DNA Materials Assembled from One DNA Strand
Abstract
1. Introduction
2. The Development of DNA Assembly
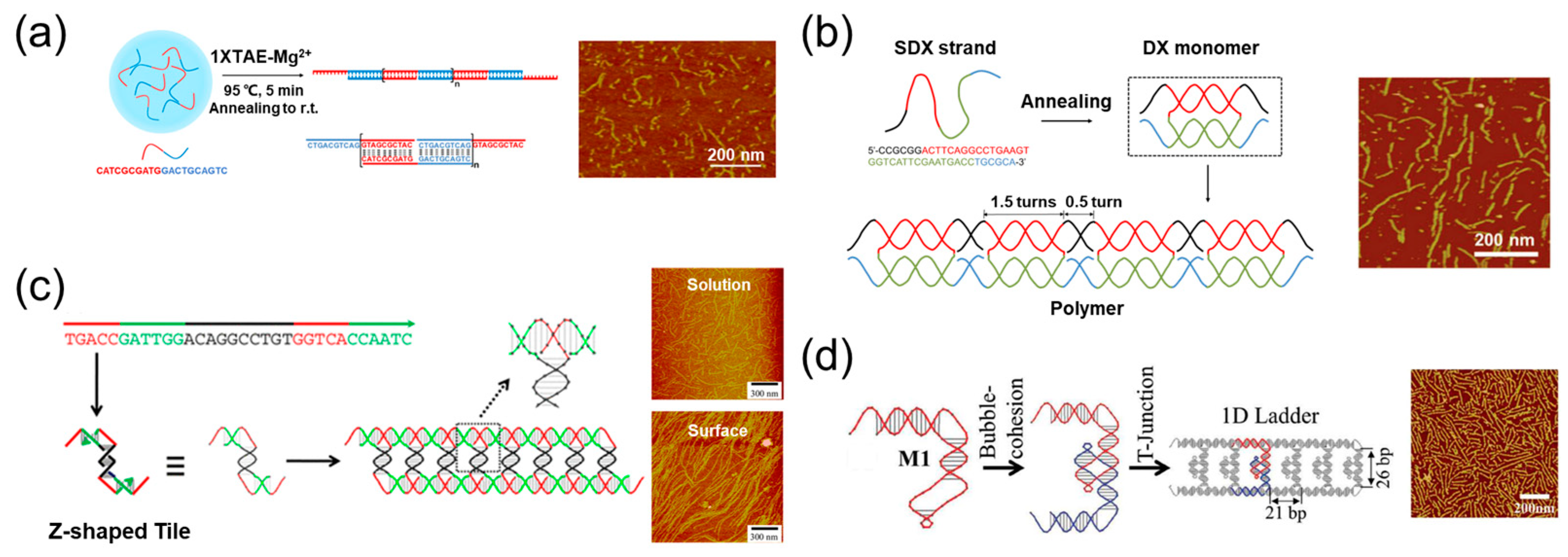
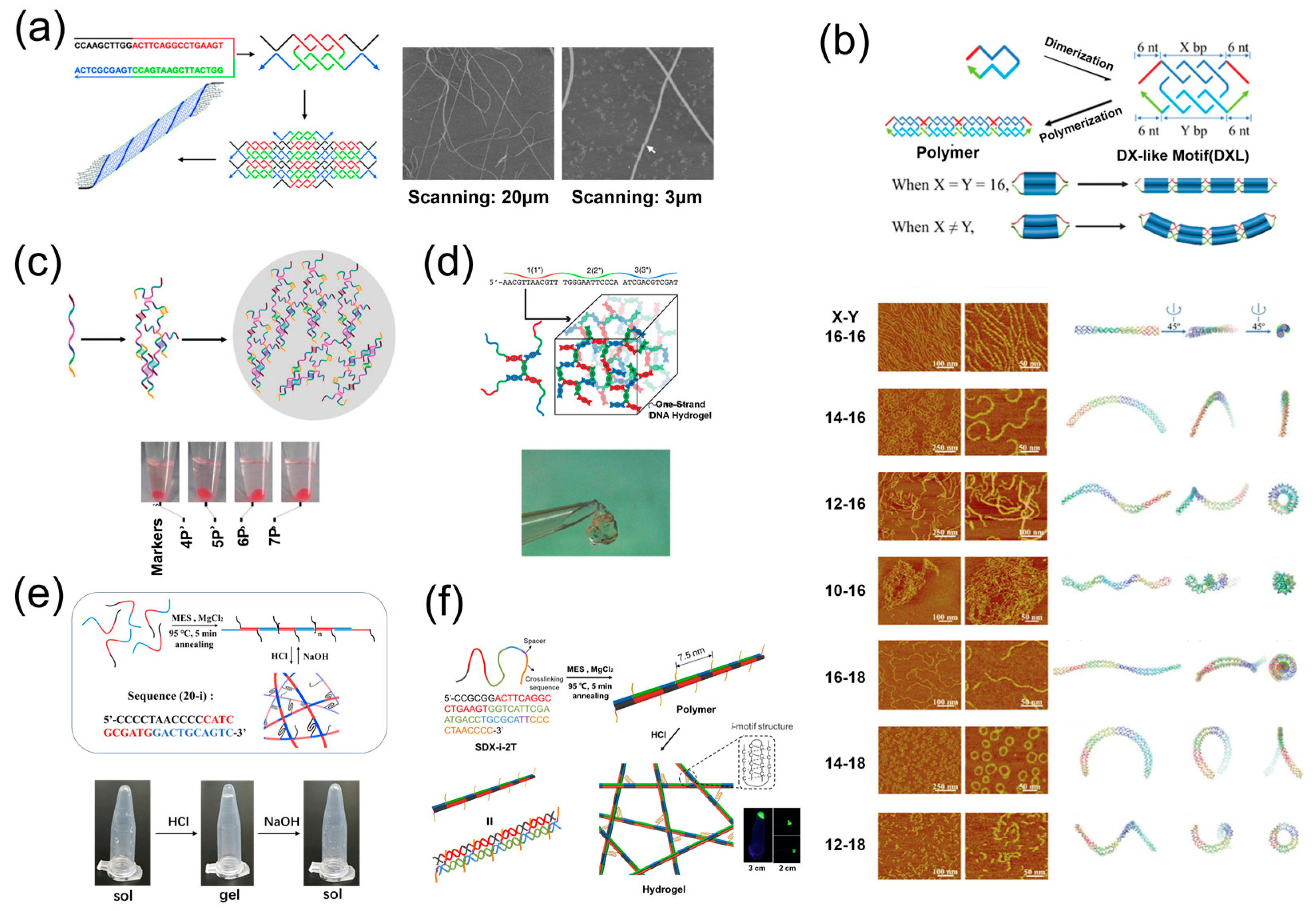
3. The 1D Materials Assembled by One DNA Strand
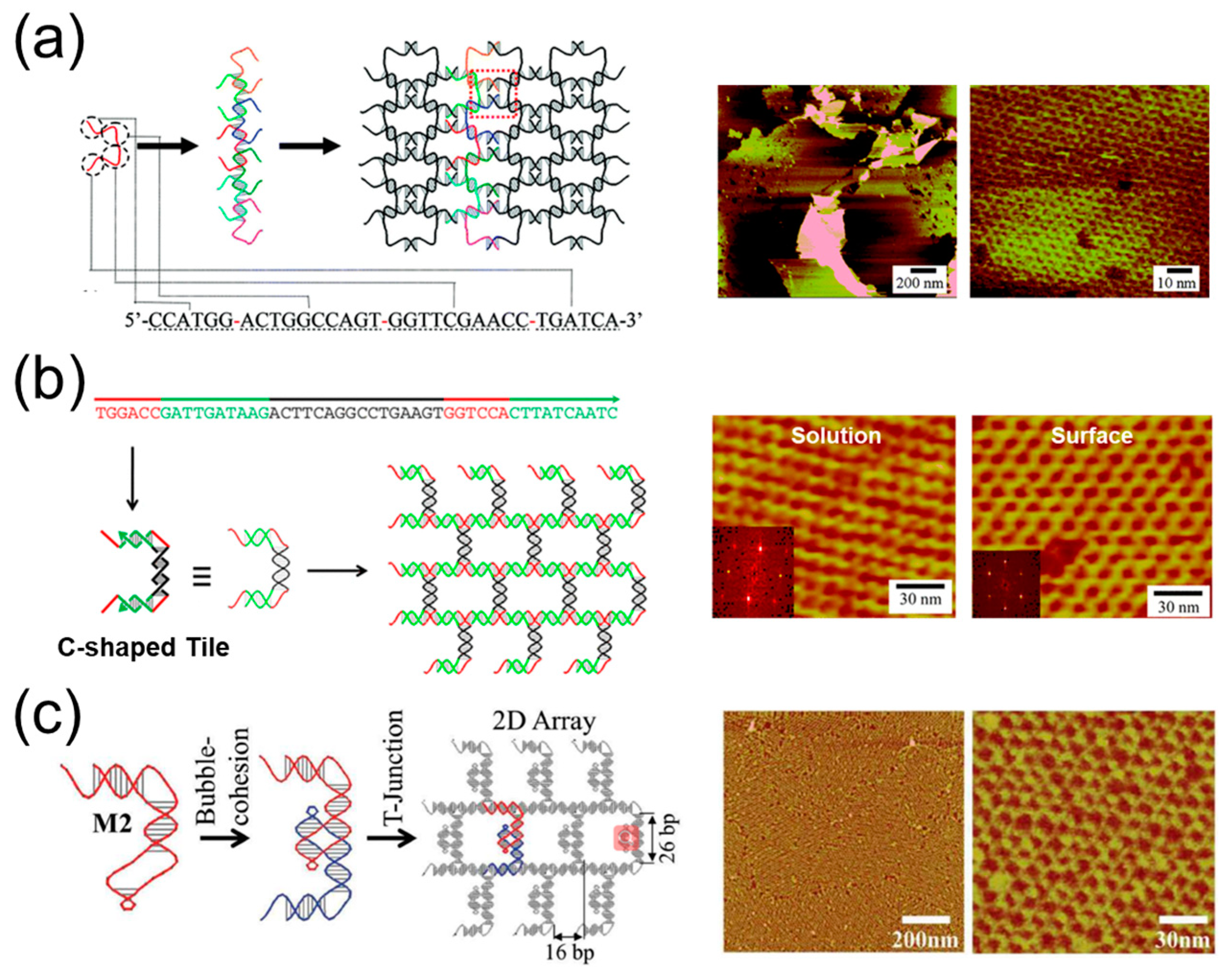
4. The 2D Materials Assembled by One DNA Strand
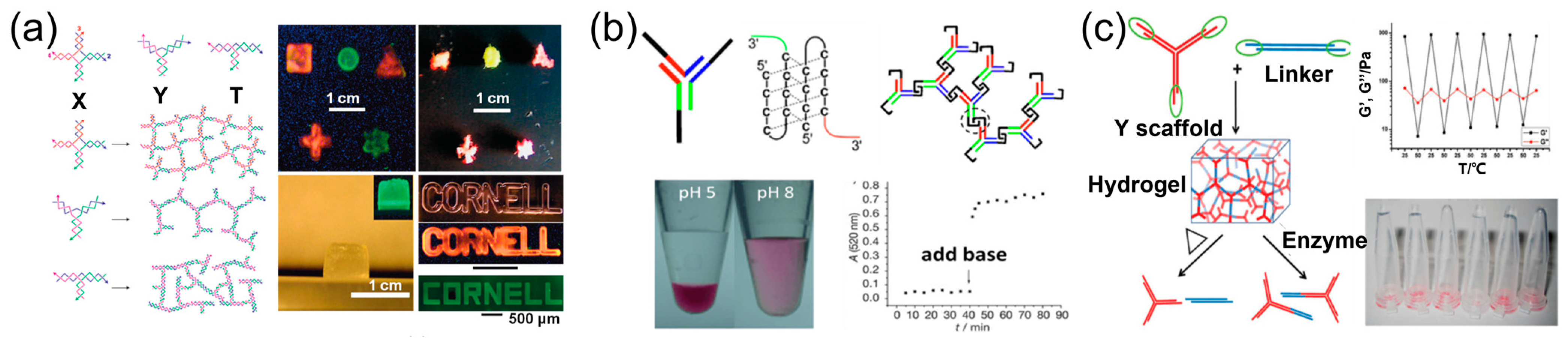
5. The 3D Materials Assembled by One DNA Strand
6. The Principle for the Construction of DNA Materials Using One DNA Strand
6.1. The DNA Sequence
6.2. DNA Length
6.3. Buffer Composition
6.4. Annealing Time
7. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Saridakis, E. The genetic informational network: How DNA conveys semantic information. Hist. Philos. Life Sci. 2021, 43, 112. [Google Scholar] [CrossRef]
- Herweijer, H.; Wolff, J.A. Progress and prospects: Naked DNA gene transfer and therapy. Gene Ther. 2003, 10, 453–458. [Google Scholar] [CrossRef]
- Song, Q.Q.; Hu, Y.M.; Yin, A.Q.; Wang, H.B.; Yin, Q.K. DNA Holliday Junction: History, Regulation and Bioactivity. Int. J. Mol. Sci. 2022, 23, 9730. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wilner, O.I.; Willner, B.; Willner, I. Advances in experimental medicine and biology. In Nano-Biotechnology for Biomedical and Diagnostic Research; Zahavy, E., Ordentlich, A., Yitzhaki, S., Shafferman, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 733, pp. 97–114. [Google Scholar]
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Sun, W.; Yang, C.Y.; Zhu, Z. Scaling Up DNA Self-Assembly. ACS Appl. Bio Mater. 2020, 3, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.D.; Wang, L.; Li, W.; Xu, X.W.; Jiang, W. DNA nanostructure-based drug delivery nanosystems in cancer therapy. Int. J. Pharm. 2017, 533, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Tu, J.; Wang, D.Q.; Zhu, H.T.; Maity, S.K.; Qu, X.M.; Bogaert, B.; Pei, H.; Zhang, H.B. Programmable and Multifunctional DNA-Based Materials for Biomedical Applications. Adv. Mater. 2018, 30, 1703658. [Google Scholar] [CrossRef]
- Shi, J.Z.; Shi, Z.W.; Dong, Y.C.; Wu, F.; Liu, D.S. Responsive DNA-Based Supramolecular Hydrogels. ACS Appl. Bio Mater. 2020, 3, 2827–2837. [Google Scholar] [CrossRef]
- Chao, J.; Liu, H.J.; Su, S.; Wang, L.H.; Huang, W.; Fan, C.H. Structural DNA Nanotechnology for Intelligent Drug Delivery. Small 2014, 10, 4626–4635. [Google Scholar] [CrossRef]
- Weiden, J.; Bastings, M.M.C. DNA origami nanostructures for controlled therapeutic drug delivery. Curr. Opin. Colloid Interface Sci. 2021, 52, 101411. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.Y.; Chen, J.; Liu, H.J. Advances in DNA Supramolecular Hydrogels for Tissue Engineering. Macromol. Biosci. 2022, 22, 2200152. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.G.; Winfree, E. Physical principles for DNA tile self-assembly. Chem. Soc. Rev. 2017, 46, 3808–3829. [Google Scholar] [CrossRef]
- Sacca, B.; Niemeyer, C.M. DNA Origami: The Art of Folding DNA. Angew. Chem. Int. Ed. 2012, 51, 58–66. [Google Scholar] [CrossRef]
- Liu, H.P.; Chen, Y.; He, Y.; Ribbe, A.E.; Mao, C.D. Approaching the limit: Can one DNA oligonucleotide assemble into large nanostructures? Angew. Chem. Int. Ed. 2006, 45, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Seeman, N.C. DNA double-crossover molecules. Biochemistry 1993, 32, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- Winfree, E.; Liu, F.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef]
- He, Y.; Chen, Y.; Liu, H.P.; Ribbe, A.E.; Mao, C.D. Self-assembly of hexagonal DNA two-dimensional (2D) arrays. J. Am. Chem. Soc. 2005, 127, 12202–12203. [Google Scholar] [CrossRef]
- He, Y.; Tian, Y.; Ribbe, A.E.; Mao, C.D. Highly connected two-dimensional crystals of DNA six-point-stars. J. Am. Chem. Soc. 2006, 128, 15978–15979. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Hogberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Han, D.R.; Pal, S.; Nangreave, J.; Deng, Z.T.; Liu, Y.; Yan, H. DNA Origami with Complex Curvatures in Three-Dimensional Space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.J.; Xing, Y.Z.; Chen, P.; Yang, Y.; Sun, Y.W.; Zhou, D.J.; Xu, L.J.; Fan, Q.H.; Liu, D.S. A pH-Triggered, Fast-Responding DNA Hydrogel. Angew. Chem. Int. Ed. 2009, 48, 7660–7663. [Google Scholar] [CrossRef]
- Xing, Y.Z.; Cheng, E.J.; Yang, Y.; Chen, P.; Zhang, T.; Sun, Y.W.; Yang, Z.Q.; Liu, D.S. Self-Assembled DNA Hydrogels with Designable Thermal and Enzymatic Responsiveness. Adv. Mater. 2011, 23, 1117–1121. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Li, C.; Boldt, F.; Wang, Y.R.; Kuan, S.L.; Tran, T.T.; Mikhalevich, V.; Fortsch, C.; Barth, H.; Yang, Z.Q.; et al. Programmable protein-DNA hybrid hydrogels for the immobilization and release of functional proteins. Chem. Commun. 2014, 50, 14620–14622. [Google Scholar] [CrossRef]
- Yan, X.; Yang, B.; Chen, Y.R.; Song, Y.F.; Ye, J.; Pan, Y.F.; Zhou, B.N.; Wang, Y.Q.; Mao, F.B.A.; Dong, Y.C.; et al. Anti-Friction MSCs Delivery System Improves the Therapy for Severe Osteoarthritis. Adv. Mater. 2021, 33, 2104758. [Google Scholar] [CrossRef]
- Li, C.; Faulkner-Jones, A.; Dun, A.R.; Jin, J.; Chen, P.; Xing, Y.Z.; Yang, Z.Q.; Li, Z.B.; Shu, W.M.; Liu, D.S.; et al. Rapid Formation of a Supramolecular Polypeptide-DNA Hydrogel for In Situ Three-Dimensional Multilayer Bioprinting. Angew. Chem. Int. Ed. 2015, 54, 3957–3961. [Google Scholar] [CrossRef]
- Dong, Y.C.; Liu, D.S. Frame-Guided Assembly of Amphiphiles. Chem. Eur. J. 2015, 21, 18018–18023. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, P.F.; Luo, D. Putting DNA to Work as Generic Polymeric Materials. Angew. Chem. Int. Ed. 2022, 61, e202110666. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Liedl, T. From DNA Tiles to Functional DNA Materials. Trends Chem. 2019, 1, 799–814. [Google Scholar] [CrossRef]
- Wang, J.B.; Li, Z.Z.; Willner, I. Dynamic Reconfigurable DNA Nanostructures, Networks and Materials. Angew. Chem. Int. Ed. 2023, 62, e202215332. [Google Scholar] [CrossRef]
- Farag, N.; Ercolani, G.; Del Grosso, E.; Ricci, F. DNA Tile Self-Assembly Guided by Base Excision Repair Enzymes. Angew. Chem. Int. Ed. 2022, 61, e202208367. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, J.Y.; Tanner, J.A.; Shiu, S.C.C. Self-Assembly of DNA Tiles with G-Quadruplex DNAzyme Catalytic Activity for Sensing Applications. ACS Appl. Bio Mater. 2022, 5, 3788–3794. [Google Scholar] [CrossRef]
- Xing, C.; Chen, Z.Y.; Dai, J.D.; Zhou, J.; Wang, L.P.; Zhang, K.L.; Yin, X.F.; Lu, C.H.; Yang, H.H. Light-Controlled, Toehold-Mediated Logic Circuit for Assembly of DNA Tiles. ACS Appl. Mater. Interfaces 2020, 12, 6336–6342. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Doi, J.D.; Huang, Y.Q.; Lin, Y.H.; Zhang, K.L.; Lu, C.H.; Yang, H.H. Active Self-Assembly of Train-Shaped DNA Nanostructures via Catalytic Hairpin Assembly Reactions. Small 2019, 15, 1901795. [Google Scholar] [CrossRef]
- Matthies, M.; Agarwal, N.P.; Poppleton, E.; Joshi, F.M.; Sulc, P.; Schmidt, T.L. Triangulated Wireframe Structures Assembled Using Single-Stranded DNA Tiles. ACS Nano 2019, 13, 1839–1848. [Google Scholar] [CrossRef]
- Zou, J.J.; Stammers, A.C.; Taladriz-Sender, A.; Withers, J.M.; Christie, I.; Vega, M.S.; Aekbote, B.L.; Peveler, W.J.; Rusling, D.A.; Burley, G.A.; et al. Fluorous-Directed Assembly of DNA Origami Nanostructures. ACS Nano 2023, 17, 752–759. [Google Scholar] [CrossRef]
- Vecchioni, S.; Lu, B.; Janowski, J.; Woloszyn, K.; Jonoska, N.; Seeman, N.C.; Mao, C.D.; Ohayon, Y.P.; Sha, R.J. The Rule of Thirds: Controlling Junction Chirality and Polarity in 3D DNA Tiles. Small 2023, 19, 2206511. [Google Scholar] [CrossRef]
- Farag, N.; Dordevic, M.; Del Grosso, E.; Ricci, F. Dynamic and Reversible Decoration of DNA-Based Scaffolds. Adv. Mater. 2023, 2211274. [Google Scholar] [CrossRef]
- Le, J.Y.; Osmanovic, D.; Klocke, M.A.; Franco, E. Fueling DNA Self-Assembly via Gel-Released Regulators. ACS Nano 2022, 16, 16372–16384. [Google Scholar] [CrossRef] [PubMed]
- Lat, P.K.; Schultz, C.W.; Yu, H.Z.; Sen, D. A Long and Reversibly Self-Assembling 1D DNA Nanostructure Built from Triplex and Quadruplex Hybrid Tiles. Angew. Chem. Int. Ed. 2021, 60, 8722–8727. [Google Scholar] [CrossRef]
- Shi, J.Z.; Jia, H.Y.; Chen, H.; Wang, X.; Xu, J.F.; Ren, W.B.; Zhao, J.; Zhou, X.; Dong, Y.C.; Liu, D.S. Concentration Insensitive Supramolecular Polymerization Enabled by Kinetically Interlocking Multiple Units Strategy. CCS Chem. 2019, 1, 296–303. [Google Scholar] [CrossRef]
- Sa-Ardyen, P.; Vologodskii, A.V.; Seeman, N.C. The flexibility of DNA double crossover molecules. Biophys. J. 2003, 84, 3829–3837. [Google Scholar] [CrossRef]
- Shi, J.Z.; Zhu, C.Y.; Li, Q.; Li, Y.J.; Chen, L.X.; Yang, B.; Xu, J.F.; Dong, Y.C.; Mao, C.D.; Liu, D.S. Kinetically Interlocking Multiple-Units Polymerization of DNA Double Crossover and Its Application in Hydrogel Formation. Macromol. Rapid Commun. 2021, 42, 2100182. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, C.; Li, X.; Hao, C.H.; Ye, S.J.; Mao, C.D. Approaching the Limit: Can One DNA Strand Assemble into Defined Nanostructures? Langmuir 2014, 30, 5859–5862. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zuo, H.; Yu, J.W.; Zhao, X.F.; Mao, C.D. One DNA strand homo-polymerizes into defined nanostructures. Nanoscale 2017, 9, 10601–10605. [Google Scholar] [CrossRef]
- Ho, P.S. Structure of the Holliday junction: Applications beyond recombination. Biochem. Soc. Trans. 2017, 45, 1149–1158. [Google Scholar] [CrossRef]
- Hamada, S.; Murata, S. Substrate-Assisted Assembly of Interconnected Single-Duplex DNA Nanostructures. Angew. Chem. Int. Ed. 2009, 48, 6820–6823. [Google Scholar] [CrossRef]
- Kim, B.; Amin, R.; Lee, J.; Yun, K.; Park, S.H. Growth and restoration of a T-tile-based 1D DNA nanotrack. Chem. Commun. 2011, 47, 11053–11055. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Hao, C.H.; Tian, C.; Wang, G.S.; Mao, C.D. DNA Polyhedra with T-Linkage. ACS Nano 2012, 6, 5138–5142. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Wu, J.Q.; Li, Z.G. Biosensing using hairpin DNA probes. Rev. Anal. Chem. 2015, 34, 1–27. [Google Scholar] [CrossRef]
- Li, M.; Yu, J.W.; Li, J.T.; Ben Wang, E.; Wang, G.S.; Mao, C.D. Self-assembly of DNA double multi-arm junctions (DMaJs). RSC Adv. 2016, 6, 76355–76359. [Google Scholar] [CrossRef]
- Suresh, G.; Padhi, S.; Patil, I.; Priyakumar, U.D. Urea Mimics Nucleobases by Preserving the Helical Integrity of B-DNA Duplexes via Hydrogen Bonding and Stacking Interactions. Biochemistry 2016, 55, 5653–5664. [Google Scholar] [CrossRef]
- Fologea, D.; Brandin, E.; Uplinger, J.; Branton, D.; Li, J. DNA conformation and base number simultaneously determined in a nanopore. Electrophoresis 2007, 28, 3186–3192. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Chen, Y.; Ribbe, A.E.; Mao, C.D. Aligning one-dimensional DNA duplexes into two-dimensional crystals. J. Am. Chem. Soc. 2007, 129, 14134–14135. [Google Scholar] [CrossRef]
- Zheng, M.X.; Li, Q.; Paluzzi, V.E.; Choi, J.H.; Mao, C.D. Engineering the Nanoscaled Morphologies of Linear DNA Homopolymers. Macromol. Rapid Commun. 2021, 42, 2100217. [Google Scholar] [CrossRef]
- Zeng, J.; Fu, W.H.; Qi, Z.P.; Zhu, Q.S.; He, H.W.; Huang, C.Z.; Zuo, H.; Mao, C.D. Self-Assembly of Microparticles by Supramolecular Homopolymerization of One Component DNA Molecule. Small 2019, 15, 1805552. [Google Scholar] [CrossRef]
- Jiang, H.L.; Pan, V.; Vivek, S.; Weeks, E.R.; Ke, Y.G. Programmable DNA Hydrogels Assembled from Multidomain DNA Strands. ChemBioChem 2016, 17, 1156–1162. [Google Scholar] [CrossRef]
- Shi, J.Z.; Jia, H.Y.; Liu, D.S. pH-Responsive Supramolecular Hydrogel Based on One Short Strand DNA. Acta Polym. Sin. 2017, 1, 135–142. [Google Scholar] [CrossRef]
- Lamprea-Burgunder, E.; Ludin, P.; Maser, P. Species-specific Typing of DNA Based on Palindrome Frequency Patterns. DNA Res. 2011, 18, 117–124. [Google Scholar] [CrossRef]
- Wemmer, D.E.; Chou, S.H.; Hare, D.R.; Reid, B.R. Duplex-hairpin transitions in DNA: NMR studies on CGCGTATACGCG. Nucleic Acids Res. 1985, 13, 3755–3772. [Google Scholar] [CrossRef]
- Gehring, K.; Leroy, J.-L.; Guéron, M. A tetrameric DNA structure with protonated cytosine-cytosine base pairs. Nature 1993, 363, 561–565. [Google Scholar] [CrossRef]
- Mergny, J.-L. Fluorescence Energy Transfer as a Probe for Tetraplex Formation: The i-Motif. Biochemistry 1999, 38, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.-L.; Guéron, M.; Mergny, J.-L.; Hélène, C. Intramolecular folding of a fragment of the cytosine-rich strand of telomeric DNA into an i-motif. Nucleic Acids Res. 1994, 22, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, S.M.; Mamiya, B.; Brian, C.; Fletcher, T.; Kern, J.T.; Thomas, P.W. G-quadruplex DNA as a target for drug discovery: Design of telomerase inhibitors based on G-quadruplex DNA structure and dynamics. Abstr. Pap. Am. Chem. Soc. 2000, 219, U6. [Google Scholar]
- Han, H.Y.; Hurley, L.H. G-quadruplex DNA: A potential target for anti-cancer drug design. Trends Pharmacol. Sci. 2000, 21, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Nakano, S.; Kirihata, T.; Fujii, S.; Sakai, H.; Kuwahara, M.; Sawai, H.; Sugimoto, N. Influence of cationic molecules on the hairpin to duplex equilibria of self-complementary DNA and RNA oligonucleotides. Nucleic Acids Res. 2007, 35, 486–494. [Google Scholar] [CrossRef]
- Xodo, L.E.; Manzini, G.; Quadrifoglio, F.; van der Marel, G.; van Boom, J.H. Hairpin structures in synthetic oligodeoxynucleotides: Sequence effects on the duplex-to-hairpin transition. Biochimie 1989, 71, 793–803. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Zhang, B.; Zheng, T.; Zhou, T.; Guo, M.; Wang, Y.; Dong, Y. DNA Materials Assembled from One DNA Strand. Int. J. Mol. Sci. 2023, 24, 8177. https://doi.org/10.3390/ijms24098177
Shi J, Zhang B, Zheng T, Zhou T, Guo M, Wang Y, Dong Y. DNA Materials Assembled from One DNA Strand. International Journal of Molecular Sciences. 2023; 24(9):8177. https://doi.org/10.3390/ijms24098177
Chicago/Turabian StyleShi, Jiezhong, Ben Zhang, Tianyi Zheng, Tong Zhou, Min Guo, Ying Wang, and Yuanchen Dong. 2023. "DNA Materials Assembled from One DNA Strand" International Journal of Molecular Sciences 24, no. 9: 8177. https://doi.org/10.3390/ijms24098177
APA StyleShi, J., Zhang, B., Zheng, T., Zhou, T., Guo, M., Wang, Y., & Dong, Y. (2023). DNA Materials Assembled from One DNA Strand. International Journal of Molecular Sciences, 24(9), 8177. https://doi.org/10.3390/ijms24098177





