, A.M.; Grutters, J.C.; Van Moorsel, C.H. Systematic review of drug effects in humans and models with surfactant-processing disease. Eur. Respir. Rev. 2018, 27, 170135. [Google Scholar] [CrossRef]
- Williamson, M.; Wallis, C. Ten-year follow up of hydroxychloroquine treatment for ABCA3 deficiency. Pediatr. Pulmonol. 2014, 49, 299–301. [Google Scholar] [CrossRef]
- Winter, J.; Essmann, S.; Kidszun, A.; Aslanidis, C.; Griese, M.; Poplawska, K.; Bartsch, M.; Schmitz, G.; Mildenberger, E. Neonatal Respiratory Insufficiency Caused by an (Homozygous) ABCA3-Stop Mutation: A Systematic Evaluation of Therapeutic Options. Klin. Padiatr. 2014, 226, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Koudriavtseva, T. Current and Future Use of Chloroquine and Hydroxychloroquine in Infectious, Immune, Neoplastic, and Neurological Diseases: A Mini-Review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.H.; Cho, K.S.; Hwang, J.J.; Lee, S.-J.; Choi, J.A.; Koh, J.-Y. Induction of Lysosomal Dilatation, Arrested Autophagy, and Cell Death by Chloroquine in Cultured ARPE-19 Cells. Investig. Opthalmol. Vis. Sci. 2010, 51, 6030–6037. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Krise, J.P. Lysosomal Sequestration of Amine-Containing Drugs: Analysis and Therapeutic Implications. J. Pharm. Sci. 2007, 96, 729–746. [Google Scholar] [CrossRef]
- Beers, M.F.; Mulugeta, S. The biology of the ABCA3 lipid transporter in lung health and disease. Cell Tissue Res. 2017, 367, 481–493. [Google Scholar] [CrossRef]
- Cheong, N.; Madesh, M.; Gonzales, L.W.; Zhao, M.; Yu, K.; Ballard, P.L.; Shuman, H. Functional and Trafficking Defects in ATP Binding Cassette A3 Mutants Associated with Respiratory Distress Syndrome. J. Biol. Chem. 2006, 281, 9791–9800. [Google Scholar] [CrossRef]
- Thavagnanam, S.; Cutz, E.; Manson, D.; Nogee, L.M.; Dell, S.D. Variable clinical outcome of ABCA3 deficiency in two siblings. Pediatr. Pulmonol. 2013, 48, 1035–1038. [Google Scholar] [CrossRef]
- Yokota, T.; Matsumura, Y.; Ban, N.; Matsubayashi, T.; Inagaki, N. Heterozygous ABCA3 mutation associated with non-fatal evolution of respiratory distress. Eur. J. Pediatr. 2007, 167, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Hallik, M.; Annilo, T.; Ilmoja, M.-L. Different course of lung disease in two siblings with novel ABCA3 mutations. Eur. J. Pediatr. 2014, 173, 1553–1556. [Google Scholar] [CrossRef]
- Kitazawa, H.; Moriya, K.; Niizuma, H.; Kawano, K.; Saito-Nanjo, Y.; Uchiyama, T.; Rikiishi, T.; Sasahara, Y.; Sakamoto, O.; Setoguchi, Y.; et al. Interstitial lung disease in two brothers with novel compound heterozygous ABCA3 mutations. Eur. J. Pediatr. 2013, 172, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Peca, D.; Campi, F.; Corsello, M.; Landolfo, F.; Boldrini, R.; Danhaive, O.; Dotta, A. New ATP-binding cassette A3 mutation causing surfactant metabolism dysfunction pulmonary type 3. Pediatr. Int. 2015, 57, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.-P.; Pinheiro, L.; Costa, M.; Silva, A.; Gonçalves, A.; Pereira, A. Novel ABCA3 mutations as a cause of respiratory distress in a term newborn. Gene 2014, 534, 417–420. [Google Scholar] [CrossRef]
- Mitsiakos, G.; Tsakalidis, C.; Karagianni, P.; Gialamprinou, D.; Chatziioannidis, I.; Papoulidis, I.; Tsanakas, I.; Soubasi, V. A New ABCA3 Gene Mutation c.3445G>A (p.Asp1149Asn) as a Causative Agent of Newborn Lethal Respiratory Distress Syndrome. Medicina 2019, 55, 389. [Google Scholar] [CrossRef] [PubMed]
- Shulenin, S.; Nogee, L.M.; Annilo, T.; Wert, S.E.; Whitsett, J.A.; Dean, M. ABCA3 Gene Mutations in Newborns with Fatal Surfactant Deficiency. New Engl. J. Med. 2004, 350, 1296–1303. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Thursfield, R.M.; Davies, J.C. Genotype-specific small-molecule therapy for cystic fibrosis. Breathe 2013, 9, 176–186. [Google Scholar] [CrossRef]
- Li, Y.; Kinting, S.; Höppner, S.; Forstner, M.E.; Uhl, O.; Koletzko, B.; Griese, M. Metabolic labelling of choline phospholipids probes ABCA3 transport in lamellar bodies. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2019, 1864, 158516. [Google Scholar] [CrossRef]
- Hofmann, N.; Galetskiy, D.; Rauch, D.; Wittmann, T.; Marquardt, A.; Griese, M.; Zarbock, R. Analysis of the Proteolytic Processing of ABCA3: Identification of Cleavage Site and Involved Proteases. PLoS ONE 2016, 11, e0152594. [Google Scholar] [CrossRef]
- Kinting, S.; Höppner, S.; Schindlbeck, U.; E Forstner, M.; Harfst, J.; Wittmann, T.; Griese, M. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Hum. Mol. Genet. 2018, 27, 943–953. [Google Scholar] [CrossRef]
- Chasset, F.; Arnaud, L.; Costedoat-Chalumeau, N.; Zahr, N.; Bessis, D.; Francès, C. The effect of increasing the dose of hydroxychloroquine (HCQ) in patients with refractory cutaneous lupus erythematosus (CLE): An open-label prospective pilot study. J. Am. Acad. Dermatol. 2016, 74, 693–699.e3. [Google Scholar] [CrossRef]
- E Bullard, J.; Nogee, L.M. Heterozygosity for ABCA3 Mutations Modifies the Severity of Lung Disease Associated with a Surfactant Protein C Gene (SFTPC) Mutation. Pediatr. Res. 2007, 62, 176–179. [Google Scholar] [CrossRef]
- Beers, M.F.; Zhao, M.; Tomer, Y.; Russo, S.J.; Zhang, P.; Gonzales, L.W.; Guttentag, S.H.; Mulugeta, S. Disruption of N-linked glycosylation promotes proteasomal degradation of the human ATP-binding cassette transporter ABCA3. Am. J. Physiol. Cell. Mol. Physiol. 2013, 305, L970–L980. [Google Scholar] [CrossRef]
- Kinting, S.; Li, Y.; Forstner, M.; Delhommel, F.; Sattler, M.; Griese, M. Potentiation of ABCA3 lipid transport function by ivacaftor and genistein. J. Cell. Mol. Med. 2019, 23, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- McCague, A.F.; Raraigh, K.S.; Pellicore, M.J.; Davis-Marcisak, E.F.; Evans, T.A.; Han, S.T.; Lu, Z.; Joynt, A.T.; Sharma, N.; Castellani, C.; et al. Correlating Cystic Fibrosis Transmembrane Conductance Regulator Function with Clinical Features to Inform Precision Treatment of Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1116–1126. [Google Scholar] [CrossRef]
- Kaltenborn, E.; Kern, S.; Frixel, S.; Fragnet, L.; Conzelmann, K.-K.; Zarbock, R.; Griese, M. Respiratory syncytial virus potentiates ABCA3 mutation-induced loss of lung epithelial cell differentiation. Hum. Mol. Genet. 2012, 21, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Schindlbeck, U.; Höppner, S.; Kinting, S.; Frixel, S.; Kröner, C.; Liebisch, G.; Hegermann, J.; Aslanidis, C.; Brasch, F.; et al. Tools to explore ABCA3 mutations causing interstitial lung disease. Pediatr. Pulmonol. 2016, 51, 1284–1294. [Google Scholar] [CrossRef]
- Forstner, M.; Lin, S.; Yang, X.; Kinting, S.; Rothenaigner, I.; Schorpp, K.; Li, Y.; Hadian, K.; Griese, M. High-Content Screening Identifies Cyclosporin A as a Novel ABCA3-Specific Molecular Corrector. Am. J. Respir. Cell Mol. Biol. 2022, 66, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Anesth. Analg. 2015, 17, 405–424. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Forstner, M.; Rapp, C.K.; Rothenaigner, I.; Li, Y.; Hadian, K.; Griese, M. ABCA3 Deficiency—Variant-Specific Response to Hydroxychloroquine. Int. J. Mol. Sci. 2023, 24, 8179. https://doi.org/10.3390/ijms24098179
Yang X, Forstner M, Rapp CK, Rothenaigner I, Li Y, Hadian K, Griese M. ABCA3 Deficiency—Variant-Specific Response to Hydroxychloroquine. International Journal of Molecular Sciences. 2023; 24(9):8179. https://doi.org/10.3390/ijms24098179
Chicago/Turabian StyleYang, Xiaohua, Maria Forstner, Christina K. Rapp, Ina Rothenaigner, Yang Li, Kamyar Hadian, and Matthias Griese. 2023. "ABCA3 Deficiency—Variant-Specific Response to Hydroxychloroquine" International Journal of Molecular Sciences 24, no. 9: 8179. https://doi.org/10.3390/ijms24098179
APA StyleYang, X., Forstner, M., Rapp, C. K., Rothenaigner, I., Li, Y., Hadian, K., & Griese, M. (2023). ABCA3 Deficiency—Variant-Specific Response to Hydroxychloroquine. International Journal of Molecular Sciences, 24(9), 8179. https://doi.org/10.3390/ijms24098179






