Detergent-Mediated Virus Inactivation in Biotechnological Matrices: More than Just CMC
Abstract
1. Introduction
2. Results
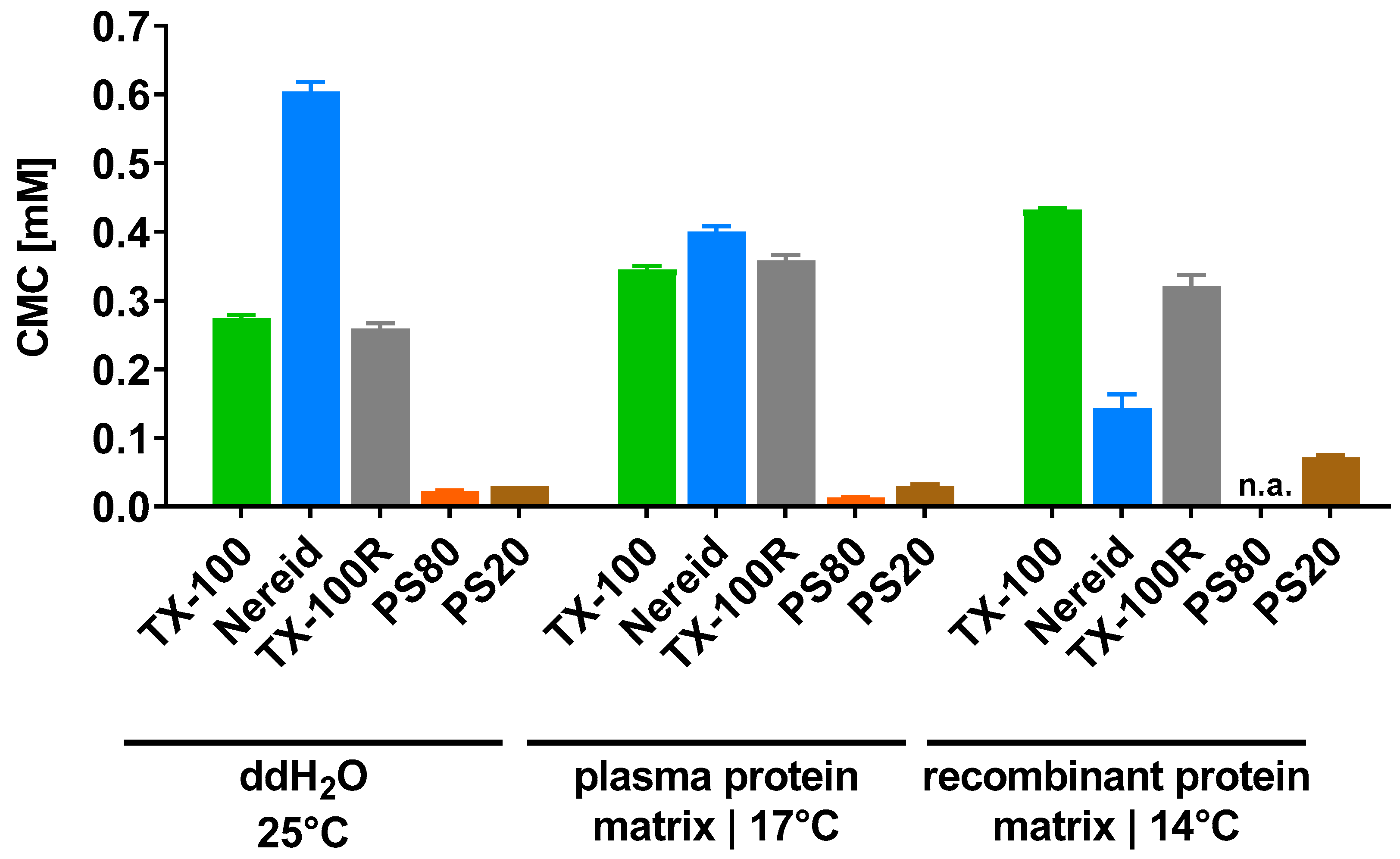
2.1. CMC Determinations
2.2. Virus Inactivation below and above Detergent CMCs
3. Discussion
4. Materials and Methods
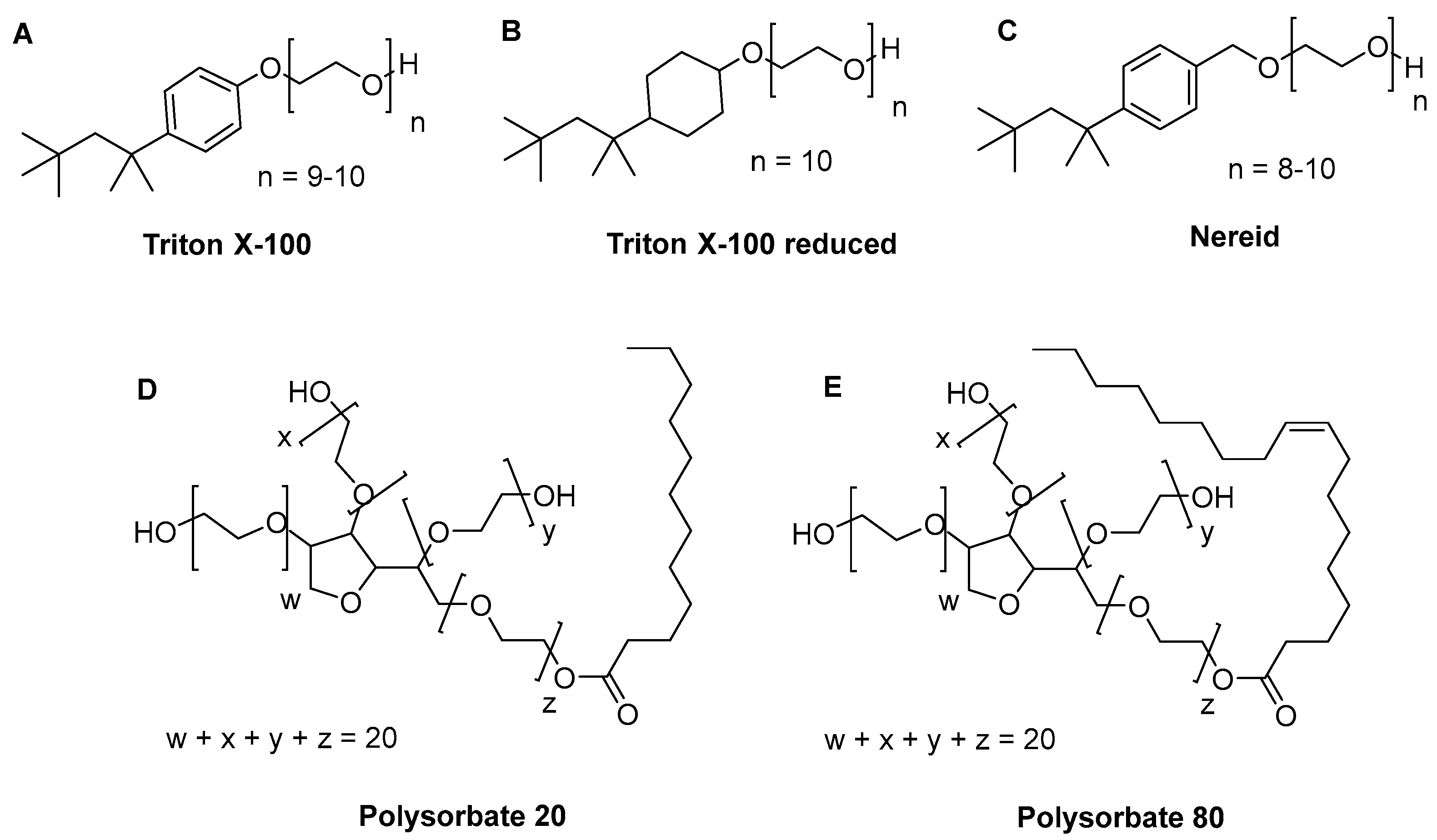
4.1. Detergents
4.2. Matrices
4.3. Force Tensiometry Measurements
4.4. Virus Propagation and Titration
4.5. Virus Inactivation Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BVDV | Bovine viral diarrhea virus |
| CMC | Critical micelle concentration |
| ddH2O | Deionized water |
| HC | Hold control |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| PS20 | Polysorbate 20 |
| PS80 | Polysorbate 80 |
| RF(s) | Reduction factor(s) |
| SC | Spike control |
| SD | Standard deviation |
| S/D | Solvent/detergent |
| TnBP | Tri-n-butyl-phosphate |
| TX-100 | Triton X-100 |
| TX-100R | Triton X-100 reduced |
References
- Remis, R.S.; O’Shaughnessy, M.V.; Tsoukas, C.; Growe, G.H.; Schechter, M.T.; Palmer, R.W.; Lawrence, D.N. HIV transmission to patients with hemophilia by heat-treated, donor-screened factor concentrate. CMAJ Can. Med. Assoc. J. 1990, 142, 1247–1254. [Google Scholar]
- Fletcher, M.L.; Trowell, J.M.; Craske, J.; Pavier, K.; Rizza, C.R. Non-A non-B hepatitis after transfusion of factor VIII in infrequently treated patients. Br. Med. J. (Clin. Res. Ed.) 1983, 287, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, B.; Wiebe, M.E.; Lippin, A.; Stryker, M.H. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion 1985, 25, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Dichtelmueller, H.; Biesert, L.; Fabbrizzi, F.; Gajardo, R.; Groner, A.; von Hoegen, I.; Jorquera, J.I.; Kempf, C.; Kreil, T.R.; Pifat, D.; et al. Robustness of solvent/detergent treatment of plasma derivatives: A data collection from Plasma Protein Therapeutics Association member companies. Transfusion 2009, 49, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Member State Committee Support Document for Identification of 4-(1,1,3,3,-Tetramethybutyl)Phenol, 4-Tert-Octylphenol as a Substance of very High Concern Because Its Endocrine Disrupting Properties Cause Probable Serious Effects to the Environment Which Gives Rise to an Equivalent Level of Concern. Available online: https://echa.europa.eu/documents/10162/4c6cccfd-d366-4a00-87e5-65aa77181fb6 (accessed on 3 March 2022).
- White, R.; Jobling, S.; Hoare, S.A.; Sumpter, J.P.; Parker, M.G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 1994, 135, 175–182. [Google Scholar] [CrossRef] [PubMed]
- ECHA. List of Substances included in Annex XIV of REACH (“Authorisation List”); 4-(1,1,3,3-Tetramethylbutyl)Phenol, Ethoxylated. Available online: https://echa.europa.eu/authorisation-list/-/dislist/details/0b0236e1807df80d (accessed on 3 March 2022).
- Conley, L.; Tao, Y.; Henry, A.; Koepf, E.; Cecchini, D.; Pieracci, J.; Ghose, S. Evaluation of eco-friendly zwitterionic detergents for enveloped virus inactivation. Biotechnol. Bioeng. 2017, 114, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.K.; Rezvani, K.; Aspelund, M.T.; Xi, G.; Gadre, D.; Linke, T.; Cai, K.; Mulagapati, S.H.R.; Witkos, T. Identification of compendial nonionic detergents for the replacement of Triton X-100 in bioprocessing. Biotechnol. Prog. 2022, 38, e3235. [Google Scholar] [CrossRef] [PubMed]
- Farcet, J.B.; Kindermann, J.; Karbiener, M.; Kreil, T.R. Development of a Triton X-100 replacement for effective virus inactivation in biotechnology processes. Eng. Rep. 2019, 1, e12078. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir ACS J. Surf. Colloids 2020, 36, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Gooran, N.; Yoon, B.K.; Jackman, J.A. Supported Lipid Bilayer Platform for Characterizing the Membrane-Disruptive Behaviors of Triton X-100 and Potential Detergent Replacements. Int. J. Mol. Sci. 2022, 23, 869. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Simons, K. Solubilization of membranes by detergents. Biochim. Biophys. Acta 1975, 415, 29–79. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Prince, A.M.; Horowitz, B.; Horowitz, M.S.; Zang, E. The development of virus-free labile blood derivatives—A review. Eur. J. Epidemiol. 1987, 3, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Farcet, J.B.; Kindermann, J.; Karbiener, M.; Scheinecker, R.; Kostner, O.; Kreil, T.R. Synthesis of “Nereid”, a new phenol-free detergent to replace Triton X-100 in virus inactivation. J. Med. Virol. 2021, 93, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Tiller, G.E.; Mueller, T.J.; Dockter, M.E.; Struve, W.G. Hydrogenation of triton X-100 eliminates its fluorescence and ultraviolet light absorption while preserving its detergent properties. Anal. Biochem. 1984, 141, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Wennerström, H. Miceles. Amphiphile aggregation in aqueous solution. Top. Curr. Chem. 1980, 87, 1–87. [Google Scholar] [PubMed]
- Nayem, J.; Zhang, Z.; Tomlinson, A.; Zarraga, I.E.; Wagner, N.J.; Liu, Y. Micellar Morphology of Polysorbate 20 and 80 and Their Ester Fractions in Solution via Small-Angle Neutron Scattering. J. Pharm. Sci. 2020, 109, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, C.; Payne, R.J.; Willis, S.H.; Doranz, B.J.; Rucker, J.B. Maturation of the Gag core decreases the stability of retroviral lipid membranes. Virology 2012, 433, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Soderlund, H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim. Biophys. Acta 1973, 307, 287–300. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Note for Guidance on Virus Validation Studies: The Design, Contribution and Interpretation of Studies Validating the Inactivation and Removal of Viruses (CPMP/BWP/268/95/rev); European Medicines Agency: Amsterdam, The Netherlands, 1996.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farcet, J.-B.; Karbiener, M.; Zelger, L.; Kindermann, J.; Kreil, T.R. Detergent-Mediated Virus Inactivation in Biotechnological Matrices: More than Just CMC. Int. J. Mol. Sci. 2023, 24, 7920. https://doi.org/10.3390/ijms24097920
Farcet J-B, Karbiener M, Zelger L, Kindermann J, Kreil TR. Detergent-Mediated Virus Inactivation in Biotechnological Matrices: More than Just CMC. International Journal of Molecular Sciences. 2023; 24(9):7920. https://doi.org/10.3390/ijms24097920
Chicago/Turabian StyleFarcet, Jean-Baptiste, Michael Karbiener, Leonhard Zelger, Johanna Kindermann, and Thomas R. Kreil. 2023. "Detergent-Mediated Virus Inactivation in Biotechnological Matrices: More than Just CMC" International Journal of Molecular Sciences 24, no. 9: 7920. https://doi.org/10.3390/ijms24097920
APA StyleFarcet, J.-B., Karbiener, M., Zelger, L., Kindermann, J., & Kreil, T. R. (2023). Detergent-Mediated Virus Inactivation in Biotechnological Matrices: More than Just CMC. International Journal of Molecular Sciences, 24(9), 7920. https://doi.org/10.3390/ijms24097920





