Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize
Abstract
1. Introduction
2. Results
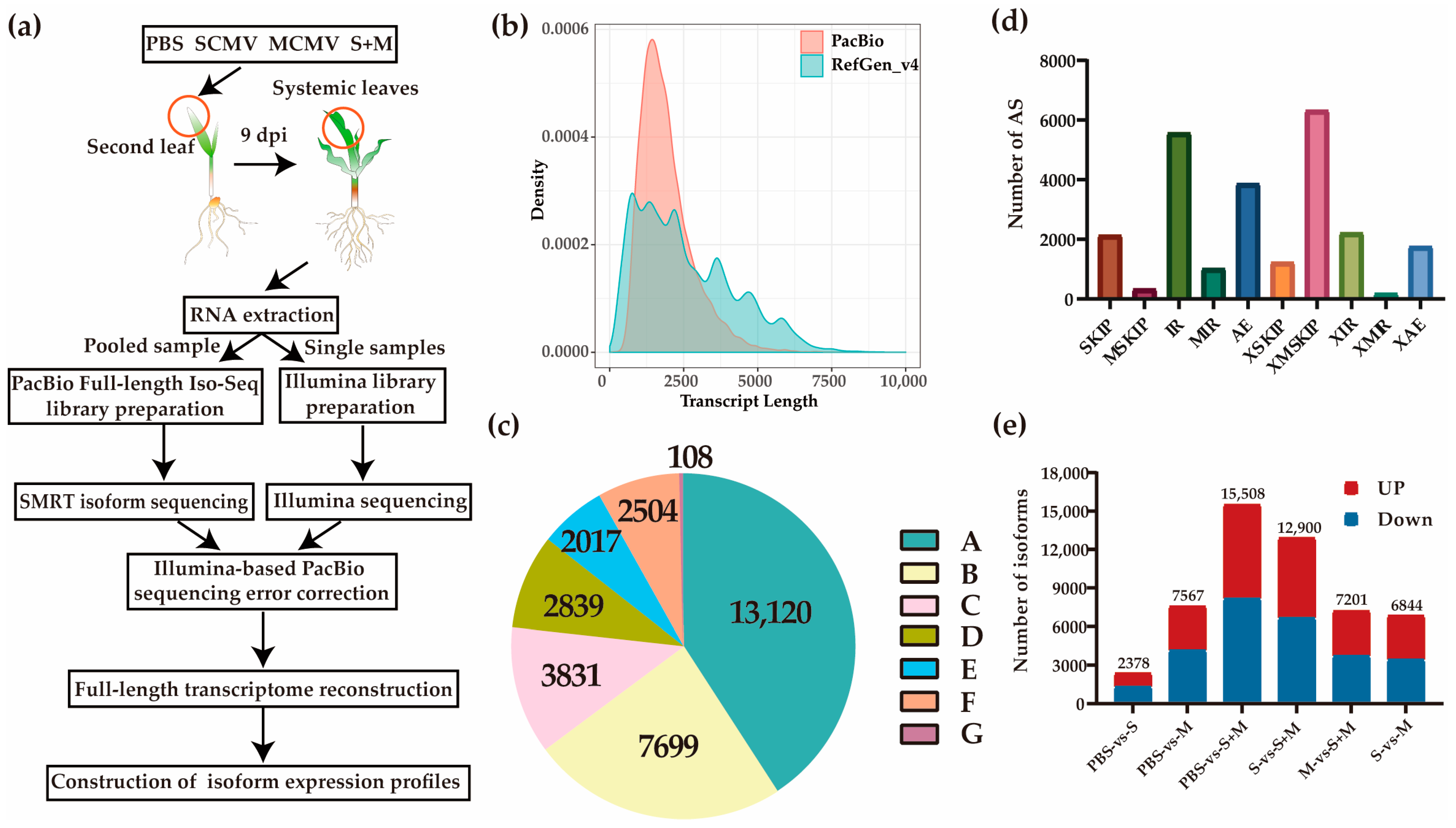
2.1. Characterization of the Full-Length Maize Transcriptome
2.2. Differentially Expressed Isoforms (DEIs)
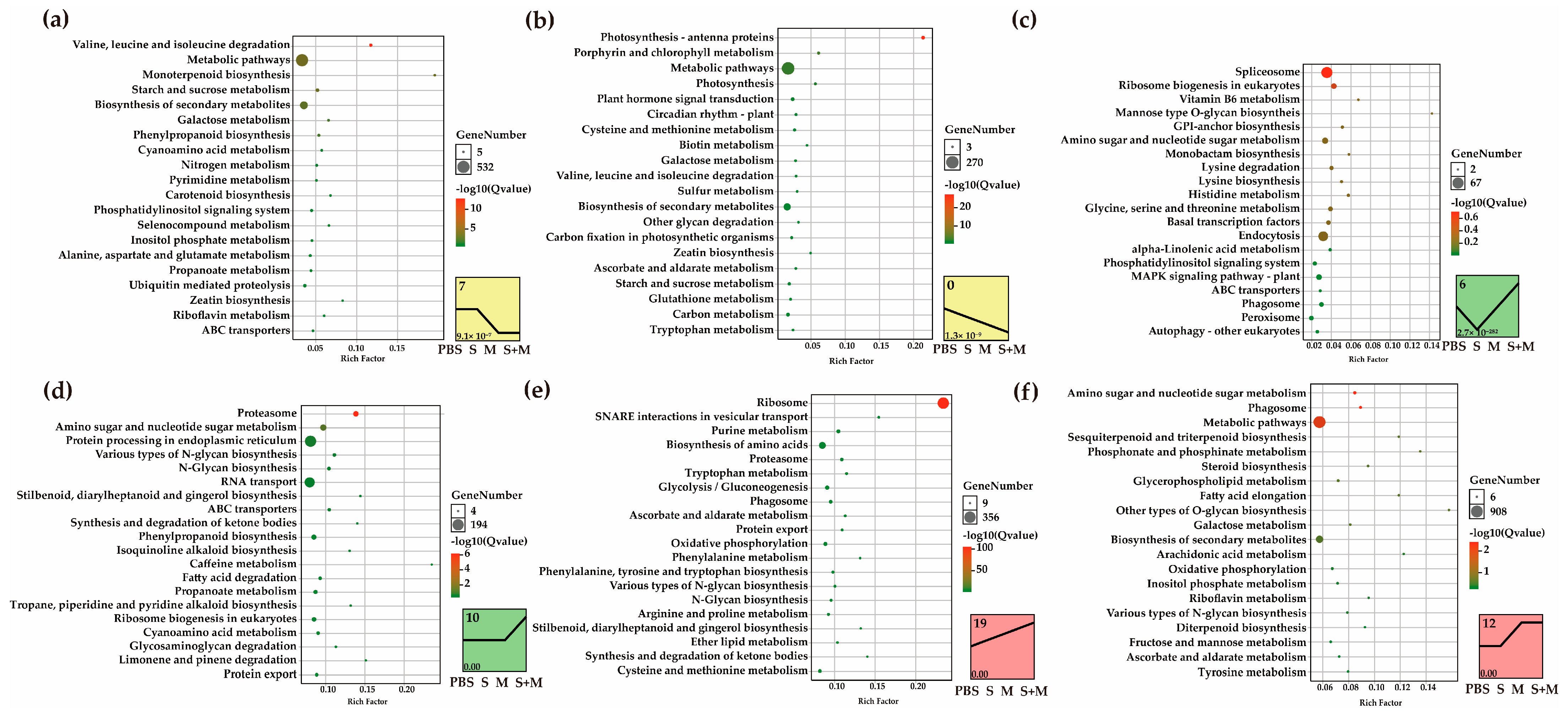
2.3. Short Time-Series Expression Miner (STEM) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
2.4. Analyses of DEIs in the 26S Proteasome
2.5. Analyses of DEIs Involved in Photosynthesis
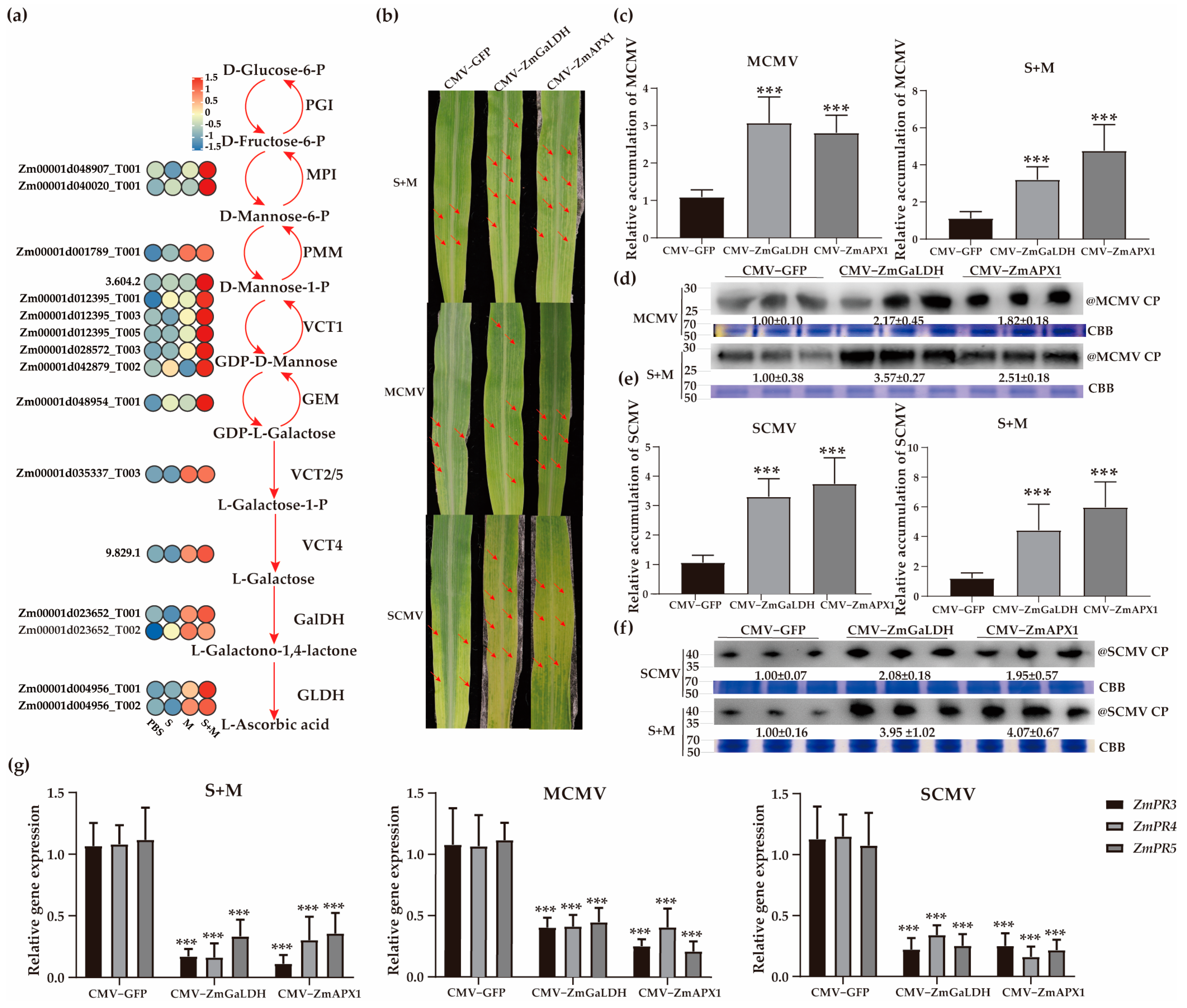
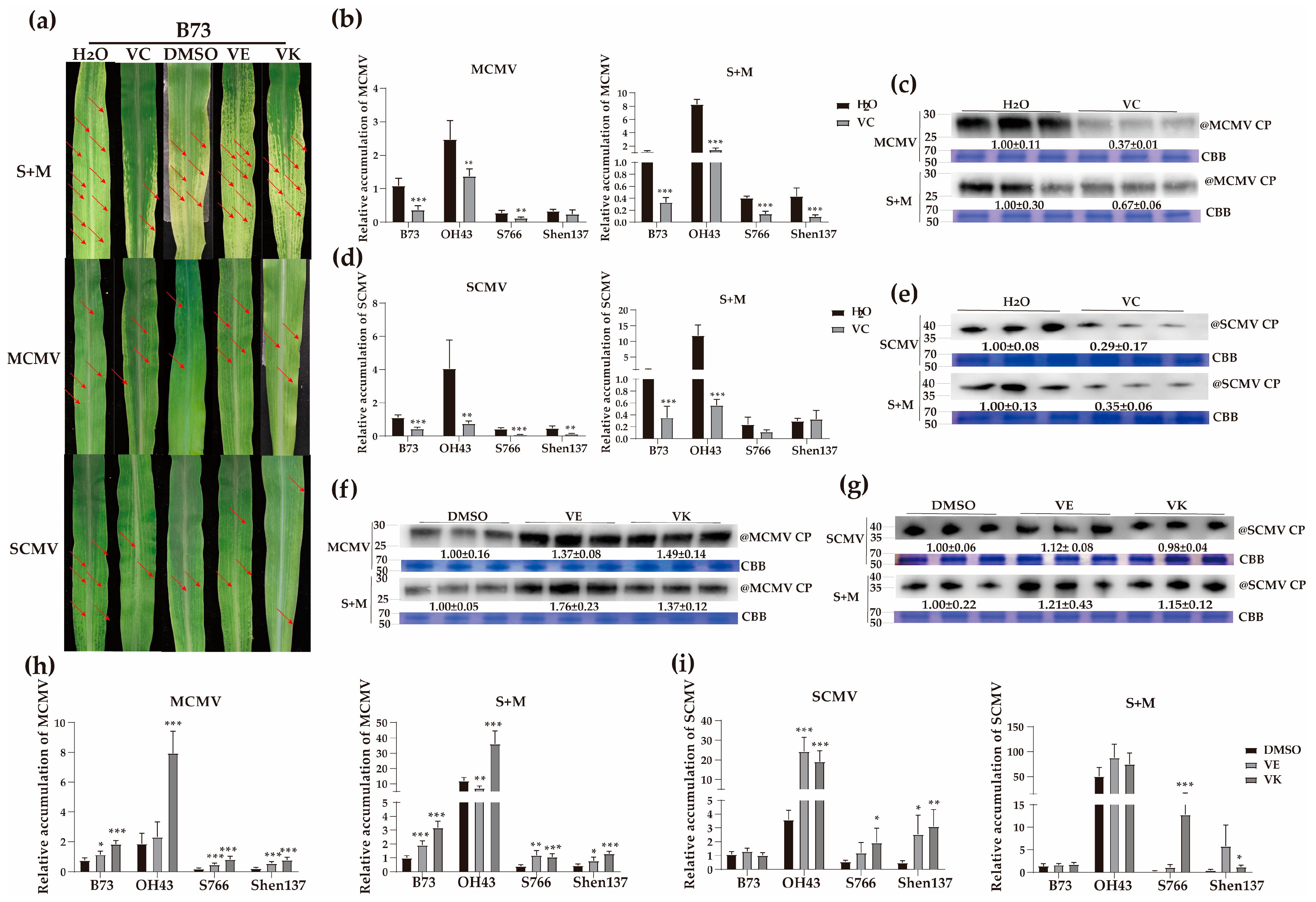
2.6. Roles of Vitamin C in Combating Viral Infection
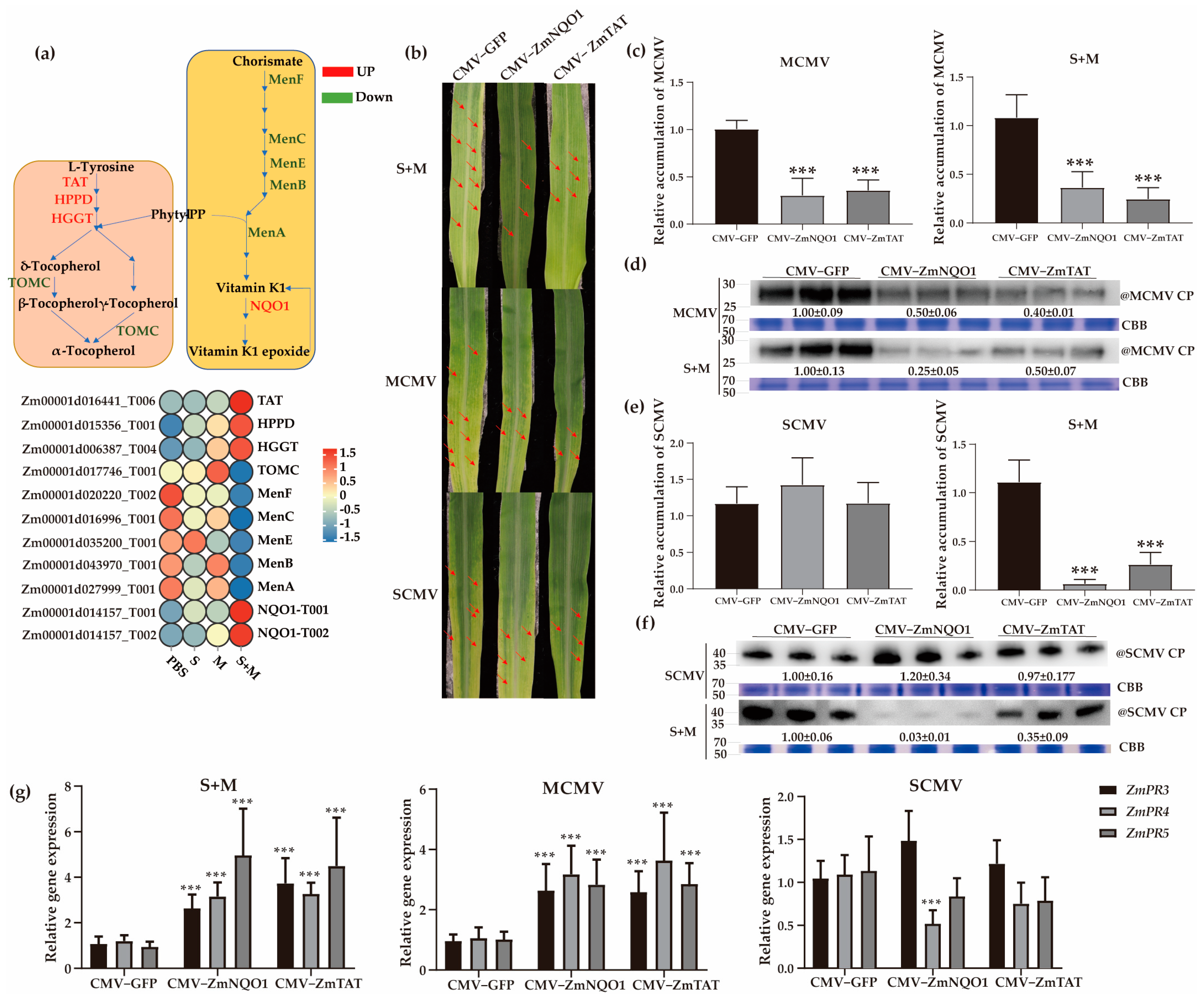
2.7. Roles of Vitamins E and K in Antiviral Responses
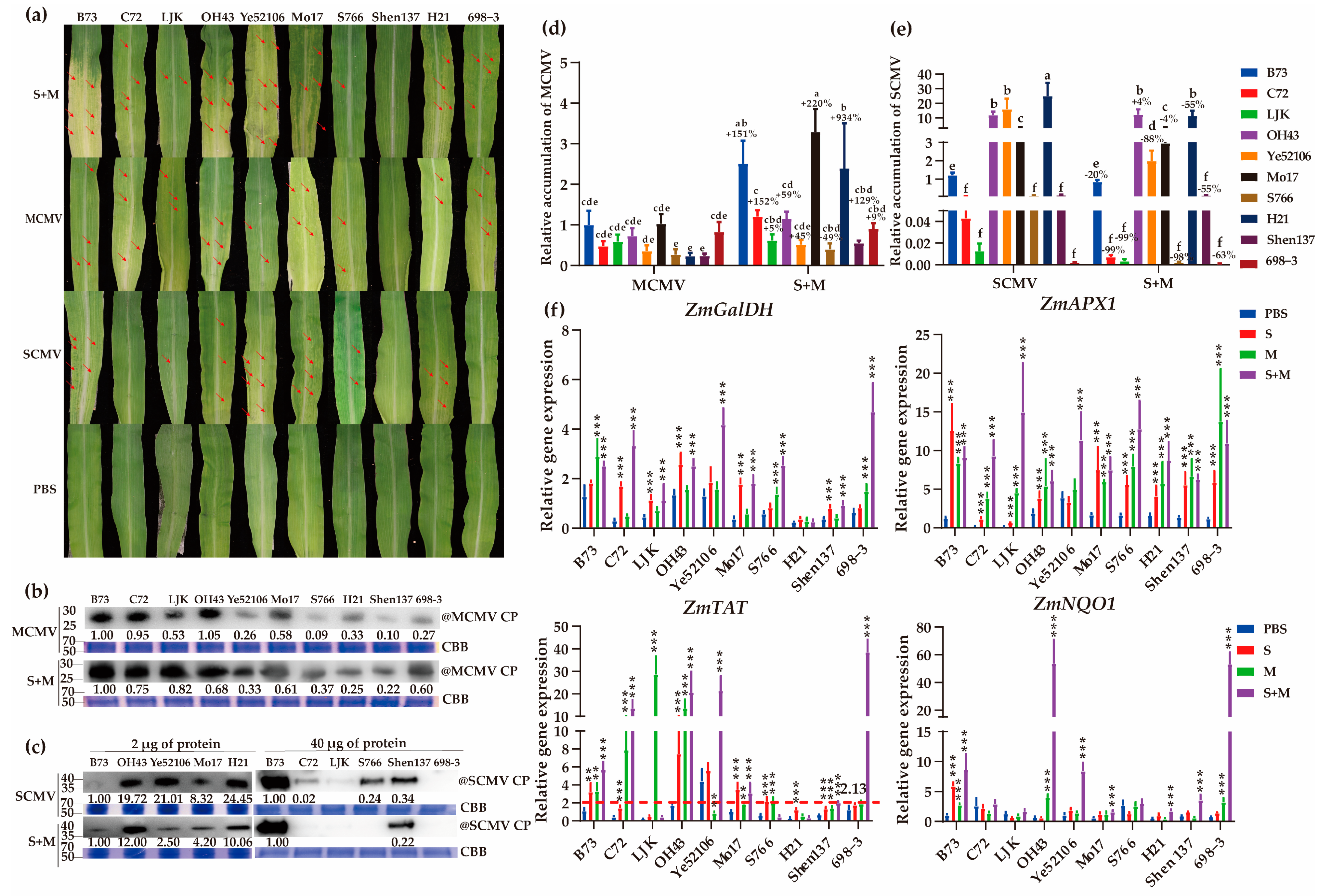
2.8. Identification of MLN-Resistant Maize Inbred Lines
2.9. Effects of Spraying Different Vitamins on Viral Infections in Resistant and Susceptible Maize Inbred Lines
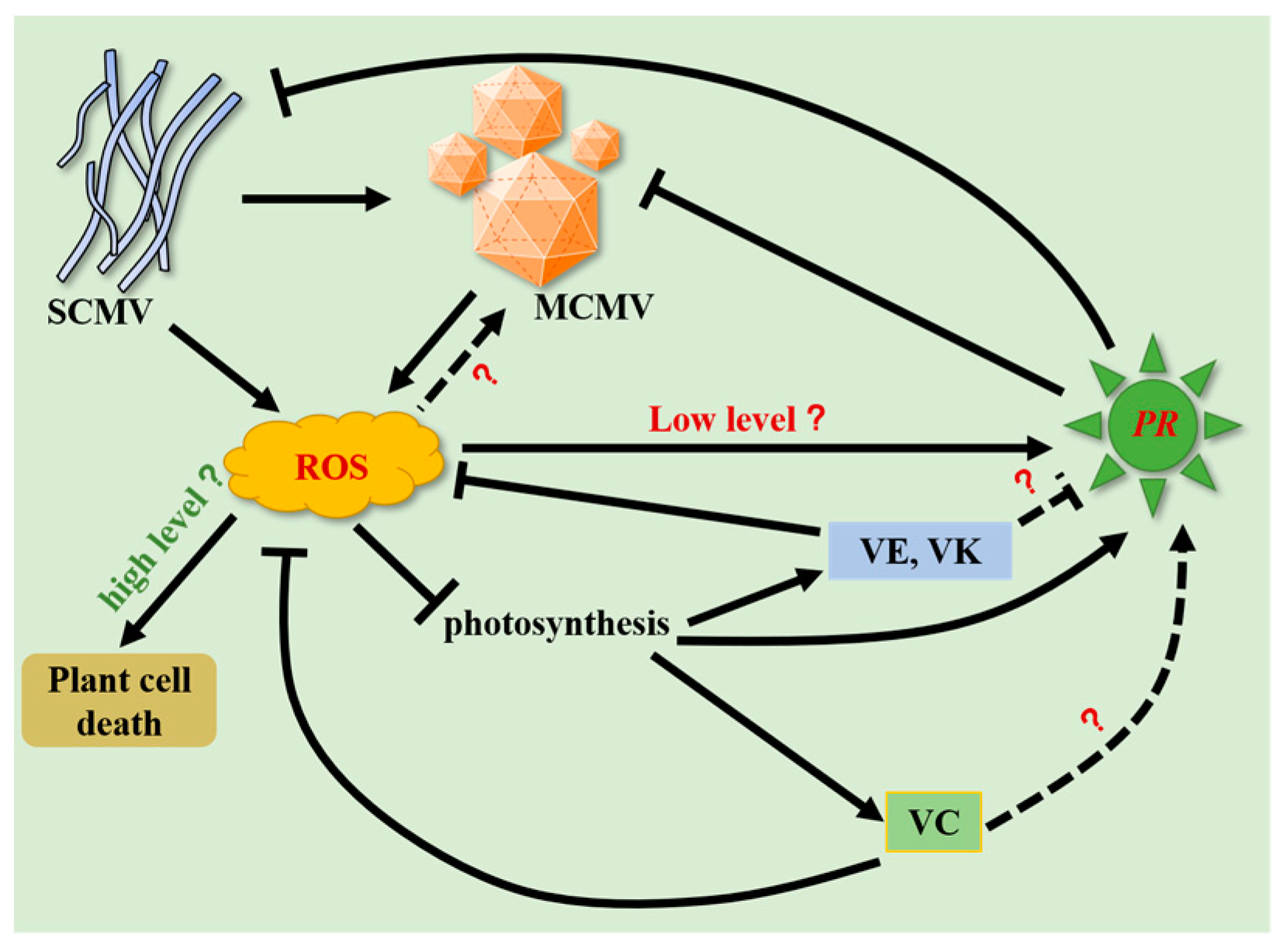
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Virus Inoculation
4.2. SMRT Iso-Seq and RNA-Seq
4.3. Mapping to the Reference Genome
4.4. Analysis of AS Events
4.5. Analysis of DEIs
4.6. Isoform Functional Annotation
4.7. STEM Analysis
4.8. CMV-Based VIGS Assays
4.9. Evaluation of Maize Germplasm for Resistance to SCMV, MCMV, and MLN in Maize
4.10. Chemical Treatments
4.11. Reverse Transcription--Quantitative PCR (RT–qPCR) Analysis
4.12. Western Blotting
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheets, K. Maize chlorotic mottle machlomovirus and wheat streak mosaic rymovirus concentrations increase in the synergistic disease corn lethal necrosis. Virology 1998, 242, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Redinbaugh, M.G.; Stewart, L.R. Maize lethal necrosis: An emerging, synergistic viral disease. Annu. Rev. Virol. 2018, 5, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, Y.; Li, Y.; Xia, Z.; Xie, J.; Carr, J.P.; Wu, B.; Fan, Z.; Zhou, T. Identification of differentially regulated maize proteins conditioning Sugarcane mosaic virus systemic infection. New Phytol. 2017, 215, 1156–1172. [Google Scholar] [CrossRef]
- Xu, X.J.; Geng, C.; Jiang, S.Y.; Zhu, Q.; Yan, Z.Y.; Tian, Y.P.; Li, X.D. A maize triacylglycerol lipase inhibits sugarcane mosaic virus infection. Plant Physiol. 2022, 189, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhao, Z.; Li, M.; Chen, L.; Jiao, Z.; Wu, Y.; Zhou, T.; Yu, W.; Fan, Z. Identification of miRNAs and their targets in maize in response to sugarcane mosaic virus infection. Plant Physiol. Biochem. 2018, 125, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, Z.; Xia, Z.; Cheng, Y.; Jiao, Z.; Sun, B.; Zhou, T.; Fan, Z. A violaxanthin deepoxidase interacts with a viral suppressor of RNA silencing to inhibit virus amplification. Plant Physiol. 2017, 175, 1774–1794. [Google Scholar] [CrossRef]
- Duble, C.; Melchinger, A.; Kuntze, L.; Stork, A.; Lubberstedt, T. Molecular mapping and gene action of Scm1 and Scm2, two major QTL contributing to SCMV resistance in maize. Plant Breed. 2000, 119, 299–303. [Google Scholar]
- Liu, Q.; Liu, H.; Gong, Y.; Tao, Y.; Jiang, L.; Zuo, W.; Yang, Q.; Ye, J.; Lai, J.; Wu, J.; et al. An atypical thioredoxin imparts early resistance to sugarcane mosaic virus in maize. Mol. Plant 2017, 10, 483–497. [Google Scholar] [CrossRef]
- Awata, L.A.O.; Ifie, B.E.; Danquah, E.; Jumbo, M.B.; Suresh, L.M.; Gowda, M.; Marchelo-Dragga, P.W.; Olsen, M.S.; Shorinola, O.; Yao, N.K.; et al. Introgression of maize lethal necrosis resistance quantitative trait loci into susceptible maize populations and validation of the resistance under field conditions in Naivasha, Kenya. Front. Plant Sci. 2021, 12, 649308. [Google Scholar] [CrossRef]
- Awata, L.A.O.; Beyene, Y.; Gowda, M.; Suresh, L.M.; Jumbo, M.B.; Tongoona, P.; Danquah, E.; Ifie, B.E.; Marchelo-Dragga, P.W.; Olsen, M.; et al. Genetic analysis of QTL for resistance to maize lethal necrosis in multiple mapping populations. Genes 2019, 11, 32. [Google Scholar] [CrossRef]
- Cao, N.; Zhan, B.; Zhou, X. Nitric oxide as a downstream signaling molecule in brassinosteroid-mediated virus susceptibility to maize chlorotic mottle virus in maize. Viruses 2019, 11, 368. [Google Scholar] [CrossRef]
- Dang, M.; Cheng, Q.; Hu, Y.; Wu, J.; Zhou, X.; Qian, Y. Proteomic changes during MCMV Infection revealed by iTRAQ quantitative proteomic analysis in maize. Int. J. Mol. Sci. 2019, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhao, Z.; Chen, L.; Li, M.; Zhou, T.; Deng, C.; Zhou, Q.; Fan, Z. Synergistic infection of two viruses MCMV and SCMV increases the accumulations of both MCMV and MCMV-derived siRNAs in maize. Sci. Rep. 2016, 6, 20520. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Z.; Gao, X.; Jiao, Z.; Wu, Y.; Zhou, T.; Fan, Z. Characterization of maize miRNAs in response to synergistic infection of maize chlorotic mottle virus and sugarcane mosaic virus. Int. J. Mol. Sci. 2019, 20, 3146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Wen, X.; Zhang, Y.; Ding, Y.; Zhang, Y.; Gao, B.; Zhang, D. PacBio full-length transcriptome of wild apple (Malus sieversii) provides insights into canker disease dynamic response. BMC Genom. 2021, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; You, X.; Langer, J.D.; Hou, J.; Rupprecht, F.; Vlatkovic, I.; Quedenau, C.; Tushev, G.; Epstein, I.; Schaefke, B.; et al. Full-length transcriptome reconstruction reveals a large diversity of RNA and protein isoforms in rat hippocampus. Nat. Commun. 2019, 10, 5009. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; He, Y.; Cao, Q.; Zhang, T.; Lou, L.; Cai, Q. Full-Length transcriptome assembly of Italian ryegrass root integrated with RNA-Seq to identify genes in response to plant cadmium stress. Int. J. Mol. Sci. 2020, 21, 1067. [Google Scholar] [CrossRef]
- Ye, W.; Wang, T.; Wei, W.; Lou, S.; Lan, F.; Zhu, S.; Li, Q.; Ji, G.; Lin, C.; Wu, X.; et al. The Full-Length transcriptome of Spartina alterniflora reveals the complexity of high salt tolerance in monocotyledonous halophyte. Plant Cell Physiol. 2020, 61, 882–896. [Google Scholar] [CrossRef]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant–virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Doke, N.; Ohashi, Y. Involvement of an O2− generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol. Mol. Plant Pathol. 1988, 32, 163–175. [Google Scholar] [CrossRef]
- Bao, G.; Zhuo, C.; Qian, C.; Xiao, T.; Guo, Z.; Lu, S. Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol. J. 2016, 14, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Schurgers, L.J.; Vermeer, C. Vitamin K: The coagulation vitamin that became omnipotent. Thromb. Haemost. 2007, 98, 120–125. [Google Scholar] [CrossRef]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, H.; Wang, Z.; Feng, T.; Yu, L.; Zhang, J.; Gao, W.; Zhou, Y.; Sun, M.; Liu, P.; et al. Wheat yellow mosaic virus NIb targets TaVTC2 to elicit broad-spectrum pathogen resistance in wheat. Plant Biotechnol. J. 2023, 21, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Mieda, T.; Yabuta, Y.; Rapolu, M.; Motoki, T.; Takeda, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Feedback inhibition of spinach L-galactose dehydrogenase by L-ascorbate. Plant Cell Physiol. 2004, 45, 1271–1279. [Google Scholar] [CrossRef]
- Gatzek, S.; Wheeler, G.L.; Smirnoff, N. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. Plant J. 2002, 30, 541–553. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Inhibition of ascorbate peroxidase by salicylic acid and 2, 6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11312–11316. [Google Scholar] [CrossRef]
- Wu, L.; Han, Z.; Wang, S.; Wang, X.; Sun, A.; Zu, X.; Chen, Y. Comparative proteomic analysis of the plant–virus interaction in resistant and susceptible ecotypes of maize infected with sugarcane mosaic virus. J. Proteom. 2013, 89, 124–140. [Google Scholar] [CrossRef]
- Fujiwara, A.; Togawa, S.; Hikawa, T.; Matsuura, H.; Masuta, C.; Inukai, T. Ascorbic acid accumulates as a defense response to turnip mosaic virus in resistant Brassica rapa cultivars. J. Exp. Bot. 2016, 67, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Chakraborty, D.; Kundu, A.; Pal, A. Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between vigna mungo and mungbean yellow mosaic India virus. Proteome Sci. 2013, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dormann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Gilliland, L.U.; Magallanes-Lundback, M.; Pollard, M.; DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 2004, 16, 1419–1432. [Google Scholar] [CrossRef]
- Ibrahim, M.; Bondok, A.; Al-Senosy, N.K.; Younis, R. Stimulation some of defense mechanisms in tomato plants under water deficit and tobacco mosaic virus (TMV). World J. Agric. Sci. 2015, 11, 289–302. [Google Scholar]
- Riewe, D.; Koohi, M.; Lisec, J.; Pfeiffer, M.; Lippmann, R.; Schmeichel, J.; Willmitzer, L.; Altmann, T. A tyrosine aminotransferase involved in tocopherol synthesis in Arabidopsis. Plant J. 2012, 71, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Sandorf, I.; Hollander-Czytko, H. Jasmonate is involved in the induction of tyrosine aminotransferase and tocopherol biosynthesis in Arabidopsis thaliana. Planta 2002, 216, 173–179. [Google Scholar] [CrossRef]
- Mukai, K.; Morimoto, H.; Kikuchi, S.; Nagaoka, S.-i. Kinetic study of free-radical-scavenging action of biological hydroquinones (reduced forms of ubiquinone, vitamin K and tocopherol quinone) in solution. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1993, 1157, 313–317. [Google Scholar] [CrossRef]
- Shimada, H.; Ohno, R.; Shibata, M.; Ikegami, I.; Onai, K.; Ohto, M.a.; Takamiya, K.i. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis. Plant J. 2005, 41, 627–637. [Google Scholar] [CrossRef]
- Eugeni Piller, L.; Besagni, C.; Ksas, B.; Rumeau, D.; Brehelin, C.; Glauser, G.; Kessler, F.; Havaux, M. Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K1 accumulation. Proc. Natl. Acad. Sci. USA 2011, 108, 14354–14359. [Google Scholar] [CrossRef]
- Nioi, P.; Hayes, J.D. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat. Res. 2004, 555, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Camborde, L.; Planchais, S.; Tournier, V.; Jakubiec, A.; Drugeon, G.; Lacassagne, E.; Pflieger, S.; Chenon, M.; Jupin, I. The ubiquitin-proteasome system regulates the accumulation of Turnip yellow mosaic virus RNA-dependent RNA polymerase during viral infection. Plant Cell 2010, 22, 3142–3152. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Tahiliani, P.; Kumar, A.; Chandu, D. The ubiquitin-proteasome system. J. Biosci. 2006, 31, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Lehto, K.; Tikkanen, M.; Hiriart, J.-B.; Paakkarinen, V.; Aro, E.-M. Depletion of the photosystem II core complex in mature tobacco leaves infected by the flavum strain of tobacco mosaic virus. Mol. Plant-Microbe Interact. 2003, 16, 1135–1144. [Google Scholar] [CrossRef]
- Wei, T.; Huang, T.-S.; McNeil, J.; Laliberté, J.-F.; Hong, J.; Nelson, R.S.; Wang, A. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J. Virol. 2010, 84, 799–809. [Google Scholar] [CrossRef]
- Foyer, C.; Descourvieres, P.; Kunert, K. Protection against oxygen radicals: An important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Jiao, Z.; Tian, Y.; Cao, Y.; Wang, J.; Zhan, B.; Zhao, Z.; Sun, B.; Guo, C.; Ma, W.; Liao, Z.; et al. A novel pathogenicity determinant hijacks maize catalase 1 to enhance viral multiplication and infection. New Phytol. 2021, 230, 1126–1141. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef]
- Roy, B.; Kirchner, J. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 2000, 54, 51–63. [Google Scholar]
- Venkatesh, J.; Park, S.W. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef]
- Mahuku, G.; Lockhart, B.E.; Wanjala, B.; Jones, M.W.; Kimunye, J.N.; Stewart, L.R.; Cassone, B.J.; Sevgan, S.; Nyasani, J.O.; Kusia, E. Maize lethal necrosis (MLN), an emerging threat to maize-based food security in sub-Saharan Africa. Phytopathology 2015, 105, 956–965. [Google Scholar] [CrossRef]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Wang, F.; Chen, Y.; Tian, Y.; Jiang, L.; Peng, J.; Zheng, H.; Lin, L.; Yan, C. Involvement of the chloroplast gene ferredoxin 1 in multiple responses of Nicotiana benthamiana to potato virus X infection. J. Exp. Bot. 2020, 71, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Q.; Liu, Z.M.; Xu, J.; Zhou, T.; Wang, M.; Chen, Y.T.; Li, H.F.; Fan, Z.F. HC-Pro protein of sugar cane mosaic virus interacts specifically with maize ferredoxin-5 in vitro and in planta. J. Gen. Virol. 2008, 89, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, A. Dissecting the molecular network of virus-plant interactions: The complex roles of host factors. Annu. Rev. Phytopathol. 2015, 53, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Luo, H. Interplay between the virus and the ubiquitin–proteasome system: Molecular mechanism of viral pathogenesis. Curr. Opin. Virol. 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Mckersie, B.D. Role of the ascorbate-glutathione antioxidant system in chilling resistance of tomato. J. Plant Physiol. 1993, 141, 234–239. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z.; et al. Maize phenylalanine ammonia-lyases contribute to resistance to sugarcane mosaic virus infection, most likely through positive regulation of salicylic acid accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef]
- Asensi-Fabado, M.A.; Munne-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Riadh, K.; Gopi, R.; Manivannan, P.; Inès, J.; Al-Juburi, H.J.; Chang-Xing, Z.; Hong-Bo, S.; Panneerselvam, R. Antioxidant defense responses: Physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plant. 2009, 31, 427–436. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Completing a pathway to plant vitamin C synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 9109–9110. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Iwamoto, T.; Kida, S.; Masushige, S.; Yamada, K.; Esashi, T. Tocopherol-associated protein is a ligand-dependent transcriptional activator. Biochem. Biophys. Res. Commun. 2001, 285, 295–299. [Google Scholar] [CrossRef]
- Elwan, M.; El-Hamahmy, M. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 2009, 122, 521–526. [Google Scholar] [CrossRef]
- Fujiwara, A.; Shimura, H.; Masuta, C.; Sano, S.; Inukai, T. Exogenous ascorbic acid derivatives and dehydroascorbic acid are effective antiviral agents against turnip mosaic virus in Brassica rapa. J. Gen. Plant Pathol. 2013, 79, 198–204. [Google Scholar] [CrossRef]
- Mukherjee, M.; Larrimore, K.E.; Ahmed, N.J.; Bedick, T.S.; Barghouthi, N.T.; Traw, M.B.; Barth, C. Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Mol. Plant-Microbe Interact. 2010, 23, 340–351. [Google Scholar] [CrossRef]
- Abreu, M.E.; Munne-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Gordon, D.; Huddleston, J.; Chaisson, M.J.; Hill, C.M.; Kronenberg, Z.N.; Munson, K.M.; Malig, M.; Raja, A.; Fiddes, I.; Hillier, L.W. Long-read sequence assembly of the gorilla genome. Science 2016, 352, aae0344. [Google Scholar] [CrossRef]
- Chin, C.-S.; Alexander, D.H.; Marks, P.; Klammer, A.A.; Drake, J.; Heiner, C.; Clum, A.; Copeland, A.; Huddleston, J.; Eichler, E.E. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods 2013, 10, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Peluso, P.; Shi, J.; Liang, T.; Stitzer, M.C.; Wang, B.; Campbell, M.S.; Stein, J.C.; Wei, X.; Chin, C.-S. Improved maize reference genome with single-molecule technologies. Nature 2017, 546, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Stamm, S.; Ben-Ari, S.; Rafalska, I.; Tang, Y.; Zhang, Z.; Toiber, D.; Thanaraj, T.; Soreq, H. Function of alternative splicing. Gene 2005, 344, 1–20. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Scholthof, K.B. Genome-wide analysis of alternative splicing landscapes modulated during plant-virus interactions in Brachypodium distachyon. Plant Cell 2015, 27, 71–85. [Google Scholar] [CrossRef]
- Langmead, B. Aligning short sequencing reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11.7.1–11.7.14. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, X.; Wang, N.; Liu, X.; Nelson, R.S.; Li, W.; Fan, Z.; Zhou, T. An efficient virus-induced gene silencing vector for maize functional genomics research. Plant J. 2016, 86, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Sitta, J.; Nzuve, F.M.; Olubayo, F.; Mutinda, C.; Muiru, W.M.; Miano, D.; Muthomi, J.W.; Leley, P. Response of assorted maize germplasm to the maize lethal necrosis disease in Kenya. J. Plant Stud. 2017, 6, 65. [Google Scholar]
- Chen, Y.; Guo, M.; Zhu, X.; Gao, W.; Dai, F.; Li, L. Identification of maize germplasm resources for resistance to maize dwarf mosaic disease. Plant Prot. 1996, 22, 13–15. [Google Scholar]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. Comput. Softw. J. Sci. 2000, 1, 270–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, K.; Yang, M.; Cui, Y.; Jiao, Z.; Gao, X.; Du, Z.; Wang, Z.; An, M.; Xia, Z.; Wu, Y. Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize. Int. J. Mol. Sci. 2023, 24, 8012. https://doi.org/10.3390/ijms24098012
Hao K, Yang M, Cui Y, Jiao Z, Gao X, Du Z, Wang Z, An M, Xia Z, Wu Y. Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize. International Journal of Molecular Sciences. 2023; 24(9):8012. https://doi.org/10.3390/ijms24098012
Chicago/Turabian StyleHao, Kaiqiang, Miaoren Yang, Yakun Cui, Zhiyuan Jiao, Xinran Gao, Zhichao Du, Zhiping Wang, Mengnan An, Zihao Xia, and Yuanhua Wu. 2023. "Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize" International Journal of Molecular Sciences 24, no. 9: 8012. https://doi.org/10.3390/ijms24098012
APA StyleHao, K., Yang, M., Cui, Y., Jiao, Z., Gao, X., Du, Z., Wang, Z., An, M., Xia, Z., & Wu, Y. (2023). Transcriptomic and Functional Analyses Reveal the Different Roles of Vitamins C, E, and K in Regulating Viral Infections in Maize. International Journal of Molecular Sciences, 24(9), 8012. https://doi.org/10.3390/ijms24098012





