Five-Year Changes in Inflammatory, Metabolic, and Oxidative Biomarkers and 10-Year Cardiovascular Disease Incidence: The REGICOR Cohort Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Study Sample Size
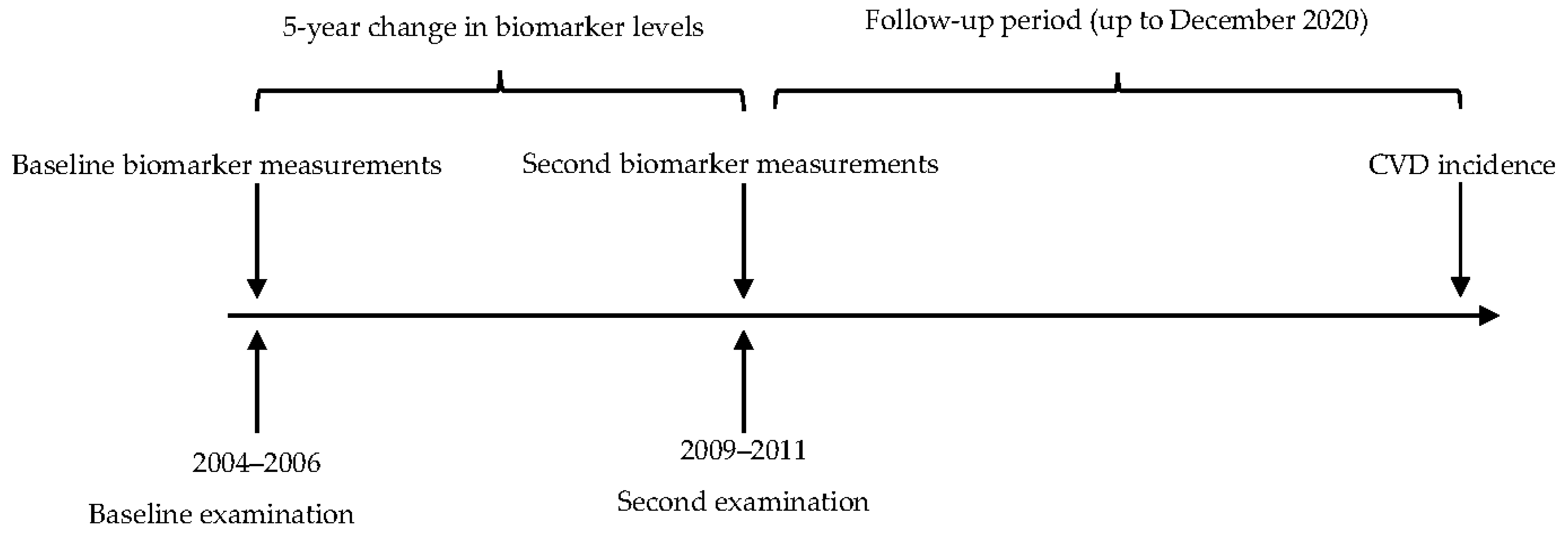
4.3. Re-Examination, Follow-Up, and Composite Endpoint
4.4. Variables and Laboratory Results
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 14 March 2023).
- European Cardiovascular Disease Statistics 2017 Edition. Available online: https://www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 13 March 2023).
- Dégano, I.R.; Salomaa, V.; Veronesi, G.; Ferriéres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.B.; Ferrario, M.M.; Meisinger, C.; et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar] [CrossRef]
- Visseren, F.L.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J.; Subirana, I.; Comín, E.; Cabezas, C.; Vila, J.; Elosua, R.; Nam, B.H.; Ramos, R.; Sala, J.; Solanas, P.; et al. Validity of an adaptation of the Framingham cardiovascular risk function: The VERIFICA study. J. Epidemiol. Community Health 2007, 61, 40–47. [Google Scholar] [CrossRef]
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.L.; Caligiuri, G.; Daemen, M.J.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications. Eur. Heart J. 2014, 35, 3013–3020. [Google Scholar] [CrossRef]
- Wiklund, O.; Borén, J. Pathogenesis of atherosclerosis: Lipid metabolism. In The ESC Textbook of Vascular Biology; Krams, R., Bäck, M., Eds.; The European Society of Cardiology: Oxford, UK, 2017; pp. 1–27. [Google Scholar]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The Road Ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef]
- Danesh, J.; Wheeler, J.G.; Hirschfield, G.M.; Eda, S.; Eiriksdottir, G.; Rumley, A.; Lowe, G.D.; Pepys, M.B.; Gudnason, V. C-Reactive Protein and Other Circulating Markers of Inflammation in the Prediction of Coronary Heart Disease. N. Engl. J. Med. 2009, 350, 1387–1397. [Google Scholar] [CrossRef]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-ageing: The role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, R.; Crea, F. Atherosclerotic Plaque Healing. N. Engl. J. Med. 2020, 383, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Subirana, I.; Fitó, M.; Diaz, O.; Vila, J.; Francés, A.; Delpon, E.; Sanchis, J.; Elosua, R.; Muñoz-Aguayo, D.; Dégano, I.R.; et al. Prediction of coronary disease incidence by biomarkers of inflammation, oxidation, and metabolism. Sci. Rep. 2018, 8, 3191. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Sattar, N.; Gudnason, V.; Danesh, J. Circulating concentrations of insulin markers and coronary heart disease: A quantitative review of 19 Western prospective studies. Eur. Heart J. 2007, 28, 2491–2497. [Google Scholar] [CrossRef]
- Georgakis, M.K.; De Lemos, J.A.; Ayers, C.; Wang, B.; Björkbacka, H.; Pana, T.A.; Thorand, B.; Sun, C.; Fani, L.; Malik, R.; et al. Association of Circulating Monocyte Chemoattractant Protein-1 Levels With Cardiovascular Mortality: A Meta-analysis of Population-Based Studies. JAMA Cardiol. 2021, 6, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, E.; Pirinen, M.; Sarin, A.P.; Havulinna, A.S.; Männistö, S.; Saltevo, J.; Lokki, M.L.; Sinisalo, J.; Lundqvist, A.; Jula, A.; et al. Genetic support for the causal role of insulin in coronary heart disease. Diabetologia 2016, 59, 2369–2377. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Röhling, M.; Kempf, K.; Kolb, H.; Martin, T.; Schneider, M.; Martin, S. The Epidemiological Boehringer Ingelheim Employee Study (Part 3): Association of Elevated Fasting Insulin Levels but Not HOMA-IR With Increased Intima Media Thickness and Arteriosclerosis in Middle-Aged Persons. Front. Cardiovasc. Med. 2021, 8, 752789. [Google Scholar] [CrossRef]
- Britton, K.A.; Mukamal, K.J.; Ix, J.H.; Siscovick, D.S.; Newman, A.B.; de Boer, I.H.; Thacker, E.L.; Biggs, M.L.; Gaziano, J.M.; Djoussé, L. Insulin resistance and incident peripheral artery disease in the Cardiovascular Health Study. Vasc. Med. 2012, 17, 85–93. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Chen, H.; Kalyani, R.R.; Sims, M.; Simpson, S.; Effoe, V.S.; Correa, A.; Bertoni, A.G.; Golden, S.H. Glycemic Markers and Subclinical Cardiovascular Disease: The Jackson Heart Study. Circ. Cardiovasc. Imaging 2019, 12, 8641. [Google Scholar] [CrossRef] [PubMed]
- Jandeleit-Dahm, K.A.M.; Gray, S.P. Insulin and cardiovascular disease: Biomarker or association? Diabetologia 2012, 55, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Rocha, V.Z. All roads lead to IL-6: A central hub of cardiometabolic signaling. Int. J. Cardiol. 2018, 259, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, D.I.; Holmes, M.V.; Kuchenbaecker, K.B.; Shah, T.; Stewart, M.; Lowe, G.; Nalls, M.A.; Chung, C.; Peasey, A.; Dunlop, M.; et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: A mendelian randomisation analysis. Lancet 2012, 379, 1214–1224. [Google Scholar]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma Concentration of Interleukin-6 and the Risk of Future Myocardial Infarction Among Apparently Healthy Men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Tracy, R.P.; Rubin, S.M.; Harris, T.B.; Pahor, M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study). Am. J. Cardiol. 2003, 92, 522–528. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Palaiopanos, K.; Björkbacka, H.; Peters, A.; de Lemos, J.A.; Seshadri, S.; Dichgans, M.; Marios, K. Circulating Interleukin-6 Levels and Incident Ischemic Stroke. Neurology 2022, 98, e1002–e1012. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, C.M.; Lutsey, P.L.; Ballantyne, C.M.; Folsom, A.R.; Pankow, J.S.; Selvin, E. Six-year change in high-sensitivity C-reactive protein and risk of diabetes, cardiovascular disease, and mortality. Am. Heart J. 2015, 170, 380–389. [Google Scholar] [CrossRef]
- Hassan, L.; Medenwald, D.; Tiller, D.; Kluttig, A.; Ludwig-Kraus, B.; Kraus, F.B.; Greiser, K.H.; Mikolajczyk, R. The association between change of soluble tumor necrosis factor receptor R1 (sTNF-R1) measurements and cardiovascular and all-cause mortality-Results from the population-based (Cardiovascular Disease, Living and Ageing in Halle) CARLA study 2002–2016. PLoS ONE 2020, 15, e0241213. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Evans, C.V.; Johnson, E.; Redmond, N.; Coppola, E.L.; Smith, N. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 281–297. [Google Scholar] [CrossRef]
- Herder, C.; Baumert, J.; Zierer, A.; Roden, M.; Meisinger, C.; Karakas, M.; Chambless, L.; Rathmann, W.; Peters, A.; Koenig, W.; et al. Immunological and Cardiometabolic Risk Factors in the Prediction of Type 2 Diabetes and Coronary Events: MONICA/KORA Augsburg Case-Cohort Study. PLoS ONE 2011, 6, e19852. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, P.; Wensley, F.; Di Angelantonio, E.; Johansson, L.; Boman, K.; Rumley, A.; Lowe, G.; Hallmans, G.; Danesh, J.; Jansson, J.H. Haemostatic and inflammatory markers are independently associated with myocardial infarction in men and women. Thromb. Res. 2012, 129, 68–73. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.; Engelmann, M.D.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef]
- Grau, M.; Subirana, I.; Elosua, R.; Solanas, P.; Ramos, R.; Masia, R.; Cordon, F.; Sala, J.; Juvinya, D.; Cerezo, C.; et al. Trends in cardiovascular risk factor prevalence (1995–2000–2005) in northeastern Spain. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 653–659. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 19 March 2023).
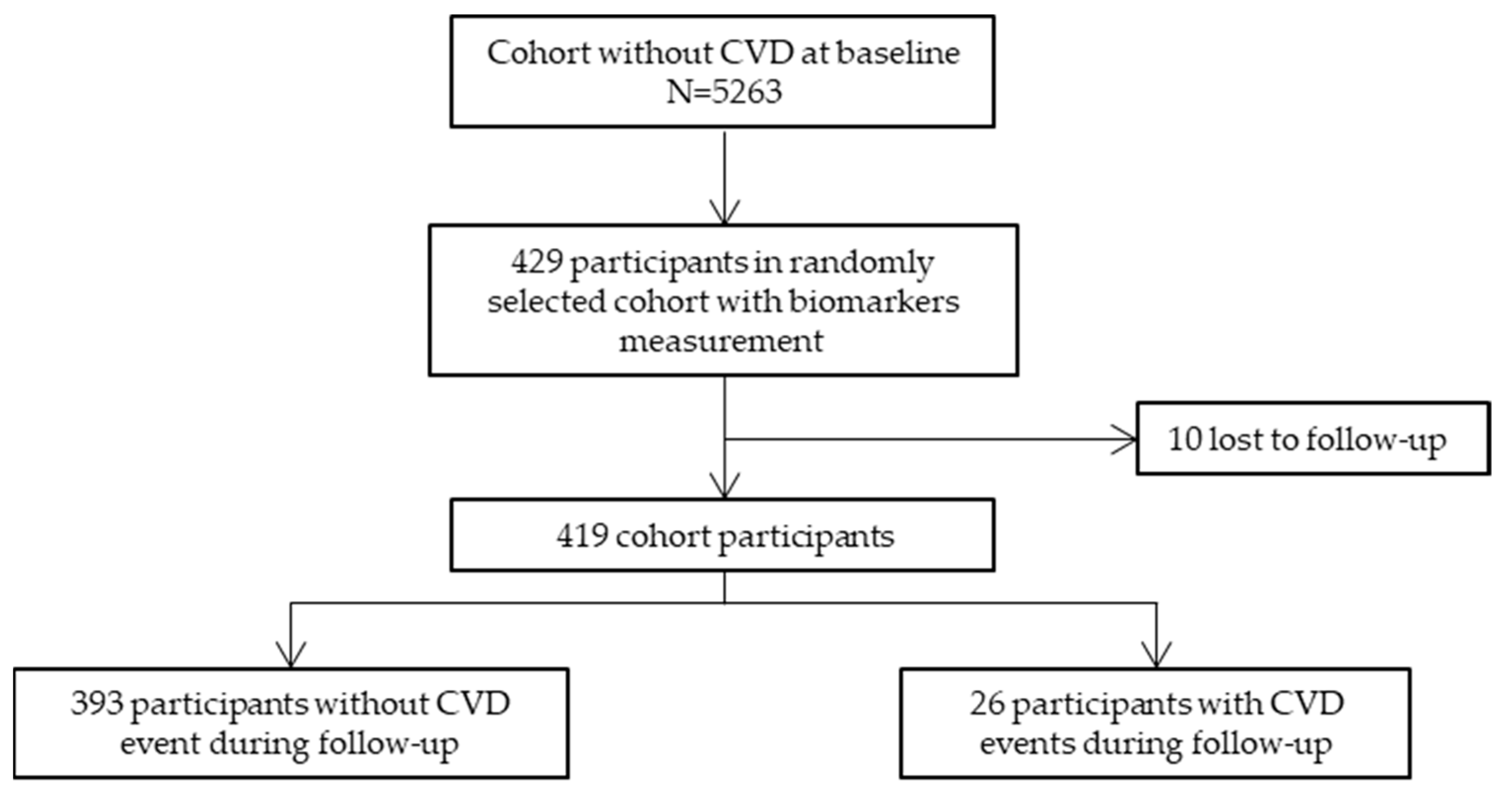
| Participants without CVD Events in the Follow-Up N = 393 | Participants with CVD Events in the Follow-Up (Cases) N = 26 | p Value | |
|---|---|---|---|
| Age, years 1 | 52 (10) | 62 (8) | <0.001 |
| Sex, women | 56.5% | 38.5% | 0.112 |
| Smoker | 18.7% | 26.9% | 0.441 |
| Diabetes | 10.2% | 34.6% | 0.001 |
| Glycaemia, mg/dL 1 | 95 (19) | 110 (38) | 0.060 |
| Glycaemia, treated | 3.97% | 12.0% | 0.093 |
| Hypertension | 38.0% | 61.5% | 0.030 |
| Hypertension, treated | 10.7% | 30.8% | 0.007 |
| Systolic blood pressure, mmHg 1 | 122 (18) | 135 (18) | 0.001 |
| Diastolic blood pressure, mmHg 1 | 78 (10) | 84 (8) | 0.004 |
| Hypercholesterolemia, treated | 14.8% | 44.4% | 0.004 |
| Total cholesterol, mg/dL 1 | 212 (44) | 213 (55) | 0.936 |
| LDL-cholesterol, mg/dL 1 | 138 (39) | 136 (38) | 0.784 |
| HDL-cholesterol, mg/dL 1 | 54 (13) | 49 (12) | 0.036 |
| Triglycerides, mg/dL 2 | 89 [66;124] | 104 [81;120] | 0.066 |
| Body mass index, kg/m2 1 | 26.9 (4.48) | 29.1 (4.25) | 0.016 |
| Participants without CVD Events in the Follow-Up N = 393 | Participants with CVD Events in the Follow-Up (Cases) N = 26 | p Value | |
|---|---|---|---|
| MCP-1 (pg/mL) 1 | |||
| 5-year change | −1.00 [−46.8;46.5] | −4.87 [−55.4;60.3] | 0.948 |
| Last value | 315 [243;396] | 352 [301;399] | 0.306 |
| TNF-α (pg/mL) 1 | |||
| 5-year change | −0.07 [−0.45;0.30] | 0.07 [−0.66;0.54] | 0.705 |
| Last value | 0.62 [0.17;1.34] | 1.29 [0.66;2.11] | 0.003 |
| IL-10 (pg/mL) 1 | |||
| 5-year change | −0.05 [−0.32;0.19] | 0.04 [−0.08;0.41] | 0.007 |
| Last value | 0.39 [0.19;0.65] | 0.40 [0.14;1.07] | 0.805 |
| IL-6 (pg/mL) 1 | |||
| 5-year change | 0.00 [−0.42;0.47] | 0.16 [−0.31;1.73] | 0.100 |
| Last value | 1.49 [0.96;2.31] | 2.21 [1.49;3.13] | 0.003 |
| CRP (mg/dL) 1 | |||
| 5-year change | 0.00 [−0.08;0.06] | 0.00 [−0.06;0.12] | 0.314 |
| Last value | 0.10 [0.04;0.24] | 0.15 [0.06;0.31] | 0.214 |
| Adiponectin (µg/mL) 1 | |||
| 5-year change | 0.29 [−0.74;1.29] | 0.58 [−0.32;1.28] | 0.606 |
| Last value | 5.57 [3.55;8.21] | 4.58 [2.91;5.90] | 0.103 |
| Leptin (ng/mL) 1 | |||
| 5-year change | 1.33 [−0.63;4.97] | 1.85 [0.04;5.03] | 0.580 |
| Last value | 8.32 [4.28;13.5] | 6.72 [4.96;16.3] | 0.759 |
| Insulin (pg/mL) 1 | |||
| 5-year change | 28.7 [−55.7;131] | 77.6 [18.5;221] | 0.029 |
| Last value | 235 [135;421] | 295 [236;467] | 0.021 |
| GHS-Px (U/L) 1 | |||
| 5-year change | −23.0 [−62.0;21.8] | −18.0 [−78.5;10.2] | 0.858 |
| Last value | 689 [623;761] | 648 [607;732] | 0.104 |
| Non-Adjusted | Adjusted for Age, Sex, Baseline Levels & Risk Factors 1 | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| MCP-1 2 | ||||
| 5-year change | 1.00 (0.96–1.04) | 0.916 | 1.01 (0.96–1.05) | 0.795 |
| Last value | 1.00 (0.98–1.03) | 0.785 | ||
| TNF-α | ||||
| 5-year change | 1.11 (0.68–1.83) | 0.666 | 1.17 (0.75–1.83) | 0.484 |
| Last value | 1.36 (1.06–1.74) | 0.016 | ||
| Interleukin-10 | ||||
| 5-year change | 1.08 (0.96–1.21) | 0.190 | 1.10 (0.96–1.26) | 0.159 |
| Last value | 1.03 (0.90–1.19) | 0.647 | ||
| Interleukin-6 | ||||
| 5-year change | 1.25 (1.05–1.49) | 0.011 | 1.32 (1.10–1.57) | 0.002 |
| Last value | 1.33 (1.12–1.57) | 0.001 | ||
| CRP | ||||
| 5-year change | 2.53 (0.72–8.84) | 0.146 | 2.73 (0.63–11.75) | 0.177 |
| Last value | 1.71 (0.46–6.29) | 0.421 | ||
| Adiponectin 2 | ||||
| 5-year change | 1.00 (1.00–1.00) | 0.975 | 1.00 (1.00–1.00) | 0.800 |
| Last value | 1.00 (1.00–1.00) | 0.124 | ||
| Leptin 2 | ||||
| 5-year change | 1.00 (1.00–1.00) | 0.157 | 1.00 (1.00–1.00) | 0.118 |
| Last value | 1.00 (1.00–1.00) | 0.427 | ||
| Insulin 2 | ||||
| 5-year change | 1.02 (1.01–1.03) | 0.002 | 1.02 (1.01–1.03) | 0.002 |
| Last value | 1.01 (1.00–1.02) | 0.010 | ||
| GHS-Px 2 | ||||
| 5-year change | 0.99 (0.94–1.05) | 0.857 | 0.99 (0.94–1.06) | 0.842 |
| Last value | 0.97 (0.93–1.01) | 0.130 | ||
| Reclassification | |
| Continuous NRI | 0.51 (0.09–0.92) |
| Categorical NRI 1 | 0.38 (0.14–0.62) |
| Discrimination (C-index) | |
| Without biomarkers | 0.65 (0.57–0.74) |
| With biomarkers | 0.74 (0.65–0.83) |
| Difference p value | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camps-Vilaro, A.; Subirana, I.; Ramos, R.; Cainzos-Achirica, M.; Tizon-Marcos, H.; Fito, M.; Degano, I.R.; Marrugat, J. Five-Year Changes in Inflammatory, Metabolic, and Oxidative Biomarkers and 10-Year Cardiovascular Disease Incidence: The REGICOR Cohort Study. Int. J. Mol. Sci. 2023, 24, 7934. https://doi.org/10.3390/ijms24097934
Camps-Vilaro A, Subirana I, Ramos R, Cainzos-Achirica M, Tizon-Marcos H, Fito M, Degano IR, Marrugat J. Five-Year Changes in Inflammatory, Metabolic, and Oxidative Biomarkers and 10-Year Cardiovascular Disease Incidence: The REGICOR Cohort Study. International Journal of Molecular Sciences. 2023; 24(9):7934. https://doi.org/10.3390/ijms24097934
Chicago/Turabian StyleCamps-Vilaro, Anna, Isaac Subirana, Rafel Ramos, Miguel Cainzos-Achirica, Helena Tizon-Marcos, Montse Fito, Irene R. Degano, and Jaume Marrugat. 2023. "Five-Year Changes in Inflammatory, Metabolic, and Oxidative Biomarkers and 10-Year Cardiovascular Disease Incidence: The REGICOR Cohort Study" International Journal of Molecular Sciences 24, no. 9: 7934. https://doi.org/10.3390/ijms24097934
APA StyleCamps-Vilaro, A., Subirana, I., Ramos, R., Cainzos-Achirica, M., Tizon-Marcos, H., Fito, M., Degano, I. R., & Marrugat, J. (2023). Five-Year Changes in Inflammatory, Metabolic, and Oxidative Biomarkers and 10-Year Cardiovascular Disease Incidence: The REGICOR Cohort Study. International Journal of Molecular Sciences, 24(9), 7934. https://doi.org/10.3390/ijms24097934






