Genome-Wide Identification of Histone Deacetylases and Their Roles Related with Light Response in Tartary Buckwheat (Fagopyrum tataricum)
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of HDACs in F. tataricum
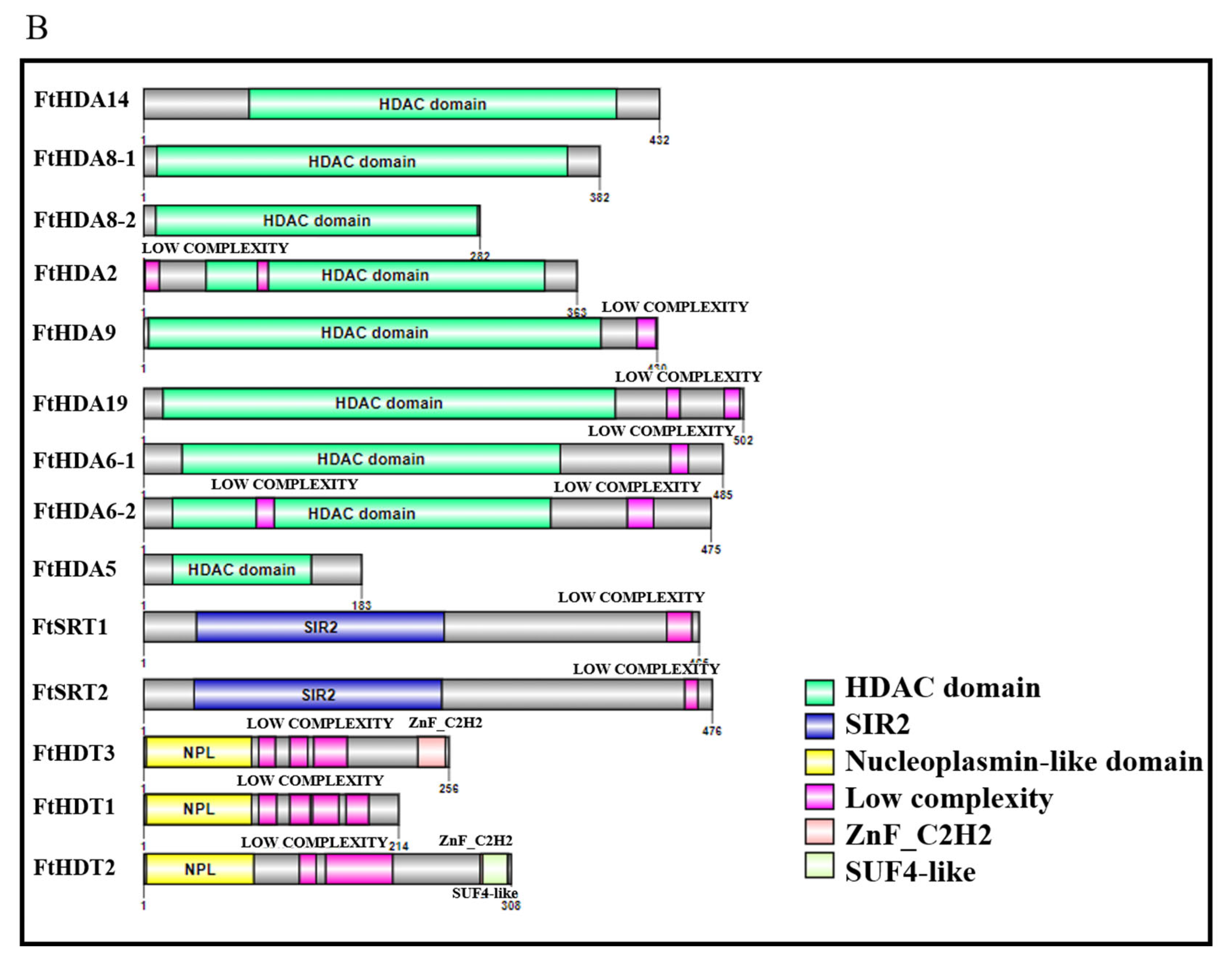
2.2. Motif and Domain Composition of the FtHDAC Genes
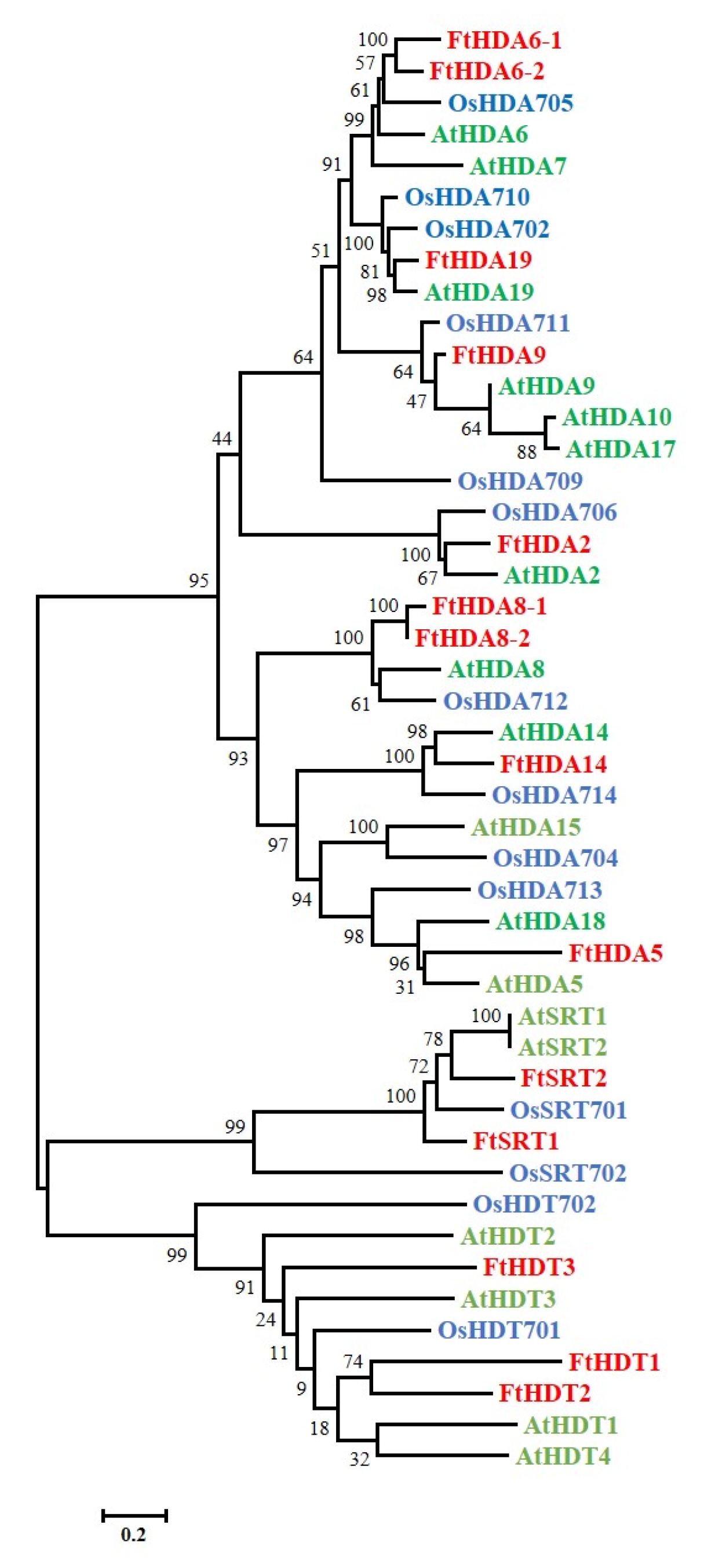
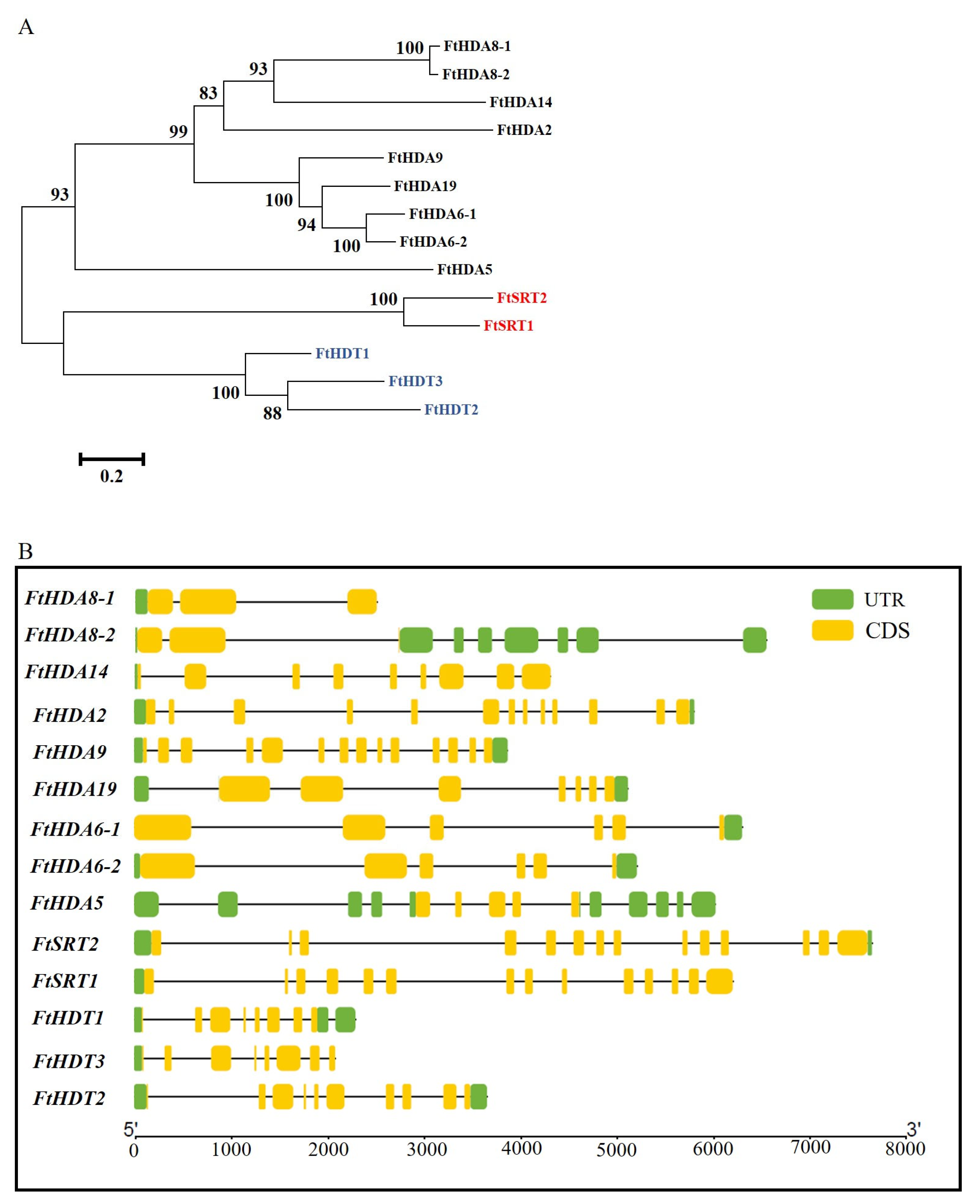
2.3. Phylogenetic Analysis and Gene Structure of the FtHDAC Gene Family
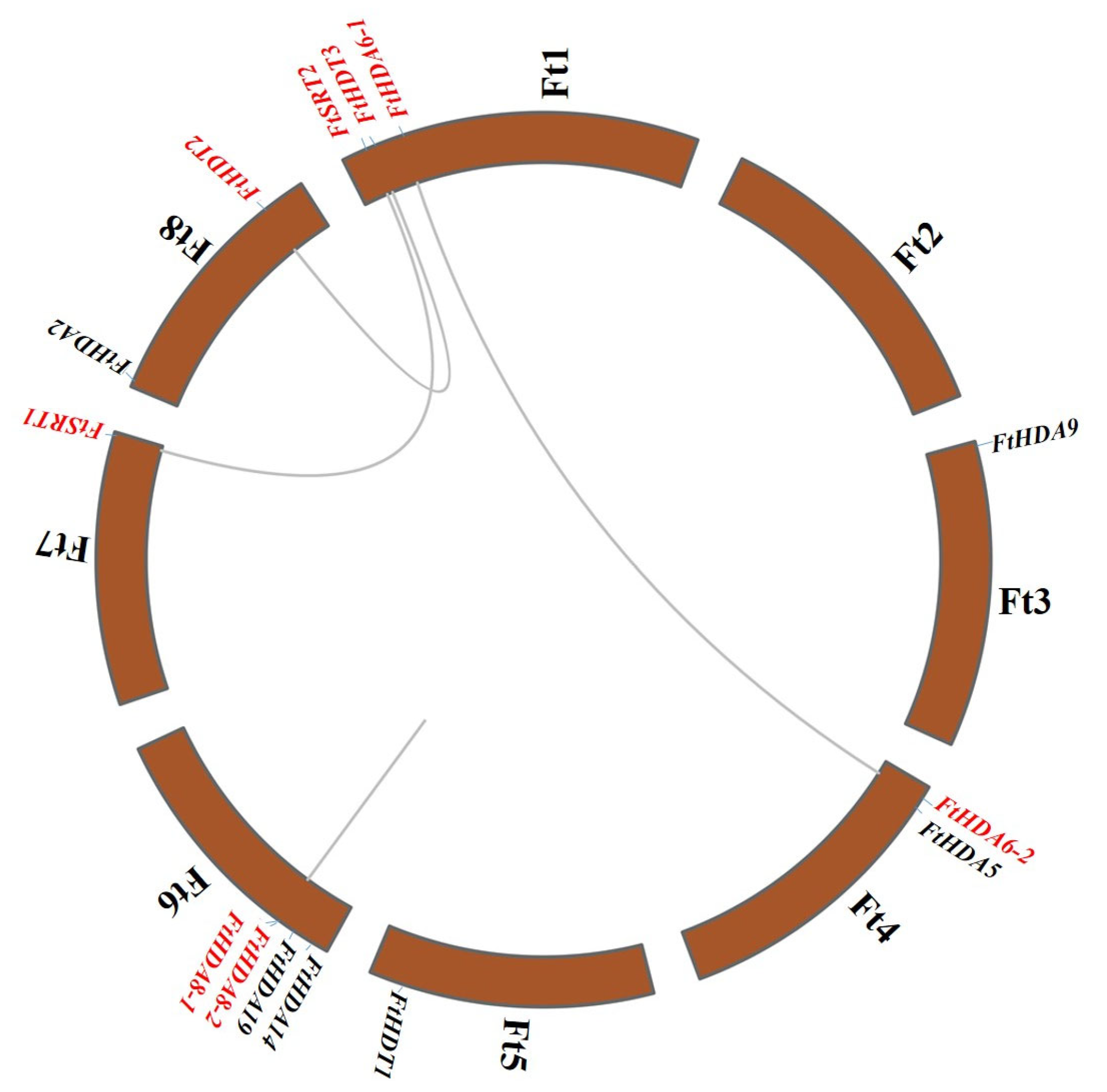
2.4. Chromosomal Location and Duplications of the FtHDACs in the F. tataricum Genome
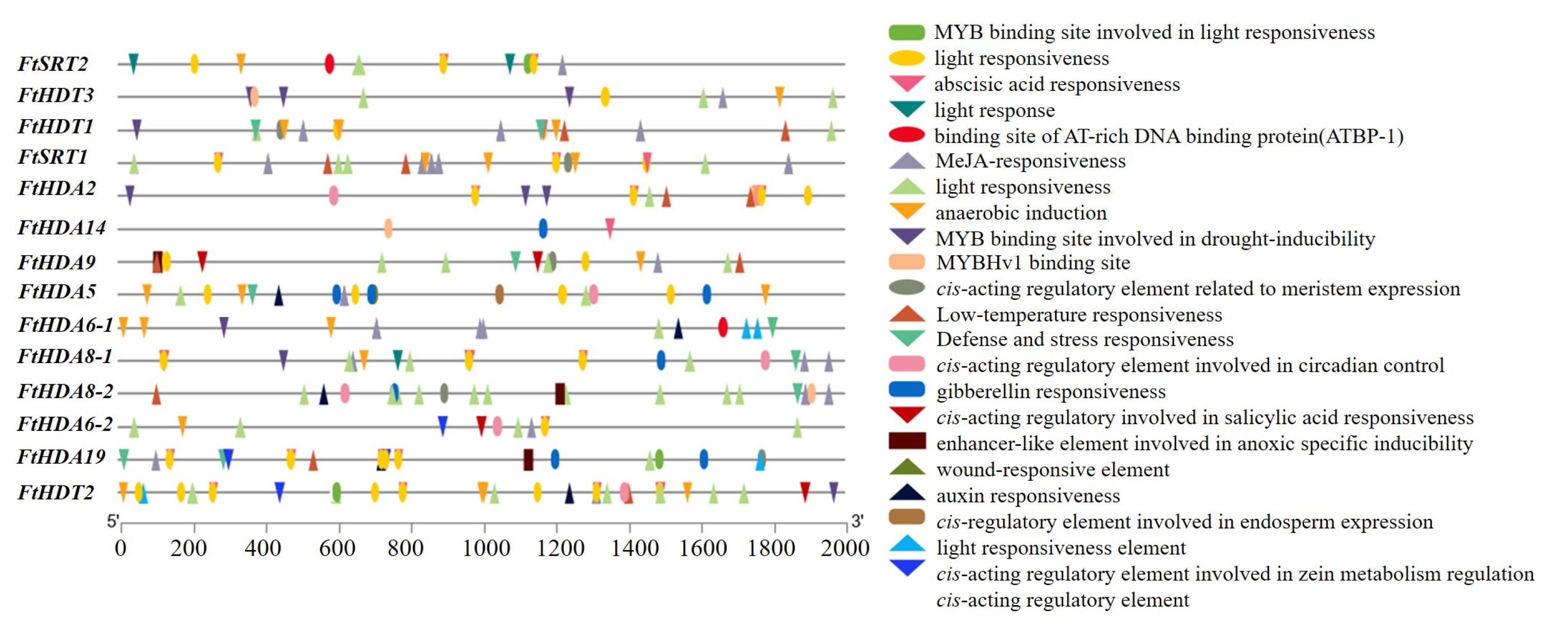
2.5. Prediction of Cis-Regulatory Elements in the Promoter Regions of the FtHDACs
2.6. Tissue-Specific Expression Profiles of the FtHDACs
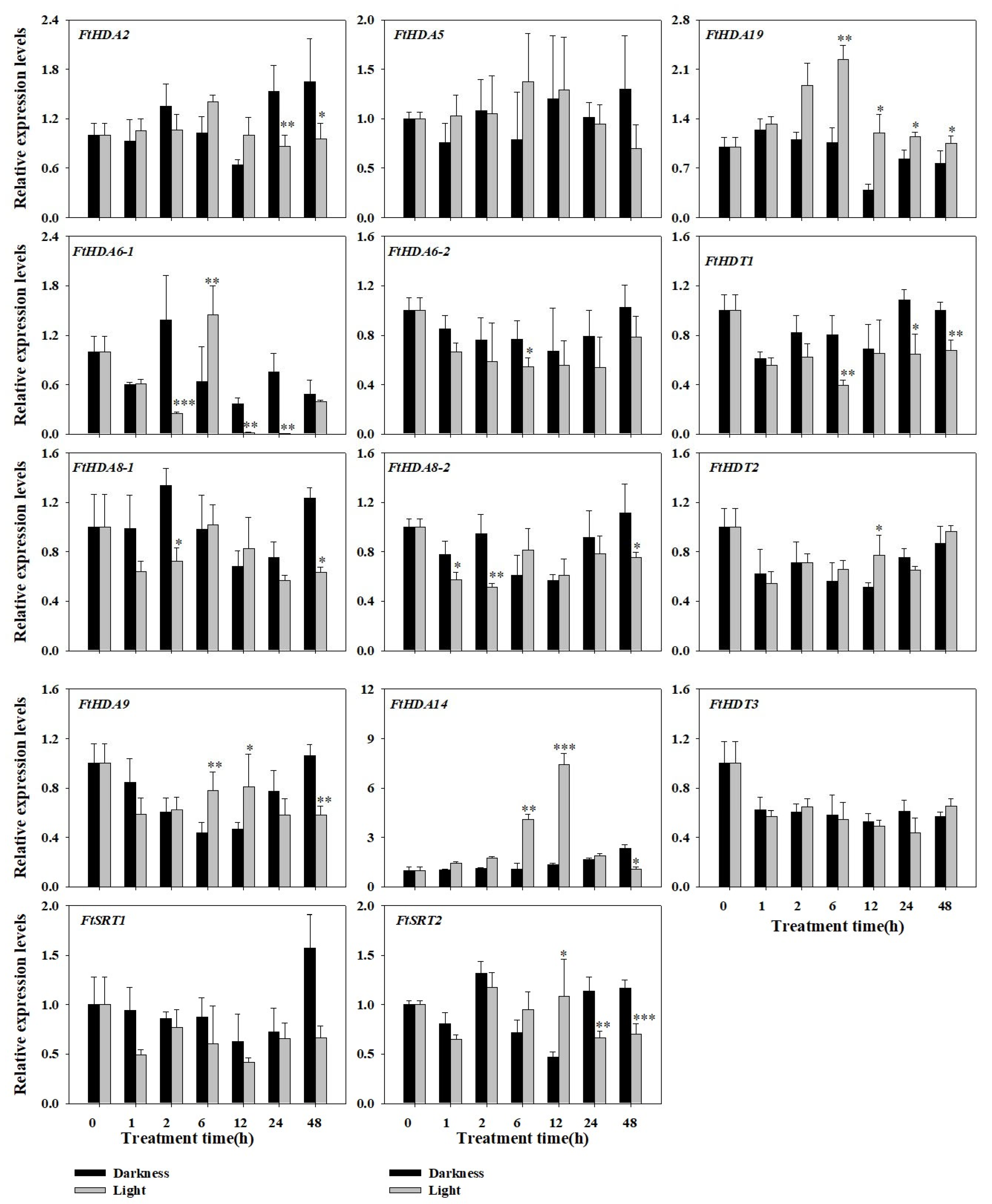
2.7. Expression Profile of the FtHDACs in Response to Diverse Light Condition
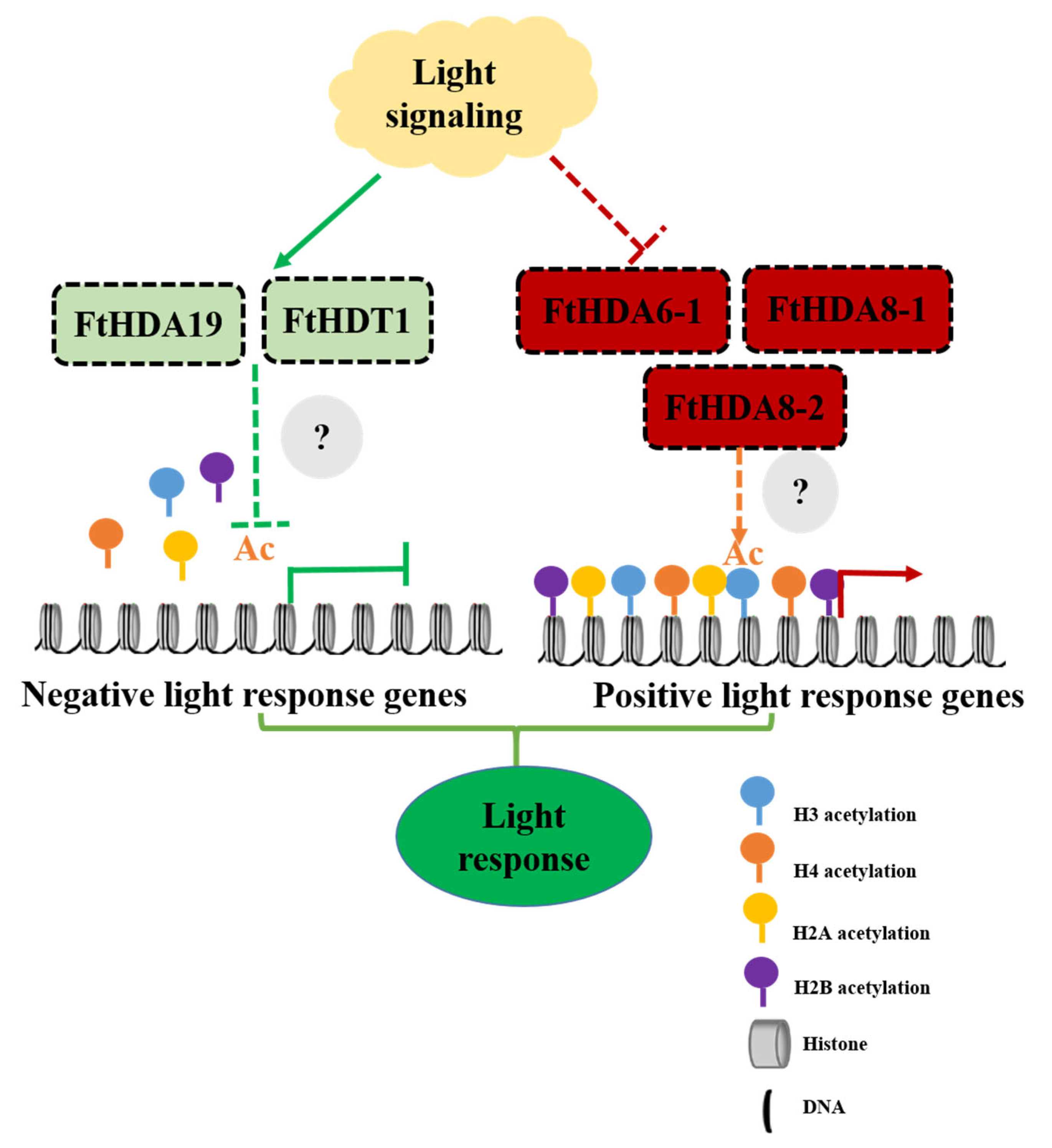
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification and Classification of FtHDAC Genes
4.3. FtHDACs Protein Motif, Domain and Gene Structure Analysis
4.4. Phylogenetic Analysis, Genome Distribution and Gene Duplication Analysis of FtHDACs
4.5. Prediction and Analysis of Cis-Regulatory Elements in the Promoter Regions of FtHDAC Genes
4.6. Transcriptome Data Analysis
4.7. RNA Isolation and Quantitative Real-Time PCR Analyses (RT-qPCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Kim, J.-M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef]
- Shahbazian, M.D.; Grunstein, M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Hollender, C.; Liu, Z. Histone deacetylase genes in Arabidopsis development. J. Integr. Plant Biol. 2008, 50, 875–885. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Schiessel, H. The nucleosome: From structure to function through physics. Curr. Opin. Struct. Biol. 2019, 56, 119–130. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Zhao, M.; Luo, M.; Yu, C.W.; Chen, C.Y.; Tai, R.; Wu, K. Transcriptional repression by histone deacetylases in plants. Mol. Plant 2014, 7, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Müller, A.; Napoli, C.A.; Selinger, D.A.; Pikaard, C.S.; Richards, E.J.; Bender, J.; Mount, D.W.; Jorgensen, R.A. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002, 30, 5036–5055. [Google Scholar] [CrossRef]
- Yang, X.-J.; Seto, E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene 2007, 26, 5310–5318. [Google Scholar] [CrossRef]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins—Emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Cho, M.H. Telomere-binding protein regulates the chromosome ends through the interaction with histone deacetylases in Arabidopsis thaliana. Nucleic Acids Res. 2016, 44, 4610–4624. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Bellizzi, M.D.R.; Ning, Y.; Meyers, B.; Wang, G.-L. HDT701, a histone H4 deacetylase, negatively regulates plant innate immunity by modulating histone H4 acetylation of defense-related genes in rice. Plant Cell 2012, 24, 3783–3794. [Google Scholar] [CrossRef]
- Luo, M.; Tai, R.; Yu, C.; Yang, S.; Chen, C.; Lin, W.; Schmidt, W.; Wu, K. Regulation of flowering time by the histone deacetylase HDA 5 in A rabidopsis. Plant J. 2015, 82, 925–936. [Google Scholar] [CrossRef]
- Ma, X.; Lv, S.; Zhang, C.; Yang, C. Histone deacetylases and their functions in plants. Plant Cell Rep. 2013, 32, 465–478. [Google Scholar] [CrossRef]
- Probst, A.V.; Fagard, M.; Proux, F.; Mourrain, P.; Boutet, S.; Earley, K.; Lawrence, R.J.; Pikaard, C.S.; Murfett, J.; Furner, I.; et al. Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 2004, 16, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-C.; Kuang, J.-F.; Chen, J.-Y.; Liu, X.-C.; Xiao, Y.-Y.; Fu, C.-C.; Wang, J.-N.; Wu, K.-Q.; Lu, W.-J. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016, 171, 1070–1084. [Google Scholar] [CrossRef]
- Kim, K.-C.; Lai, Z.; Fan, B.; Chen, Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 2008, 20, 2357–2371. [Google Scholar] [CrossRef]
- Zheng, Y.; Ding, Y.; Sun, X.; Xie, S.; Wang, D.; Liu, X.; Su, L.; Wei, W.; Pan, L.; Zhou, D.-X. Histone deacetylase HDA9 negatively regulates salt and drought stress responsiveness in Arabidopsis. J. Exp. Bot. 2016, 67, 1703–1713. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.-C.; Chen, W.-Q.; Chen, X.; Xu, Z.-H.; Bai, S.-N. HDA18 affects cell fate in Arabidopsis root epidermis via histone acetylation at four kinase genes. Plant Cell 2013, 25, 257–269. [Google Scholar] [CrossRef]
- Kuang, J.-F.; Chen, J.-Y.; Luo, M.; Wu, K.-Q.; Sun, W.; Jiang, Y.-M.; Lu, W.-J. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 2011, 63, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yu, C.-W.; Chen, F.-F.; Zhao, L.; Tian, G.; Liu, X.; Cui, Y.; Yang, J.-Y.; Wu, K. Histone deacetylase HDA6 is functionally associated with AS1 in repression of KNOX genes in arabidopsis. PLoS Genet. 2012, 8, e1003114. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, L.; Duan, J.; Miki, B.; Wu, K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell 2005, 17, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Chen, C.-Y.; Zhao, M.; Zhao, L.; Duan, X.; Duan, J.; Wu, K.; Liu, X. Identification of HDA15-PIF1 as a key repression module directing the transcriptional network of seed germination in the dark. Nucleic Acids Res. 2017, 45, 7137–7150. [Google Scholar] [CrossRef]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar]
- Fisher, A.J.; Franklin, K.A. Chromatin remodelling in plant light signalling. Physiol. Plant. 2011, 142, 305–313. [Google Scholar] [CrossRef]
- Charron, J.-B.F.; He, H.; Elling, A.A.; Deng, X.W. Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 2009, 21, 3732–3748. [Google Scholar] [CrossRef]
- Bertrand, C.; Benhamed, M.; Li, Y.-F.; Ayadi, M.; Lemonnier, G.; Renou, J.-P.; Delarue, M.; Zhou, D.-X. Arabidopsis HAF2 gene encoding TATA-binding protein (TBP)-associated factor TAF1, is required to integrate light sig-nals to regulate gene expression and growth. J. Biol. Chem. 2005, 280, 1465–1473. [Google Scholar] [CrossRef]
- Benhamed, M.; Bertrand, C.; Servet, C.; Zhou, D.X. Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 2006, 18, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Benhamed, M.; Martin-Magniette, M.-L.; Taconnat, L.; Bitton, F.; Servet, C.; De Clercq, R.; De Meyer, B.; Buysschaert, C.; Rombauts, S.; Villarroel, R.; et al. Genome-scale Arabidopsis promoter array identifies targets of the histone acetyltransferase GCN5. Plant J. 2008, 56, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.-Y.; Wang, K.-C.; Luo, M.; Tai, R.; Yuan, L.; Zhao, M.; Yang, S.; Tian, G.; Cui, Y.; et al. PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chloro-phyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell 2013, 25, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, X.; Liu, X.; Li, Y.; Wu, K.; Hou, X. Arabidopsis NF-YCs mediate the light-controlled hypocotyl elongation via modulating histone acetylation. Mol. Plant 2016, 10, 260–273. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2023, 63, 657–673. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Huang, C.; Wen, D.; Lu, J.; Chen, S.; Zhang, T.; Shi, Y.; Xue, J.; Ma, W.; et al. The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum. Plant Cell Environ. 2018, 42, 1340–1351. [Google Scholar] [CrossRef]
- Yao, P.; Huang, Y.; Dong, Q.; Wan, M.; Wang, A.; Chen, Y.; Li, C.; Wu, Q.; Chen, H.; Zhao, H. FtMYB6, a light-induced SG7 R2R3-MYB transcription factor, promotes flavonol biosynthesis in Tartary buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2020, 68, 13685–13696. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, J.; Deng, R.-Y.; Zhao, H.-X.; Li, C.-L.; Chen, H.; Suzuki, T.; Park, S.-U.; Wu, Q. Overexpression of a tartary buckwheat R2R3-MYB transcription factor gene, FtMYB9, enhances tolerance to drought and salt stresses in transgenic Arabidopsis. J. Plant Physiol. 2017, 214, 81–90. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, H.; Gao, F.; Yao, P.; Deng, R.; Li, C.; Chen, H.; Wu, Q. A R2R3-MYB transcription factor gene, FtMYB13, from Tartary buckwheat improves salt/drought tolerance in Arabidopsis. Plant Physiol. Biochem. 2018, 132, 238–248. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Wang, X.; Kang, J.; Lv, B.; Dong, Q.; Li, C.; Chen, H.; Wu, Q. Tartary buckwheat transcription factor FtbZIP5, regulated by FtSnRK2.6, can improve salt/drought resistance in transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 1123. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, Q.; Wang, A.; Lv, B.; Dong, Q.; Yao, Y.; Wu, Q.; Zhao, H.; Li, C.; Chen, H.; et al. Tartary buckwheat transcription factor FtbZIP83 improves the drought/salt tolerance of Arabidopsis via an ABA-mediated pathway. Plant Physiol. Biochem. 2019, 144, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yao, H.; Zhao, H.; Zhou, J.; Luo, X.; Huang, Y.; Li, C.; Chen, H.; Wu, Q. Tartary buckwheat FtMYB10 encodes an R2R3-MYB transcription factor that acts as a novel negative regulator of salt and drought response in transgenic Arabidopsis. Plant Physiol. Biochem. 2016, 109, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, A.B.; Zhong, X. Functions and mechanisms of plant histone deacetylases. Sci. China Life Sci. 2019, 63, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ren, R.; Xiao, H.; Chen, Z.; Yu, J.; Zhang, H.; Shi, Q.; Hou, H.; He, S.; Li, L. Characteristic and evolution of HAT and HDAC genes in Gramineae genomes and their expression analysis under diverse stress in Oryza sativa. Planta 2021, 253, 1–22. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, J.; Zhang, J.; Wu, P.-Y.; Yang, S.; Wu, K. Identification and characterization of histone deacetylases in tomato (Solanum lycopersicum). Front. Plant Sci. 2015, 5, 760. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, H.; Zheng, S.; Huang, R.; Tong, H. Genome-wide identification of the HDAC family proteins and functional characterization of CsHD2C, a HD2-type histone deacetylase gene in tea plant (Camellia sinensis L. O. Kuntze). Plant Physiol. Biochem. 2020, 155, 898–913. [Google Scholar] [CrossRef]
- Aquea, F.; Timmermann, T.; Arce-Johnson, P. Analysis of histone acetyltransferase and deacetylase families of Vitis vinifera. Plant Physiol. Biochem. 2010, 48, 194–199. [Google Scholar] [CrossRef]
- Eom, S.H.; Hyun, T.K. Comprehensive analysis of the histone deacetylase gene family in Chinese cabbage (Brassica rapa): From evolution and expression pattern to functional analysis of BraHDA3. Agriculture 2021, 11, 244. [Google Scholar] [CrossRef]
- Yang, C.; Shen, W.; Chen, H.; Chu, L.; Xu, Y.; Zhou, X.; Liu, C.; Chen, C.; Zeng, J.; Liu, J.; et al. Characterization and subcellular localization of histone deacetylases and their roles in response to abiotic stresses in soybean. BMC Plant Biol. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Chung, P.J.; Kim, Y.S.; Jeong, J.S.; Park, S.-H.; Nahm, B.H.; Kim, J.-K. The histone deacetylase OsHDAC1 epigenetically regulates the OsNAC6 gene that controls seedling root growth in rice. Plant J. 2009, 59, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Labbe, H.; Sridha, S.; Wang, L.; Tian, L.; Latoszek-Green, M.; Yang, Z.; Brown, D.; Miki, B.; Wu, K. Expression and function of HD2-type histone deacetylases in Arabidopsis development. Plant J. 2004, 38, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Schölkopf, B.; Weigel, D.; Lohmann, J. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef]
- Gao, M.-J.; Li, X.; Huang, J.; Gropp, G.M.; Gjetvaj, B.; Lindsay, D.L.; Wei, S.; Coutu, C.; Wan, X.-C.; Hannoufa, A.; et al. SCARECROW-LIKE15 interacts with HISTONE DEACETYLASE19 and is essential for repressing the seed maturation programme. Nat. Commun. 2015, 6, 7243. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, B.; Luo, M.; Li, Y.; Liu, C.; Chen, C.; Yu, C.-W.; Yang, S.; Dong, S.; Ruan, J.; et al. HISTONE DEACETYLASE19 Interacts with HSL1 and Participates in the Repression of Seed Maturation Genes in Arabidopsis Seedlings. Plant Cell 2013, 25, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Guo, Q.; Lin, R. The SNL-HDA19 histone deacetylase complex antagonizes HY5 activity to repress photomorphogenesis in Arabidopsis. New Phytol. 2020, 229, 3221–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, T.; Chen, C.-Y.; Ji, R.; Gu, D.; Li, T.; Zhang, D.; Tu, Y.-T.; Wu, K.; Liu, X. HY5 interacts with the histone deacetylase HDA15 to repress hypocotyl cell elongation in photomorphogenesis. Plant Physiol. 2019, 180, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; van Zanten, M.; Pavlova, P.; Clifton, R.; Pontvianne, F.; Snoek, L.B.; Millenaar, F.F.; Schulkes, R.K.; van Driel, R.; Voesenek, L.A.C.J.; et al. hytochrome B and histone deacetylase 6 control light-induced chromatin compaction in Arabidopsis thaliana. PLoS Genet. 2009, 5, e1000638. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Li, D.; Meyer, T.; Muller, R.; Ploetz, L.; et al. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2008, 36, D1009–D1014. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Ivica, L.; Supriya, K.; Peer, B. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Jon, I.; Matús, K.; Inge, J.; Dan, B.; Mahmut, U.; Hamish, M.; James, M.; Rodrigo, L.; Steve, P.; Peter, R. EDAM: An ontology of bioinformatics operations, types of data and identifiers, topics and formats. Bioinformatics 2013, 29, 1325–1332. [Google Scholar] [CrossRef]
- Timothy, L.B.; Nadya, W.; Chris, M.; Wilfred, W.L. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, Y.B.; Ma, J.Y.; Luo, X.T.; Nie, P.; Zuo, Z.X.; Lahrmann, U.; Zhao, Q.; Zheng, Y.Y.; Zhao, Y.; et al. IBS: An illustrator for the presentation and visualization of biological sequences. Bioinformatics 2015, 31, 3359–3361. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Liu, M.Y.; Sun, W.J.; Ma, Z.T.; Hu, Y.; Chen, H. Tartary buckwheat database (TBD): An integrative platform for gene analysis of and biological information on Tartary buckwheat. J. Zhejiang Univ. Sci. B 2021, 22, 954–958. [Google Scholar] [CrossRef]
- Yan, H.; Liu, C.; Zhao, J.; Ye, X.; Wu, Q.; Yao, T.; Peng, L.; Zou, L.; Zhao, G. Genome-wide analysis of the NF-Y gene family and their roles in relation to fruit development in Tartary buckwheat (Fagopyrum tataricum). Int. J. Biol. Macromol. 2021, 190, 487–498. [Google Scholar] [CrossRef]

| Name | Gene ID | Chromosome Localization | CDS | Length (AA) | Mw | Name | Gene ID |
|---|---|---|---|---|---|---|---|
| FtHDA2 | FtPinG0002233600.01 | Ft8: 2509180–2515001 | 1092 | 363 | 39.98 | 8.44 | AtHDA2 |
| FtHDA5 | FtPinG0004507000.01 | Ft4: 4862314–4868357 | 552 | 183 | 20.68 | 9.39 | AtHDA5 |
| FtHDA6-1 | FtPinG0005824600.01 | Ft1: 11816747–11823065 | 1458 | 485 | 54.11 | 5.74 | AtHDA6 |
| FtHDA6-2 | FtPinG0006680200.01 | Ft4: 2793974–2799198 | 1428 | 475 | 53.65 | 5.39 | AtHDA6 |
| FtHDA8-1 | FtPinG0006335400.01 | Ft6: 10766017–10768527 | 1149 | 382 | 41.25 | 5.28 | AtHDA8 |
| FtHDA8-2 | FtPinG0006335800.01 | Ft6: 10775588–10782216 | 849 | 282 | 30.21 | 5.34 | AtHDA8 |
| FtHDA9 | FtPinG0003240400.01 | Ft3: 1572336–1576218 | 1293 | 430 | 49.19 | 5.06 | AtHDA9 |
| FtHDA14 | FtPinG0002696900.01 | Ft6: 3383062–3387385 | 1299 | 432 | 46.83 | 6.10 | AtHDA14 |
| FtHDA19 | FtPinG0007059500.01 | Ft6: 7283288–7288420 | 1509 | 502 | 56.19 | 5.08 | AtHDA19 |
| FtSRT1 | FtPinG0001808100.01 | Ft7: 50614511–50620732 | 1398 | 465 | 51.76 | 8.50 | AtSRT1/2 |
| FtSRT2 | FtPinG0000318300.01 | Ft1: 5066323–5073990 | 1431 | 476 | 52.75 | 9.35 | AtSRT1/2 |
| FtHDT1 | FtPinG0001683500.01 | Ft5: 47668125–47670424 | 645 | 214 | 23.55 | 4.11 | AtHDT1/3/2 |
| FtHDT2 | FtPinG0007109100.01 | Ft8: 41374550–41378216 | 927 | 308 | 33.35 | 4.67 | AtHDT2/1/3 |
| FtHDT3 | FtPinG0001411500.01 | Ft1: 6259288–6261377 | 771 | 256 | 27.83 | 5.10 | AtHDT3/2/1 |
| The Paralogous Gene | Ka | Ks | Ka/Ks | Date | |
|---|---|---|---|---|---|
| (Million Year Ago) | |||||
| FtHDA8-1 | FtHDA8-2 | 0.03 | 0.07 | 0.42 | 2.47 |
| FtHDA6-1 | FtHDA6-2 | 0.13 | 1.16 | 0.11 | 38.77 |
| FtHDT2 | FtHDT3 | 0.42 | NaN | NaN | NaN |
| FtSRT1 | FtSRT2 | 0.32 | 2.66 | 0.12 | 88.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Chen, H.; Liao, Q.; Xia, M.; Yao, T.; Peng, L.; Zou, L.; Zhao, G.; Zhao, J.; Wu, D.-T. Genome-Wide Identification of Histone Deacetylases and Their Roles Related with Light Response in Tartary Buckwheat (Fagopyrum tataricum). Int. J. Mol. Sci. 2023, 24, 8090. https://doi.org/10.3390/ijms24098090
Yan H, Chen H, Liao Q, Xia M, Yao T, Peng L, Zou L, Zhao G, Zhao J, Wu D-T. Genome-Wide Identification of Histone Deacetylases and Their Roles Related with Light Response in Tartary Buckwheat (Fagopyrum tataricum). International Journal of Molecular Sciences. 2023; 24(9):8090. https://doi.org/10.3390/ijms24098090
Chicago/Turabian StyleYan, Huiling, Hongxu Chen, Qingxia Liao, Mengying Xia, Tian Yao, Lianxin Peng, Liang Zou, Gang Zhao, Jianglin Zhao, and Ding-Tao Wu. 2023. "Genome-Wide Identification of Histone Deacetylases and Their Roles Related with Light Response in Tartary Buckwheat (Fagopyrum tataricum)" International Journal of Molecular Sciences 24, no. 9: 8090. https://doi.org/10.3390/ijms24098090
APA StyleYan, H., Chen, H., Liao, Q., Xia, M., Yao, T., Peng, L., Zou, L., Zhao, G., Zhao, J., & Wu, D.-T. (2023). Genome-Wide Identification of Histone Deacetylases and Their Roles Related with Light Response in Tartary Buckwheat (Fagopyrum tataricum). International Journal of Molecular Sciences, 24(9), 8090. https://doi.org/10.3390/ijms24098090










