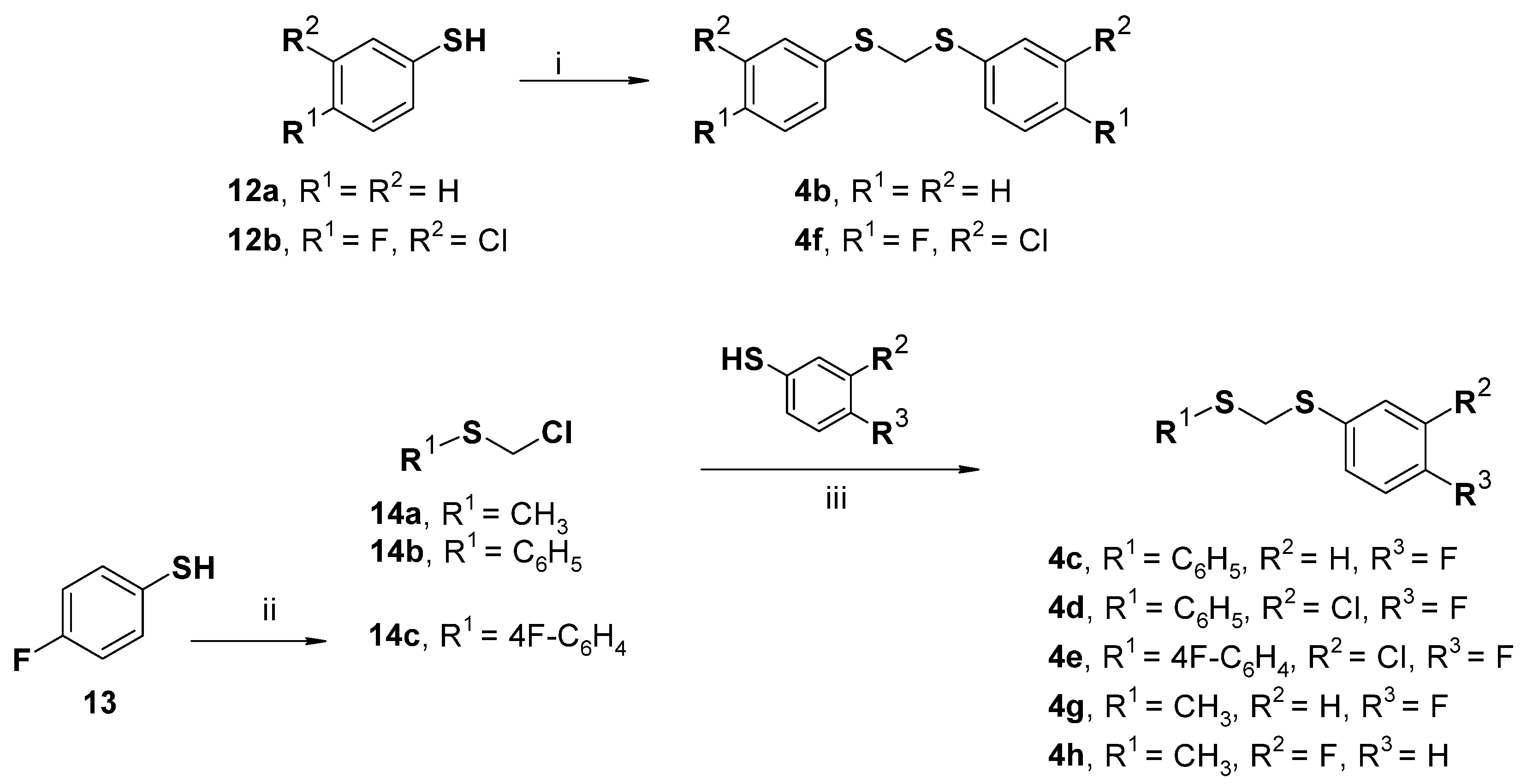Leveraging the 3-Chloro-4-fluorophenyl Motif to Identify Inhibitors of Tyrosinase from Agaricus bisporus
Abstract
1. Introduction
2. Results
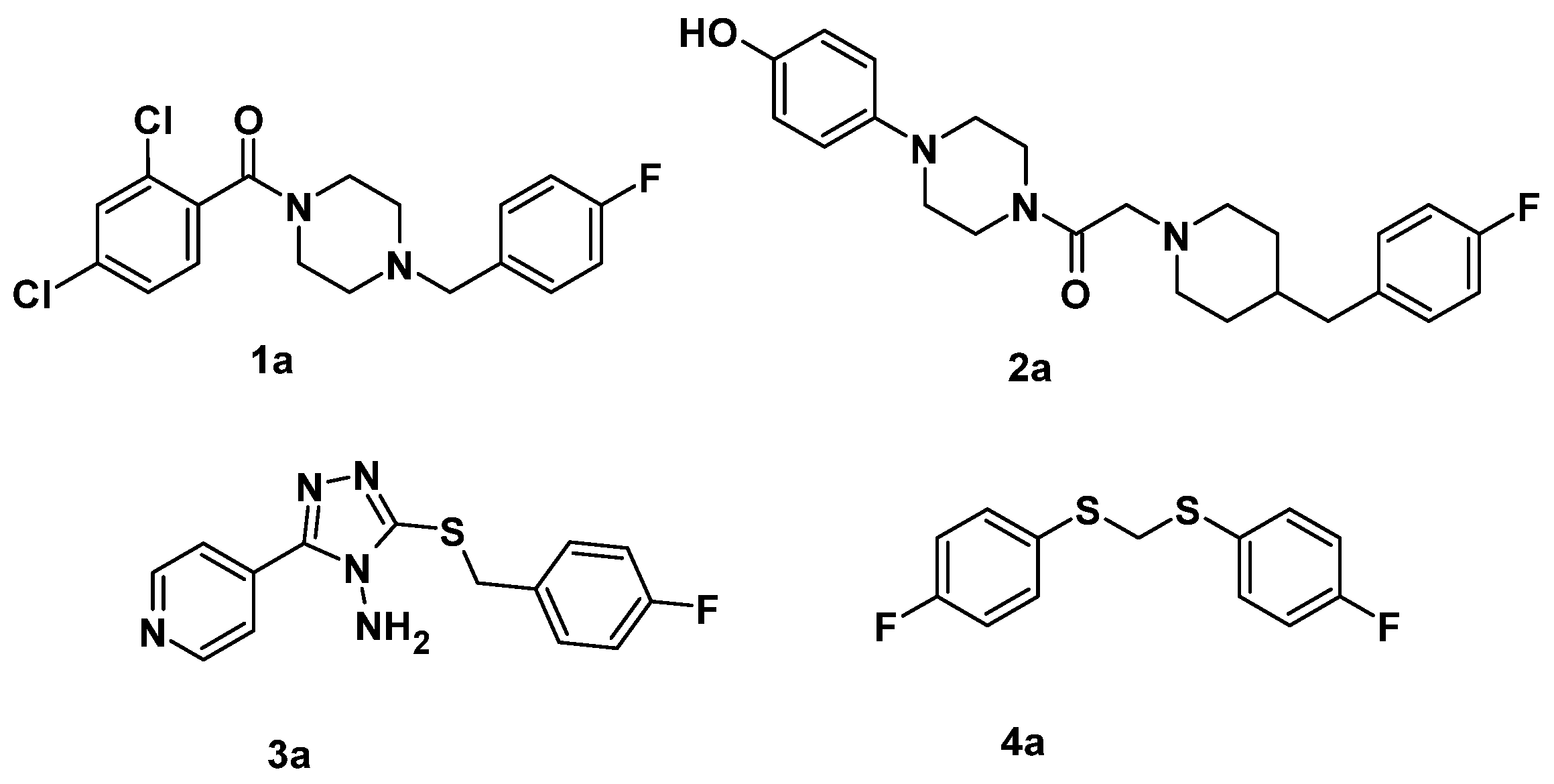
2.1. Lead Optimization Strategy
2.2. In Vitro Assay Determination of AbTYR Inhibition

| Entry | IC50 (μM) a ± SD b |
|---|---|
| 1a c | 0.79 ± 0.11 |
| 1b d | 0.24 ± 0.03 |
| 1c e | 3.60 ± 0.33 |
| 1d | 0.42 ± 0.03 |
| 1e | 0.19 ± 0.07 |
| 1f | 1.72 ± 0.11 |
| 2a f | 4.49 ± 0.13 |
| 2b | 4.43 ± 0.54 |
| 2c | 1.73 ± 0.28 |
| 2d | 1.38 ± 0.15 |
| 3a g | 83.61 ± 15.65 |
| 3b | 24.15 ± 1.02 |
| 3c | >350 |
| 3d | 67.32 ± 6.67 |
| 4a h | 48.42 ± 0.76 |
| 4b | >350 |
| 4c | >350 |
| 4d | 6.26 ± 0.37 |
| 4e | 10.65 ± 1.51 |
| 4f | 2.96 ± 0.34 |
| 4g | 26.02 ± 2.63 |
| 4h | 183.46 ± 6.12 |
| Kojic Acid (KA) c | 17.76 ± 0.18 |
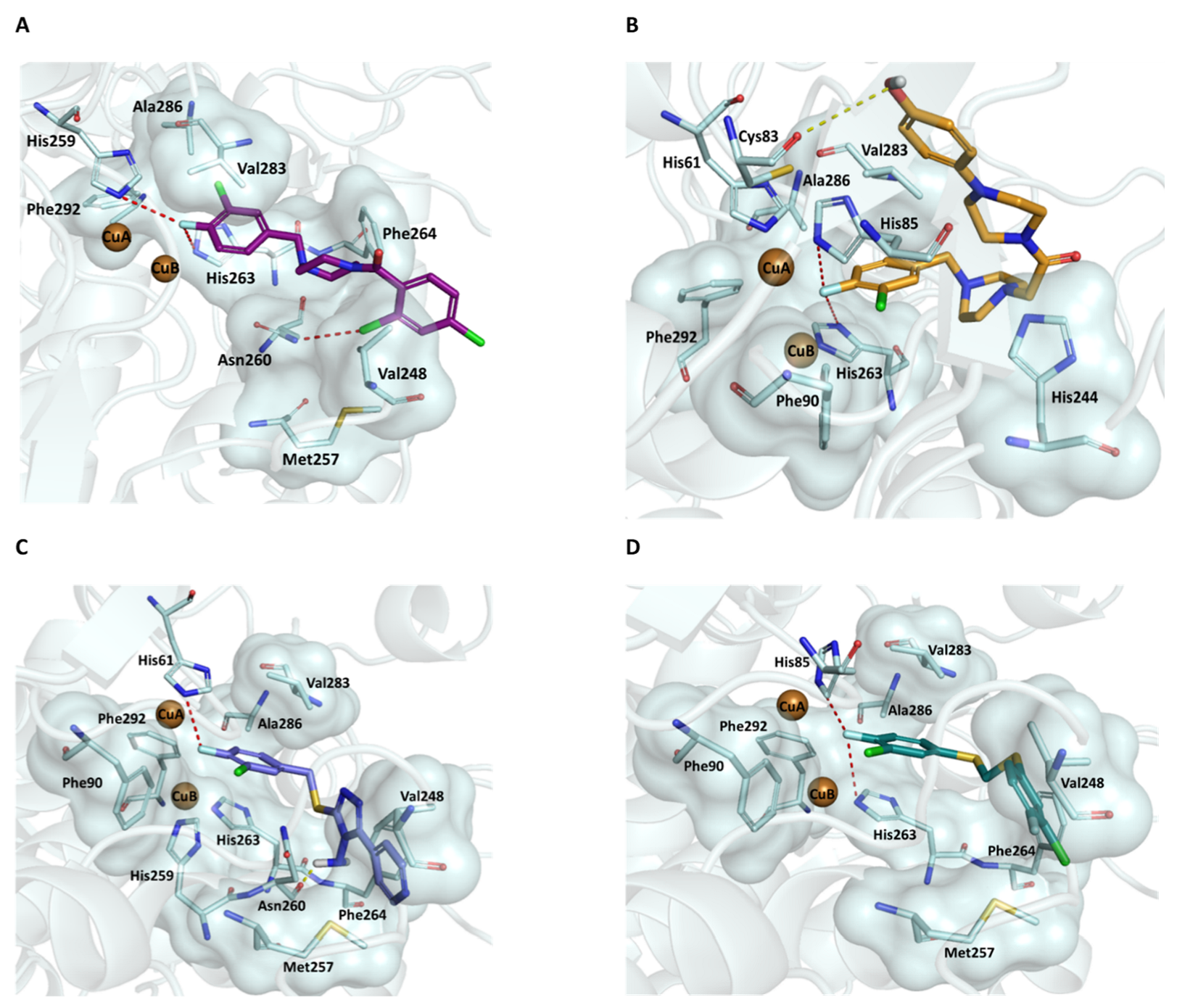
2.3. Docking Studies on Mushroom Tyrosinase
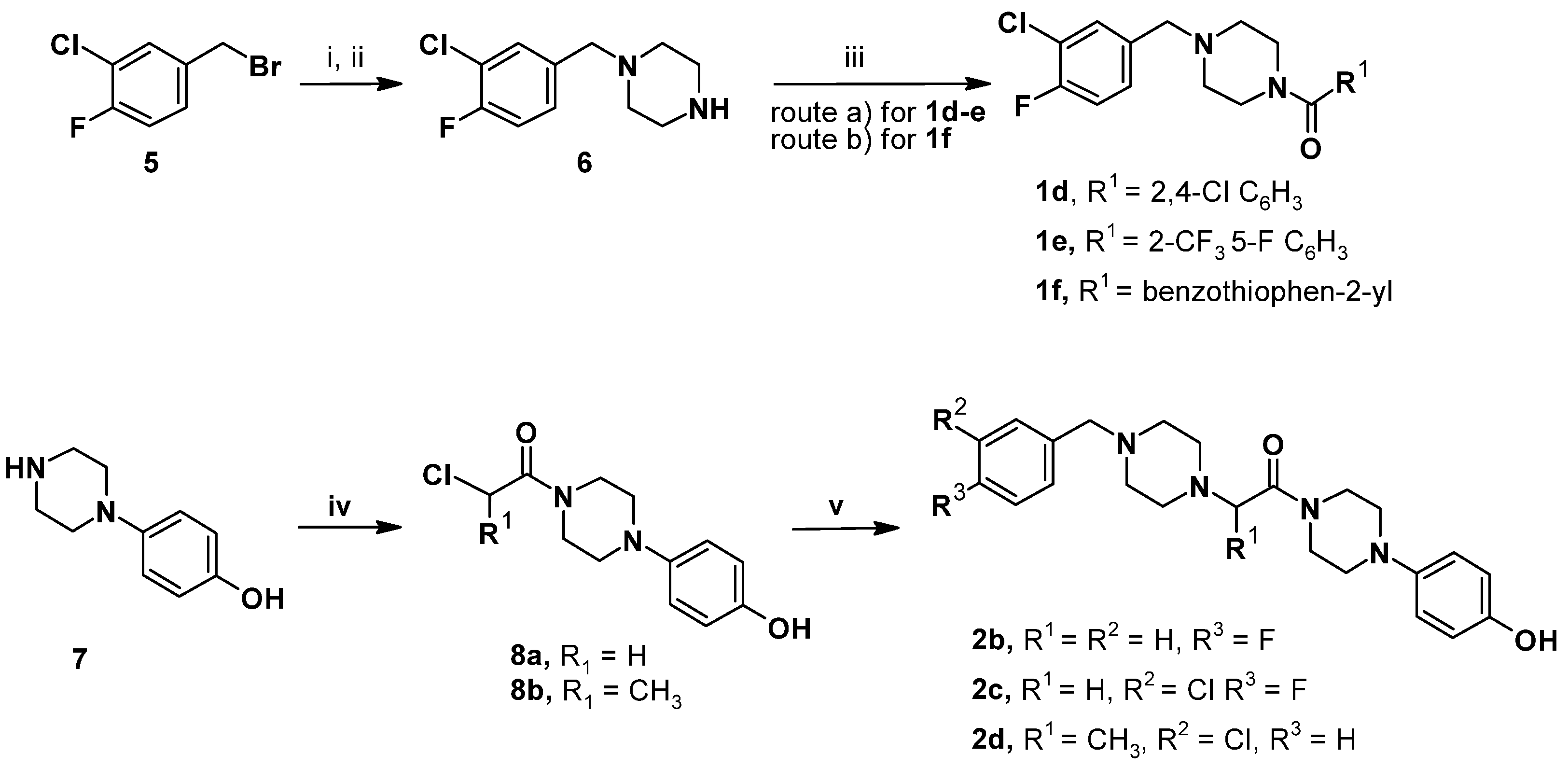
2.4. Chemistry
3. Materials and Methods
3.1. Biochemical Assays
3.2. Molecular Modelling
3.3. Chemistry
3.3.1. General Procedures for the Synthesis of Key Intermediate 1-[(3-Chloro-4-fluorophenyl)methyl]piperazine (6)
3.3.2. General Procedures for the Synthesis of Amides 1d–f
3.3.3. General Procedures for the Synthesis of Amides 2b–d
3.3.4. General Procedure for the Synthesis of the 4H-1,2,4-Triazol-4-amines (3b–d)
3.3.5. General Procedures for the Synthesis of Dithioacetals 4b and 4f
3.3.6. Synthesis of 1-[(Chloromethyl)sulfanyl]-4-fluorobenzene (14c)
3.3.7. General Procedure for the Preparation of the Dithioacetals 4c–e and 4g–h
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghani, U. Azole inhibitors of mushroom and human tyrosinases: Current advances and prospects of drug development for melanogenic dermatological disorders. Eur. J. Med. Chem. 2022, 239, 114525. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M. Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: Review and new insight. J. Biomol. Struct. Dyn. 2022; 1–13, ahead of print. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Deniz, F.S.S. Inhibition of Melanogenesis by Some Well-Known Polyphenolics: A Review. Curr. Pharm. Biotechnol. 2021, 22, 1412–1423. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Stelmach, J.; Zajdel, K.; Kucharska, E.; Zajdel, R. Plants as Modulators of Melanogenesis: Role of Extracts, Pure Compounds and Patented Compositions in Therapy of Pigmentation Disorders. Int. J. Mol. Sci. 2022, 23, 14787. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Peng, X.; Li, S.; Liu, B.; Chu, C. Recent discovery of tyrosinase inhibitors in traditional Chinese medicines and screening methods. J. Ethnopharmacol. 2023, 303, 115951. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Gebalski, J.; Graczyk, F.; Zaluski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure-activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef]
- Roulier, B.; Peres, B.; Haudecoeur, R. Advances in the Design of Genuine Human Tyrosinase Inhibitors for Targeting Melanogenesis and Related Pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Nuramrum, N.; Rungrotmongkol, T.; Kongtaworn, N.; Sabuakham, S. Structural dynamics and susceptibility of isobutylamido thiazolyl resorcinol (Thiamidol(TM)) against human and mushroom tyrosinases. J. Biomol. Struct. Dyn. 2023; 1–8, ahead of print. [Google Scholar] [CrossRef]
- De Luca, L.; Mirabile, S.; Ricci, F.; Adornato, I.; Cacciola, A.; Gitto, M.P.G.R. Synthesis and biochemical evaluation of 5-(pyridin-4-yl)-3-(alkylsulfanyl)-4H-1,2,4-triazol-4-amine-based inhibitors of tyrosinase from Agaricus bisporus. ARKIVOC 2022, 2022, 156–166. [Google Scholar] [CrossRef]
- Mirabile, S.; Vittorio, S.; Paola Germano, M.; Adornato, I.; Ielo, L.; Rapisarda, A.; Gitto, R.; Pintus, F.; Fais, A.; De Luca, L. Evaluation of 4-(4-Fluorobenzyl)piperazin-1-yl]-Based Compounds as Competitive Tyrosinase Inhibitors Endowed with Antimelanogenic Effects. ChemMedChem 2021, 16, 3083–3093. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, S.; Seidel, T.; Germano, M.P.; Gitto, R.; Ielo, L.; Garon, A.; Rapisarda, A.; Pace, V.; Langer, T.; De Luca, L. A Combination of Pharmacophore and Docking-based Virtual Screening to Discover new Tyrosinase Inhibitors. Mol. Inform. 2020, 39, e1900054. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, S.; Ielo, L.; Mirabile, S.; Gitto, R.; Fais, A.; Floris, S.; Rapisarda, A.; Germano, M.P.; De Luca, L. 4-Fluorobenzylpiperazine-Containing Derivatives as Efficient Inhibitors of Mushroom Tyrosinase. ChemMedChem 2020, 15, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Ielo, L.; Deri, B.; Germano, M.P.; Vittorio, S.; Mirabile, S.; Gitto, R.; Rapisarda, A.; Ronsisvalle, S.; Floris, S.; Pazy, Y.; et al. Exploiting the 1-(4-fluorobenzyl)piperazine fragment for the development of novel tyrosinase inhibitors as anti-melanogenic agents: Design, synthesis, structural insights and biological profile. Eur. J. Med. Chem. 2019, 178, 380–389. [Google Scholar] [CrossRef]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Germano, M.P.; De Luca, L.; Ricci, F.; Corallo, D.; Aveic, S.; Mariotto, E.; et al. Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem. 2022, 231, 114147. [Google Scholar] [CrossRef]
- De Luca, L.; Germano, M.P.; Fais, A.; Pintus, F.; Buemi, M.R.; Vittorio, S.; Mirabile, S.; Rapisarda, A.; Gitto, R. Discovery of a new potent inhibitor of mushroom tyrosinase (Agaricus bisporus) containing 4-(4-hydroxyphenyl)piperazin-1-yl moiety. Bioorg. Med. Chem. 2020, 28, 115497. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Sankar, U.; Mahalakshmi, S.; Balasubramanian, K.K. A One-Pot Stereoselective Synthesis of Electron-Deficient 4-Substituted (E,E)-1-Arylsulfonylbuta-1,3-dienes and Their Chemoselective [3+2] Cycloaddition with Azomethine Ylides—A Simple Synthesis of 1,3,4-Trisubstituted Pyrrolidines and Pyrroles. Synlett 2013, 24, 1533–1540. [Google Scholar] [CrossRef]
- Carbonnel, E.; Pannecoucke, X.; Besset, T.; Jubault, P.; Poisson, T. An electrophilic reagent for the synthesis of OCHFMe-containing molecules. Chem. Commun. 2018, 54, 2491–2493. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, J.I.; Clark, B.A.; Ford, D.; Macdonald, R.J.; Macpherson, A.D.; McNab, H.; Nicolson, I.S.; Reed, D.; Sommerville, C.C. Reactions of 2-(pyrrol-1-yl)benzyl radicals and related species under flash vacuum pyrolysis conditions. Org. Biomol. Chem. 2009, 7, 5173–5183. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes. BIOVIA Workbook, Release 2020; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Wolber, G.; Langer, T. LigandScout: 3-d pharmacophores derived from protein-bound Ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirabile, S.; Ielo, L.; Lombardo, L.; Ricci, F.; Gitto, R.; Germanò, M.P.; Pace, V.; De Luca, L. Leveraging the 3-Chloro-4-fluorophenyl Motif to Identify Inhibitors of Tyrosinase from Agaricus bisporus. Int. J. Mol. Sci. 2023, 24, 7944. https://doi.org/10.3390/ijms24097944
Mirabile S, Ielo L, Lombardo L, Ricci F, Gitto R, Germanò MP, Pace V, De Luca L. Leveraging the 3-Chloro-4-fluorophenyl Motif to Identify Inhibitors of Tyrosinase from Agaricus bisporus. International Journal of Molecular Sciences. 2023; 24(9):7944. https://doi.org/10.3390/ijms24097944
Chicago/Turabian StyleMirabile, Salvatore, Laura Ielo, Lisa Lombardo, Federico Ricci, Rosaria Gitto, Maria Paola Germanò, Vittorio Pace, and Laura De Luca. 2023. "Leveraging the 3-Chloro-4-fluorophenyl Motif to Identify Inhibitors of Tyrosinase from Agaricus bisporus" International Journal of Molecular Sciences 24, no. 9: 7944. https://doi.org/10.3390/ijms24097944
APA StyleMirabile, S., Ielo, L., Lombardo, L., Ricci, F., Gitto, R., Germanò, M. P., Pace, V., & De Luca, L. (2023). Leveraging the 3-Chloro-4-fluorophenyl Motif to Identify Inhibitors of Tyrosinase from Agaricus bisporus. International Journal of Molecular Sciences, 24(9), 7944. https://doi.org/10.3390/ijms24097944