Abstract
In the epithelial–mesenchymal transition (EMT) process, cells lose their epithelial phenotype and gain mesenchymal features. This phenomenon was observed in the metastatic phase of neoplastic diseases, e.g., cervical cancer. There are specific markers that are expressed in the EMT. The aim of this study was to determine the localization of and associations between the immunohistochemical (IHC) expression of TWIST, SNAIL, and SLUG proteins in precancerous lesions and cervical cancer. The IHC analysis disclosed higher expressions of EMT markers in precancerous lesions and cervical cancer than in the control group. Moreover, stronger expression of TWIST, SNAIL, and SLUG was observed in cervical intraepithelial neoplasia grade 3 (CIN3) vs. CIN1, CIN3 vs. CIN2, and CIN2 vs. CIN1 cases (p < 0.05). In cervical cancer, IHC reactions demonstrated differences in TWIST, SNAIL, and SLUG expression in grade 1 (G1) vs. grade 2 (G2) (p < 0.0011; p < 0.0017; p < 0.0001, respectively) and in G1 vs. grade 3 (G3) (p < 0.0029; p < 0.0005; p < 0.0001, respectively). The results of our study clearly showed that existing differences in the expression of the tested markers in precancerous vs. cancerous lesions may be utilized in the diagnosis of cervical cancer. Further studies on bigger populations, as well as in comparison with well-known markers, may improve our outcomes.
Keywords:
SNAIL; TWIST; SLUG; EMT; cervical cancer; cervical intraepithelial neoplasia; immunohistochemistry; HSIL; LSIL 1. Introduction
Cervical cancer is one of the leading malignancies of the female genital system. In the United States alone, in 2023, over 13,960 women will be diagnosed with cervical cancer, and 4280 of them will die. There are still 341,831 deaths among women per year worldwide, despite reductions in the mortality and incidence of cervical cancer brought on by the human papilloma virus (HPV) vaccine and increased screening [1,2]. A squamous intraepithelial lesion is formed by the abnormal growth of squamous cells on the surface of the cervix. The degree of dysplasia is determined by the percentage of cervical epithelium that contains dysplastic cells. When compared to the more serious CIN2 and CIN3 (high-grade), which proceed to involve the full thickness of the epithelium, CIN1 (low-grade) only affects the lower one-third or less of the epithelium. When dysplasia penetrates the basement membrane, it develops into cancer. Cervical cancer is usually diagnosed between the ages of 35 and 44, with a reported average 5-year survival rate of 66% [3]. There are several factors that influence this value. One of them is the clinical stage at diagnosis. When diagnosed at an early stage, the 5-year survival rate is 92%; if the cancer has spread regionally to surrounding tissue and lymph nodes, then the rate is 58%, but it dramatically declines to 18% when distant metastases occur [4]. Patients with locally advanced cervical cancer (stage IB3 to IVA) have a higher rate of recurrence. The early stage of the disease limited to the cervix and uterus (stage IA to IB2) can be treated by radical surgery or concomitant chemotherapy, which is based on patient characteristics and the volume of the disease [5]. After surgery alone, the probability of relapse is at least 30% [6,7]. Although it is not common at initial diagnosis, metastases develop in 15% to 61% of women with cervical cancer, usually within the first two years after finishing treatment [8]. The presence of invasion and metastasis is the major cause of most cancer-related deaths. The epithelial–mesenchymal transition (EMT) process is closely related to tumor metastasis. During the process of EMT, polarized epithelial tumor cells gain invasive and migratory characteristics, leave the primary site, invade the basement membrane, intravasate into blood or lymph vessels, transport through the circulation, extravasate from the circulation, disseminate into a secondary site, and finally, grow at the metastatic site. EMT can be triggered by the dysregulation of oncogenes, tumor suppressors, miRNAs, and growth factor signals. Several transcription factors influence the process of EMT: i.a., twist family bHLH transcription factor 1 (TWIST), snail family zinc finger 1 (SNAIL), and snail family zinc finger 2 (SLUG) [9].
TWIST belongs to the helix–loop–helix transcription factors engaged in the EMT process. Its expression leads to a loss of E-cadherin-mediated cell–cell adhesion, activates mesenchymal markers, and initiates cell motility. Li et al. showed that the expression of TWIST is crucial for the activation of the β-catenin and Akt pathway in HeLa cells to maintain the EMT process [10]. High expression of TWIST was linked with chemo- and radiotherapy resistance [11,12,13]. Moreover, TWIST overexpression is associated with lower patient survival rates and cervical cancer progression [9,11,14,15,16].
The SNAIL family is composed of zinc-finger-containing transcription factors and includes SNAIL, SLUG, and SMUC. SNAIL is one of the most widely studied regulators of the EMT process. Its expression is controlled at many levels: transcriptional, translational, and post-translational [11]. As a transcriptional factor, SNAIL rules genes related to EMT-independent functions such as cell survival, motility, anti-apoptosis, immune suppression, stem cell properties, and chemo-resistance [17,18,19,20,21].
SLUG is a member of the SNAIL superfamily and has a pivotal role in the EMT process. Increased expression of SLUG can lead to reduced E-cadherin expression and the onset of EMT. There are reports in the literature that SLUG initiates EMT and promotes metastasis through its trans-repression effect on E-cadherin regulation in cervical cancer [22,23]. Xian Liu et al. showed that exogenously expressed SLUG in HeLa and SiHa cells significantly enhanced cell motility in vitro and promoted distant metastasis in vivo [24]. On the other hand, Nan Cui et al. demonstrated that SLUG acts as a suppressor gene, inhibiting the proliferation of cervical cancer in vitro and tumor formation in vivo [25]. The scientific findings presented above indicate a strong need to expand research on EMT marker expression in cervical cancer.
The aim of this study was, thus, to determine the localization of and associations between immunohistochemical (IHC) expression of the TWIST, SNAIL, and SLUG proteins in precancerous lesions and cervical cancer.
2. Results
We determined the IHC expression of TWIST, SNAIL, and SLUG in 124 cervical cancer cases, 229 CIN cases, and 145 patients in the control group. We observed interesting cellular expression patterns. For TWIST, cytoplasmic localization in cancer (Figure 1E), CIN lesions (Figure 1B–D), and normal tissue (Figure 1A) were found. The expression of the SNAIL protein in normal tissue (Figure 2A) was nuclear, but in cervical cancer (Figure 2E) and CIN lesions (Figure 2B–D), the reaction was nuclear–cytoplasmic. The expression of the SLUG protein was nuclear–cytoplasmic in cancer cells (Figure 3E) and nuclear in CIN lesions (Figure 3B–D) and normal tissue (Figure 3A).
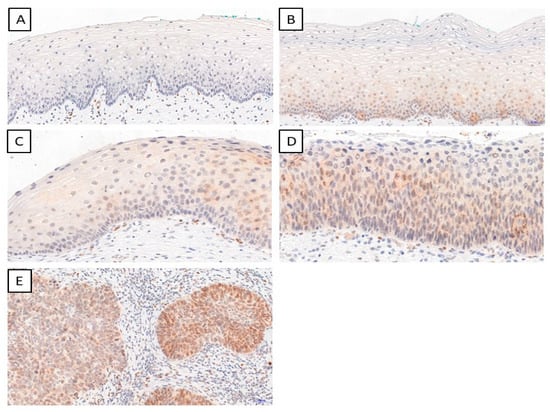
Figure 1.
The IHC expression of TWIST (normal cervical tissue (A); CIN1 (B); CIN2 (C); CIN3 (D); cervical cancer (E)); magnification ×200.
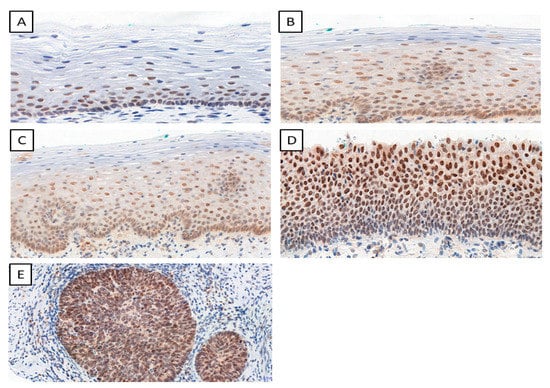
Figure 2.
The IHC expression of SNAIL (normal cervical tissue (A); CIN1 (B); CIN2 (C); CIN3 (D); cervical cancer (E)); magnification ×200.
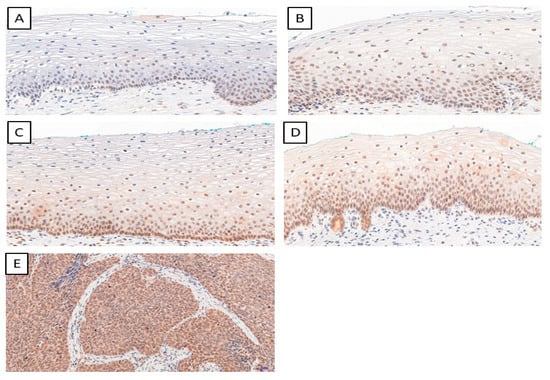
Figure 3.
The IHC expression of SLUG (normal cervical tissue (A); CIN1 (B); CIN2 (C); CIN3 (D); cervical cancer (E)); magnification ×200.
2.1. Precancerous Lesions
The highest TWIST, SNAIL, and SLUG expression was noted in CIN3 and was significantly higher as compared to that in CIN1 and CIN2 (respectively, TWIST p < 0.0001, SNAIL p < 0.0013; SLUG p < 0.0001, Figure 4A; Mann–Whitney test). Moreover, expression was significantly higher in the high-grade squamous intraepithelial lesion (HSIL) group than in the low-grade squamous intraepithelial lesion (LSIL) group (p < 0.0001; Mann–Whitney test; Figure 4B). In addition, significantly stronger expression of TWIST, SNAIL, and SLUG was observed in CIN3 vs. CIN1, CIN3 vs. CIN2, and CIN2 vs. CIN1 cases (p < 0.05 for all; Dunn’s multiple comparison tests). Moreover, the Spearman correlation test revealed a significant correlation between EMT markers in CIN lesions (Table 1; Figure 5A–C).
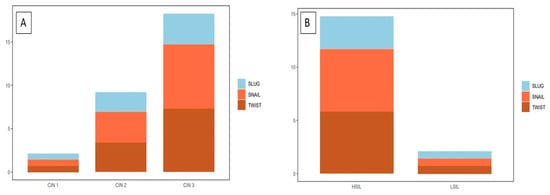
Figure 4.
A comparison of the IHC expression of the tested markers in precancerous lesions: (A) TWIST expression in CIN1, CIN2, and CIN3; SNAIL expression in CIN1, CIN2, and CIN3; SLUG expression in CIN1, CIN2, and CIN3); (B) TWIST expression in HSIL and LSIL; SNAIL expression in HSIL and LSIL; SLUG expression in HSIL and LSIL Mann–Whitney test).

Table 1.
Spearman correlation test results. All correlations except SLUG vs. TWIST in cervical cancer were statistically significant.
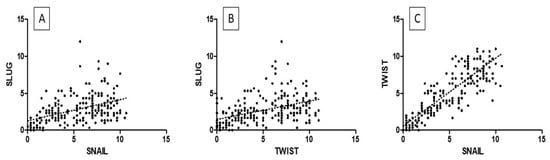
Figure 5.
The correlations between IHC expression levels in precancerous lesions: (A) SLUG vs. SNAIL; (B) SLUG vs. TWIST; (C) TWIST vs. SNAIL (Spearman correlation test).
2.2. Cervical Cancer
In cervical cancer cases, the expression of EMT markers was higher than in the control group (p < 0.0001 for TWIST, SNAIL, and SLUG; Mann–Whitney test; Figure 6A). Moreover, we demonstrated a considerable difference in TWIST, SNAIL, and SLUG expression between G1 and G2 (respectively, p < 0.0011, Figure 6B; p < 0.0017, p < 0.0001, Mann–Whitney test), as well as between G1 and G3 (accordingly, p < 0.0029, Figure 6B; p < 0.0005, p < 0.0001; Mann–Whitney test). Between G2 and G3, we found no significant differences in the expression of EMT markers (TWIST: p < 0.8304, Figure 6B; SNAIL: p < 0.1208, SLUG: p < 0.7947; Mann–Whitney test). The Spearman correlation test showed that the expression of TWIST positively correlated with the expression of the SNAIL protein in cervical cancer (r = 0.4338, p < 0.0001; Figure 7C; Table 1). A positive correlation was also shown between SLUG protein expression and SNAIL (r = 0.3764, p < 0.0001; Figure 7A; Table 1). There were no significant correlations between SLUG protein expression and TWIST protein expression in cervical cancer (r = 0.1066, p < 0.2483; Figure 7B; Table 1). The expression of all EMT markers in the LSIL, HSIL, G1, G2 and G3 was significantly different in Kruskal–Wallis test (Figure 8; p < 0.0001). Dunn’s multiple comparison test showed statistically significant difference of TWIST expression between groups: LSIL vs. HSIL, LSIL vs. G1, LSIL vs. G2, LSIL vs. G3; SNAIL expression were different in groups: LSIL vs. HSIL, LSIL vs. G1, LSIL vs. G2, LSIL vs. G3, HSIL vs. G2, HSIL vs. G3 and the SLUG expression were statistically different in the groups: LSIL vs. HSIL, LSIL vs. G1, LSIL vs. G2, LSIL vs. G3, HSIL vs. G1, HSIL vs. G2, HSIL vs. G3, G1 vs. G2, G1 vs. G3.
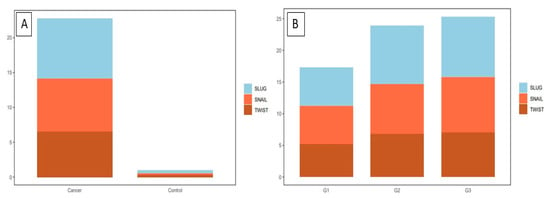
Figure 6.
The IHC expression of the tested markers in cervical cancer: (A) TWIST expression in cervical cancer and control group; SNAIL expression in cervical cancer and control group; SLUG expression in cervical cancer and control group (Mann–Whitney test). (B) A comparison of the IHC expression of TWIST, SNAIL, and SLUG in regard to the histological malignancy G (Mann–Whitney test).
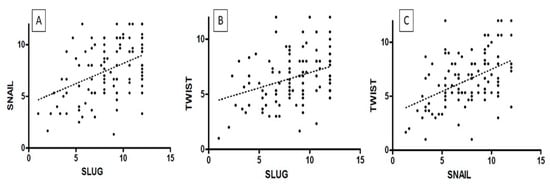
Figure 7.
The correlations between IHC expression levels in cervical cancer: (A) SLUG vs. SNAIL; (B) SLUG vs. TWIST; (C) SNAIL vs. TWIST (Spearman correlation test).
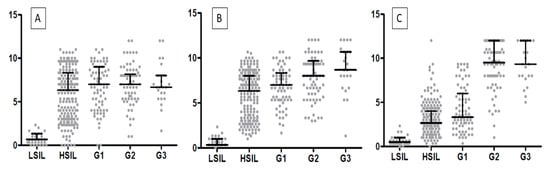
Figure 8.
A comparison of the IHC expression of (A) TWIST, (B) SNAIL, and (C) SLUG in regard to the LSIL, HSIL, and histological malignancy G (p < 0.05, Mann–Whitney test).
3. Discussion
The epithelial–mesenchymal transition phenomenon plays an important role in cervical cancer progression. Many studies were conducted to investigate the mechanism of the progression of cervical cancer from CIN. The formation of metastases from cervical cancer is a process that involves multiple steps and a cascade of reactions. The prognosis significantly decreases when distant metastases occur because the treatment of local lesions is more effective than systemic therapy [26]. The cadherins are key components that contribute to cell motility and invasiveness via EMT. A reduction in E-cadherin expression leads to a loss of cell polarity and decreased cell adhesion [27]. In many human malignancies, downregulation of E-cadherin is associated with a poor prognosis and is a key feature of cancerogenesis, such as tumor spreading [28,29]. Several important genes induce EMT and act as E-cadherin repressors, such as SNAIL, SLUG, and TWIST. The risk of developing invasive cervical cancer from HSIL is approximately 20% (10–40% according to the literature) [30,31,32]. Previous studies described the expression of EMT markers in cervical cancer; however, there were no prior data on their expression in preinvasive lesions. Due to the lack of such studies, we decided to examine whether there were any differences in EMT marker expression between precancerous lesions, cervical cancer, and normal cervical epithelium. We showed that the expression of SLUG, SNAIL, and TWIST was significantly higher in CIN lesions than in the control group. In addition, we demonstrated that EMT marker expression changed with the histological stage and rose with the stage (TWIST: p < 0.0001, Figure 4; SNAIL: p < 0.0013, Figure 4; SLUG: p < 0.0001, Figure 4; Mann–Whitney test). This may suggest that EMT has a role in the progression of CIN lesions into cervical cancer. Several studies suggested the involvement of SNAIL, SLUG, and TWIST in the development of cervical cancer [15,33,34,35]. The analysis of IHC expression showed higher expression of SLUG, SNAIL, and TWIST in cervical cancer than in the control group. Tian et al. showed that high SNAIL expression predicts a lower survival rate and is correlated with highly aggressive FIGO stage and LNM (lymph node metastasis) status in cervical cancer patients [36]. The overexpression of SLUG observed in cervical cancer is consistent with the work of Liu et al., who revealed a significant effect of SLUG on the EMT process [24]. In clinical practice, markers of EMT play an increasingly important role and are crucial for many treatments of cervical cancer. Dai et al. described novel therapeutic strategies based on negative regulation of the Wnt signaling pathway and reversing the EMT process. HMQ-T-F2 (F2) was shown to suppress the expression of SNAIL [37]. Due to EMT-induced anti-apoptotic abilities, enhanced DNA damage repair, and a changed drug metabolism route, tumor cells become resistant to therapy and cytotoxicity [38]. Tumor radiation sensitivity rapidly declines as EMT advances because EMT is inextricably linked to tumor radiation resistance. Increased radiation resistance in cervical cancer may be caused by TRIP4 overexpression in tumor tissues and cancerous cells, which may encourage EMT and activate the PI3K/Akt and MAPK/ERK signaling pathways [39]. XAV939 is an inhibitor of the Wnt signaling pathway that was shown to increase cervical cancer cells’ radiation sensitivity [40,41]. The expression of EMT markers seems to be an important aspect in planning the treatment of patients and predicting the response to treatment. Work on finding efficient therapeutic targets for cervical cancer metastasis seems to be justified.
4. Materials and Methods
This study was performed on selected archival paraffin-embedded specimens. Patients were operated on between 2014 and 2017 at the Polish Mother’s Memorial Hospital in Lodz. The patients, aged from 25 to 86 years old, were of the female sex. The control group consisted of normal cervical tissue obtained from patients who underwent total hysterectomy due to uterine leiomyomas. The study group consisted of CIN1 (31), CIN2 (75), CIN3 (123), and cervical cancer (124) cases, whereas the control group was composed of 145 cases. The study was approved by the Ethics Committee of Medical University in Wroclaw (21 December 2022; protocol code 1003/2022).
4.1. TMA Construction
To confirm the histological diagnosis and determine whether the material was suitable for further examination, 6 m thick paraffin sections were produced and stained with hematoxylin and eosin (HE). Briefly, slides were scanned using the histologic Pannoramic MIDI scanner (3DHistech Ltd., Sysmex Suisse AG, Horgen, Switzerland). The sites of CIN in the altered cervix epithelium were then selected and digitally tagged after two independent pathologists reviewed the scans. Next, duplicate tissue core punches (2 mm) for each case were taken from the appropriate paraffin donor blocks for use in the preparation of TMAs (TMA Grand Master; 3DHistech). Normal epithelial tissue from the cervix was designated as the control group.
4.2. Immunohistochemistry (IHC)
All IHC reactions were performed on 4 µm thick paraffin slides from TMA using a Dako Autostainer Link48 (Dako, Glostrup, Denmark). The following primary antibodies were used: SLUG (1:50, sc-166476, Santa Cruz Biotechnology, Santa Cruz, CA, USA), TWIST (1:50, ab-50887, Abcam, Cambridge, UK), and SNAIL (1:400, 13099-1-AP, Proteintech, Rosemont, IL, USA). All procedures were conducted as previously described [42]. EnVision FLEX (Dako) was used for the visualization of antibodies, in accordance with the manufacturer’s instructions. Cytoplasmic reactions of SLUG, SNAIL, and TWIST were evaluated via Pannoramic Viewer Digital (3DHistech) image analysis and the routinely used immunoreactive scale (IRS) by Remmele and Stegner. This scale evaluates the percentage of positive cancer cells (A) and the staining intensity of the reaction (B). The final result is the product of these two values (AxB). The nuclear expression of SNAIL and SLUG was evaluated semi-quantitatively based on the percentage of positively stained cells of the whole section (3 slides per case) and encoded as follows: 0: absence of staining; 1: 1–10% cells stained; 2: 11–25% cells stained; 3: 26–50% cells stained; and 4: over 50% cells stained.
4.3. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad, La Jolla, CA, USA) with Spearman correlation, Kruskal–Wallis, Dunn’s multiple comparison, and Mann–Whitney tests. p-values < 0.05 were considered to be statistically significant. The image GP program was used to create the diagrams [43].
5. Conclusions
In our study, the expression of TWIST, SNAIL, and SLUG increased gradually as lesions progressed from LSIL to HSIL. We are the first to show a gradual increase in EMT markers in CIN lesions. Moreover, we confirmed a higher expression of TWIST, SNAIL, and SLUG in cervical cancer than in the control group. The aforementioned data support the idea that EMT elements play a role in the development of cancer.
Author Contributions
Conceptualization, A.P.-K. and C.K.; methodology, A.P.; software, P.S.-G.; validation, A.P.-K., P.D. and M.P.-O.; formal analysis, A.P.-K.; investigation, A.P.-K.; resources, H.R. and B.S.; data curation, P.S.-G.; writing—original draft preparation, A.P.-K.; writing—review and editing, A.P.-K. and C.K.; visualization, A.P.; supervision, P.D., C.K. and M.P.-O.; project administration, A.P.-K.; funding acquisition, A.P.-K., P.D. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, grant number, 2018/29/N/NZ5/01911. The APC was funded by the National Science Centre.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Medical University in Wroclaw (21 December 2022; protocol code 1003/2022).
Informed Consent Statement
Patient consent was waived due to use of anonymized archival material only, which in no way influenced the diagnostic and therapeutic process.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.; Fu, Y.; Cheng, B.; Xie, X.; Wang, X. A Comparative Study on the Accuracy and Efficacy Between Dalton and CINtec® PLUS p16/Ki-67 Dual Stain in Triaging HPV-Positive Women. Front. Oncol. 2021, 11, 815213. [Google Scholar] [CrossRef]
- Cervical Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 22 May 2022).
- Popiel, A.; Kobierzycki, C.; Dzięgiel, P. The Role of Testin in Human Cancers. Pathol. Oncol. Res. 2019, 25, e12770. [Google Scholar] [CrossRef]
- Cervical Cancer: Statistics|Cancer.Net. Available online: https://www.cancer.net/cancer-types/cervical-cancer/statistics (accessed on 13 June 2022).
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.-U.; Creasman, W.T.; Major, F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef]
- SEER Cancer Statistics Review 1975–2003—Previous Version—SEER Cancer Statistics. Available online: https://seer.cancer.gov/archive/csr/1975_2003/ (accessed on 13 June 2022).
- Lee, M.-Y.; Chou, C.-Y.; Tang, M.-J.; Shen, M.-R. Epithelial-Mesenchymal Transition in Cervical Cancer: Correlation with Tumor Progression, Epidermal Growth Factor Receptor Overexpression, and Snail Up-Regulation. Clin. Cancer Res. 2008, 14, 4743–4750. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.P. Activation of β-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer 2011, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-Y.; Shen, M.-R. Epithelial-mesenchymal transition in cervical carcinoma. Am. J. Transl. Res. 2012, 4, 1–13. [Google Scholar] [PubMed]
- Shibata, K.; Kajiyama, H.; Ino, K.; Terauchi, M.; Yamamoto, E.; Nawa, A.; Nomura, S.; Kikkawa, F. Twist expression in patients with cervical cancer is associated with poor disease outcome. Ann. Oncol. 2008, 19, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Chen, L.; Han, X.; Wang, J.; Wang, J. Short hairpin RNA targeting Twist1 suppresses cell proliferation and improves chemosensitivity to cisplatin in HeLa human cervical cancer cells. Oncol. Rep. 2012, 27, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Wang, W.; Tuerhanjiang, A.; Wu, Z.; Yang, R.; Yuan, M.; Ma, D.; Wang, W.; Wang, S. Twist2, the key Twist isoform related to prognosis, promotes invasion of cervical cancer by inducing epithelial-mesenchymal transition and blocking senescence. Hum. Pathol. 2014, 45, 1839–1846. [Google Scholar] [CrossRef]
- Fan, Q.; Qiu, M.-T.; Zhu, Z.; Zhou, J.-H.; Chen, L.; Zhou, Y.; Gu, W.; Wang, L.-H.; Li, Z.-N.; Xu, Y.; et al. Twist induces epithelial-mesenchymal transition in cervical carcinogenesis by regulating the TGF-β/Smad3 signaling pathway. Oncol. Rep. 2015, 34, 1787–1794. [Google Scholar] [CrossRef]
- Kyo, S.; Sakaguchi, J.; Ohno, S.; Mizumoto, Y.; Maida, Y.; Hashimoto, M.; Nakamura, M.; Takakura, M.; Nakajima, M.; Masutomi, K. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum. Pathol. 2006, 37, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Baygi, M.E.; Soheili, Z.S.; Schmitz, I.; Sameie, S.; Schulz, W.A. Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines. Cell Biol. Toxicol. 2010, 26, 553–567. [Google Scholar] [CrossRef]
- Zha, Y.-H.; He, J.-F.; Mei, Y.-W.; Yin, T.; Mao, L. Zinc-finger transcription factor Snail accelerates survival, migration and expression of matrix metalloproteinase-2 in human bone mesenchymal stem cells. Cell Biol. Int. 2007, 31, 1089–1096. [Google Scholar] [CrossRef]
- Vega, S.; Morales, A.V.; Ocaña, O.H.; Valdés, F.; Fabregat, I.; Nieto, M.A. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004, 18, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.P. Snail: More than EMT. Cell Adhes. Migr. 2010, 4, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Sun, R.; Fang, S.; Qin, C.; Pan, X.; Peng, L.; Li, Y.; Li, G. Lnc-CC3 increases metastasis in cervical cancer by increasing Slug expression. Oncotarget 2016, 7, 41650–41661. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-Y.; Guan, H.-M.; Liu, J.; Huang, L.-J.; Hu, X.-L.; Chen, Y.-H.; Wu, Y.-H.; Wang, Y.; Yang, Q.; Zhou, J.-Y. Long noncoding RNA SNHG12 modulated by human papillomavirus 16 E6/E7 promotes cervical cancer progression via ERK/Slug pathway. J. Cell. Physiol. 2020, 235, 7911–7922. [Google Scholar] [CrossRef]
- Liu, X.; Feng, Q.; Zhang, Y.; Zheng, P.; Cui, N. Absence of EpCAM in cervical cancer cells is involved in slug induced epithelial-mesenchymal transition. Cancer Cell Int. 2021, 21, 163. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Yang, W.-T.; Zheng, P.-S. Slug inhibits the proliferation and tumor formation of human cervical cancer cells by up-regulating the p21/p27 proteins and down-regulating the activity of the Wnt/β-catenin signaling pathway via the trans-suppression Akt1/p-Akt1 expression. Oncotarget 2016, 7, 26152–26167. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Huang, J.; Liu, P.; Zhang, C.; Liu, J.; Xia, M.; Shang, C.; Ooi, S.; Chen, Y.; Qin, S.; et al. Downregulation of LNMAS orchestrates partial EMT and immune escape from macrophage phagocytosis to promote lymph node metastasis of cervical cancer. Oncogene 2022, 41, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Hugo, H.J.; Kokkinos, M.I.; Blick, T.; Ackland, M.L.; Thompson, E.W.; Newgreen, D.F. Defining the E-Cadherin Repressor Interactome in Epithelial-Mesenchymal Transition: The PMC42 Model as a Case Study. Cells Tissues Organs 2011, 193, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef]
- Bourboulia, D.; Han, H.; Jensen-Taubman, S.; Gavil, N.; Isaac, B.; Wei, B.; Neckers, L.; Stetler-Stevenson, W.G. TIMP-2 modulates cancer cell transcriptional profile and enhances E-cadherin/beta-catenin complex expression in A549 lung cancer cells. Oncotarget 2013, 4, 166–176. [Google Scholar] [CrossRef]
- Rosty, C.; Hewett, D.G.; Brown, I.S.; Leggett, B.A.; Whitehall, V.L.J. Serrated polyps of the large intestine: Current understanding of diagnosis, pathogenesis, and clinical management. J. Gastroenterol. 2012, 48, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Christensen, P.A.; Luna, E.; Armylagos, D.; Xu, J.; Hsu, W.-C.; Zhou, H.; Schwartz, M.R.; Mody, D.R. Age-specific 3-year cumulative risk of cervical cancer and high-grade dysplasia on biopsy in 9434 women who underwent HPV cytology cotesting. Cancer Cytopathol. 2019, 127, 757–764. [Google Scholar] [CrossRef]
- Raab, S.S.; Bishop, N.S.; Zaleski, M.S. Long-term outcome and relative risk in women with atypical squamous cells of undetermined significance. Am. J. Clin. Pathol. 1999, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, Y.; Xu, H.; Cheng, Y.; Kong, B. Snail family proteins in cervical squamous carcinoma: Expression and significance. Clin. Investig. Med. 2013, 36, E223–E233. [Google Scholar] [CrossRef]
- Qureshi, R.; Arora, H.; Rizvi, M. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2015, 356, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Zhang, C.; Breuer, E.K. EMT-Inducing Molecular Factors in Gynecological Cancers. Biomed Res Int. 2015, 2015, 420891. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, P.; Niu, Q.; Hu, X. Combined Snail and E-cadherin Predicts Overall Survival of Cervical Carcinoma Patients: Comparison Among Various Epithelial-Mesenchymal Transition Proteins. Front. Mol. Biosci. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Yu, R.; Fan, M.; Yang, T.; Wang, B.; Zhang, Y. HMQ-T-F2 suppresses migration of the human cervical cancer HeLa cells by reversing EMT via the PI3K/Akt signaling pathway. Oncol. Rep. 2019, 42, 1451–1458. [Google Scholar] [CrossRef]
- Weadick, B.; Nayak, D.; Persaud, A.K.; Hung, S.W.; Raj, R.; Campbell, M.J.; Chen, W.; Li, J.; Williams, T.M.; Govindarajan, R. EMT-Induced Gemcitabine Resistance in Pancreatic Cancer Involves the Functional Loss of Equilibrative Nucleoside Transporter 1. Mol. Cancer Ther. 2021, 20, 410–422. [Google Scholar] [CrossRef]
- Alafate, W.; Li, X.; Zuo, J.; Zhang, H.; Xiang, J.; Wu, W.; Xie, W.; Bai, X.; Wang, M.; Wang, J. Elevation of CXCL1 indicates poor prognosis and radioresistance by inducing mesenchymal transition in glioblastoma. CNS Neurosci. Ther. 2020, 26, 475–485. [Google Scholar] [CrossRef]
- Zhang, J.; Si, J.; Gan, L.; Guo, M.; Yan, J.; Chen, Y.; Wang, F.; Xie, Y.; Wang, Y.; Zhang, H. Inhibition of Wnt signalling pathway by XAV939 enhances radiosensitivity in human cervical cancer HeLa cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 479–487. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, X.; Xu, H.; Yi, M.; Liu, S.; Wen, F. WNT974 Inhibits Proliferation, Induces Apoptosis, and Enhances Chemosensitivity to Doxorubicin in Lymphoma Cells by Inhibiting Wnt/β-Catenin Signaling. Experiment 2020, 26, e923799. [Google Scholar] [CrossRef]
- Popiel-Kopaczyk, A.; Grzegrzolka, J.; Piotrowska, A.; Olbromski, M.; Smolarz, B.; Romanowicz, H.; Rusak, A.; Mrozowska, M.; Dziegiel, P.; Podhorska-Okolow, M.; et al. The Expression of Testin, Ki-67 and p16 in Cervical Cancer Diagnostics. Curr. Issues Mol. Biol. 2023, 45, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. Imeta 2022, 1, e5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).