The β-1,3-Glucanase Degrades Callose at Plasmodesmata to Facilitate the Transport of the Ribonucleoprotein Complex in Pyrus betulaefolia
Abstract
1. Introduction
2. Results
2.1. Screening and Identification of the PbANK Interacting Proteins
2.2. Characterization of the PbANK Interaction Protein PbPDBG
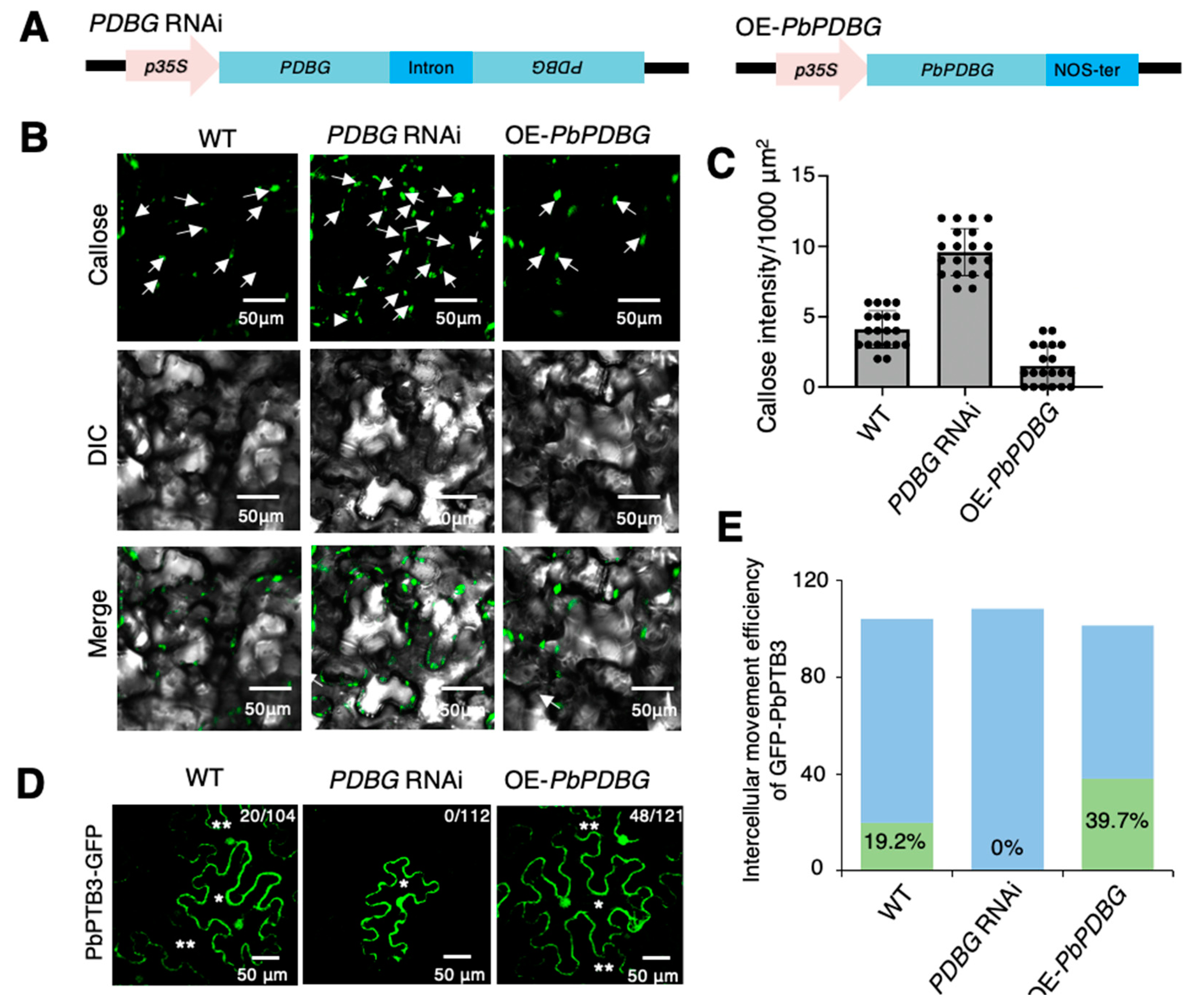
2.3. PbPDBG Regulated Callose Deposition at Plasmodesmata
2.4. PbANK Regulated the Localization of PbPDBG
2.5. PbPDBG Facilitated the Long-Distance Transport of the Ribonucleoprotein Complex
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Conserved Domain and Phylogenetic Analysis
4.3. RNA Extraction, Reverse Transcription, RT-qPCR Analysis
4.4. Extraction Protein of Plasmodesmata
4.5. Co-Immunoprecipitation/Mass Spectrometry (CO-IP/MS)
4.6. Yeast Two-Hybrid Assay
4.7. Bimolecular Fluorescence Complementation (BiFC) Assays
4.8. Luciferase Complementation Imaging (LCI) Assays
4.9. Subcellular Co-Localization
4.10. β-1,3-Glucanase Activity Assay
4.11. Bombardment
4.12. Callose Staining
4.13. dCAPS Analysis
4.14. The Transformation of Tobacco
4.15. The Transient Expression of P. betulaefolia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PbWoxT1 | Wuschel-related homeobox transport1 |
| PDBG | Plasmodesmata-localized β-1,3-glucanase |
| PTB | polypyrimidine tract binding |
| ANK | ankyrin repeat domain |
| TTG1 | Transpareet testa glabra1 |
| GAI | Gibberellic acid insensitive |
| BiFC | Bimolecular fluorescence complementation |
| RBP | RNA-binding protein |
| VIGS | Virus-induced gene silencing |
References
- Hao, L.; Zhang, Y.; Wang, S.; Zhang, W.; Wang, S.; Xu, C.; Yu, Y.; Li, T.; Jiang, F.; Li, W. A constitutive and drought-responsive mRNA undergoes long-distance transport in pear (Pyrus betulaefolia) phloem. Plant Sci. 2020, 293, 110419. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Zhang, W.; Zhang, Q.; Hao, L.; Zhang, Y.; Xu, C.; Yu, Y.; Wang, B.; Li, T.; et al. PbTTG1 forms a ribonucleoprotein complex with polypyrimidine tract-binding protein PbPTB3 to facilitate the long-distance trafficking of PbWoxT1 mRNA. Plant Sci. 2019, 280, 424–432. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Xu, C.; Xiang, L.; Huang, W.; Zhang, C.; Sun, S.; Li, T.; Wang, S. PbANK facilitates the long-distance movement of the PbWoxT1-PbPTB3 RNP complex by degrading deposited callose. Plant Sci. 2022, 318, 111232. [Google Scholar] [CrossRef]
- Notaguchi, M.; Wolf, S.; Lucas, W.J. Phloem-mobile Aux/IAA transcripts target to the root tip and modify root architecture. J. Integr. Plant Biol. 2012, 54, 760–772. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Li, M.; Harada, T.; Han, Z.; Li, T. Gibberellic acid insensitive mRNA transport in both directions between stock and scion in Malus. Tree Genet. Genomes 2010, 6, 1013–1019. [Google Scholar] [CrossRef]
- Lu, K.J.; Huang, N.C.; Liu, Y.S.; Lu, C.A.; Yu, T.S. Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol. 2012, 9, 653–662. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.G.; Miller, W.A.; Hannapel, D.J. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vera, M.; Gandin, V.; Singer, R.H.; Tutucci, E. Intracellular mRNA transport and localized translation. Nat. Rev. Mol. Cell Biol. 2021, 22, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Ephrussi, A. mRNA localization: Gene expression in the spatial dimension. Cell 2009, 136, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.K.; Brandom, J.L.; Xoconostle-Cazares, B.; Ringgold, V.; Lough, T.J.; Lucas, W.J. A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 2009, 21, 197–215. [Google Scholar] [CrossRef]
- Cho, S.K.; Sharma, P.; Butler, N.M.; Kang, I.H.; Shah, S.; Rao, A.G.; Hannapel, D.J. Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J. Exp. Bot. 2015, 66, 6835–6847. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, W.; Huang, J.; Hao, L.; Wang, S.; Wang, A.; Meng, D.; Zhang, Q.; Chen, Q.; Li, T. PbWoxT1 mRNA from pear (Pyrus betulaefolia) undergoes long-distance transport assisted by a polypyrimidine tract binding protein. New Phytol. 2016, 210, 511–524. [Google Scholar] [CrossRef]
- Lucas, W.J.; Ham, B.K.; Kim, J.Y. Plasmodesmata—Bridging the gap between neighboring plant cells. Trends Cell Biol. 2009, 19, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, C. Plasmodesmata and the symplast. Curr. Biol. 2018, 28, R1374–R1378. [Google Scholar] [CrossRef]
- Stadler, R.; Wright, K.M.; Lauterbach, C.; Amon, G.; Gahrtz, M.; Feuerstein, A.; Oparka, K.J.; Sauer, N. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005, 41, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Naumann, M.; Falter, C.; Zwikowics, C.; Jamrow, T.; Manisseri, C.; Somerville, S.C.; Voigt, C.A. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013, 161, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Gomann, J.; Herrfurth, C.; Zienkiewicz, A.; Ischebeck, T.; Haslam, T.M.; Hornung, E.; Feussner, I. Sphingolipid long-chain base hydroxylation influences plant growth and callose deposition in Physcomitrium patens. New Phytol. 2021, 231, 297–314. [Google Scholar] [CrossRef]
- Simpson, C.; Thomas, C.; Findlay, K.; Bayer, E.; Maule, A.J. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell 2009, 21, 581–594. [Google Scholar] [CrossRef]
- Vatén, A.; Dettmer, J.; Wu, S.; Stierhof, Y.D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R.; et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Wang, L.; Wu, M.; Zhang, D.; Fang, W.; Chen, F.; Teng, N. Cytological and Molecular Characteristics of Pollen Abortion in Lily with Dysplastic Tapetum. Hortic. Plant J. 2019, 5, 281–294. [Google Scholar] [CrossRef]
- Amor, Y.; Haigler, C.H.; Johnson, S.; Wainscott, M.; Delmer, D.P. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. USA 1995, 92, 9353–9357. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kim, J.Y. Callose synthesis in higher plants. Plant Signal. Behav. 2009, 4, 489–492. [Google Scholar] [CrossRef]
- Jacobs, A.K.; Lipka, V.; Burton, R.A.; Panstruga, R.; Strizhov, N.; Schulze-Lefert, P.; Fincher, G.B. An Arabidopsis Callose Synthase, GSL5, Is Required for Wound and Papillary Callose Formation. Plant Cell 2003, 15, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Köhle, H.; Jeblick, W.; Poten, F.; Blaschek, W.; Kauss, H. Chitosan-Elicited Callose Synthesis in Soybean Cells as a Ca2+-Dependent Process. Plant Physiol. 1985, 77, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of callose (beta-1,3-glucan) turnover at plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F., Jr. Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Lucas, W.J.; Ding, B.; Vanderschoot, C. Plasmodesmata and the supracellular nature of plants. New Phytol. 1993, 125, 435–476. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.E.; Vesk, M.; Overall, R.L. Callose deposition at plasmodesmata. Protoplasma 1998, 201, 30–37. [Google Scholar] [CrossRef]
- Allison, A.V. The Ultrastructure of Local Lesions Induced by Potato Virus X: A Sequence of Cytological Events in the Course of Infection. Phytopathology 1974, 64, 784. [Google Scholar] [CrossRef]
- Elvira, M.I.; Galdeano, M.M.; Gilardi, P.; Garcia-Luque, I.; Serra, M.T. Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. J. Exp. Bot. 2008, 59, 1253–1265. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Functions and regulation of β-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci. Res. 2003, 13, 17–34. [Google Scholar] [CrossRef]
- Bucciaglia, P.A.; Zimmermann, E.; Smith, A.G. Functional analysis of a beta-1,3-glucanase gene (Tag1) with anther-specific RNA and protein accumulation using antisense RNA inhibition. J. Plant Physiol. 2003, 160, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Erlanger, M.; Rosenthal, M.; Epel, B.L. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 2007, 49, 669–682. [Google Scholar] [CrossRef]
- Levy, A.; Guenoune-Gelbart, D.; Epel, B.L. beta-1,3-Glucanases: Plasmodesmal Gate Keepers for Intercellular Communication. Plant Signal. Behav. 2007, 2, 404–407. [Google Scholar] [CrossRef]
- Rinne, P.L.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjarvi, J.; van der Schoot, C. Chilling of dormant buds hyperinduces FLOWERING LOCUS T and recruits GA-inducible 1,3-beta-glucanases to reopen signal conduits and release dormancy in Populus. Plant Cell 2011, 23, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Levy, A.; Gera, A.; Epel, B.L. Subcellular dynamics and role of Arabidopsis beta-1,3-glucanases in cell-to-cell movement of tobamoviruses. Mol. Plant Microbe Interact. 2013, 26, 1016–1030. [Google Scholar] [CrossRef]
- Payne, G.; Ward, E.; Gaffney, T.; Goy, P.A.; Moyer, M.; Harper, A.; Meins, F., Jr.; Ryals, J. Evidence for a third structural class of p-l,3-glucanase in tobacco. Plant Mol. Biol. 1990, 15, 797–808. [Google Scholar] [CrossRef]
- Escobar, C.; Hernandez, L.E.; Jimenez, A.; Creissen, G.; Ruiz, M.T.; Mullineaux, P.M. Transient expression of Arabidopsis thaliana ascorbate peroxidase 3 in Nicotiana benthamiana plants infected with recombinant potato virus X. Plant Cell Rep. 2003, 21, 699–704. [Google Scholar] [CrossRef]
- Romero, G.O.; Simmons, C.; Yaneshita, M.; Doan, M.; Thomas, B.R.; Rodriguez, R.L. Characterization of rice endo-b-glucanase genes (Gns2–Gns14) defines a new subgroup within the gene family. Gene 1998, 223, 311–320. [Google Scholar] [CrossRef]
- Liu, B.; Xue, X.; Cui, S.; Zhang, X.; Han, Q.; Zhu, L.; Liang, X.; Wang, X.; Huang, L.; Chen, X.; et al. Cloning and characterization of a wheat beta-1,3-glucanase gene induced by the stripe rust pathogen Puccinia striiformis f. sp. tritici. Mol. Biol. Rep. 2010, 37, 1045–1052. [Google Scholar] [CrossRef]
- Jondle, D.J.; Coors, J.G.; Duke, S.H. Maize leaf 0-1,3-glucanase activity in relation to resistance to Exserohilum turcicum. Can. J. Bot. 1989, 67, 263–266. [Google Scholar] [CrossRef]
- Lee, J.Y.; Wang, X.; Cui, W.; Sager, R.; Modla, S.; Czymmek, K.; Zybaliov, B.; van Wijk, K.; Zhang, C.; Lu, H.; et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 2011, 23, 3353–3373. [Google Scholar] [CrossRef]
- Wu, S.W.; Kumar, R.; Iswanto, A.B.B.; Kim, J.Y. Callose balancing at plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef]
- Mestre, P.; Arista, G.; Piron, M.C.; Rustenholz, C.; Ritzenthaler, C.; Merdinoglu, D.; Chich, J.F. Identification of a Vitis vinifera endo-beta-1,3-glucanase with antimicrobial activity against Plasmopara viticola. Mol. Plant Pathol. 2017, 18, 708–719. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhang, W.J.; Zhai, H.C.; Lv, Y.Y.; Cai, J.P.; Jia, F.; Wang, J.S.; Hu, Y.S. Expression of a wheat beta-1,3-glucanase in Pichia pastoris and its inhibitory effect on fungi commonly associated with wheat kernel. Protein Expr. Purif. 2019, 154, 134–139. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. Callose homeostasis at plasmodesmata: Molecular regulators and developmental relevance. Front. Plant Sci. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Fridborg, I.; Grainger, J.; Page, A.; Coleman, M.; Findlay, K.; Angell, S. TIP, A Novel Host Factor Linking Callose Degradation with the Cell-to-Cell Movement of Potato virus X. Mol. Plant-Microbe Interact. 2003, 16, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Simenel, C.; Garnaud, C.; Clavaud, C.; Tada, R.; Barbin, L.; Mouyna, I.; Heddergott, C.; Popolo, L.; Ohya, Y.; et al. The Dual Activity Responsible for the Elongation and Branching of beta-(1,3)-Glucan in the Fungal Cell Wall. mBio 2017, 8, e00619-17. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant beta-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Epel, B.L. Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host beta-1,3-glucanases. Semin. Cell Dev. Biol. 2009, 20, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Krabel, D.; Eschrich, W.; Wirth, S.; Wolf, G. Callase-(1,3-β-D-glucanase) activity during spring reactivation in deciduous trees. Plant Sci. 1993, 93, 19–23. [Google Scholar] [CrossRef]
- Laulhere, J.P.; Briat, J.F. Iron release and uptake by plant ferritin: Effects of pH, reduction and chelation. Biochem. J. 1993, 290, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Yang, N.; Jiang, S.; Ma, C.; Li, H. Overexpression of a Monodehydroascorbate Reductase Gene from Sugar Beet M14 Increased Salt Stress Tolerance. Sugar Tech 2020, 23, 45–56. [Google Scholar] [CrossRef]
- Faulkner, C. Isolation of Plasmodesmata. Methods Mol. Biol. 2017, 1511, 187–198. [Google Scholar] [PubMed]
- Sundararaj, P.; Kathiresan, T. Induction of -1,3-glucanase and chitinase activities in resistant and susceptible sugarcane clones inoculated with Pratylenchuszeae. Int. J. Nematol. 2012, 22, 47–56. [Google Scholar]
- Duan, X.; Zhao, L.; Zhang, W.; Huang, J.; Ma, C.; Hao, L.; Harada, T.; Li, T. KNOTTED1 mRNA undergoes long-distance transport and interacts with movement protein binding protein 2C in pear (Pyrus betulaefolia). Plant Cell Tiss Organ Cult. 2015, 121, 109–119. [Google Scholar] [CrossRef]
- Makhzoum, A.; Petit-Paly, G.; St. Pierre, B.; Bernards, M.A. Functional analysis of the DAT gene promoter using transient Catharanthus roseus and stable Nicotiana tabacum transformation systems. Plant Cell Rep. 2011, 30, 1173–1182. [Google Scholar] [CrossRef]
- Unver, T.; Budak, H. Virus-induced gene silencing, a post transcriptional gene silencing method. Int. J. Plant Genom. 2009, 2009, 198680. [Google Scholar] [CrossRef]
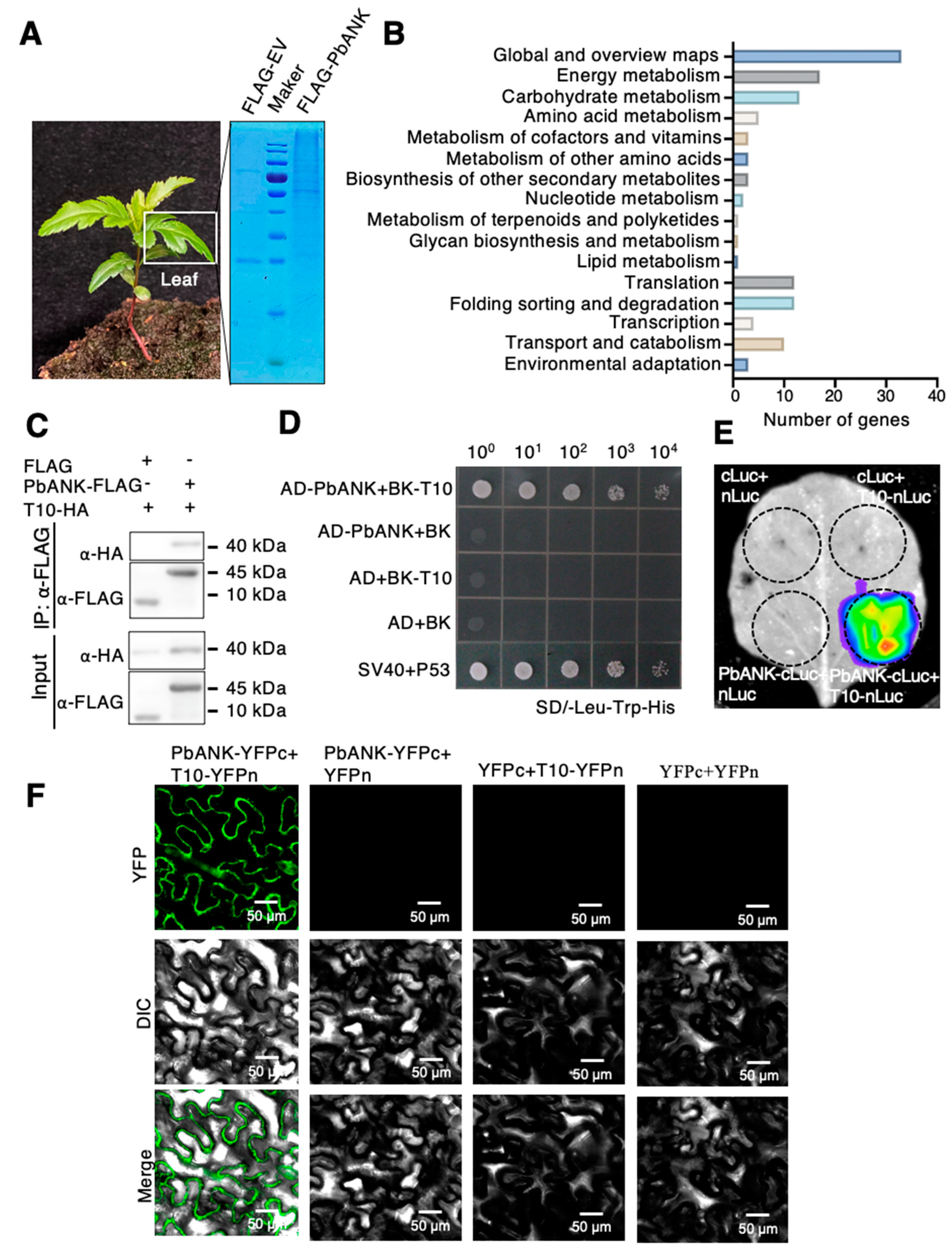



| NO. | Description |
|---|---|
| T1 | Glycerate dehydrogenase-like protein |
| T2 | Carbonic anhydrase isoform 1 |
| T3 | Ferritin OS = Pyrus x bretschneideri |
| T4 | Pgip protein |
| T5 | Fructokinase |
| T6 | Monodehydroascorbate reductase |
| T7 | Glutathione peroxidase |
| T8 | Spermidine synthase |
| T9 | Ascorbate peroxidase |
| T10 | Beta-1,3-glucanase |
| T11 | Aspartate aminotransferase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, S.; Xu, C.; Xiang, L.; Huang, W.; Zhang, X.; Tian, B.; Mao, C.; Li, T.; Wang, S. The β-1,3-Glucanase Degrades Callose at Plasmodesmata to Facilitate the Transport of the Ribonucleoprotein Complex in Pyrus betulaefolia. Int. J. Mol. Sci. 2023, 24, 8051. https://doi.org/10.3390/ijms24098051
Yu Y, Wang S, Xu C, Xiang L, Huang W, Zhang X, Tian B, Mao C, Li T, Wang S. The β-1,3-Glucanase Degrades Callose at Plasmodesmata to Facilitate the Transport of the Ribonucleoprotein Complex in Pyrus betulaefolia. International Journal of Molecular Sciences. 2023; 24(9):8051. https://doi.org/10.3390/ijms24098051
Chicago/Turabian StyleYu, Yunfei, Shengyuan Wang, Chaoran Xu, Ling Xiang, Wenting Huang, Xiao Zhang, Baihui Tian, Chong Mao, Tianzhong Li, and Shengnan Wang. 2023. "The β-1,3-Glucanase Degrades Callose at Plasmodesmata to Facilitate the Transport of the Ribonucleoprotein Complex in Pyrus betulaefolia" International Journal of Molecular Sciences 24, no. 9: 8051. https://doi.org/10.3390/ijms24098051
APA StyleYu, Y., Wang, S., Xu, C., Xiang, L., Huang, W., Zhang, X., Tian, B., Mao, C., Li, T., & Wang, S. (2023). The β-1,3-Glucanase Degrades Callose at Plasmodesmata to Facilitate the Transport of the Ribonucleoprotein Complex in Pyrus betulaefolia. International Journal of Molecular Sciences, 24(9), 8051. https://doi.org/10.3390/ijms24098051





