Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches
Abstract
1. Introduction
2. Therapeutic or Preventive Management of Stroke in Cancer Patients
3. Reperfusion Therapy for Stroke in Cancer Patients
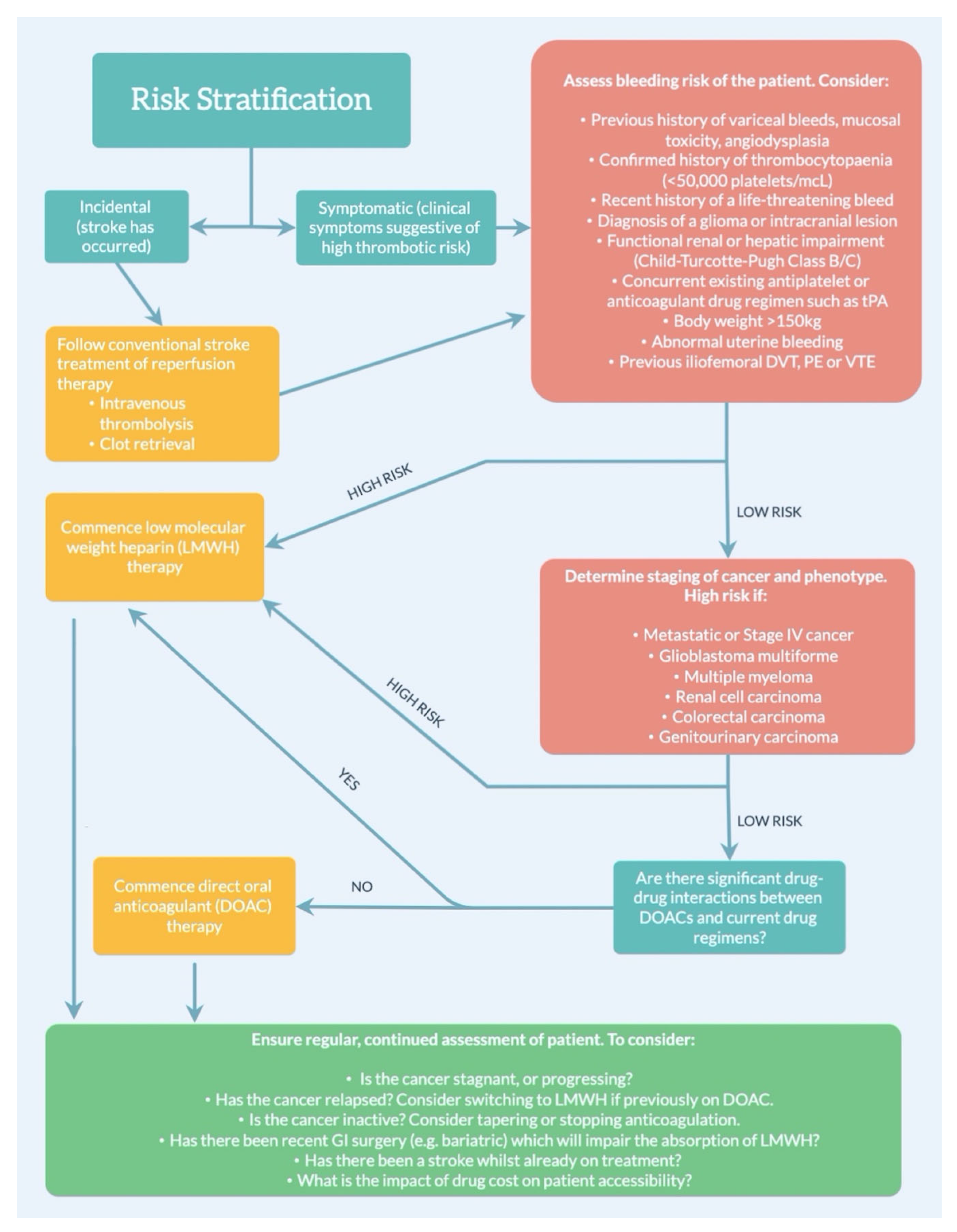
4. Anticoagulation
5. Antiplatelet Therapy
6. Lifestyle Management
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dardiotis, E.; Aloizou, A.M.; Markoula, S.; Siokas, V.; Tsarouhas, K.; Tzanakakis, G.; Libra, M.; Kyritsis, A.P.; Brotis, A.G.; Aschner, M.; et al. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int. J. Oncol. 2019, 54, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-Y.; Bhaskar, S.M.M. When Two Maladies Meet: Disease Burden and Pathophysiology of Stroke in Cancer. Int. J. Mol. Sci. 2022, 23, 15769. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Chung, J.W.; Lee, M.J.; Seo, W.K.; Kim, G.M.; Ahn, M.J. Cancer-Related Stroke: An Emerging Subtype of Ischemic Stroke with Unique Pathomechanisms. J. Stroke 2020, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Hong, J.M.; Kim, H.Y.; Lee, J.; Chung, P.W.; Park, K.Y.; Kim, G.M.; Lee, K.H.; Chung, C.S.; Bang, O.Y. Ischemic stroke in cancer patients with and without conventional mechanisms: A multicenter study in Korea. Stroke 2010, 41, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Selvik, H.A.; Thomassen, L.; Logallo, N.; Næss, H. Prior cancer in patients with ischemic stroke: The Bergen NORSTROKE study. J. Stroke Cerebrovasc. Dis. 2014, 23, 919–925. [Google Scholar] [CrossRef]
- Sanossian, N.; Djabiras, C.; Mack, W.J.; Ovbiagele, B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 1146–1150. [Google Scholar] [CrossRef]
- Rolleston, J.D. Jean Baptiste Bouillaud (1796–1881). A Pioneer in Cardiology and Neurology. Proc. R. Soc. Med. 1931, 24, 1253–1262. [Google Scholar] [CrossRef]
- Bouillaud, S.; Bouillaud, J. De l’Obliteration des veines et de son influence sur la formation des hydropisies partielles: Consideration sur la hydropisies passive et general. Arch. Gen. Med. 1823, 1, 188–204. [Google Scholar]
- Graus, F.; Rogers, L.R.; Posner, J.B. Cerebrovascular complications in patients with cancer. Mediciine 1985, 64, 16–35. [Google Scholar] [CrossRef]
- Fitzpatrick, T.; Carrier, M.; Le Gal, G. Cancer, atrial fibrillation, and stroke. Thromb. Res. 2017, 155, 101–105. [Google Scholar] [CrossRef]
- Lun, R.; Siegal, D.; Ramsay, T.; Dowlatshahi, D. Cancer and stroke: What do we know and where do we go? Thromb. Res. 2022, 219, 133–140. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Stefan, O.; Vera, N.; Otto, B.; Heinz, L.; Wolfgang, G. Stroke in cancer patients: A risk factor analysis. J. Neurooncol. 2009, 94, 221–226. [Google Scholar] [CrossRef]
- Navi, B.B.; Iadecola, C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann. Neurol. 2018, 83, 873–883. [Google Scholar] [CrossRef]
- Zoller, B.; Ji, J.; Sundquist, J.; Sundquist, K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: A nationwide follow-up study from Sweden. Eur. J. Cancer 2012, 48, 1875–1883. [Google Scholar] [CrossRef]
- Patell, R.; Gutierrez, A.; Rybicki, L.; Khorana, A.A. Usefulness of CHADS2 and CHA2DS2-VASc scores for stroke prediction in patients with cancer and atrial fibrillation. Am. J. Cardiol. 2017, 120, 2182–2186. [Google Scholar] [CrossRef]
- Sorigue, M.; Gual-Capllonch, F.; Garcia, O.; Sarrate, E.; Franch-Sarto, M.; Ibarra, G.; Grau, J.; Orna, E.; Ribera, J.-M.; Sancho, J.-M. Incidence, predictive factors, management, and survival impact of atrial fibrillation in non-Hodgkin lymphoma. Ann. Hematol. 2018, 97, 1633–1640. [Google Scholar] [CrossRef]
- Khamis, A.; Shaban, A.E.; Altamimi, T.S.; Shkoukani, Z.W.; Hamam, I. Atrial fibrillation in cancer patients who develop stroke. Cardiooncology 2022, 8, 12. [Google Scholar] [CrossRef]
- Bhaskar, S.; Stanwell, P.; Cordato, D.; Attia, J.; Levi, C. Reperfusion therapy in acute ischemic stroke: Dawn of a new era? BMC Neurol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Ozaki, T.; Nicholson, P.; Schaafsma, J.D.; Agid, R.; Krings, T.; Pikula, A.; Pereira, V.M. Endovascular Therapy of Acute Ischemic Stroke in Patients with Large-Vessel Occlusion Associated with Active Malignancy. J. Stroke Cerebrovasc. Dis. 2021, 30, 105455. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.C.; Grewal, P.; Beer-Furlan, A.; Vargas, A.; Osteraas, N.; Dafer, R.; Chen, M. Endovascular thrombectomy for acute ischemic stroke in patients with cancer: A propensity-matched analysis. J. Neurointerv. Surg. 2022, 14, 1161–1165. [Google Scholar] [CrossRef]
- Verschoof, M.A.; Groot, A.E.; de Bruijn, S.F.T.M.; Roozenbeek, B.; van der Worp, H.B.; Dippel, D.W.J.; Emmer, B.J.; Roosendaal, S.D.; Majoie, C.B.L.M.; Roos, Y.B.W.E.M.; et al. Clinical Outcome After Endovascular Treatment in Patients with Active Cancer and Ischemic Stroke. Neurology 2022, 98, e993–e1001. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, D.H.; Suh, D.C.; Kwon, H.S.; Jeong, D.E.; Kim, J.G.; Lee, J.S.; Kim, J.S.; Kang, D.W.; Jeon, S.B.; et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J. Neurol. 2019, 266, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.H.; Yoon, W.; Kim, J.T.; Choi, K.H.; Kang, K.W.; Lee, J.H.; Cho, K.H.; Park, M.S. Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Neurol. Sci. 2020, 41, 379–385. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Zaorsky, N.G.; Zhang, Y.; Tchelebi, L.T.; Mackley, H.B.; Chinchilli, V.M.; Zacharia, B.E. Stroke among cancer patients. Nat. Commun. 2019, 10, 5172. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, Y.D.; Park, H.; Kim, B.M.; Bang, O.Y.; Kim, H.C.; Han, E.; Kim, D.J.; Heo, J.; Kim, M.; et al. Immediate and Long-Term Outcomes of Reperfusion Therapy in Patients with Cancer. Stroke 2021, 52, 2026–2034. [Google Scholar] [CrossRef]
- Aloizou, A.M.; Richter, D.; Charles James, J.; Lukas, C.; Gold, R.; Krogias, C. Mechanical Thrombectomy for Acute Ischemic Stroke in Patients with Malignancy: A Systematic Review. J. Clin. Med. 2022, 11, 4696. [Google Scholar] [CrossRef]
- Eun, M.Y.; Jeon, E.T.; Seo, K.D.; Lee, D.; Jung, J.M. Reperfusion Therapy in Acute Ischemic Stroke with Active Cancer: A Meta-Analysis Aided by Machine Learning. J. Stroke Cerebrovasc. Dis. 2021, 30, 105742. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Vazquez, S.; Das, A.; Dominguez, J.F.; Kamal, H.; Chong, J.; Mayer, S.A.; Kaur, G.; Gandhi, C.; Al-Mufti, F. Investigating Outcomes Post Endovascular Thrombectomy in Acute Stroke Patients with Cancer. Neurology 2022, 99, e2583–e2592. [Google Scholar] [CrossRef]
- Santana Baskar, P.; Cordato, D.; Wardman, D.; Bhaskar, S. In-hospital acute stroke workflow in acute stroke—Systems-based approaches. Acta Neurol. Scand. 2021, 143, 111–120. [Google Scholar] [CrossRef]
- Chowdhury, S.Z.; Baskar, P.S.; Bhaskar, S. Effect of prehospital workflow optimization on treatment delays and clinical outcomes in acute ischemic stroke: A systematic review and meta-analysis. Acad. Emerg. Med. 2021, 28, 781–801. [Google Scholar] [CrossRef]
- Khan, U.T.; Walker, A.J.; Baig, S.; Card, T.R.; Kirwan, C.C.; Grainge, M.J. Venous thromboembolism and mortality in breast cancer: Cohort study with systematic review and meta-analysis. BMC Cancer 2017, 17, 747. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Bhaskar, S.M.M. Venous Thromboembolism in Cancer Patients Undergoing Chemotherapy: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 2954. [Google Scholar] [CrossRef]
- Navi, B.B.; Sherman, C.P.; Genova, R.; Mathias, R.; Lansdale, K.N.; LeMoss, N.M.; Wolfe, J.; Skakodub, A.; Kamel, H.; Tagawa, S.T.; et al. Mechanisms of Ischemic Stroke in Patients with Cancer: A Prospective Study. Ann. Neurol. 2021, 90, 159–169. [Google Scholar] [CrossRef]
- Navi, B.B.; Zhang, C.; Sherman, C.P.; Genova, R.; LeMoss, N.M.; Kamel, H.; Tagawa, S.T.; Saxena, A.; Ocean, A.J.; Kasner, S.E.; et al. Ischemic stroke with cancer: Hematologic and embolic biomarkers and clinical outcomes. J. Thromb. Haemost. 2022, 20, 2046–2057. [Google Scholar] [CrossRef]
- Fuellen, G.; Walter, U.; Henze, L.; Böhmert, J.; Palmer, D.; Lee, S.; Schmitt, C.A.; Rudolf, H.; Kowald, A. Protein Biomarkers in Blood Reflect the Interrelationships Between Stroke Outcome, Inflammation, Coagulation, Adhesion, Senescence and Cancer. Cell. Mol. Neurobiol. 2023, 43, 1413–1424. [Google Scholar] [CrossRef]
- Carrier, M.; Abou-Nassar, K.; Mallick, R.; Tagalakis, V.; Shivakumar, S.; Schattner, A.; Kuruvilla, P.; Hill, D.; Spadafora, S.; Marquis, K.; et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2019, 380, 711–719. [Google Scholar] [CrossRef]
- Khorana, A.A.; Soff, G.A.; Kakkar, A.K.; Vadhan-Raj, S.; Riess, H.; Wun, T.; Streiff, M.B.; Garcia, D.A.; Liebman, H.A.; Belani, C.P.; et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N. Engl. J. Med. 2019, 380, 720–728. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.; Escalante, C.P.; Goldhaber, S.Z.; McBane, R.; Connors, J.M.; Raskob, G.E. Treatment of Cancer-Associated Venous Thromboembolism with Low-Molecular-Weight Heparin or Direct Oral Anticoagulants: Patient Selection, Controversies, and Caveats. Oncologist 2021, 26, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O’Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Ibeh, C.; Elkind, M.S.V. Stroke Prevention After Cryptogenic Stroke. Curr. Cardiol. Rep. 2021, 23, 174. [Google Scholar] [CrossRef]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef]
- Simanek, R.; Vormittag, R.; Ay, C.; Alguel, G.; Dunkler, D.; Schwarzinger, I.; Steger, G.; Jaeger, U.; Zielinski, C.; Pabinger, I. High platelet count associated with venous thromboembolism in cancer patients: Results from the Vienna Cancer and Thrombosis Study (CATS). J. Thromb. Haemost. 2010, 8, 114–120. [Google Scholar] [CrossRef]
- Del Brutto, V.J.; Chaturvedi, S.; Diener, H.C.; Romano, J.G.; Sacco, R.L. Antithrombotic Therapy to Prevent Recurrent Strokes in Ischemic Cerebrovascular Disease: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 786–803. [Google Scholar] [CrossRef]
- Radmilovic, J.; Di Vilio, A.; D’Andrea, A.; Pastore, F.; Forni, A.; Desiderio, A.; Ragni, M.; Quaranta, G.; Cimmino, G.; Russo, V. The pharmacological approach to oncologic patients with acute coronary syndrome. J. Clin. Med. 2020, 9, 3926. [Google Scholar] [CrossRef]
- Tsigkas, G.; Vakka, A.; Apostolos, A.; Bousoula, E.; Vythoulkas-Biotis, N.; Koufou, E.-E.; Vasilagkos, G.; Tsiafoutis, I.; Hamilos, M.; Aminian, A.; et al. Dual Antiplatelet Therapy and Cancer; Balancing between Ischemic and Bleeding Risk: A Narrative Review. J. Cardiovasc. Dev. Dis. 2023, 10, 135. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V. The coagulopathy of cancer. Curr. Opin. Hematol. 2014, 21, 423–429. [Google Scholar] [CrossRef]
- Ozaki, Y.; Garcia-Garcia, H.M.; Mintz, G.S.; Waksman, R. Supporting evidence from optical coherence tomography for shortening dual antiplatelet therapy after drug-eluting stents implantation. Expert Rev. Cardiovasc. Ther. 2020, 18, 261–267. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Ha, J.; Hwang, I.G.; Song, T.J.; Yoo, J.; Ahn, S.H.; Kim, K.; Kim, B.M.; Kim, D.J.; et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann. Neurol. 2019, 86, 143–149. [Google Scholar] [CrossRef]
- Sun, Y.E.; Na, H.K.; Kwak, S.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Different Thrombus Histology in a Cancer Patient with Deep Vein Thrombosis and Recurrent Strokes. J. Stroke 2022, 24, 300–302. [Google Scholar] [CrossRef]
- Bhaskar, S.; Cordato, D.; Cappelen-Smith, C.; Cheung, A.; Ledingham, D.; Celermajer, D.; Levi, C. Clarion call for histopathological clot analysis in “cryptogenic” ischemic stroke: Implications for diagnosis and treatment. Ann. Clin. Transl. Neurol. 2017, 4, 926–930. [Google Scholar] [CrossRef]
- Bhaskar, S.; Saab, J.; Cappelen-Smith, C.; Killingsworth, M.; Wu, X.J.; Cheung, A.; Manning, N.; Aouad, P.; McDougall, A.; Hodgkinson, S.; et al. Clot Histopathology in Ischemic Stroke with Infective Endocarditis. Can. J. Neurol. Sci. 2019, 46, 331–336. [Google Scholar] [CrossRef]
- Dearborn, J.L.; Urrutia, V.C.; Zeiler, S.R. Stroke and Cancer- A Complicated Relationship. J. Neurol. Transl. Neurosci. 2014, 2, 1039. [Google Scholar]
- Johansson, A.; Drake, I.; Engström, G.; Acosta, S. Modifiable and Non-Modifiable Risk Factors for Atherothrombotic Ischemic Stroke among Subjects in the Malmö Diet and Cancer Study. Nutrients 2021, 13, 1952. [Google Scholar] [CrossRef]
- Abdol Razak, N.B.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-Associated Thrombosis: An Overview of Mechanisms, Risk Factors, and Treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Arboix, A. Cerebrovascular disease in the cancer patient. Rev. Neurol. 2000, 31, 1250–1252. [Google Scholar] [PubMed]
- Arboix, A.; Jiménez, C.; Massons, J.; Parra, O.; Besses, C. Hematological disorders: A commonly unrecognized cause of acute stroke. Expert Rev. Hematol. 2016, 9, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hull, R.D.; Pineo, G.F.; Brant, R.F.; Mah, A.F.; Burke, N.; Dear, R.; Wong, T.; Cook, R.; Solymoss, S.; Poon, M.C.; et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am. J. Med. 2006, 119, 1062–1072. [Google Scholar] [CrossRef]
- Lee, A.Y.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A. Tinzaparin vs Warfarin for Treatment of Acute Venous Thromboembolism in Patients with Active Cancer: A Randomized Clinical Trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef]
- Deitcher, S.R.; Kessler, C.M.; Merli, G.; Rigas, J.R.; Lyons, R.M.; Fareed, J. Secondary prevention of venous thromboembolic events in patients with active cancer: Enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin. Appl. Thromb. Hemost. 2006, 12, 389–396. [Google Scholar] [CrossRef]
- Meyer, G.; Marjanovic, Z.; Valcke, J.; Lorcerie, B.; Gruel, Y.; Solal-Celigny, P.; Le Maignan, C.; Extra, J.M.; Cottu, P.; Farge, D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch. Intern. Med. 2002, 162, 1729–1735. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1181–1201. [Google Scholar]
- Moik, F.; Posch, F.; Zielinski, C.; Pabinger, I.; Ay, C. Direct oral anticoagulants compared to low-molecular-weight heparin for the treatment of cancer-associated thrombosis: Updated systematic review and meta-analysis of randomized controlled trials. Res. Pract. Thromb. Haemost. 2020, 4, 550–561. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Aguiar, V.C.R.; Junior, V.C.; Casella, I.B.; Zerati, A.E.; Fareed, J. Edoxaban for Venous Thromboembolism Treatment-The New Kid on The Block for Latin America. A Practical Guide. Clin. Appl. Thromb. Hemost. 2018, 24, 340S–349S. [Google Scholar] [CrossRef]
- Ceresetto, J.M. Venous thromboembolism in Latin America: A review and guide to diagnosis and treatment for primary care. Clinics 2016, 71, 36–46. [Google Scholar] [CrossRef]
- Athanazio, R.A.; Ceresetto, J.M.; Marfil Rivera, L.J.; Cesarman-Maus, G.; Galvez, K.; Marques, M.A.; Tabares, A.H.; Ortiz Santacruz, C.A.; Santini, F.C.; Corrales, L.; et al. Direct Oral Anticoagulants for the Treatment of Cancer-Associated Venous Thromboembolism: A Latin American Perspective. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221082988. [Google Scholar] [CrossRef]
- Pabinger, I.; Riedl, J. Direct oral anticoagulants: Now also for prevention and treatment of cancer-associated venous thromboembolism? Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 136–143. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Foerster, K.I.; Hermann, S.; Mikus, G.; Haefeli, W.E. Drug-Drug Interactions with Direct Oral Anticoagulants. Clin. Pharmacokinet. 2020, 59, 967–980. [Google Scholar] [CrossRef]
- Say, R.E.; Thomson, R. The importance of patient preferences in treatment decisions—Challenges for doctors. BMJ 2003, 327, 542–545. [Google Scholar] [CrossRef]
- Zalunardo, B.; Panzavolta, C.; Bigolin, P.; Visonà, A. Multidisciplinary Care for the Prevention and Treatment of Venous Thromboembolism in Patients with Cancer-Associated Thrombosis (CAT): Impact of Educational Interventions on CAT-Related Events and on Patients’ and Clinicians’ Awareness. Life 2022, 12, 1594. [Google Scholar] [CrossRef]
- Balanescu, D.V.; Aziz, M.K.; Donisan, T.; Palaskas, N.; Lopez-Mattei, J.; Hassan, S.; Kim, P.; Song, J.; Ntim, W.; Cilingiroglu, M. Cancer treatment resumption in patients with new-generation drug-eluting stents. Coron. Artery Dis. 2021, 32, 295–301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.-Y.; Bhaskar, S.M.M. Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches. Int. J. Mol. Sci. 2023, 24, 7981. https://doi.org/10.3390/ijms24097981
Sun M-Y, Bhaskar SMM. Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches. International Journal of Molecular Sciences. 2023; 24(9):7981. https://doi.org/10.3390/ijms24097981
Chicago/Turabian StyleSun, Ming-Yee, and Sonu M. M. Bhaskar. 2023. "Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches" International Journal of Molecular Sciences 24, no. 9: 7981. https://doi.org/10.3390/ijms24097981
APA StyleSun, M.-Y., & Bhaskar, S. M. M. (2023). Bridging the Gap in Cancer-Related Stroke Management: Update on Therapeutic and Preventive Approaches. International Journal of Molecular Sciences, 24(9), 7981. https://doi.org/10.3390/ijms24097981








