Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene
Abstract
1. Introduction
2. Results and Discussion
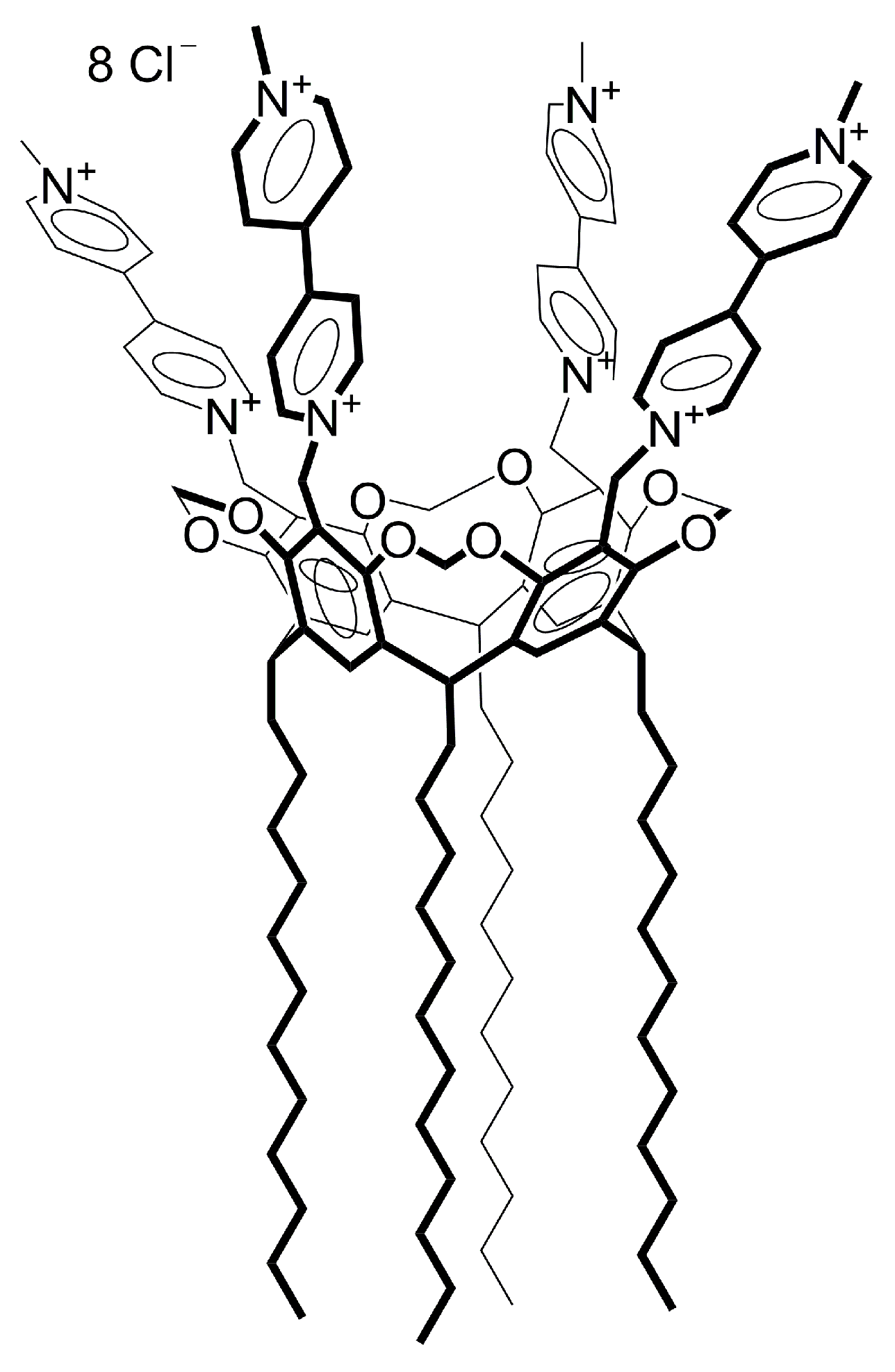
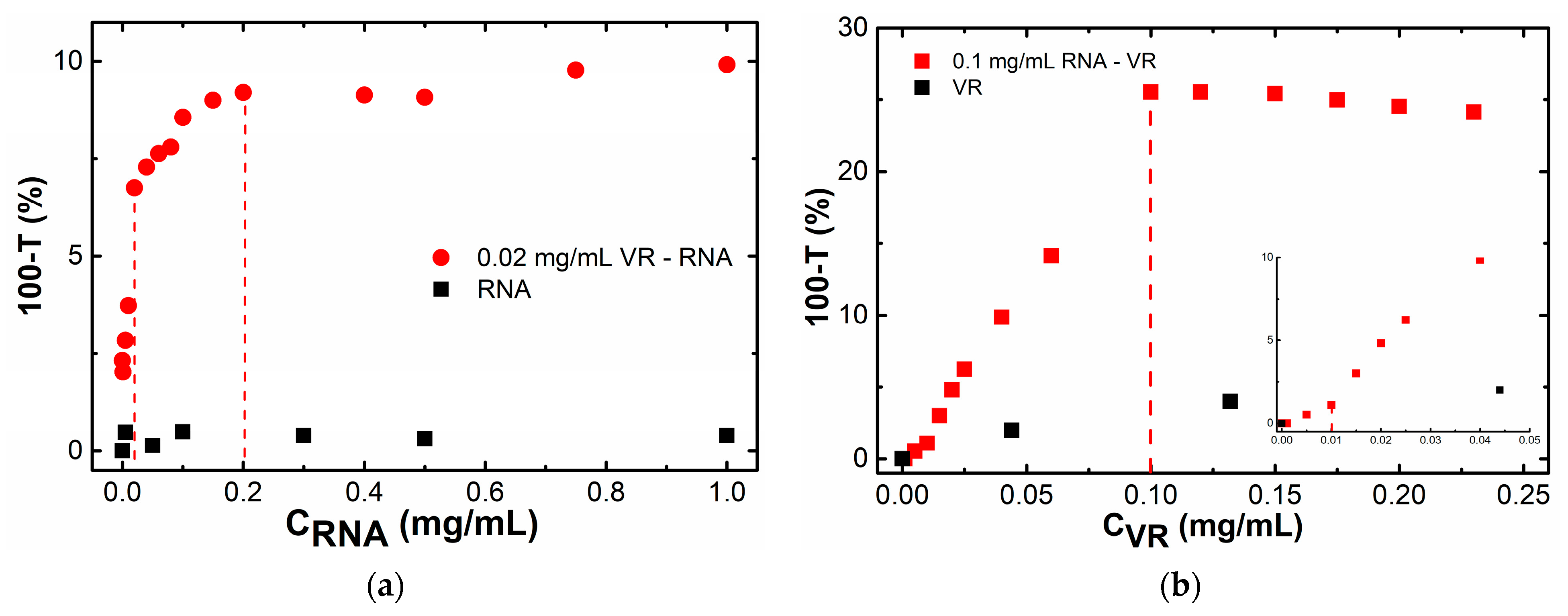
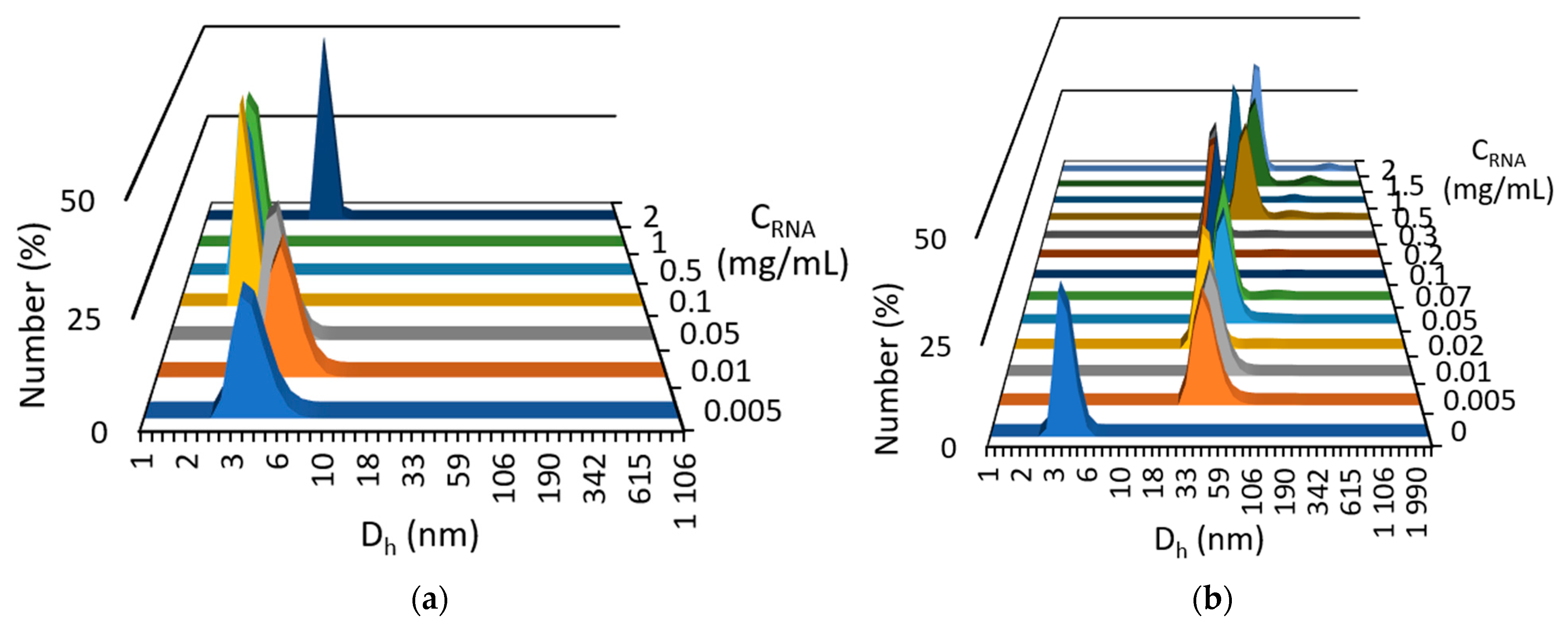
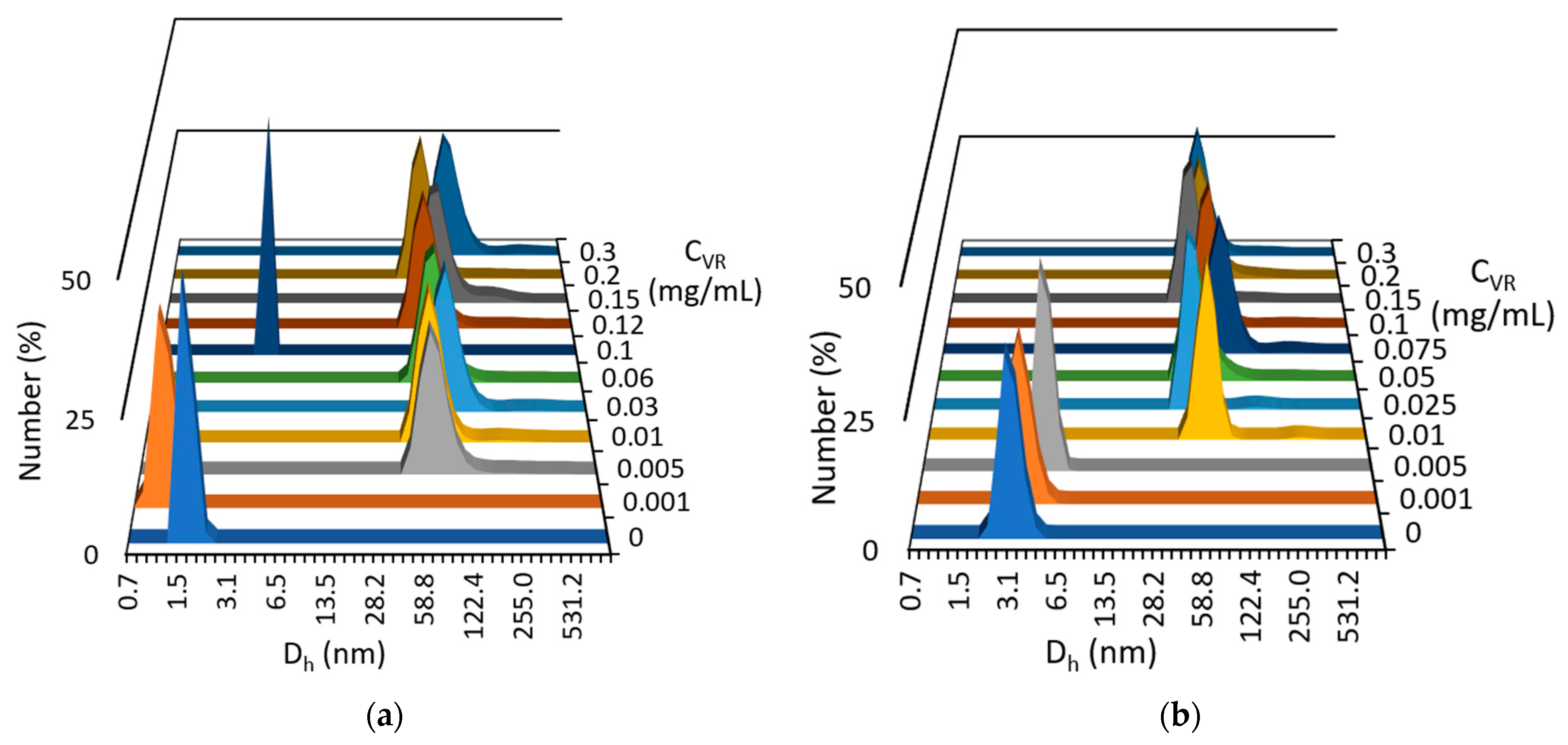
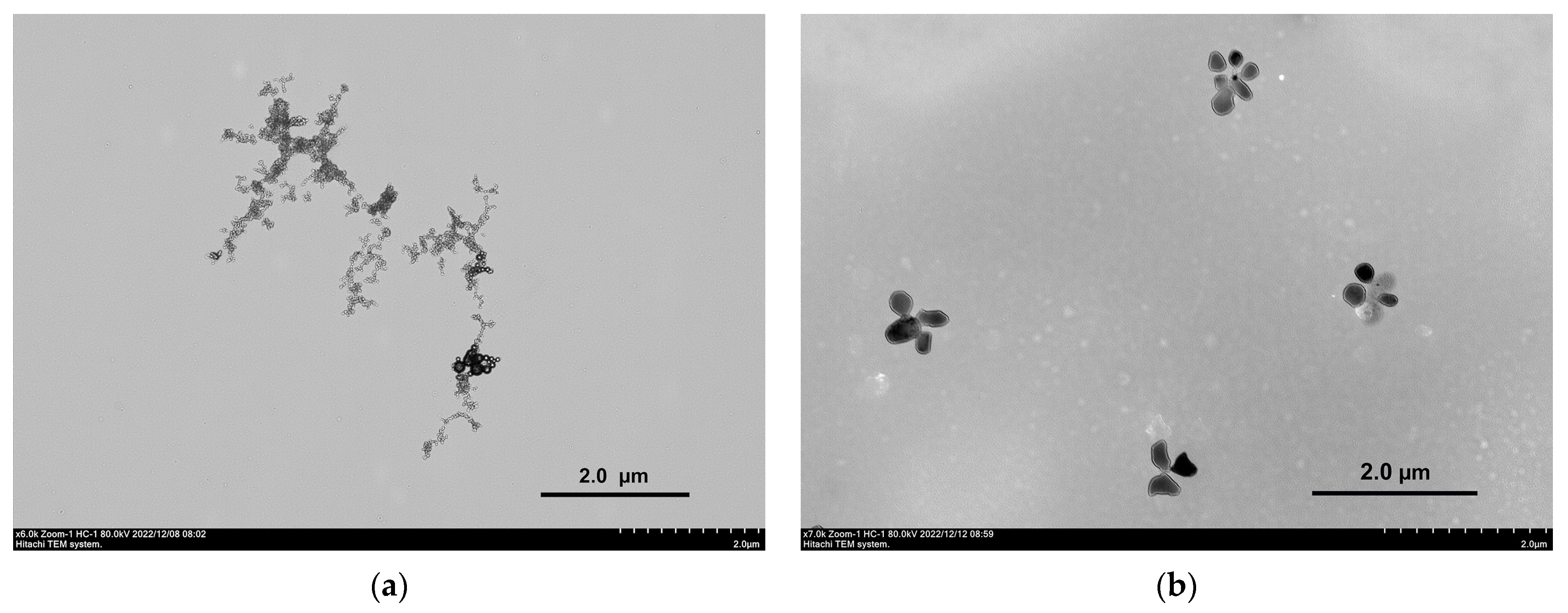
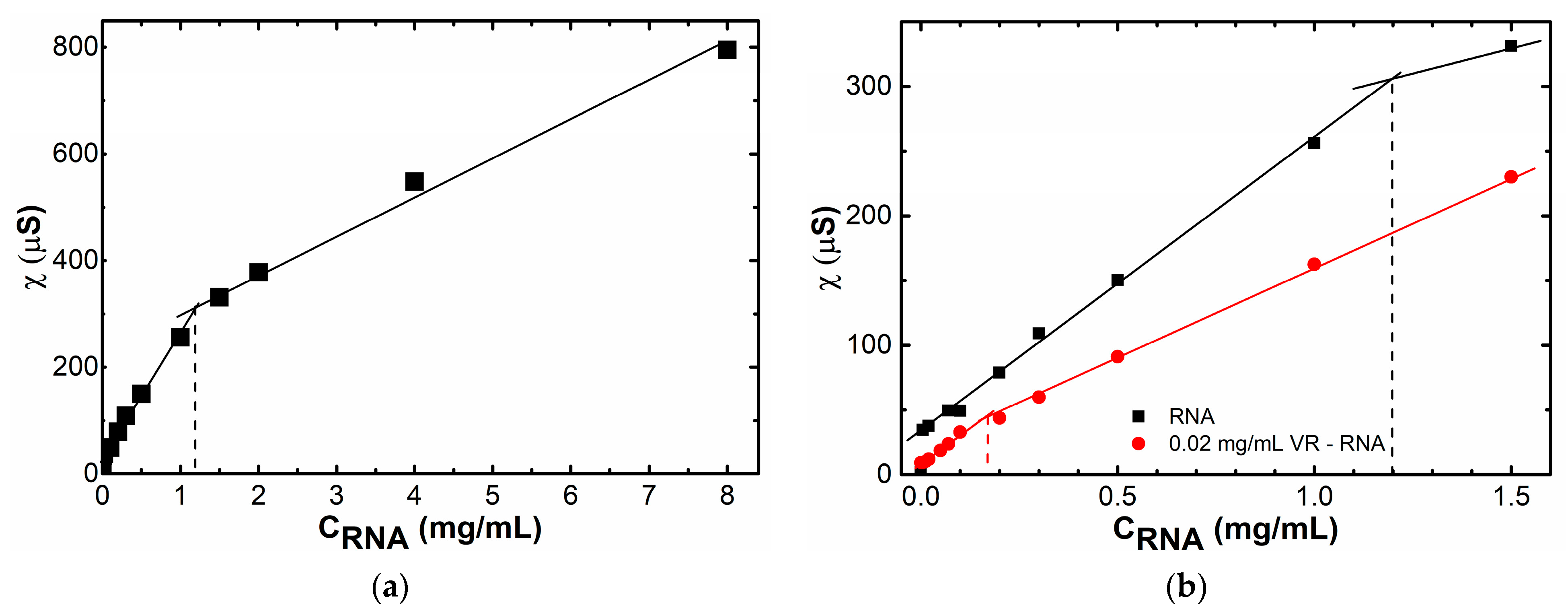
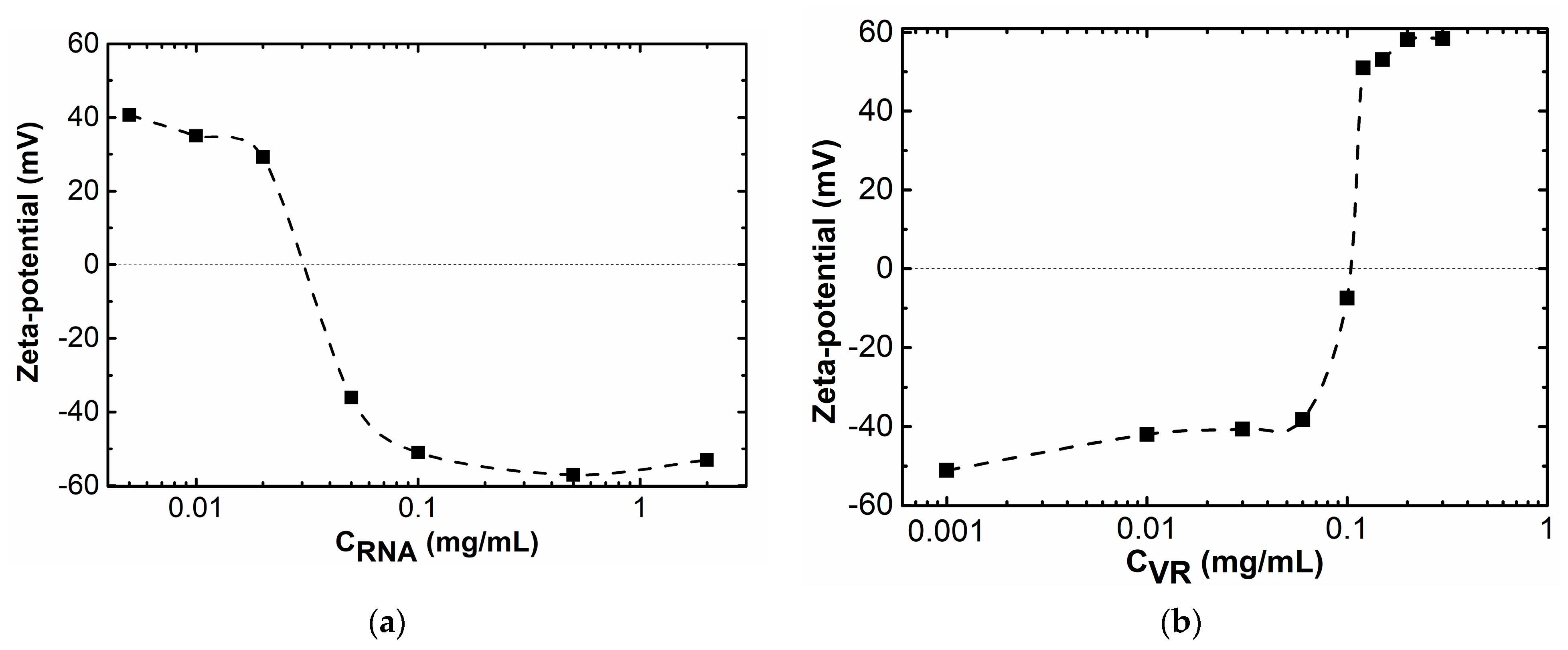
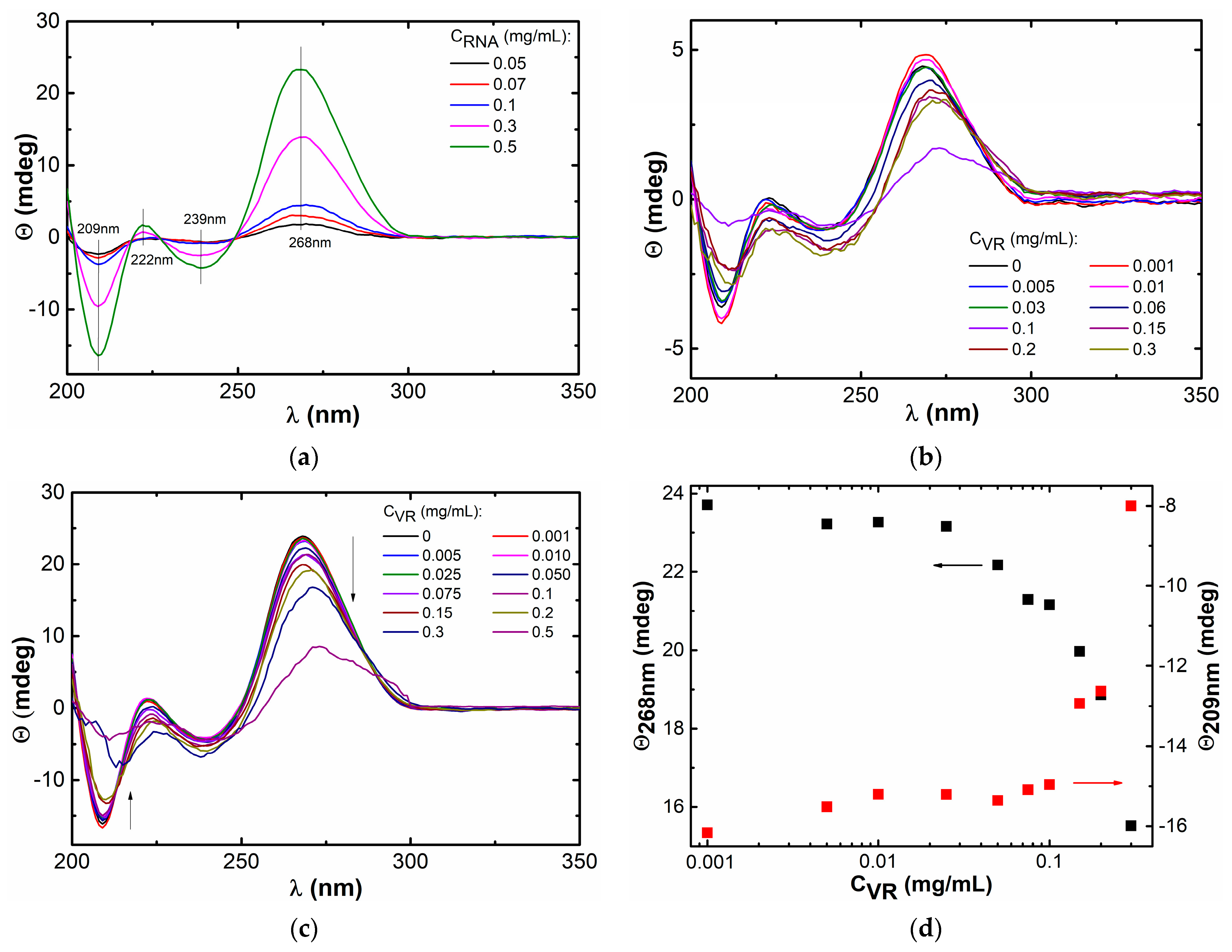
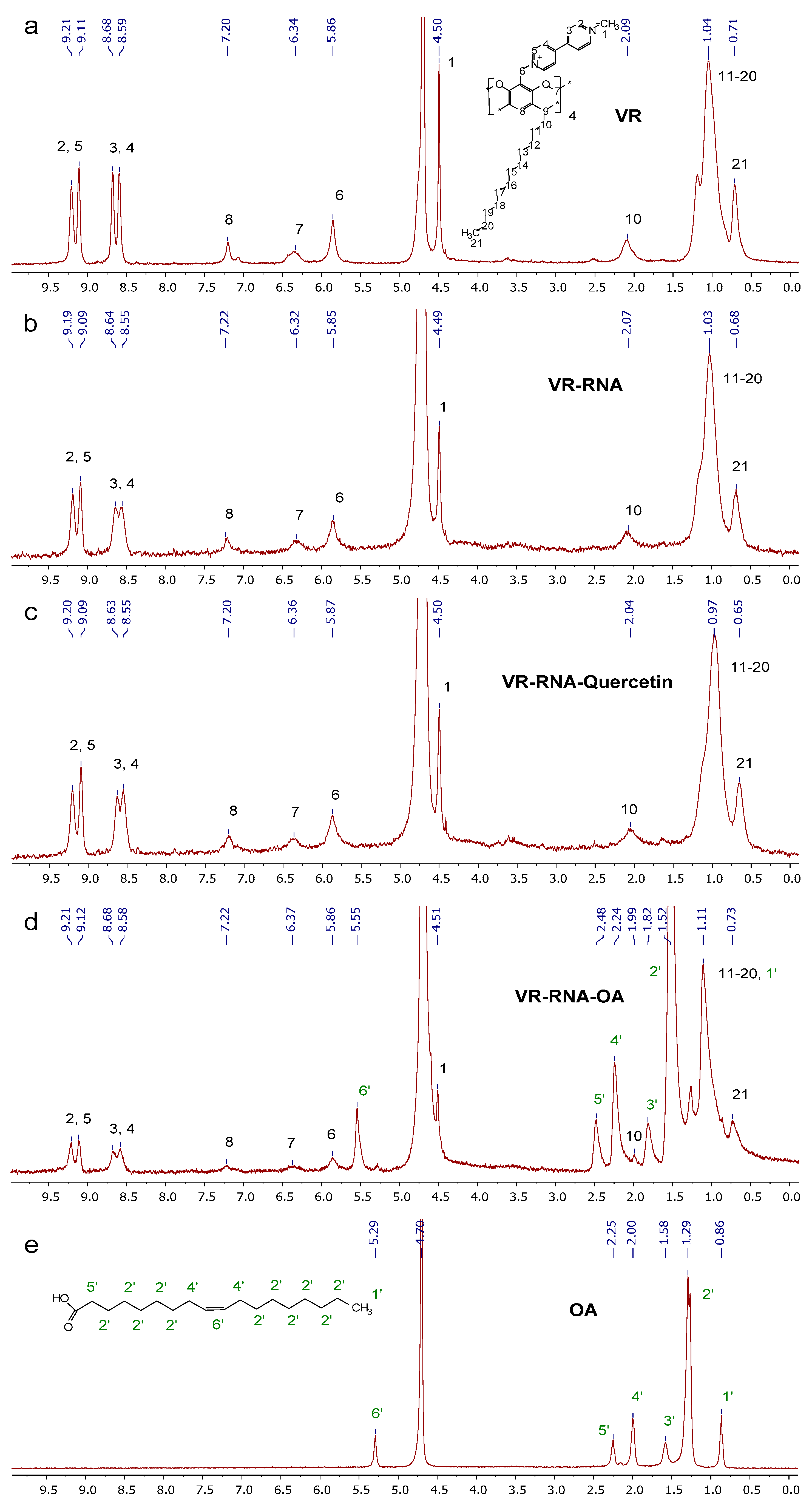
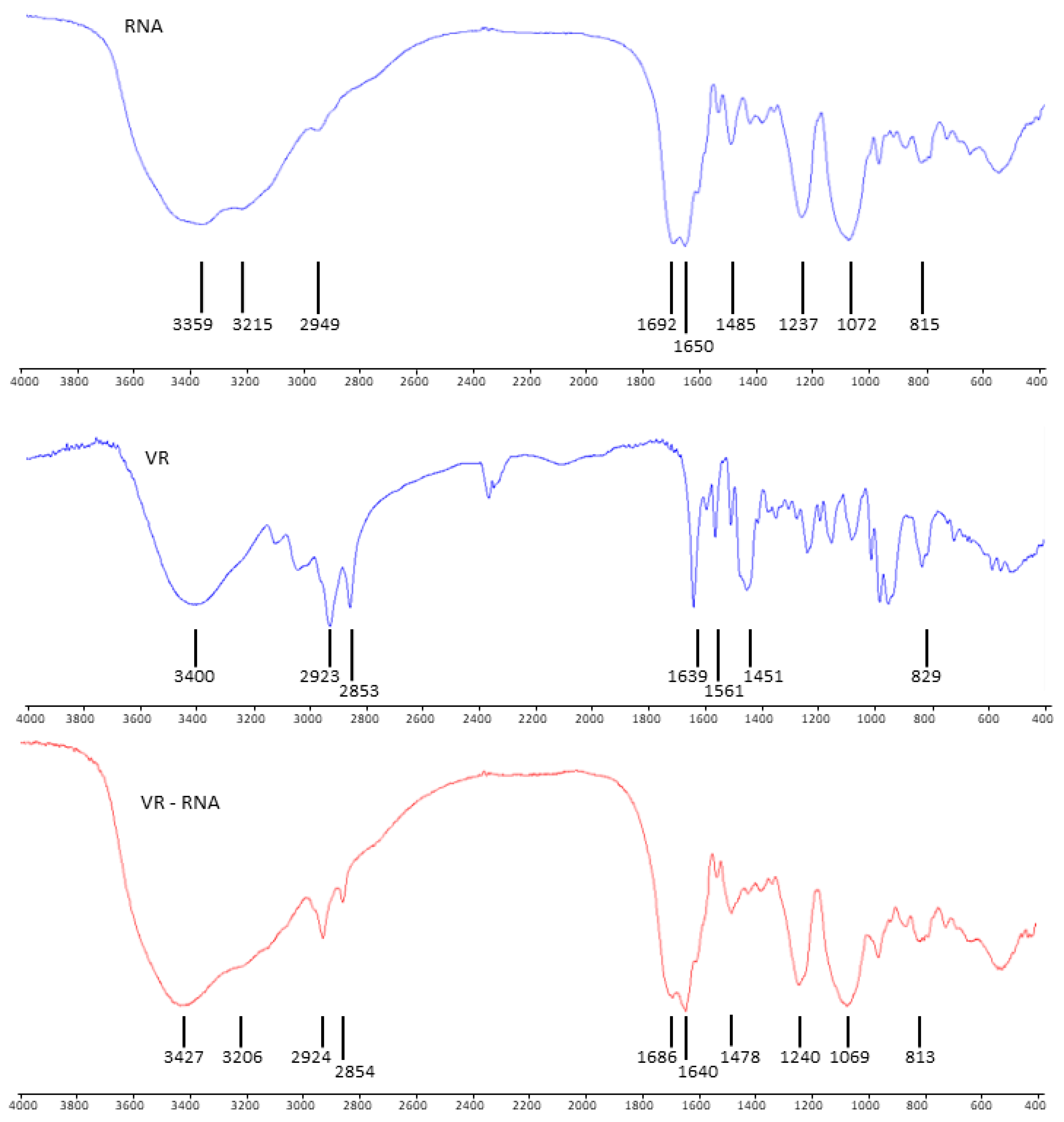
2.1. Mixed Self-Assembly of VR and RNA
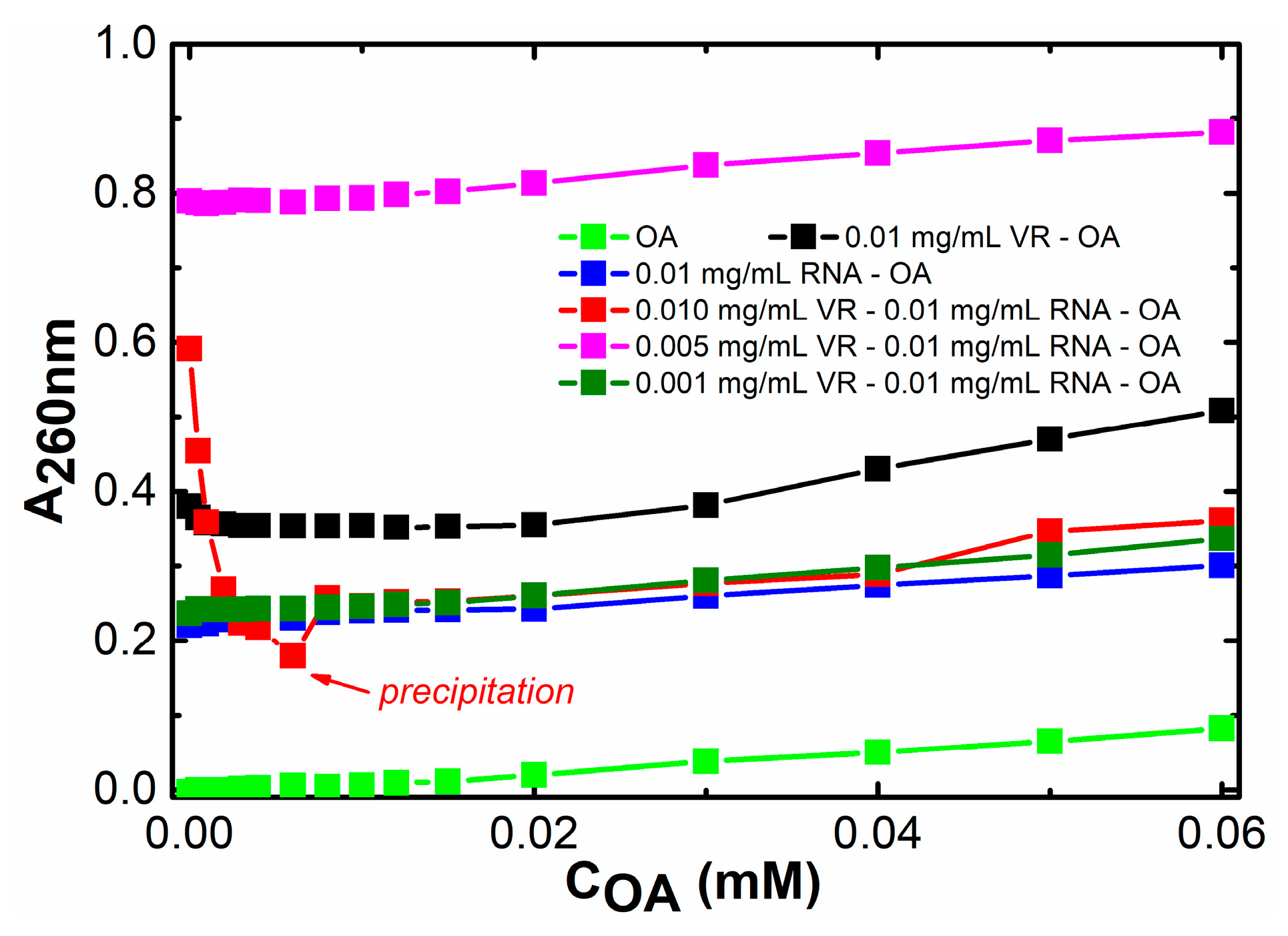
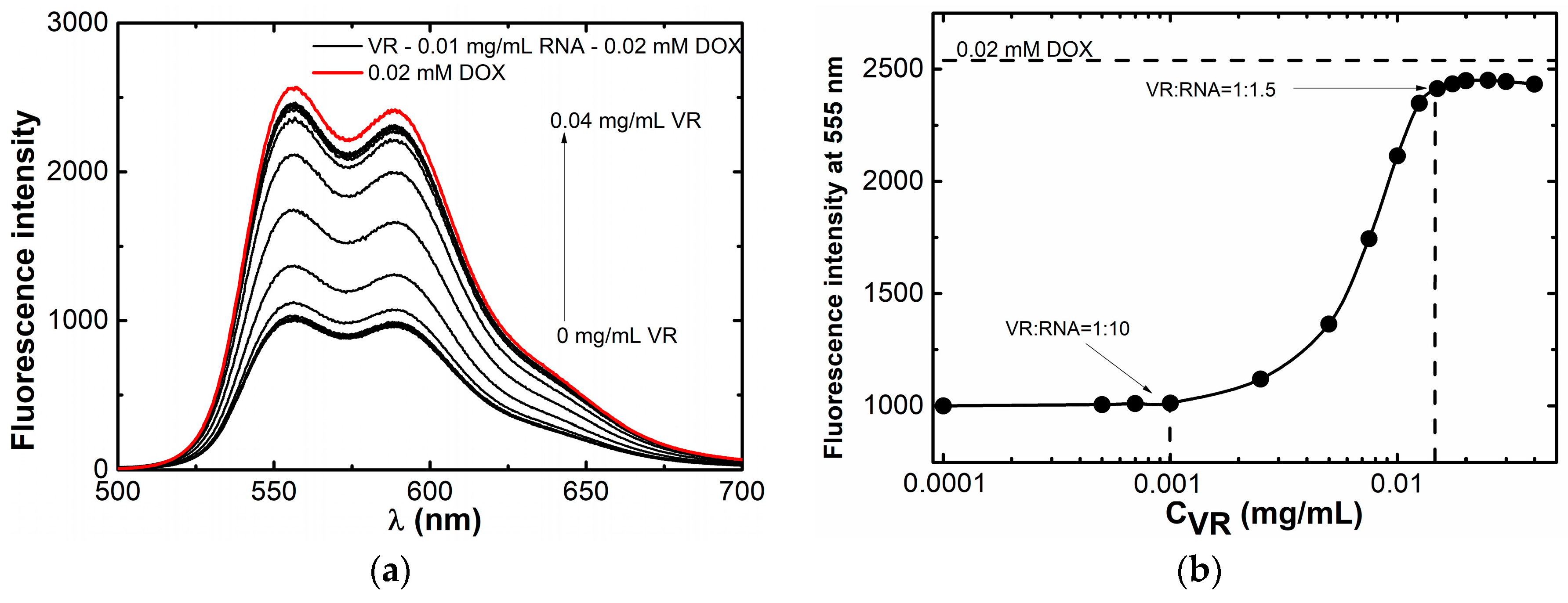
2.2. Binding of Biologically Active Substances in VR–RNA Aggregates
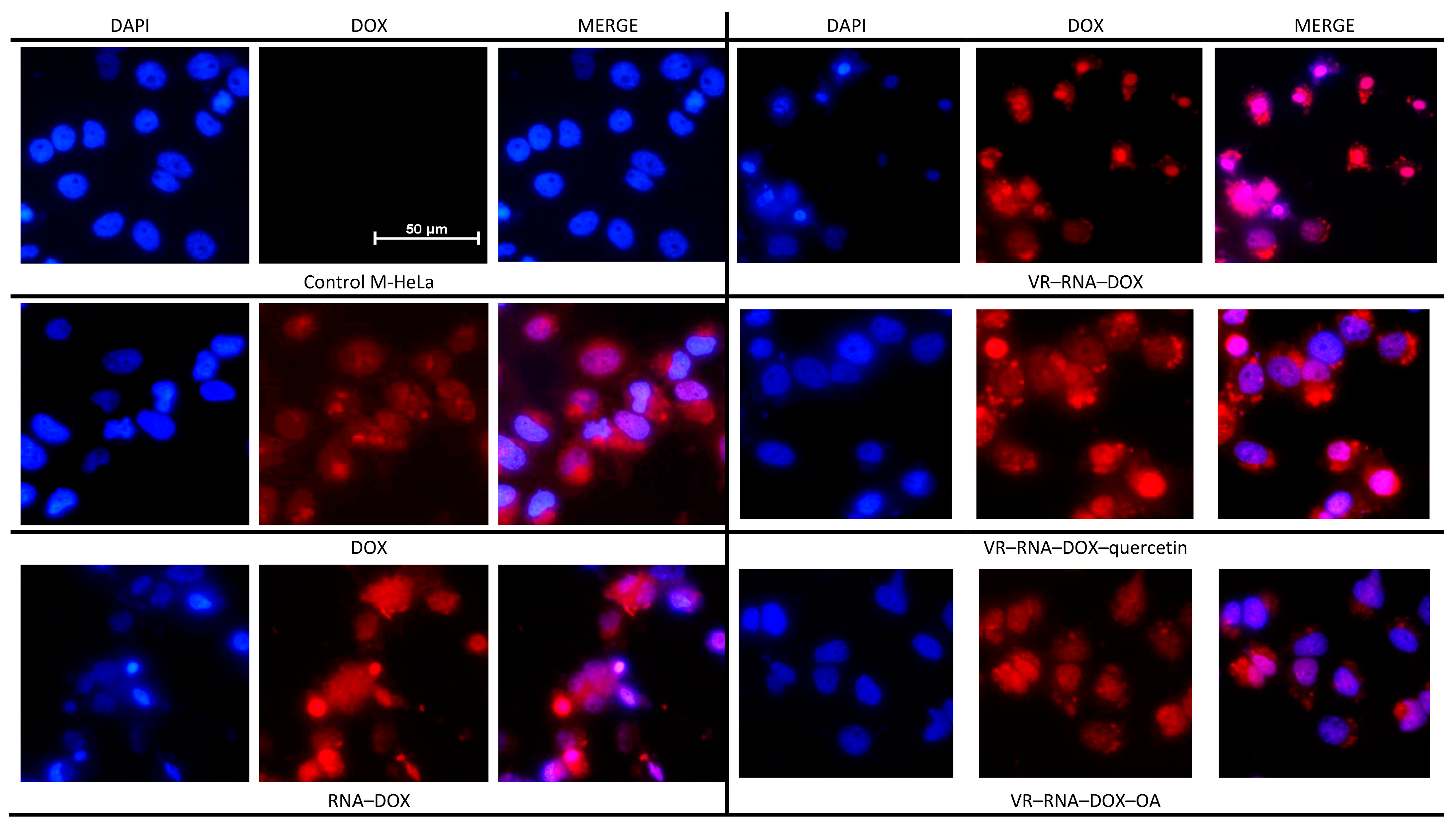
2.3. Biological Properties
3. Materials and Methods
3.1. Chemicals and Solution Preparation
3.2. Absorption Spectrophotometry
3.3. Fluorescence Spectroscopy
3.4. Dynamic and Electrophoretic Light Scattering
3.5. Transmission Electron Microscopy
3.6. Circular Dichroism (CD)
3.7. Conductometry
3.8. FTIR-Spectroscopy
3.9. NMR Spectroscopy
3.10. Substrate Release
3.11. Cytotoxic Activity
3.12. Flow Cytometry
3.13. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, P.; Siddiqui, J.A.; Lakshmanan, I.; Ganti, A.K.; Salgia, R.; Jain, M.; Batra, S.K.; Nasser, M.W. RNA-based therapies: A cog in the wheel of lung cancer defense. Mol. Cancer 2021, 20, 54. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Liang, X.; Li, D.; Leng, S.; Zhu, X. RNA-based pharmacotherapy for tumors: From bench to clinic and back. Biomed. Pharmacother. 2020, 125, 109997. [Google Scholar] [CrossRef]
- Pecot, C.V.; Wu, S.Y.; Bellister, S.; Filant, J.; Rupaimoole, R.; Hisamatsu, T.; Bhattacharya, R.; Maharaj, A.; Azam, S.; Rodriguez-Aguayo, C.; et al. Therapeutic silencing of KRAS using systemically delivered siRNAs. Mol. Cancer Ther. 2014, 13, 2876–2885. [Google Scholar] [CrossRef]
- Zhu, H.B.; Yang, K.; Xie, Y.Q.; Lin, Y.W.; Mao, Q.Q.; Xie, L.P. Silencing of mutant P53 by siRNA induces cell cycle arrest and apoptosis in human bladder cancer cells. World J. Surg. Oncol. 2013, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, Z.; Pan, S.; Shi, M.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Overcoming multidrug resistance by codelivery of MDR1-targeting siRNA and doxorubicin using EphA10-mediated pH-sensitive lipoplexes: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 21590–21600. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guan, W.; Peng, J.; Chen, Y.; Xu, G.; Dou, H. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020, 103, 247–258. [Google Scholar] [CrossRef]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan, S.T.; Kim, N.Y.; et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.D.; Reidenbach, D.; Salomon, N.; Sahin, U.; Türeci, Ö.; Vormehr, M.; Kranz, L.M. MRNA therapeutics in cancer immunotherapy. Mol. Cancer 2021, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tian, Y.; Song, J.; An, G.; Yang, P. MRNA vaccines: The dawn of a new era of cancer immunotherapy. Front. Immunol. 2022, 13, 8871251. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhaori, G. Potential clinical applications of siRNA technique: Benefits and limitations. Eur. J. Clin. Investig. 2011, 41, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Abernathy, E.; Glaunsinger, B. Emerging roles for RNA Degradation in Viral Replication and Antiviral Defense. Virology 2015, 479–480, 600–608. [Google Scholar] [CrossRef]
- Kondili, M.; Roux, M.; Vabret, N.; Bailly-Bechet, M. Innate immune system activation by viral RNA: How to predict It? Virology 2016, 488, 169–178. [Google Scholar] [CrossRef]
- Nishino, M.; Matsuzaki, I.; Musangil, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef]
- Kim, T.K.; Eberwine, J.H. Mammalian cell transfection: The present and the future. Anal. Bioanal. Chem. 2010, 397, 3173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-M.; Wang, Y.-Y.; Zhao, M.-X.; Tan, C.-P.; Li, Y.-Q.; Le, X.-Y.; Ji, L.-N.; Mao, Z.-W. Multifunctional QD-based co-delivery of siRNA and doxorubicin to HeLa cells for reversal of multidrug resistance and real-time tracking. Biomaterials 2012, 33, 2780–2790. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Sabirsh, A.; Ye, L.; Wu, X.; Lu, H.; Liu, J. A Novel graphene quantum dot-based mRNA delivery platform. ChemistryOpen 2021, 10, 666–671. [Google Scholar] [CrossRef]
- Ahir, M.; Upadhyay, P.; Ghosh, A.; Sarker, S.; Bhattacharya, S.; Gupta, P.; Ghosh, S.; Chattopadhyay, S.; Adhikary, A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater. Sci. 2020, 8, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Abeyratne, E.; Tharmarajah, K.; Freitas, J.R.; Mostafavi, H.; Mahalingam, S.; Zaid, A.; Zaman, M.; Taylor, A. Liposomal delivery of the RNA genome of a live-attenuated chikungunya virus vaccine candidate provides local, but not systemic protection after one dose. Front. Immunol. 2020, 11, 304. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, M.; Wang, N.; Li, S.; Liu, Z.; Li, Z.; Ji, Z.; Li, B. Intracellular delivery of messenger RNA to macrophages with surfactant-derived lipid nanoparticles. Mater. Today Adv. 2022, 16, 100295. [Google Scholar] [CrossRef]
- Xu, C.; Li, D.; Cao, Z.; Xiong, M.; Yang, X.; Wang, J. Facile hydrophobization of siRNA with anticancer drug for non-cationic nanocarrier-mediated systemic delivery. Nano Lett. 2019, 19, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The roles of polymers in mRNA delivery. Matter 2022, 5, 1670–1699. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Q.; Shen, Y.; Gao, X.; Li, L.; Yan, Y.; Wang, H.; Cheng, Y. Green tea catechin dramatically promotes RNAi mediated by low-molecular-weight polymers. ACS Cent. Sci. 2018, 4, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Krasheninina, O.; Apartsin, E.; Fuentes, E.; Szulc, A.; Ionov, M.; Venyaminova, A.; Shcharbin, D.; de la Mata, F.; Bryszewska, M.; Gόmez, R. Complexes of pro-apoptotic siRNAs and carbosilane dendrimers: Formation and effect on cancer cells. Pharmaceutics 2019, 11, 25. [Google Scholar] [CrossRef]
- Michanek, A.; Kristen, N.; Höök, F.; Nylander, T.; Sparr, E. RNA and DNA interactions with zwitterionic and charged lipid membranes—A DSC and QCM-D study. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, R.B.; Anderson, D.G.; Green, J.J. Electrostatic surface modifications to improve gene delivery. Expert Opin. Drug Deliv. 2010, 7, 535–550. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, D.; Cao, Z.; Zhang, C.; Cheng, D.; Liu, J.; Shuai, X. Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 2015, 3, 1035–1049. [Google Scholar] [CrossRef]
- Khelghati, N.; Soleimanpour Mokhtarvand, J.; Mir, M.; Alemi, F.; Asemi, Z.; Sadeghpour, A.; Maleki, M.; Samadi Kafil, H.; Jadidi-niaragh, F.; Majidinia, M.; et al. The importance of co-delivery of nanoparticle-siRNA and anticancer agents in cancer therapy. Chem. Biol. Drug Des. 2021, 97, 997–1015. [Google Scholar] [CrossRef]
- Kumar, K.; Rani, V.; Mishra, M.; Chawla, R. New Paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci. Rep. 2017, 7, 46186. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Li, J.Q.; Wang, Z.Z.; Dong, D.W.; Qi, X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Liu, H.; Jain, A.; Patel, P.V.; Cheng, K. Co-delivery of IKBKE siRNA and cabazitaxel by hybrid nanocomplex inhibits invasiveness and growth of triple-negative breast cancer. Sci. Adv. 2020, 6, eabb0616. [Google Scholar] [CrossRef]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Multifunctional lipid-based nanoparticles for codelivery of anticancer drugs and siRNA for treatment of non-small cell lung cancer with different level of resistance and EGFR mutations. Pharmaceutics 2021, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.C.; Huang, Q.; Xu, Z.; Wang, R.; Guo, D.S. Gene delivery based on macrocyclic amphiphiles. Theranostics 2019, 9, 3094–3106. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Zhang, L.; Wang, Z.; Zhou, Q.; Huang, T.; Ge, C.; Xu, H.; Zhu, M.; Zhao, F.; et al. Cyclodextrin-mediated formation of porous RNA nanospheres and their application in synergistic targeted therapeutics of hepatocellular carcinoma. Biomaterials 2020, 261, 120304. [Google Scholar] [CrossRef]
- Kont, A.; Mendonça, M.C.P.; Cronin, M.F.; Cahill, M.R.; O’Driscoll, C.M. Co-formulation of amphiphilic cationic and anionic cyclodextrins forming nanoparticles for siRNA delivery in the treatment of acute myeloid leukaemia. Int. J. Mol. Sci. 2022, 23, 9791. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef] [PubMed]
- Krošl, I.; Otković, E.; Nikšić-Franjić, I.; Colasson, B.; Reinaud, O.; Višnjevac, A.; Piantanida, I. Impact of positive charge and ring-size on the interactions of calixarenes with DNA, RNA and nucleotides. New J. Chem. 2022, 46, 6860–6869. [Google Scholar] [CrossRef]
- Gasparello, J.; Lomazzi, M.; Papi, C.; D’Aversa, E.; Sansone, F.; Casnati, A.; Donofrio, G.; Gambari, R.; Finotti, A. Efficient delivery of microRNA and antimiRNA molecules using an argininocalix[4]arene macrocycle. Mol. Ther. Nucleic Acids 2019, 18, 748–763. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.Y.; Gao, G.J.; Ren, X.W.; Cai, X.Y.; Yu, Q.K.; Xing, S.; Zhu, B. Multistimuli responsive RNA amphiphilic polymeric assembly constructed by calixpyridinium-based supramolecular interactions. Tetrahedron 2020, 76, 131620. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, B.H. Syntheses and structural studies of calix[4]arene-nucleoside and calix[4]arene-oligonucleotide hybrids. Nucleic Acids Res. 2003, 31, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.; Razuvayeva, Y.; Ziganshina, A.; Sapunova, A.; Lyubina, A.; Amerhanova, S.; Kulik, N.; Voloshina, A.; Nizameev, I.; Salnikov, V.; et al. Effect of preorganization and amphiphilicity of calix[4]arene platform on functional properties of viologen derivatives. J. Mol. Liq. 2022, 345, 117801. [Google Scholar] [CrossRef]
- Kashapov, R.; Razuvayeva, Y.; Ziganshina, A.; Lyubina, A.; Amerhanova, S.; Sapunova, A.; Voloshina, A.; Nizameev, I.; Salnikov, V.; Zakharova, L. Formation of supramolecular structures in aqueous medium by noncovalent interactions between surfactant and resorcin[4]arene. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129330. [Google Scholar] [CrossRef]
- Zhao, G.; Cao, K.; Xu, C.; Sun, A.; Lu, W.; Zheng, Y.; Li, H.; Hong, G.L.; Wu, B.; Qiu, Q.M.; et al. Crosstalk between Mitochondrial Fission and Oxidative Stress in Paraquat-Induced Apoptosis in Mouse Alveolar Type II Cells. Int. J. Biol. Sci. 2017, 13, 888–900. [Google Scholar] [CrossRef]
- Yu, G.; Yu, W.; Shao, L.; Zhang, Z.; Chi, X.; Mao, Z.; Gao, C.; Huang, F. Fabrication of a Targeted Drug Delivery System from a Pillar[5]Arene-Based Supramolecular Diblock Copolymeric Amphiphile for Effective Cancer Therapy. Adv. Funct. Mater. 2016, 26, 8999–9008. [Google Scholar] [CrossRef]
- Kharkovsky, A.V.; Panin, L.E. Specific Stimulator of Protein Biosynthesis in Hematopoietic and Immunocompetent Organs. RU Patent 2 045 270 C1, 13 January 1992. [Google Scholar]
- Routh, P.; Mukherjee, P.; Dawn, A.; Nandi, A.K. Self assembly of poly(o-methoxy aniline) with RNA and RNA/DNA hybrids: Physical properties and conformational change of poly(o-methoxy aniline). Biophys. Chem. 2009, 143, 145–153. [Google Scholar] [CrossRef]
- Nasalean, L.; Baudrey, S.; Leontis, N.B.; Jaeger, L. Controlling RNA Self-Assembly to Form Filaments. Nucleic Acids Res. 2006, 34, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Cafferty, B.J.; Gállego, I.; Chen, M.C.; Farley, K.I.; Eritja, R.; Hud, N.V. Efficient self-assembly in water of long noncovalent polymers by nucleobase analogues. J. Am. Chem. Soc. 2013, 135, 2447–2450. [Google Scholar] [CrossRef] [PubMed]
- Van Treeck, B.; Protter, D.S.W.; Matheny, T.; Khong, A.; Link, C.D.; Parker, R. RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. USA 2018, 115, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Szabat, M.; Pedzinski, T.; Czapik, T.; Kierzek, E.; Kierzek, R. Structural aspects of the antiparallel and parallel duplexes formed by DNA, 2′-O-methyl RNA and RNA oligonucleotides. PLoS ONE 2015, 10, e0143354. [Google Scholar] [CrossRef]
- Geinguenaud, F.; Militello, V.; Arluison, V. Application of FTIR Spectroscopy to Analyze RNA Structure. In RNA Spectroscopy; Arluison, V., Wien, F., Eds.; Springer: New York, NY, USA, 2020; pp. 119–134. [Google Scholar] [CrossRef]
- Routh, P.; Garai, A.; Nandi, A.K. Optical and Electronic Properties of Polyaniline Sulfonic Acid-Ribonucleic Acid-Gold Nanobiocomposites. Phys. Chem. Chem. Phys. 2011, 13, 13670–13682. [Google Scholar] [CrossRef]
- Doudna, J.A.; Doherty, E.A. Emerging themes in RNA folding. Fold Des. 1997, 2, 65–70. [Google Scholar] [CrossRef]
- Pradhan, A.B.; Bhuiya, S.; Haque, L.; Das, S. Role of hydroxyl groups in the B-ring of flavonoids in stabilization of the hoogsteen paired third strand of poly(U).poly(A)*poly(U) Triplex. Arch. Biochem. Biophys. 2018, 637, 9–20. [Google Scholar] [CrossRef]
- Friedman, R.A.; Honig, B. A Free energy analysis of nucleic acid base stacking in aqueous solution. Biophys. J. 1995, 69, 1528–1535. [Google Scholar] [CrossRef]
- Rubio, A.R.; Busto, N.; Leal, J.M.; García, B. Doxorubicin binds to duplex RNA with higher affinity than ctDNA and favours the isothermal denaturation of triplex RNA. RSC Adv. 2016, 6, 101142–101152. [Google Scholar] [CrossRef]
- Rhee, S.; Han, Z.J.; Liu, K.; Miles, H.T.; Davies, D.R. Structure of a triple helical DNA with a triplex-duplex junction. Biochemistry 1999, 38, 16810–16815. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Tate, W.P. Primordial soup or vinaigrette: Did the RNA world evolve at acidic pH? Biol. Direct 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, S.; Chen, X.; Pan, S.; Qian, H.; Zhu, X. Effects of quercetin on the efficacy of various chemotherapeutic drugs in cervical cancer cells. Drug Des. Dev. Ther. 2021, 15, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Setyono, B.; Grossmann, M.; Liautard, J.-P. Evidence that proteins bound to the polysomal messenger rna exist also free in the cytoplasm of HeLa cells. Biochimie 1977, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Munnier, E.; Tewes, F.; Cohen-Jonathan, S.; Linassier, C.; Douziech-Eyrolles, L.; Marchais, H.; Soucé, M.; Hervé, K.; Dubois, P.; Chourpa, I. On the interaction of doxorubicin with oleate ions: Fluorescence spectroscopy and liquid-liquid extraction study. Chem. Pharm. Bull. 2007, 55, 1006–1010. [Google Scholar] [CrossRef]
- Bronich, T.K.; Nehls, A.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Novel Drug Delivery Systems Based on the Complexes of Block Ionomers and Surfactants of Opposite Charge. Colloids Surf. B Biointerfaces 1999, 16, 243–251. [Google Scholar] [CrossRef]
- Burns, C.P.; North, J.A.; Petersen, E.S.; Ingraham, L.M. Subcellular Distribution of Doxorubicin: Comparison of Fatty Acid-Modified and Unmodified Cells. Proc. Soc. Exp. Biol. Med. 1988, 188, 455–460. [Google Scholar] [CrossRef] [PubMed]


| Compound | M HeLa | Chang Liver | ||
|---|---|---|---|---|
| IC50 (RNA), mg/mL | IC50 (DOX), µM | IC50 (RNA), mg/mL | IC50 (DOX), µM | |
| RNA | >0.1 | - | >0.1 | - |
| VR:RNA = 1:1 | >0.01 | - | >0.01 | - |
| VR:RNA = 1:10 | >0.1 | - | >0.1 | - |
| VR:RNA = 1:10 | >0.1 | - | >0.1 | - |
| + quercetin | ||||
| VR:RNA = 1:10 + OA | >0.1 | - | >0.1 | - |
| DOX | - | 4.7 | - | >10 |
| RNA + DOX | 0.09 | 9.0 | >0.1 | >10 |
| VR:RNA = 1:10 + DOX | 0.045 | 4.5 | 0.02 | 2.0 |
| VR:RNA = 1:10 | 0.068 | 6.8 | 0.023 | 2.3 |
| + quercetin + DOX | ||||
| VR:RNA = 1:10 | 0.039 | 3.9 | 0.024 | 2.4 |
| + OA + DOX | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashapov, R.; Razuvayeva, Y.; Kashapova, N.; Ziganshina, A.; Salnikov, V.; Sapunova, A.; Voloshina, A.; Zakharova, L. Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene. Int. J. Mol. Sci. 2023, 24, 7911. https://doi.org/10.3390/ijms24097911
Kashapov R, Razuvayeva Y, Kashapova N, Ziganshina A, Salnikov V, Sapunova A, Voloshina A, Zakharova L. Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene. International Journal of Molecular Sciences. 2023; 24(9):7911. https://doi.org/10.3390/ijms24097911
Chicago/Turabian StyleKashapov, Ruslan, Yuliya Razuvayeva, Nadezda Kashapova, Albina Ziganshina, Vadim Salnikov, Anastasiia Sapunova, Alexandra Voloshina, and Lucia Zakharova. 2023. "Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene" International Journal of Molecular Sciences 24, no. 9: 7911. https://doi.org/10.3390/ijms24097911
APA StyleKashapov, R., Razuvayeva, Y., Kashapova, N., Ziganshina, A., Salnikov, V., Sapunova, A., Voloshina, A., & Zakharova, L. (2023). Emergence of Nanoscale Drug Carriers through Supramolecular Self-Assembly of RNA with Calixarene. International Journal of Molecular Sciences, 24(9), 7911. https://doi.org/10.3390/ijms24097911








