Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis
Abstract
1. Introduction
2. Results
2.1. MTS1338-Overexpressing Mtb Strain Has Increased Viability during Macrophage Infection
2.2. Stress Tolerance of the MTS1338-Overexpressing Mtb Strain
2.3. Transcriptome Changes Induced by MTS1338 Overexpression in the Logarithmic Growth Phase
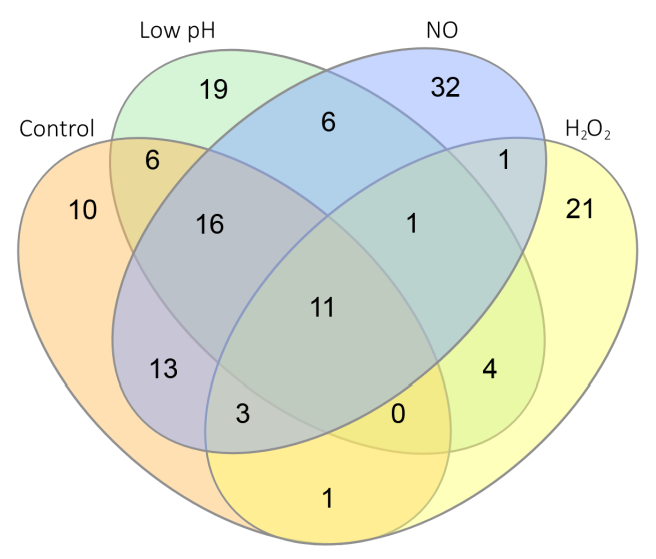
2.4. Transcriptome Changes Induced by Macrophage-like Stresses
2.4.1. Stress-Specific Transcriptional Changes
2.4.2. MTS1338-Specific Transcriptional Response
3. Discussion
- mpt70, encoding a secreted immunodominant antigen, Mpt70, which is upregulated in response to IFN-γ and nutrient or oxygen deprivation of infected macrophages [25,26] and is known to promote T cell differentiation [27]. Mpt70 is mainly expressed at the later stages of in vivo infection and could be functionally linked to the genes involved in Mtb persistence, such as DosR [27].
- Rv2034, encoding the ArsR repressor, which senses heavy metal ions and causes derepression of stress-induced operons. In Mtb, Rv2034 upregulates phoP and dosR genes [28,29]. PhoP controls the expression of Mtb key virulence factors such as ESX-1, cell wall components, and Ag85 antigen via sRNA Mcr7 and messenger metabolite c-di-AMP, and is essential for Mtb virulence and persistence [8,30]; furthermore, it plays a major role in Mtb survival under hypoxia and acidic pH [31,32]. DosR, a key player in Mtb adaptation to non-replicating survival in the hypoxic environment [22,33], acts together with PhoP in sensing macrophage-like stresses [32]. However, although MTS1338 is regulated by DosR, it is not fully DosR-dependent [7], and neither dosR nor any other Dos-regulated genes were differentially expressed in the MTS1338-over strain. Similarly, the PhoP-encoding gene (Rv0757) also was not identified as a DEG in this study.
- Rv2035, together with Rv2034, is predicted to constitute a novel toxin–antitoxin system involved in Mtb latency during macrophage infection [34].
- cmtR (Rv1994c), a member of the ArsR family, functions as a redox sensor and can be significantly activated by H2O2 stress [35]. CmtR can physically interact with the negative regulator Zur and de-repress the expression of the esx-3 operon, leading to Zn2+ accumulation and the promotion of ROS detoxification in mycobacteria [35]. Consequently, CmtR contributes to bacterial survival in macrophages and in the lungs of infected mice [35].
- The Rv2641–Rv2642 operon. Rv2641 (cadI) encodes a cadmium-induced protein [36] that could be also sensitive to copper and zinc in Mycobacterium bovis BCG [37,38]. The function of CadI is similar to that of metallothioneins—low-molecular-weight cysteine-rich proteins that protect Mtb against metal toxicity [37]. Rv2642 belongs to the ArsR family, and regulates cadI and its own expression through binding to a conserved motif [39]. Rv2642 and Rv2643 are immunogenic and induce significant IFN-γ response in individuals with latent tuberculosis infection [40].
4. Materials and Methods
4.1. MTS1338-Overexpressing Strain
4.2. Bacterial Growth Conditions
4.3. Stress Effects on Mtb Survival
4.4. [3H]-Uracil Incorporation
4.5. RNA Isolation
4.6. RNA-Seq and Data Analysis
4.7. Mtb Infection of Macrophages
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stiens, J.; Tan, Y.Y.; Joyce, R.; Arnvig, K.B.; Kendall, S.L.; Nobeli, I. Using a whole genome co-expression network to inform the functional characterisation of predicted genomic elements from Mycobacterium tuberculosis Transcriptomic Data. Mol. Microbiol. 2023, 119, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Yan, B.; Casey, R.; Hingley-Wilson, S.; Ettwiller, L.; Jacobs, W.R.J. Commonalities of Mycobacterium tuberculosis Transcriptomes in Response to Defined Persisting Macrophage Stresses. Front. Immunol. 2022, 13, 3386. [Google Scholar] [CrossRef] [PubMed]
- Arnvig, K.B.; Comas, I.; Thomson, N.; Houghton, J.; Boshoff, H.I.; Croucher, N.; Rose, G.; Perkins, T.T.; Parkhill, J.; Dougan, G.; et al. Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of Mycobacterium tuberculosis. PLoS Pathog. 2011, 7, e1002342. [Google Scholar] [CrossRef] [PubMed]
- Gerrick, E.R.; Barbier, T.; Chase, M.R.; Xu, R.; François, J.; Lin, V.H.; Szucs, M.J.; Rock, J.M.; Ahmad, R.; Tjaden, B.; et al. Small RNA profiling in Mycobacterium tuberculosis identifies MrsI as necessary for an anticipatory iron sparing response. Proc. Natl. Acad. Sci. USA 2018, 115, 6464–6469. [Google Scholar] [CrossRef]
- Girardin, R.C.; McDonough, K.A. Small RNA Mcr11 requires the transcription factor AbmR for stable expression and regulates genes involved in the central metabolism of Mycobacterium tuberculosis. Mol. Microbiol. 2019, 113, 504–520. [Google Scholar] [CrossRef]
- Mai, J.; Rao, C.; Watt, J.; Sun, X.; Lin, C.; Zhang, L.; Liu, J. Mycobacterium tuberculosis 6C sRNA binds multiple mRNA targets via C-rich loops independent of RNA chaperones. Nucleic Acids Res. 2019, 47, 4292–4307. [Google Scholar] [CrossRef]
- Moores, A.; Riesco, A.B.; Schwenk, S.; Arnvig, K.B. Expression, maturation and turnover of DrrS, an unusually stable, DosR regulated small RNA in Mycobacterium tuberculosis. PLoS ONE 2017, 12, e0174079. [Google Scholar] [CrossRef]
- Solans, L.; Gonzalo-Asensio, J.; Sala, C.; Benjak, A.; Uplekar, S.; Rougemont, J.; Guilhot, C.; Malaga, W.; Martin, C.; Cole, S.T. The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in Mycobacterium tuberculosis. PLoS Pathog. 2014, 10, e1004183. [Google Scholar] [CrossRef]
- Arnvig, K.B.; Young, D.B. Identification of small RNAs in Mycobacterium tuberculosis. Mol. Microbiol. 2009, 73, 397–408. [Google Scholar] [CrossRef]
- Ignatov, D.V.; Salina, E.G.; Fursov, M.V.; Skvortsov, T.A.; Azhikina, T.L.; Kaprelyants, A.S. Dormant non-culturable Mycobacterium tuberculosis retains stable low-abundant mRNA. BMC Genom. 2015, 16, 954. [Google Scholar] [CrossRef]
- Salina, E.G.; Grigorov, A.; Skvortsova, Y.; Majorov, K.; Bychenko, O.; Ostrik, A.; Logunova, N.; Ignatov, D.; Kaprelyants, A.; Apt, A.; et al. MTS1338, A Small Mycobacterium tuberculosis RNA, Regulates Transcriptional Shifts Consistent with Bacterial Adaptation for Entering into Dormancy and Survival Within Host Macrophages. Front. Cell. Infect. Microbiol. 2019, 9, 405. [Google Scholar] [CrossRef]
- Bychenko, O.; Skvortsova, Y.; Ziganshin, R.; Grigorov, A.; Aseev, L.; Ostrik, A.; Kaprelyants, A.; Salina, E.G.; Azhikina, T. Mycobacterium tuberculosis Small RNA MTS1338 Confers Pathogenic Properties to Non-Pathogenic Mycobacterium smegmatis. Microorganisms 2021, 9, 414. [Google Scholar] [CrossRef]
- Kondratieva, E.; Majorov, K.; Grigorov, A.; Skvortsova, Y.; Kondratieva, T.; Rubakova, E.; Linge, I.; Azhikina, T.; Apt, A. An In Vivo Model of Separate M. tuberculosis Phagocytosis by Neutrophils and Macrophages: Gene Expression Profiles in the Parasite and Disease Development in the Mouse Host. Int. J. Mol. Sci. 2022, 23, 2961. [Google Scholar] [CrossRef]
- Gottesman, S.; McCullen, C.; Guillier, M.; Vanderpool, C.; Majdalani, N.; Benhammou, J.; Thompson, K.; Fitzgerald, P.; Sowa, N.; Fitzgerald, D. Small RNA Regulators and the Bacterial Response to Stress. Cold Spring Harb. Symp. Quant. Biol. 2006, 71, 1–11. [Google Scholar] [CrossRef]
- Brosse, A.; Guillier, M. Bacterial Small RNAs in Mixed Regulatory Networks. Microbiol. Spectr. 2018, 6, 6.3.05. [Google Scholar] [CrossRef]
- Ahmed, W.; Zheng, K.; Liu, Z.-F. Small Non-Coding RNAs: New Insights in Modulation of Host Immune Response by Intracellular Bacterial Pathogens. Front. Immunol. 2016, 7, 431. [Google Scholar] [CrossRef]
- Schwenk, S.; Arnvig, K.B. Regulatory RNA in Mycobacterium tuberculosis, back to basics. Pathog. Dis. 2018, 76, 1–12. [Google Scholar] [CrossRef]
- Ostrik, A.A.; Azhikina, T.L.; Salina, E.G. Small Noncoding RNAs and Their Role in the Pathogenesis of Mycobacterium tuberculosis Infection. Biochemistry 2021, 86, S109–S119. [Google Scholar] [CrossRef]
- Pisu, D.; Huang, L.; Grenier, J.K.; Russell, D.G. Dual RNA-Seq of Mtb-Infected Macrophages In Vivo Reveals Ontologically Distinct Host-Pathogen Interactions. Cell Rep. 2020, 30, 335–350.e4. [Google Scholar] [CrossRef]
- Arnvig, K.B.; Young, D.B. Non-Coding RNA and Its Potential Role in Mtb Pathogenesis. RNA Biol. 2012, 9, 427–436. [Google Scholar] [CrossRef]
- Ignatov, D.V.; Timoshina, O.Y.; Logunova, N.N.; Skvortsov, T.A.; Azhikina, T.L. Expression of small RNAs of Mycobacterium tuberculosis in murine models of tuberculosis infection. Russ. J. Bioorg. Chem. 2014, 40, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Rustad, T.R.; Sherrid, A.M.; Minch, K.J.; Sherman, D.R. Hypoxia: A window into Mycobacterium tuberculosis latency. Cell. Microbiol. 2009, 11, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Nazarova, E.V.; Tan, S.; Liu, Y.; Russell, D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215, 1135–1152. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.C.; Lukey, P.T.; Robb, L.C.; McAdam, R.A.; Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 2002, 43, 717–731. [Google Scholar] [CrossRef]
- Bertholet, S.; Ireton, G.C.; Kahn, M.; Guderian, J.; Mohamath, R.; Stride, N.; Laughlin, E.M.; Baldwin, S.L.; Vedvick, T.S.; Coler, R.N.; et al. Identification of Human T Cell Antigens for the Development of Vaccines against Mycobacterium tuberculosis. J. Immunol. 2008, 181, 7948–7957. [Google Scholar] [CrossRef]
- Clemmensen, H.S.; Dube, J.-Y.; McIntosh, F.; Rosenkrands, I.; Jungersen, G.; Aagaard, C.; Andersen, P.; Behr, M.A.; Mortensen, R. In Vivo Antigen Expression Regulates CD4 T Cell Differentiation and Vaccine Efficacy against Mycobacterium tuberculosis Infection. Mbio 2021, 12, e00226-21. [Google Scholar] [CrossRef]
- Gao, C.-H.; Yang, M.; He, Z.-G. An ArsR-like transcriptional factor recognizes a conserved sequence motif and positively regulates the expression of phoP in mycobacteria. Biochem. Biophys. Res. Commun. 2011, 411, 726–731. [Google Scholar] [CrossRef]
- Gao, C.-H.; Yang, M.; He, Z.-G. Characterization of a Novel ArsR-Like Regulator Encoded by Rv2034 in Mycobacterium tuberculosis. PLoS ONE 2012, 7, e36255. [Google Scholar] [CrossRef]
- Ryndak, M.; Wang, S.; Smith, I. PhoP, a key player in Mycobacterium tuberculosis virulence. Trends Microbiol. 2008, 16, 528–534. [Google Scholar] [CrossRef]
- Bansal, R.; Kumar, V.A.; Sevalkar, R.R.; Singh, P.R.; Sarkar, D. Mycobacterium tuberculosis virulence-regulator PhoP interacts with alternative sigma factor SigE during acid-stress response. Mol. Microbiol. 2017, 104, 400–411. [Google Scholar] [CrossRef]
- Singh, P.R.; Vijjamarri, A.K.; Sarkar, D. Metabolic Switching of Mycobacterium tuberculosis during Hypoxia Is Controlled by the Virulence Regulator PhoP. J. Bacteriol. 2020, 202, e00705-19. [Google Scholar] [CrossRef]
- Park, H.-D.; Guinn, K.M.; Harrell, M.I.; Liao, R.; Voskuil, M.I.; Tompa, M.; Schoolnik, G.K.; Sherman, D.R. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 833–843. [Google Scholar] [CrossRef]
- Bajaj, R.A.; Arbing, M.A.; Shin, A.; Cascio, D.; Miallau, L. Crystal structure of the toxin Msmeg_6760, the structural homolog of Mycobacterium tuberculosis Rv2035, a novel type II toxin involved in the hypoxic response. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 863–869. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Liao, J.; Hui, J.; Li, W.; He, Z.-G. A novel stress-inducible CmtR-ESX3-Zn2+ regulatory pathway essential for survival of Mycobacterium bovis under oxidative stress. J. Biol. Chem. 2020, 295, 17083–17099. [Google Scholar] [CrossRef]
- Hotter, G.S.; Wilson, T.; Collins, D.M. Identification of a cadmium-induced gene in Mycobacterium bovis and Mycobacterium tuberculosis. FEMS Microbiol. Lett. 2001, 200, 151–155. [Google Scholar] [CrossRef]
- Ma, R.; Farrell, D.; Gonzalez, G.; Browne, J.A.; Nakajima, C.; Suzuki, Y.; Gordon, S.V. The TbD1 Locus Mediates a Hypoxia-Induced Copper Response in Mycobacterium bovis. Front. Microbiol. 2022, 13, 817952. [Google Scholar] [CrossRef]
- Salina, E.G.; Huszár, S.; Zemanová, J.; Keruchenko, J.; Riabova, O.; Kazakova, E.; Grigorov, A.; Azhikina, T.; Kaprelyants, A.; Mikušová, K.; et al. Copper-related toxicity in replicating and dormant Mycobacterium tuberculosis caused by 1-hydroxy-5-R-pyridine-2(1H)-thiones. Metallomics 2018, 10, 992–1002. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Xie, L.; Zhang, C.; Feng, Y.; Xie, J. Characterization of a Putative ArsR Transcriptional Regulator Encoded by Rv2642 from Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2017, 35, 2031–2039. [Google Scholar] [CrossRef]
- Serra-Vidal, M.M.; Latorre, I.; Franken, K.L.C.M.; Dãaz, J.; Galvão, M.L.D.S.; Casas, I.; Maldonado, J.; Milã, C.; Solsona, J.; Jimenez-Fuentes, M.Ã.; et al. Immunogenicity of 60 novel latency-related antigens of Mycobacterium tuberculosis. Front. Microbiol. 2014, 5, 517. [Google Scholar] [CrossRef]
- Stover, C.K.; De La Cruz, V.F.; Fuerst, T.R.; Burlein, J.E.; Benson, L.A.; Bennett, L.T.; Bansal, G.P.; Young, J.F.; Lee, M.H.; Hatfull, G.F.; et al. New use of BCG for recombinant vaccines. Nature 1991, 351, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Connell, N.D. Chapter 6: Mycobacterium: Isolation, Maintenance, Transformation, and Mutant Selection. Methods Cell Biol. 1995, 45, 107–125. [Google Scholar] [CrossRef]
- Hu, Y.; Mangan, J.A.; Dhillon, J.; Sole, K.M.; Mitchison, D.A.; Butcher, P.D.; Coates, A.R.M. Detection of mRNA Transcripts and Active Transcription in Persistent Mycobacterium tuberculosis Induced by Exposure to Rifampin or Pyrazinamide. J. Bacteriol. 2000, 182, 6358–6365. [Google Scholar] [CrossRef] [PubMed]
- Majorov, K.B.; Lyadova, I.V.; Kondratieva, T.K.; Eruslanov, E.B.; Rubakova, E.I.; Orlova, M.O.; Mischenko, V.V.; Apt, A.S. Different Innate Ability of I/St and A/Sn Mice to Combat Virulent Mycobacterium tuberculosis: Phenotypes Expressed in Lung and Extrapulmonary Macrophages. Infect. Immun. 2003, 71, 697–707. [Google Scholar] [CrossRef]
- Rustad, T.R.; Roberts, D.M.; Liao, R.P.; Sherman, D.R. Isolation of Mycobacterial RNA. Methods Mol. Biol. 2009, 465, 13–22. [Google Scholar] [CrossRef]
- Huang, Y.; Sheth, R.U.; Kaufman, A.; Wang, H.H. Scalable and cost-effective ribonuclease-based rRNA depletion for transcriptomics. Nucleic Acids Res. 2020, 48, E20. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
| Stress Factor | Functional Category According to Mycobrowser (https://mycobrowser.epfl.ch/, accessed on 16 May 2022) | Genes | Up/Down |
|---|---|---|---|
| pH | Regulatory proteins | Rv0260c | ↓ |
| Rv0792c Rv1395 furA Rv1990c cmtR Rv2034 Rv2250c Rv2642 Rv3183 Rv3334 | ↑ | ||
| Information pathways | Rv0516c Rv2464c | ↑ | |
| Cell wall and cell processes | iniB Rv0841 arsC mpt70 | ↑ | |
| esxK esxL rocE | ↓ | ||
| Intermediary metabolism and respiration | glbN nirB nirD Rv3741c Rv3742c | ↓ | |
| cyp138 galK Rv0793 grcC2 frdA Rv1990A | ↑ | ||
| Lipid metabolism | papA4 | ↑ | |
| PE/PPE | pe13 ppe18 ppe19 ppe43 pe27 | ↓ | |
| ppe29 ppe37 | ↑ | ||
| Virulence, detoxification, adaptation | hsp vapB30 vapC30 higB mazE6 mazF6 | ↑ | |
| Insertion seqs and phages | Rv3428c | ↑ | |
| Conserved hypotheticals (unknown function) | Rv1954A Rv0140 Rv0142 Rv0826 Rv1044 Rv1357c Rv1989c Rv1993c Rv2035 Rv2466c cadI Rv3054c Rv3182 Rv3188 Rv3189 Rv3659c | ↑ | |
| Rv0259c | ↓ | ||
| H2O2 | Regulatory proteins | Rv0260c | ↓ |
| Rv1129c Rv1395 Rv1473A cmtR Rv0792c Rv2034 Rv2642 whiB6 | ↑ | ||
| Information pathways | Rv2464c | ↑ | |
| Rv3201c | ↓ | ||
| Cell wall and cell processes | Rv0188 Rv2617c Rv3289c | ↓ | |
| Rv1473 mpt70 | ↑ | ||
| Intermediary metabolism and respiration | galK Rv0793 prpD prpC | ↑ | |
| Rv1279 glbN lat | ↓ | ||
| Lipid metabolism | pks11 desA3 Rv3371 | ↓ | |
| Pe/ppe | ppe27 | ↑ | |
| pe20 ppe31 | ↓ | ||
| Virulence, detoxification, adaptation | Rv3660c | ↑ | |
| Insertion seqs and phages | Rv0095c Rv0829 | ↓ | |
| Conserved hypotheticals (unknown function) | Rv0259c Rv2015c Rv1278 Rv1765c | ↓ | |
| Rv0791c Rv0826 cadI Rv3659c Rv2035 Rv2422 | ↑ | ||
| NO | Regulatory proteins | Rv0196 Rv0792c kmtR csoR Rv1219c Rv1395 Rv1674c furA Rv1990c cmtR Rv2034 Rv2250c Rv2642 Rv2989 Rv3183 Rv3334 Rv3840 whiB6 | ↑ |
| Cell wall and cell processes | lprK | ↓ | |
| Rv0841 arsC mpt70 Rv2963 Rv3657c | ↑ | ||
| Intermediary metabolism and respiration | bioF2 lipF | ↓ | |
| galK Rv0793 cysD hisE Rv2250A ethA | ↑ | ||
| Lipid metabolism | acpA fadD34 | ↓ | |
| Rv0830 papA4 lipX | ↑ | ||
| PE/PPE | pe15 ppe20 ppe29 pe20 ppe31 ppe37 | ↑ | |
| pe31 | ↓ | ||
| Virulence, detoxification, adaptation | mce1A mce1B mce1D | ↓ | |
| mymT hsp clpB vapB30 vapC30 yrbE3A mazE6 mazF6 Rv3660c | ↑ | ||
| Insertion seqs and phages | Rv2013 Rv3428c Rv3751 | ↑ | |
| Conserved hypotheticals (unknown function) | Rv0034 Rv1157c Rv1158c Rv1697 | ↓ | |
| Rv0448c Rv0724A Rv0826 Rv0968 Rv1044 Rv1048c Rv1989c Rv1995 Rv2016 Rv2035 Rv2036 Rv2327 cadI Rv2662 Rv3054c Rv3182 Rv3188 Rv3189 Rv3659c Rv3839 | ↑ |
| Gene | Product | Log2FC | |||
|---|---|---|---|---|---|
| Ctrl | pH | H2O2 | NO | ||
| galK | Probable galactokinase GalK (galactose kinase) | 1.50 | 2.00 | 2.14 | 2.15 |
| Rv0792c | Probable transcriptional regulatory protein | 1.59 | 1.70 | 1.97 | 1.80 |
| Rv0826 | Conserved hypothetical protein | 2.38 | 1.95 | 2.32 | 2.60 |
| Rv1395 | Transcriptional regulatory protein | 2.30 | 2.01 | 1.65 | 2.53 |
| cmtR | Metal sensor transcriptional regulator CmtR (ArsR-SmtB family) | 2.14 | 2.17 | 1.58 | 1.88 |
| Rv2034 | ArsR repressor protein | 3.08 | 2.68 | 2.58 | 3.45 |
| Rv2035 | Conserved hypothetical protein | 2.13 | 1.90 | 1.56 | 2.41 |
| cadI | Cadmium-inducible protein CadI | 2.21 | 2.00 | 2.17 | 3.26 |
| Rv2642 | Possible transcriptional regulatory protein | 2.20 | 2.23 | 1.91 | 2.79 |
| mpt70 | Major secreted immunogenic protein Mpt70 | 2.51 | 2.36 | 2.09 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, B.A.; Grigorov, A.S.; Skvortsova, Y.V.; Bychenko, O.S.; Salina, E.G.; Azhikina, T.L. Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis. Int. J. Mol. Sci. 2023, 24, 7928. https://doi.org/10.3390/ijms24097928
Martini BA, Grigorov AS, Skvortsova YV, Bychenko OS, Salina EG, Azhikina TL. Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2023; 24(9):7928. https://doi.org/10.3390/ijms24097928
Chicago/Turabian StyleMartini, Billy A., Artem S. Grigorov, Yulia V. Skvortsova, Oksana S. Bychenko, Elena G. Salina, and Tatyana L. Azhikina. 2023. "Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis" International Journal of Molecular Sciences 24, no. 9: 7928. https://doi.org/10.3390/ijms24097928
APA StyleMartini, B. A., Grigorov, A. S., Skvortsova, Y. V., Bychenko, O. S., Salina, E. G., & Azhikina, T. L. (2023). Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis. International Journal of Molecular Sciences, 24(9), 7928. https://doi.org/10.3390/ijms24097928







