Metal-Organic Framework (MOF)—A Universal Material for Biomedicine
Abstract
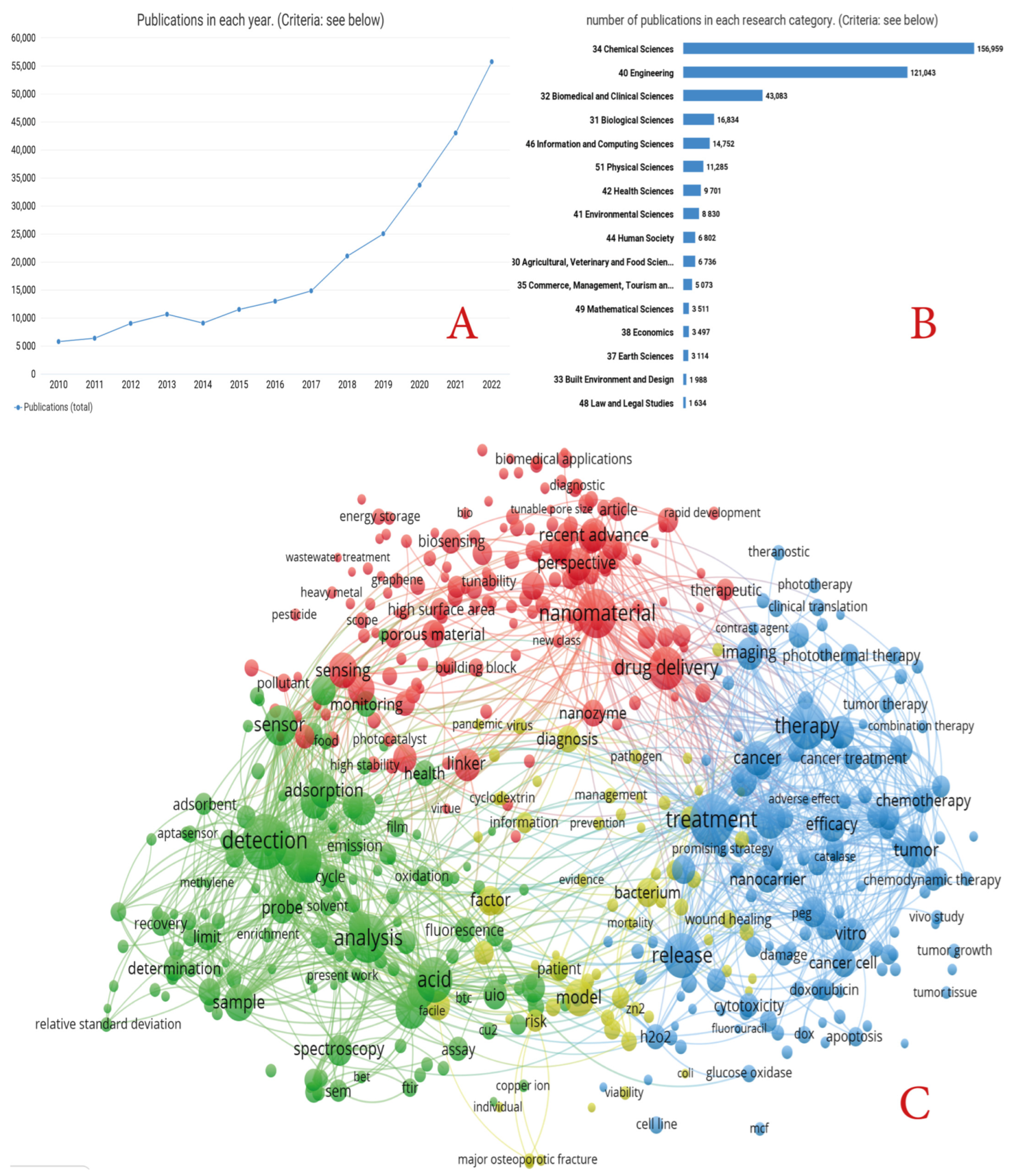
1. Introduction
2. General Approaches and Methods of Synthesizing MOFs for Biomedical Purposes
2.1. Solvothermal and Hydrothermal Methods
2.2. Microwave Synthesis
2.3. Ultrasonic Method
2.4. Mechanochemical Synthesis of MOFs
2.5. Other Synthesis Methods
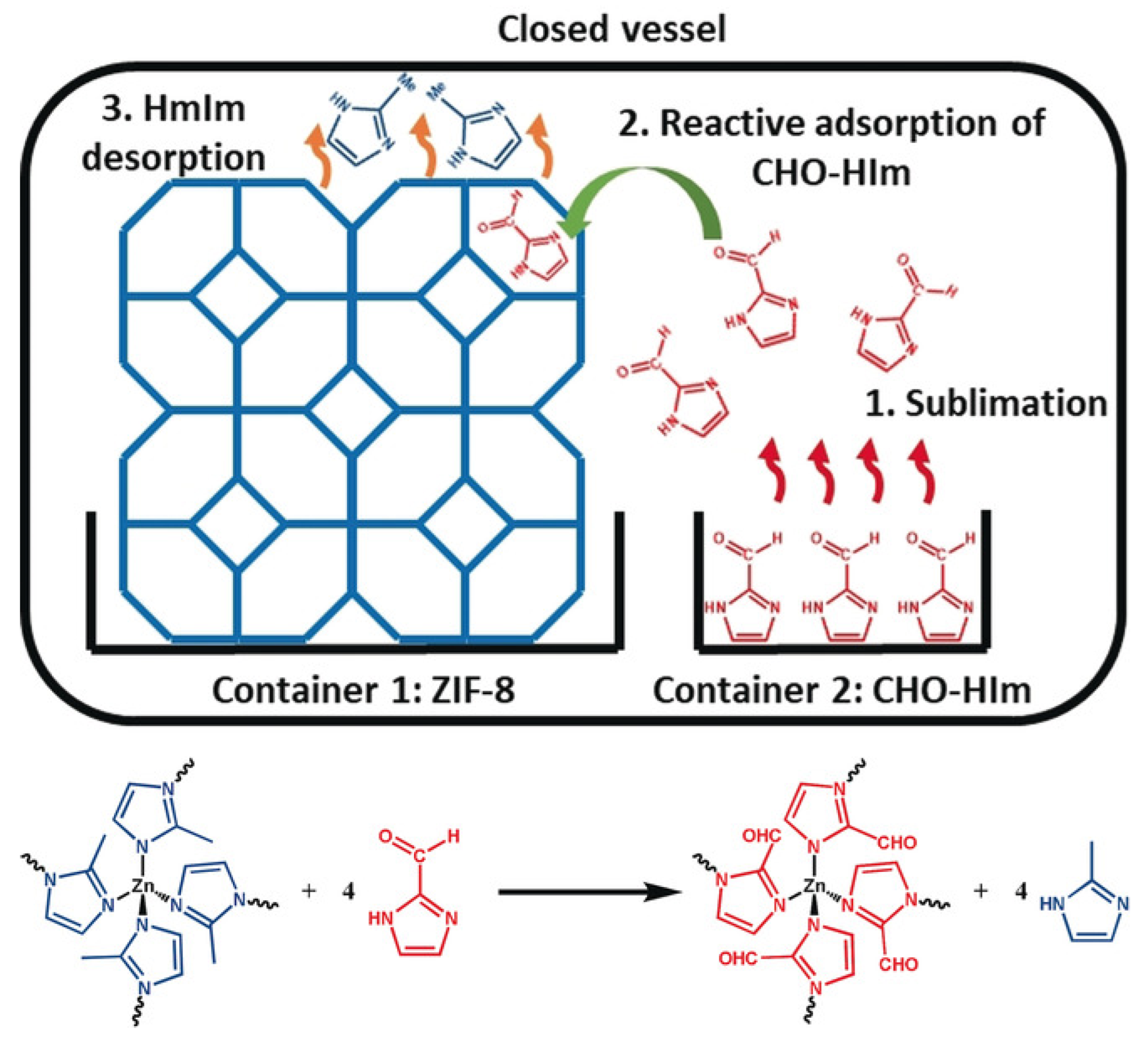
2.6. Post-Synthetic Modification of MOFs for Biomedical Application
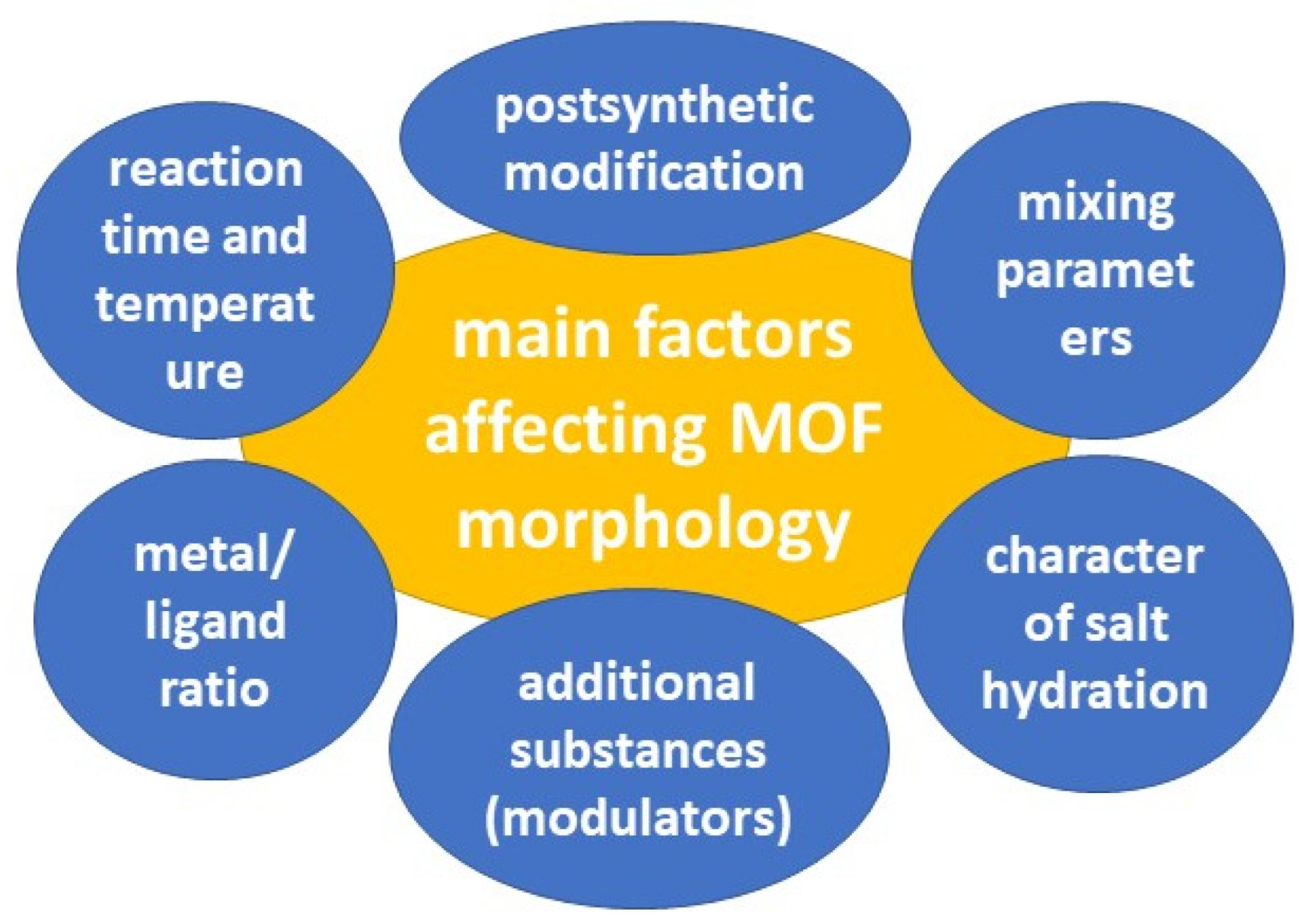
2.7. Adjustment of Main Factors for Biomedical Applications of the MOFs
2.7.1. Size
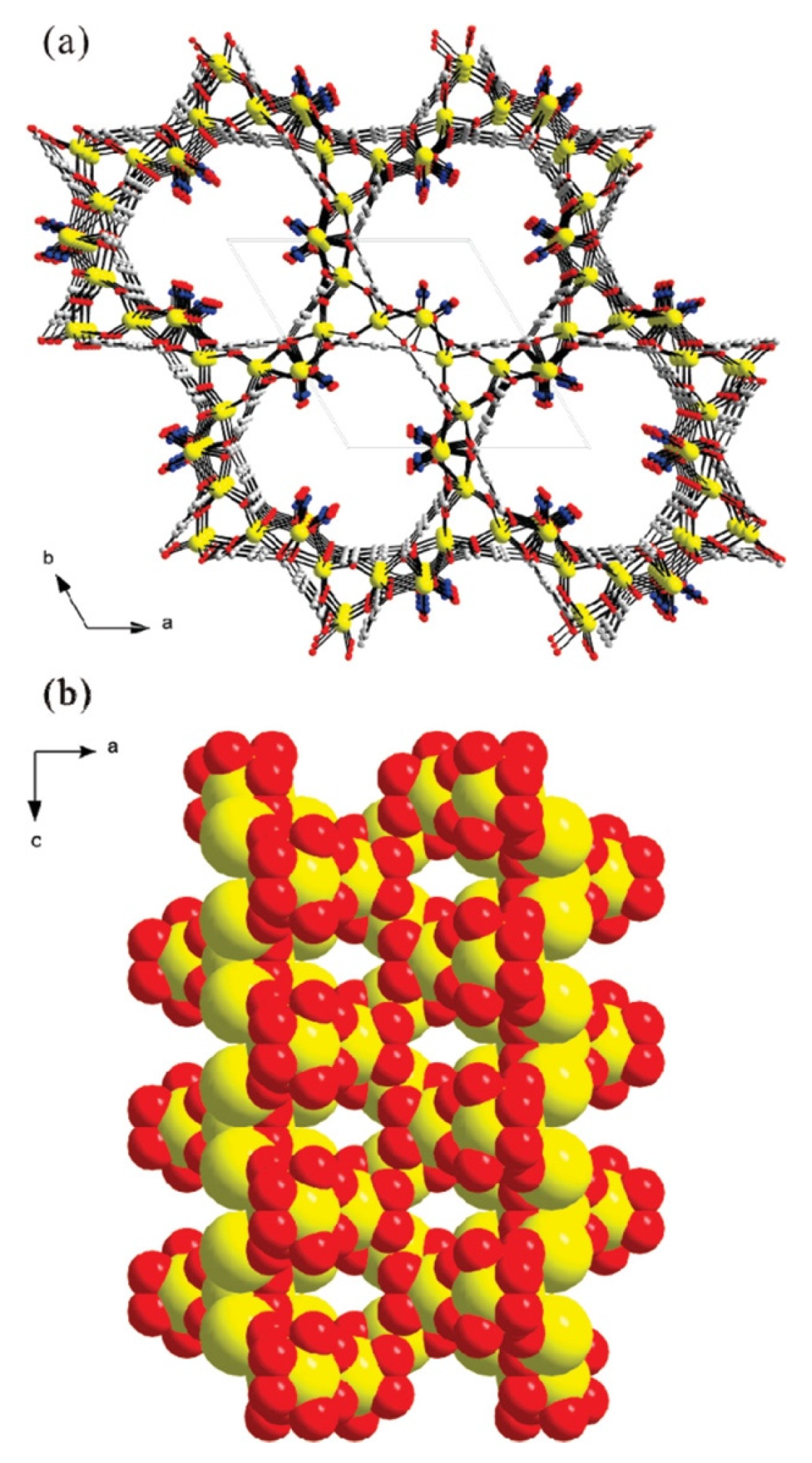
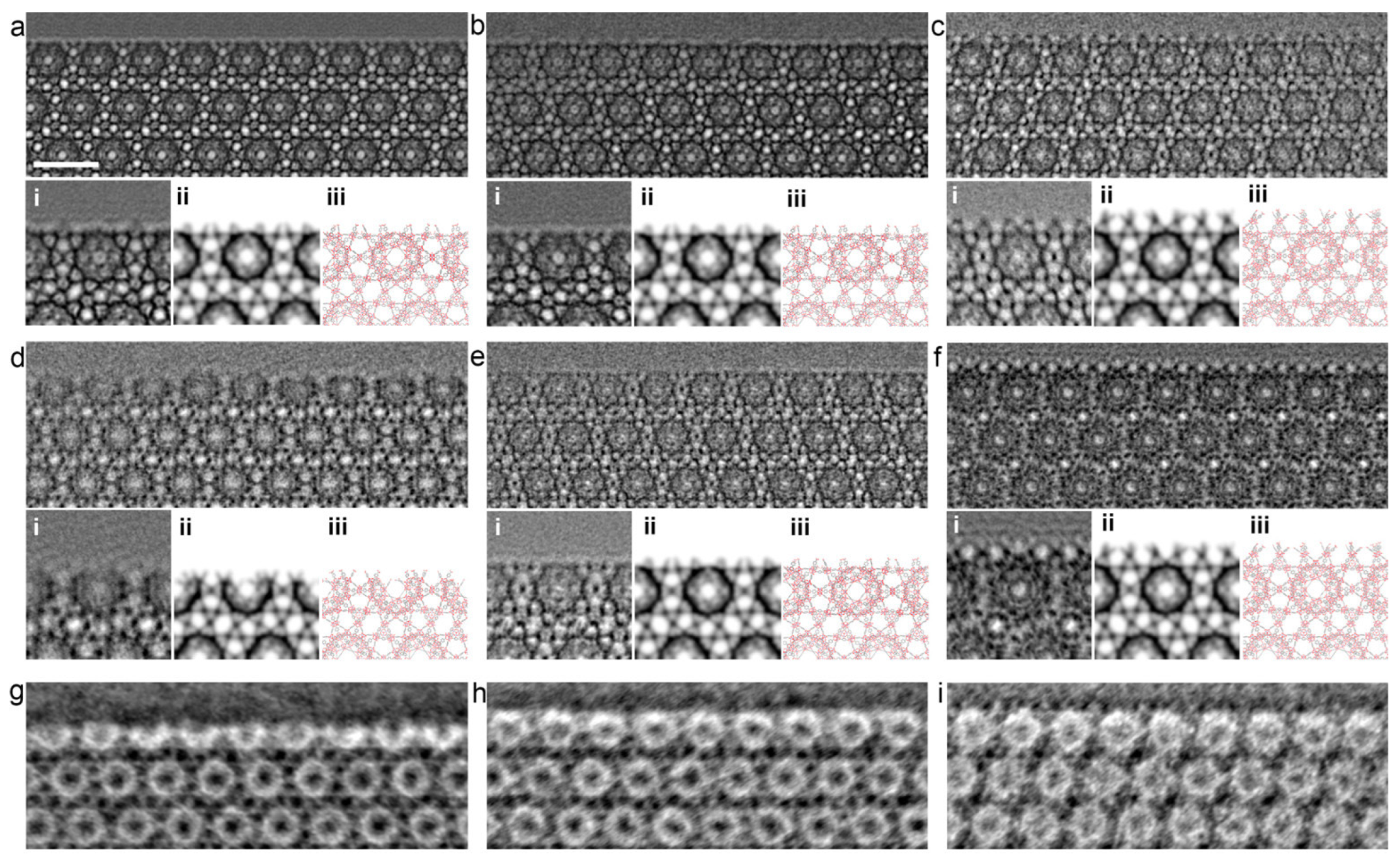
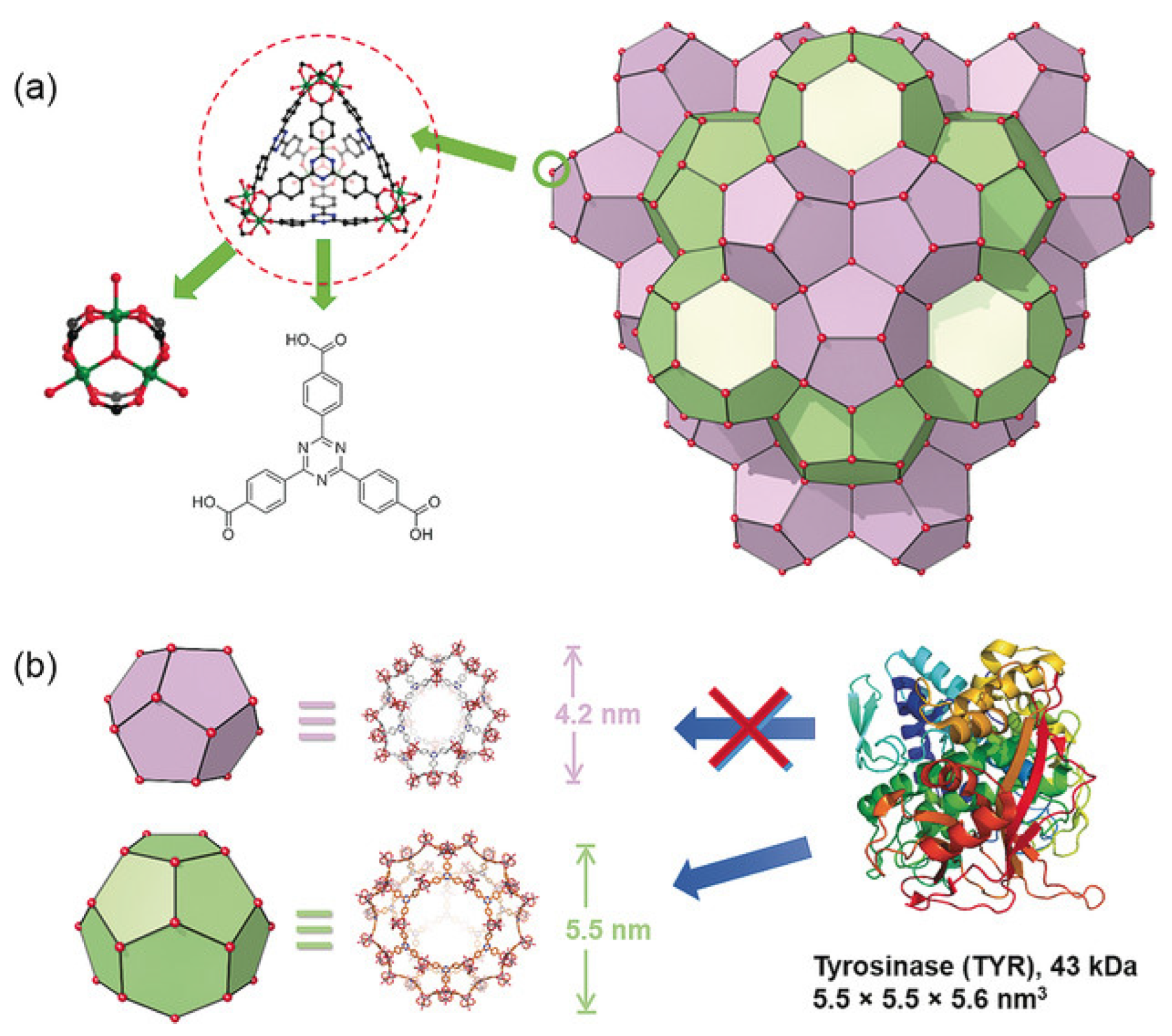
2.7.2. Porosity; Morphology
3. Biomedical Applications of MOFs
3.1. MOFs as a Therapeutic Agent
3.2. Drug Delivery
3.2.1. MOF Carrier
3.2.2. Oral Delivery
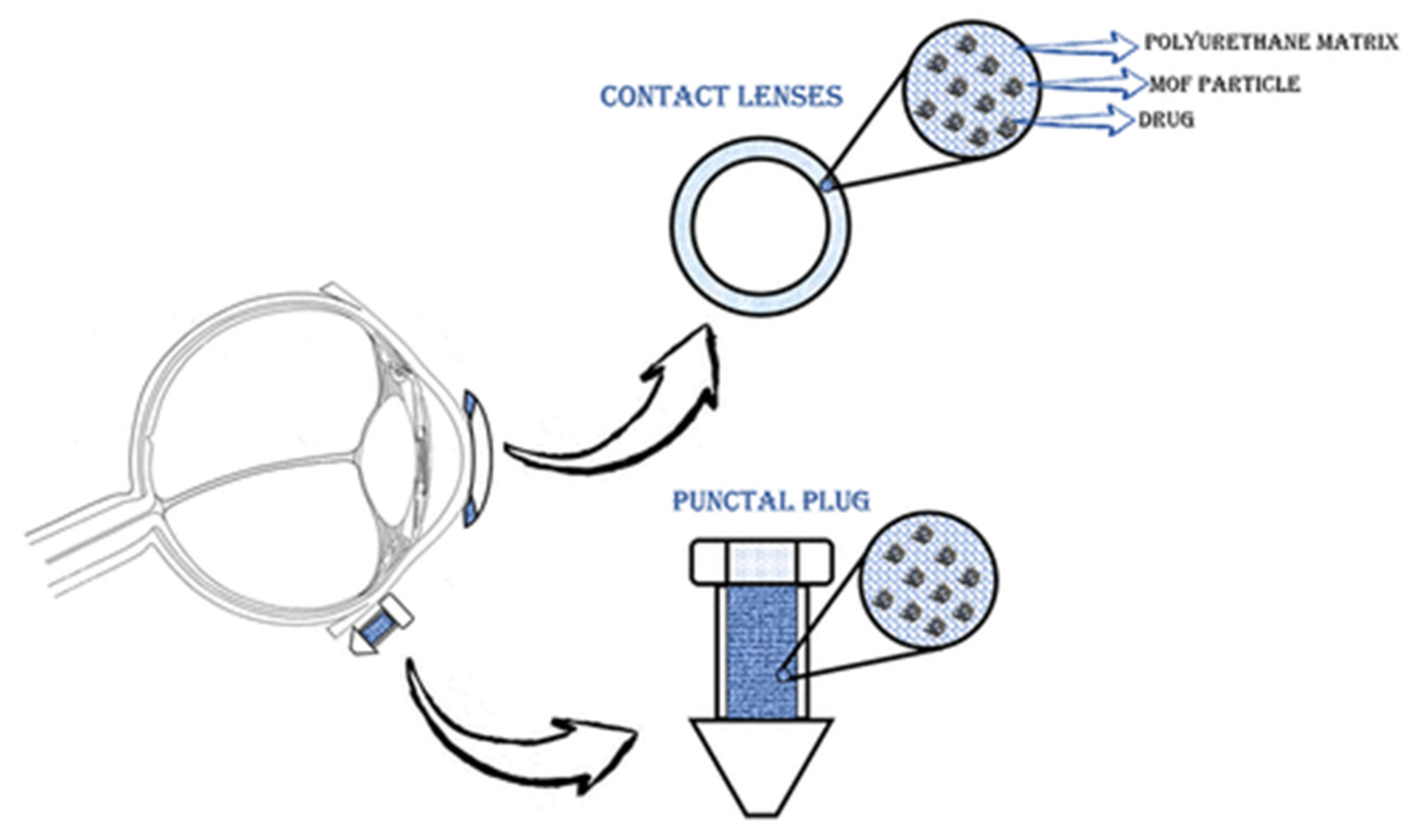
3.2.3. Eye Delivery
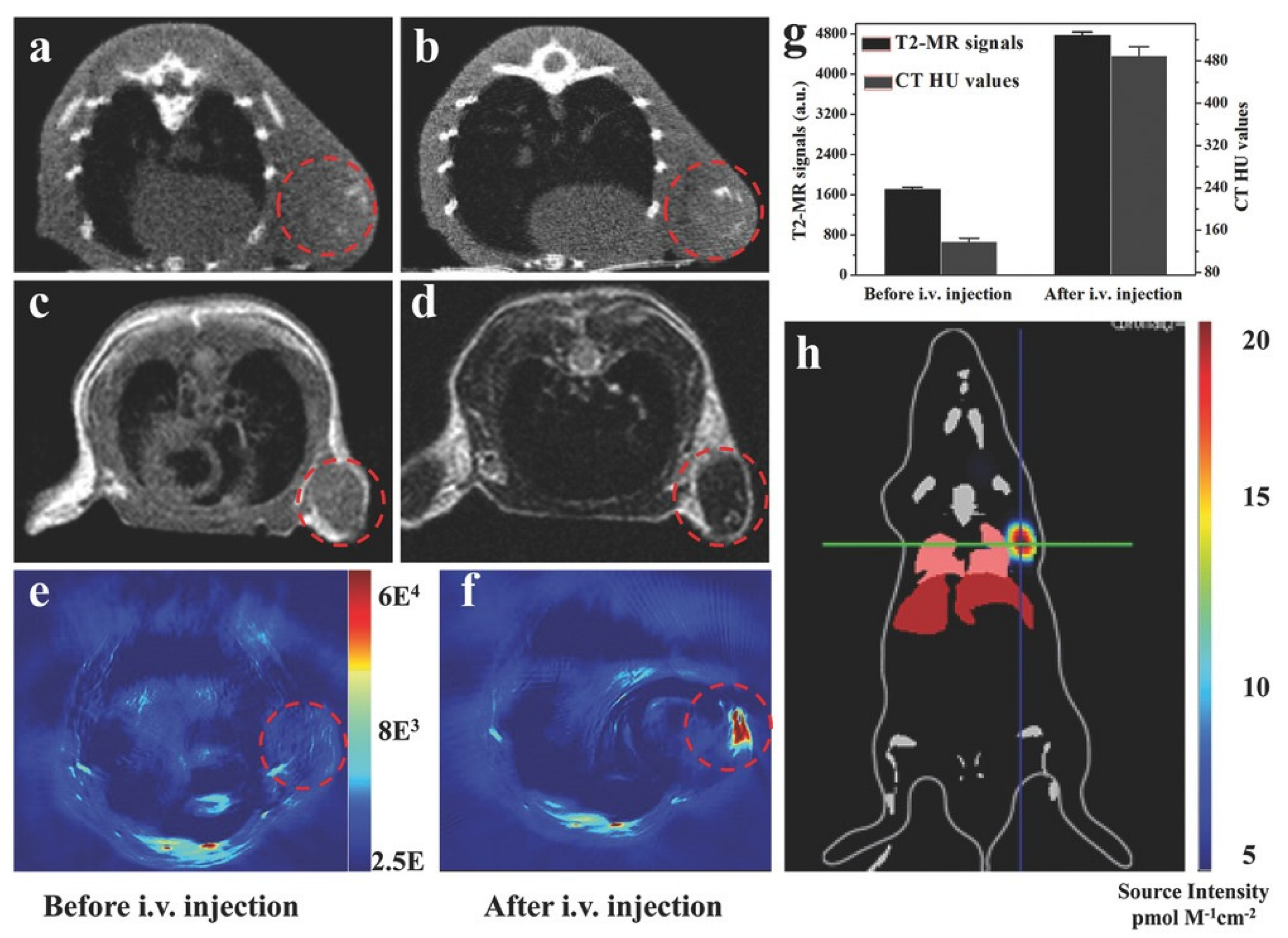
3.3. Diagnostic Systems Based on the MOFs
3.4. Other Biomedical Applications of MOFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| 5-FU | 5-fluorouracil |
| ALP | Alkaline phosphatase |
| ARS | alizarin red S |
| BDC | benzoldicarboxylate |
| CT | Computerized tomography |
| DA | dodecanoic acid |
| DAPI | 4′,6-diamidino-2-phenylindole, a fluorescent stain that binds strongly to adenine–thymine-rich regions in DNA |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| EPR spectroscopy | Electron paramagnetic resonance spectroscopy |
| GSH | glutathione depletion |
| HRTEM | high-resolution transmission electron microscopy |
| IL | ionic liquid |
| MOF | Metal-organic framework |
| MRI (fMRI) | Magnetic resonance imaging (functional MRI) |
| MTT | is a colorimetric assay for assessing cell metabolic activity |
| NMOF | nano MOFs |
| PAI | Photoacoustic imaging |
| Pca | Prostate cancer |
| PDT | Photodynamic therapy |
| PEG | polyethylene glycol |
| PLGA | Copolymer, poly(lactic-co-glycolic acid) |
| PSM | Post-synthetic modification |
| PTT | Photothermal therapy |
| RES | Reticuloendothelial system |
| ROS | reactive oxygen species |
| SDT | Sonodynamic therapy |
| SEM | scanning electron microscopy |
| SOSG | singlet oxygen sensor green |
| VEGF | vascular endothelial growth factor |
| X-ray | nondestructive technique that provides detailed information about the crystallographic structure |
References
- Diez-Pascual, A.M.; Rahdar, A. Functional Nanomaterials in Biomedicine: Current Uses and Potential Applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Gupta, S.; Narayan, M.; Prasad, R.; Krishnan, A. (Eds.) Engineered Nanomaterials for Innovative Therapies and Biomedicine; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-82917-9. [Google Scholar]
- Li, S.; Yang, Y.; Wang, S.; Gao, Y.; Song, Z.; Chen, L.; Chen, Z. Advances in Metal Graphitic Nanocapsules for Biomedicine. Exploration 2022, 2, 20210223. [Google Scholar] [CrossRef]
- Ferreira Soares, D.C.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-Hybrid Nanoparticles: Current Advances in Biomedical Applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Vetcher, A.A.; Stanishevskiy, Y.M. Biopolymeric Nanoparticles—Multifunctional Materials of the Future. Polymers 2022, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical Applications of Polymer-Composite Materials: A Review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Kezimana, P.; Prokonov, F.Y.; Vasilenko, I.A.; Stanishevskiy, Y.M. Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review. Crystals 2022, 12, 272. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Rizk, M.G.H.; Kezimana, P.; Kirichuk, A.A.; Stanishevskiy, Y.M. Application of Gold Nanoparticle-Based Materials in Cancer Therapy and Diagnostics. ChemEngineering 2021, 5, 69. [Google Scholar] [CrossRef]
- Rejepov, D.T.; Vodyashkin, A.A.; Sergorodceva, A.V.; Stanishevskiy, Y.M. Biomedical Applications of Silver Nanoparticles (Review). Drug Dev. Regist. 2021, 10, 176–187. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical Applications of Metallic Nanoparticles in Cancer: Current Status and Future Perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica Nanoparticles: Biomedical Applications and Toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xuan, W.; Cui, Y. Engineering Homochiral Metal-Organic Frameworks for Heterogeneous Asymmetric Catalysis and Enantioselective Separation. Adv. Mater. 2010, 22, 4112–4135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of Metal–Organic Frameworks in Heterogeneous Supramolecular Catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-Organic Frameworks: Versatile Heterogeneous Catalysts for Efficient Catalytic Organic Transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-B.; Liang, J.; Wang, X.-S.; Cao, R. Multifunctional Metal–Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-D.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent Advances in Gas Storage and Separation Using Metal-Organic Frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Zhou, W.; Chen, B. Porous Metal-Organic Frameworks for Gas Storage and Separation: What, How, and Why? J. Phys. Chem. Lett. 2014, 5, 3468–3479. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Xue, H.; Pang, H. Metal-Organic Frameworks for Direct Electrochemical Applications. Coord. Chem. Rev. 2018, 376, 292–318. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
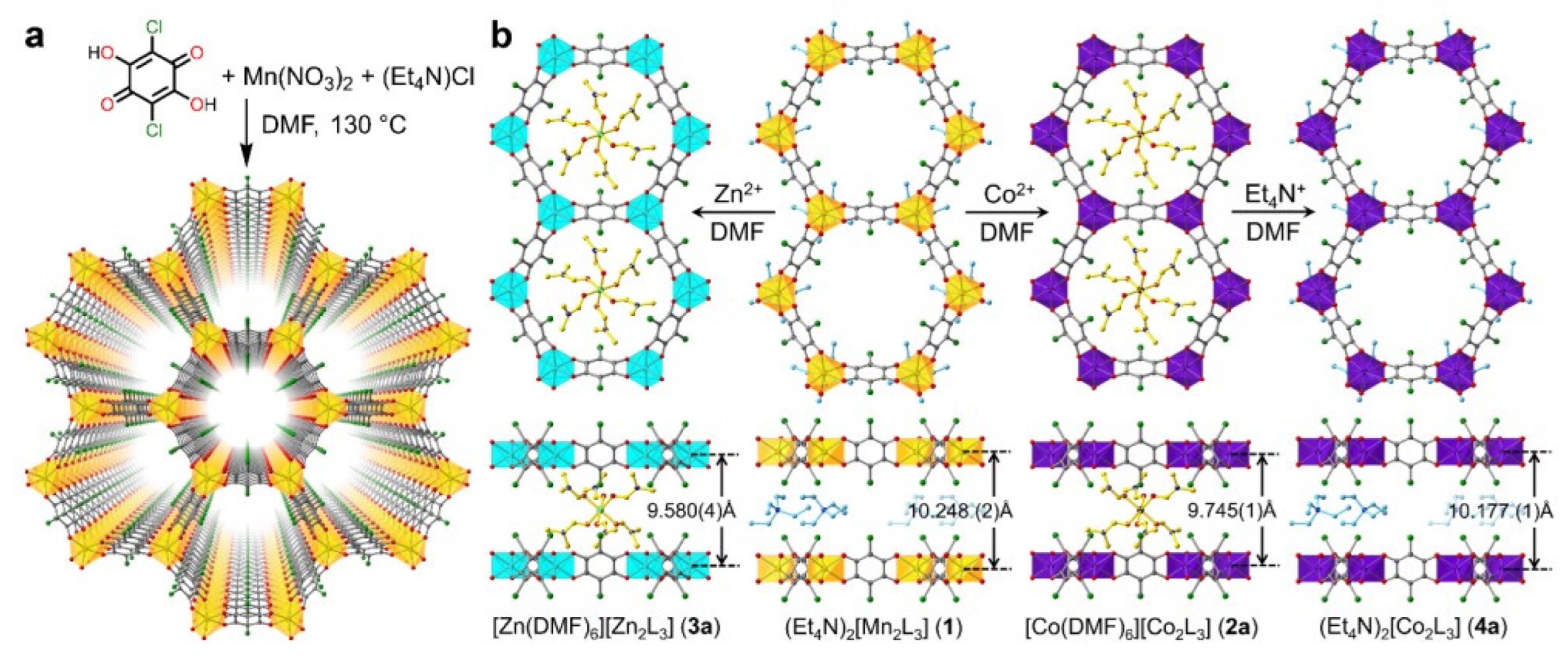
- Zhao, S.-N.; Zhang, Y.; Song, S.-Y.; Zhang, H.-J. Design Strategies and Applications of Charged Metal Organic Frameworks. Coord. Chem. Rev. 2019, 398, 113007. [Google Scholar] [CrossRef]
- Qin, J.; Cho, M.; Lee, Y. Ferrocene-Encapsulated Zn Zeolitic Imidazole Framework (ZIF-8) for Optical and Electrochemical Sensing of Amyloid-β Oligomers and for the Early Diagnosis of Alzheimer’s Disease. ACS Appl. Mater. Interfaces 2019, 11, 11743–11748. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.; Zhang, L.; Drout, R.J.; Li, P.; Haney, C.R.; Brikha, A.; Noh, H.; Mehdi, B.L.; Browning, N.D.; Dravid, V.P.; et al. A Bismuth Metal—Organic Framework as a Contrast Agent for X-Ray Computed Tomography. ACS Appl. Bio Mater. 2019, 2, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Chen, J.; Deng, J.; Xing, X. Thermal Expansion, Ferroelectric and Magnetic Properties in (1 − x)PbTiO3 − x Bi(Ni1/2 Ti1/2)O3. J. Am. Chem. Soc. 2010, 132, 1925–1928. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF Thin Films: Existing and Future Applications. Chem. Soc. Rev. 2011, 40, 1081. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tan, G.; Fang, Y.; Liu, S.; Zhou, Y.; Kumar, A.; Trivedi, M.; Liu, D.; Liu, J. Biomedical Applications of Metal—Organic Framework (MOF)-Based Nano-Enzymes. New J. Chem. 2021, 45, 20987–21000. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef]
- Ge, X.; Wong, R.; Anisa, A.; Ma, S. Recent Development of Metal-Organic Framework Nanocomposites for Biomedical Applications. Biomaterials 2022, 281, 121322. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Rezaei, M.; Yazdani, M.; Mogharabi-Manzari, M. Opportunities and Challenges in Biomedical Applications of Metal-Organic Frameworks. J. Inorg. Organomet. Polym. 2021, 31, 4443–4462. [Google Scholar] [CrossRef]
- Yang, J.; Grzech, A.; Mulder, F.M.; Dingemans, T.J. Methyl Modified MOF-5: A Water Stable Hydrogen Storage Material. Chem. Commun. 2011, 47, 5244. [Google Scholar] [CrossRef]
- Umapathi, A.; Kumawat, M.; Daima, H.K. Engineered Nanomaterials for Biomedical Applications and Their Toxicity: A Review. Environ. Chem. Lett. 2022, 20, 445–468. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Vellingiri, K.; Jo, S.-H.; Kumar, P.; Ok, Y.S.; Kim, K.-H. Recent Advances in Controlled Modification of the Size and Morphology of Metal-Organic Frameworks. Nano Res. 2018, 11, 4441–4467. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of Solvents, PH, Molar Ratio and Temperature in Tuning Metal Organic Framework Architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Xuan, W.; Ramachandran, R.; Zhao, C.; Wang, F. Influence of Synthesis Temperature on Cobalt Metal-Organic Framework (Co-MOF) Formation and Its Electrochemical Performance towards Supercapacitor Electrodes. J. Solid State Electrochem. 2018, 22, 3873–3881. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, J.; Wang, C.; Li, Y.; Chen, Y.; Zhang, M. Effect of Cosolvent and Temperature on the Structures and Properties of Cu-MOF-74 in Low-Temperature NH3-SCR. Ind. Eng. Chem. Res. 2017, 56, 3542–3550. [Google Scholar] [CrossRef]
- Rezaee, R.; Montazer, M.; Mianehro, A.; Mahmoudirad, M. Biomedical Applicable Cellulose Fabric Modified with Zirconium-Based Metal-Organic Frameworks (Zr-MOFs). Starch Stärke 2021, 73, 2100120. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, W.; Rao, C.; Li, B.; Wang, X.; Liu, D.; Pan, Y.; Liu, J. Recent Advances in Fe-MOF Compositions for Biomedical Applications. CMC 2021, 28, 6179–6198. [Google Scholar] [CrossRef]
- Pangestu, A.; Lestari, W.W.; Wibowo, F.R.; Larasati, L. Green Electro-Synthesized MIL-101(Fe) and Its Aspirin Detoxification Performance Compared to MOF-808. J. Inorg. Organomet. Polym. 2022, 32, 1828–1839. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Gizer, G.; Sahiner, M.; Yildirim, Y.; Demirci, S.; Can, M.; Sahiner, N. Rod-like l-Aspartic Acid-Cu(II) Metal Organic Frameworks; Synthesis, Characterization and Biomedical Properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100110. [Google Scholar] [CrossRef]
- Rabiee, N.; Ghadiri, A.M.; Alinezhad, V.; Sedaghat, A.; Ahmadi, S.; Fatahi, Y.; Makvandi, P.; Saeb, M.R.; Bagherzadeh, M.; Asadnia, M.; et al. Synthesis of Green Benzamide-Decorated UiO-66-NH2 for Biomedical Applications. Chemosphere 2022, 299, 134359. [Google Scholar] [CrossRef] [PubMed]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Remya, V.R.; Kurian, M. Synthesis and Catalytic Applications of Metal-Organic Frameworks: A Review on Recent Literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Guo, X. Synthesis Methods and Crystallization of MOFs. Synth. Methods Cryst. 2020, 1–23. [Google Scholar] [CrossRef]
- McKinstry, C.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Effect of Synthesis Conditions on Formation Pathways of Metal Organic Framework (MOF-5) Crystals. Cryst. Growth Des. 2013, 13, 5481–5486. [Google Scholar] [CrossRef]
- Lee, D.W.; Jo, V.; Ok, K.M. Sr2[C6H3(CO2)3(NO3)]·DMF: One-Dimensional Nano-Channel in a New Non-Centrosymmetric Strontium–Organic Framework with High Thermal Stability. Cryst. Growth Des. 2011, 11, 2698–2701. [Google Scholar] [CrossRef]
- Krishnan, R.; Shibu, S.N.; Poelman, D.; Badyal, A.K.; Kunti, A.K.; Swart, H.C.; Menon, S.G. Recent Advances in Microwave Synthesis for Photoluminescence and Photocatalysis. Mater. Today Commun. 2022, 32, 103890. [Google Scholar] [CrossRef]
- Reddy, B.R.; Sridevi, V.; Kumar, T.H.; Rao, C.S.; Palla, V.C.S.; Suriapparao, D.V.; Undi, G.S. Synthesis of Renewable Carbon Biorefinery Products from Susceptor Enhanced Microwave-Assisted Pyrolysis of Agro-Residual Waste: A Review. Process. Saf. Environ. Prot. 2022, 164, 354–372. [Google Scholar] [CrossRef]
- Ren, S.; Yu, H.; Wang, L.; Huang, Z.; Lin, T.; Huang, Y.; Yang, J.; Hong, Y.; Liu, J. State of the Art and Prospects in Metal-Organic Framework-Derived Microwave Absorption Materials. Nano-Micro Lett. 2022, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Jhung, S.H.; Lee, J.-H.; Forster, P.M.; Férey, G.; Cheetham, A.K.; Chang, J.-S. Microwave Synthesis of Hybrid Inorganic–Organic Porous Materials: Phase-Selective and Rapid Crystallization. Chem. Eur. J. 2006, 12, 7899–7905. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A Review on Contemporary Metal–Organic Framework Materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Wu, X.; Bao, Z.; Yuan, B.; Wang, J.; Sun, Y.; Luo, H.; Deng, S. Microwave Synthesis and Characterization of MOF-74 (M = Ni, Mg) for Gas Separation. Microporous Mesoporous Mater. 2013, 180, 114–122. [Google Scholar] [CrossRef]
- Thi Dang, Y.; Hoang, H.T.; Dong, H.C.; Bui, K.-B.T.; Nguyen, L.H.T.; Phan, T.B.; Kawazoe, Y.; Doan, T.L.H. Microwave-Assisted Synthesis of Nano Hf- and Zr-Based Metal-Organic Frameworks for Enhancement of Curcumin Adsorption. Microporous Mesoporous Mater. 2020, 298, 110064. [Google Scholar] [CrossRef]
- Doan, T.L.H.; Dao, T.Q.; Tran, H.N.; Tran, P.H.; Le, T.N. An Efficient Combination of Zr-MOF and Microwave Irradiation in Catalytic Lewis Acid Friedel–Crafts Benzoylation. Dalton Trans. 2016, 45, 7875–7880. [Google Scholar] [CrossRef]
- Taddei, M.; Dau, P.V.; Cohen, S.M.; Ranocchiari, M.; van Bokhoven, J.A.; Costantino, F.; Sabatini, S.; Vivani, R. Efficient Microwave Assisted Synthesis of Metal–Organic Framework UiO-66: Optimization and Scale Up. Dalton Trans. 2015, 44, 14019–14026. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A.; Retailleau, P. Effect of Two Sonochemical Procedures on Achieving to Different Morphologies of Lead(II) Coordination Polymer Nano-Structures. Ultrason. Sonochem. 2013, 20, 1428–1435. [Google Scholar] [CrossRef]
- Toukoniitty, B.; Mikkola, J.-P.; Murzin, D.Y.; Salmi, T. Utilization of Electromagnetic and Acoustic Irradiation in Enhancing Heterogeneous Catalytic Reactions. Appl. Catal. A Gen. 2005, 279, 1–22. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical Synthesis of MOF-5. Chem. Commun. 2008, 6336. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Khan, N.A.; Park, J.H.; Jhung, S.H. Synthesis of a Metal-Organic Framework Material, Iron Terephthalate, by Ultrasound, Microwave, and Conventional Electric Heating: A Kinetic Study. Chem. A Eur. J. 2010, 16, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-W.; Yang, D.-A.; Kim, J.; Kim, J.; Ahn, W.-S. Facile Synthesis of MOF-177 by a Sonochemical Method Using 1-Methyl-2-Pyrrolidinone as a Solvent. Dalton Trans. 2010, 39, 2883. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, J.; Zhang, P.; Dai, S. Mechanochemical Synthesis of Metal-Organic Frameworks. Polyhedron 2019, 162, 59–64. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267. [Google Scholar] [CrossRef]
- Al-Terkawi, A.-A.; Scholz, G.; Emmerling, F.; Kemnitz, E. Strontium-Coordination Polymers Based on Tetrafluorophthalic and Phthalic Acids: Mechanochemical Synthesis, Ab Initio Structures Determination, and Spectroscopic Characterization. Dalton Trans. 2017, 46, 12574–12587. [Google Scholar] [CrossRef]
- Al-Terkawi, A.-A.; Scholz, G.; Emmerling, F.; Kemnitz, E. Mechanochemical Synthesis, Characterization, and Structure Determination of New Alkaline Earth Metal-Tetrafluoroterephthalate Frameworks: Ca(p BDC-F4)·4H2O, Sr(p BDC-F4)·4H2O, and Ba(p BDC-F4). Cryst. Growth Des. 2016, 16, 1923–1933. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Song, X.; Yang, T.; Liang, Z.; Fan, C. In Situ Electrochemical Synthesis of MOF-5 and Its Application in Improving Photocatalytic Activity of BiOBr. Trans. Nonferrous Met. Soc. China 2015, 25, 3987–3994. [Google Scholar] [CrossRef]
- Hackler, R.A.; Pandharkar, R.; Ferrandon, M.S.; Kim, I.S.; Vermeulen, N.A.; Gallington, L.C.; Chapman, K.W.; Farha, O.K.; Cramer, C.J.; Sauer, J.; et al. Isomerization and Selective Hydrogenation of Propyne: Screening of Metal-Organic Frameworks Modified by Atomic Layer Deposition. J. Am. Chem. Soc. 2020, 142, 20380–20389. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Wang, Y.-L.; Zhang, N.; Jiang, Y.-L.; Wei, J.-J.; Luo, F. Spontaneous Resolution in the Ionothermal Synthesis of Homochiral Zn(II) Metal-Organic Frameworks with (10,3)-a Topology Constructed from Achiral 5-Sulfoisophthalate. Cryst. Growth Des. 2011, 11, 3717–3720. [Google Scholar] [CrossRef]
- Lu, K.; Liebman Peláez, A.; Wu, L.; Cao, Y.; Zhu, C.; Fu, H. Ionothermal Synthesis of Five Keggin-Type Polyoxometalate-Based Metal-Organic Frameworks. Inorg. Chem. 2019, 58, 1794–1805. [Google Scholar] [CrossRef]
- Mehta, J.P.; Tian, T.; Zeng, Z.; Divitini, G.; Connolly, B.M.; Midgley, P.A.; Tan, J.-C.; Fairen-Jimenez, D.; Wheatley, A.E.H. Sol-Gel Synthesis of Robust Metal-Organic Frameworks for Nanoparticle Encapsulation. Adv. Funct. Mater. 2018, 28, 1705588. [Google Scholar] [CrossRef]
- Sumida, K.; Liang, K.; Reboul, J.; Ibarra, I.A.; Furukawa, S.; Falcaro, P. Sol–Gel Processing of Metal-Organic Frameworks. Chem. Mater. 2017, 29, 2626–2645. [Google Scholar] [CrossRef]
- Buso, D.; Nairn, K.M.; Gimona, M.; Hill, A.J.; Falcaro, P. Fast Synthesis of MOF-5 Microcrystals Using Sol−Gel SiO2 Nanoparticles. Chem. Mater. 2011, 23, 929–934. [Google Scholar] [CrossRef]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.-C.; Moghadam, P.Z.; Fairen-Jimenez, D. A Sol–Gel Monolithic Metal–Organic Framework with Enhanced Methane Uptake. Nat. Mater. 2018, 17, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K. Supercritical Fluid Processing for Metal-Organic Frameworks, Porous Coordination Polymers, and Covalent Organic Frameworks. J. Supercrit. Fluids 2018, 134, 197–203. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Liu, M.; Bai, Y.; Wang, X.; Shang, S.; Chen, J.; Liu, Y. Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes. Angew. Chem. Int. Ed. 2021, 60, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- Martinez Joaristi, A.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal-Organic Frameworks—Prospective Industrial Applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Abbasi, Z.; Cseri, L.; Zhang, X.; Ladewig, B.P.; Wang, H. Metal-Organic Frameworks (MOFs) and MOF-Derived Porous Carbon Materials for Sustainable Adsorptive Wastewater Treatment. In Sustainable Nanoscale Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–194. ISBN 978-0-12-814681-1. [Google Scholar]
- Kiran, E. Supercritical Fluids and Polymers—The Year in Review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Bai, H.; Li, L.; Li, J. Polymeric Nanoporous Materials Fabricated with Supercritical CO2 and CO2-Expanded Liquids. Chem. Soc. Rev. 2014, 43, 6938–6953. [Google Scholar] [CrossRef] [PubMed]
- Sui, R.; Charpentier, P. Synthesis of Metal Oxide Nanostructures by Direct Sol–Gel Chemistry in Supercritical Fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Vitillo, J.G.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Functionalizing the Defects: Postsynthetic Ligand Exchange in the Metal Organic Framework UiO-66. Chem. Mater. 2016, 28, 7190–7193. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Wang, Z.; Cohen, S.M. Systematic Functionalization of a Metal−Organic Framework via a Postsynthetic Modification Approach. J. Am. Chem. Soc. 2008, 130, 8508–8517. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Hoseinzadeh, H.; Hayati, B.; Mahmoodi, N.M.; Mehraeen, E. Post-Synthetic Functionalization of the Metal-Organic Framework: Clean Synthesis, Pollutant Removal, and Antibacterial Activity. J. Environ. Chem. Eng. 2021, 9, 104590. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal-Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef]
- An, J.; Rosi, N.L. Tuning MOF CO2 Adsorption Properties via Cation Exchange. J. Am. Chem. Soc. 2010, 132, 5578–5579. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Ma, D.; Li, L.; Li, G.; Li, G.; Shi, Z.; Feng, S. A Strategy toward Constructing a Bifunctionalized MOF Catalyst: Post-Synthetic Modification of MOFs on Organic Ligands and Coordinatively Unsaturated Metal Sites. Chem. Commun. 2012, 48, 6151. [Google Scholar] [CrossRef]
- Karagiaridi, O.; Bury, W.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Solvent-Assisted Linker Exchange: An Alternative to the De Novo Synthesis of Unattainable Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2014, 53, 4530–4540. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A. Development of Citric Anhydride Anchored Mesoporous MOF through Post Synthesis Modification to Sequester Potentially Toxic Lead (II) from Water. Microporous Mesoporous Mater. 2018, 261, 198–206. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal-Organic Frameworks toward Applications. Adv. Funct. Mater. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Cohen, S.M. Postsynthetic Modification of Metal-Organic Frameworks—A Progress Report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; DeGayner, J.A.; Winegar, P.H.; Fang, Y.; Harris, T.D. Harnessing Structural Dynamics in a 2D Manganese–Benzoquinoid Framework To Dramatically Accelerate Metal Transport in Diffusion-Limited Metal Exchange Reactions. J. Am. Chem. Soc. 2018, 140, 11444–11453. [Google Scholar] [CrossRef] [PubMed]
- Marreiros, J.; Van Dommelen, L.; Fleury, G.; Oliveira-Silva, R.; Stassin, T.; Iacomi, P.; Furukawa, S.; Sakellariou, D.; Llewellyn, P.L.; Roeffaers, M.; et al. Vapor-Phase Linker Exchange of the Metal–Organic Framework ZIF-8: A Solvent-Free Approach to Post-synthetic Modification. Angew. Chem. 2019, 131, 18642–18646. [Google Scholar] [CrossRef]
- Huang, J.-W.; Chen, Y.-B.; Yang, J.-M.; Zhu, H.-B.; Yang, H. Boosting the Oxygen Reduction Performance of MOF-5-Derived Fe-N-C Electrocatalysts via a Dual Strategy of Cation-Exchange and Guest-Encapsulation. Electrochim. Acta 2021, 366, 137408. [Google Scholar] [CrossRef]
- Grancha, T.; Acosta, A.; Cano, J.; Ferrando-Soria, J.; Seoane, B.; Gascon, J.; Pasán, J.; Armentano, D.; Pardo, E. Cation Exchange in Dynamic 3D Porous Magnets: Improvement of the Physical Properties. Inorg. Chem. 2015, 54, 10834–10840. [Google Scholar] [CrossRef]
- Park, J.; Feng, D.; Zhou, H.-C. Dual Exchange in PCN-333: A Facile Strategy to Chemically Robust Mesoporous Chromium Metal–Organic Framework with Functional Groups. J. Am. Chem. Soc. 2015, 137, 11801–11809. [Google Scholar] [CrossRef]
- Liu, T.; Yan, B. A Stable Broad-Range Fluorescent PH Sensor Based on Eu3+ Post-Synthetic Modification of a Metal–Organic Framework. Ind. Eng. Chem. Res. 2020, 59, 1764–1771. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S. An Investigation of Affecting Factors on MOF Characteristics for Biomedical Applications: A Systematic Review. Heliyon 2021, 7, e06914. [Google Scholar] [CrossRef]
- Gao, X.; Cui, R.; Ji, G.; Liu, Z. Size and Surface Controllable Metal-Organic Frameworks (MOFs) for Fluorescence Imaging and Cancer Therapy. Nanoscale 2018, 10, 6205–6211. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Yang, J.; Liang, A.; Li, Y.; Zhuang, Q.; Gu, J. Nanoscale Zr-Based MOFs with Tailorable Size and Introduced Mesopore for Protein Delivery. Adv. Funct. Mater. 2018, 28, 1707356. [Google Scholar] [CrossRef]
- Nguyen Thi, H.P.; Ninh, H.D.; Tran, C.V.; Le, B.T.; Bhosale, S.V.; La, D.D. Size-Control and Surface Modification of Flexible Metal-Organic Framework MIL-53(Fe) by Polyethyleneglycol for 5- Fluorouracil Anticancer Drug Delivery. ChemistrySelect 2019, 4, 2333–2338. [Google Scholar] [CrossRef]
- Cravillon, J.; Münzer, S.; Lohmeier, S.-J.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412. [Google Scholar] [CrossRef]
- Lim, I.H.; Schrader, W.; Schüth, F. Insights into the Molecular Assembly of Zeolitic Imidazolate Frameworks by ESI-MS. Chem. Mater. 2015, 27, 3088–3095. [Google Scholar] [CrossRef]
- Marshall, C.R.; Staudhammer, S.A.; Brozek, C.K. Size Control over Metal–Organic Framework Porous Nanocrystals. Chem. Sci. 2019, 10, 9396–9408. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous Metal–Organic-Framework Nanoscale Carriers as a Potential Platform for Drug Delivery and Imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Duren, T. Opening the Gate: Framework Flexibility in ZIF-8 Explored by Experiments and Simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Liu, X.; Liu, L.; Cha, D.; Zheng, X.; Yousef, A.A.; Song, K.; Zhu, Y.; Zhang, D.; et al. Direct Imaging of Tunable Crystal Surface Structures of MOF MIL-101 Using High-Resolution Electron Microscopy. J. Am. Chem. Soc. 2019, 141, 12021–12028. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Muhammad, F.; Sun, F.; Tian, Y.; Zhu, G. Size, Shape, and Porosity Control of Medi-MOF-1 via Growth Modulation under Microwave Heating. Cryst. Growth Des. 2019, 19, 889–895. [Google Scholar] [CrossRef]
- Ansari, S.M.; Bhor, R.D.; Pai, K.R.; Sen, D.; Mazumder, S.; Ghosh, K.; Kolekar, Y.D.; Ramana, C.V. Cobalt Nanoparticles for Biomedical Applications: Facile Synthesis, Physiochemical Characterization, Cytotoxicity Behavior and Biocompatibility. Appl. Surf. Sci. 2017, 414, 171–187. [Google Scholar] [CrossRef]
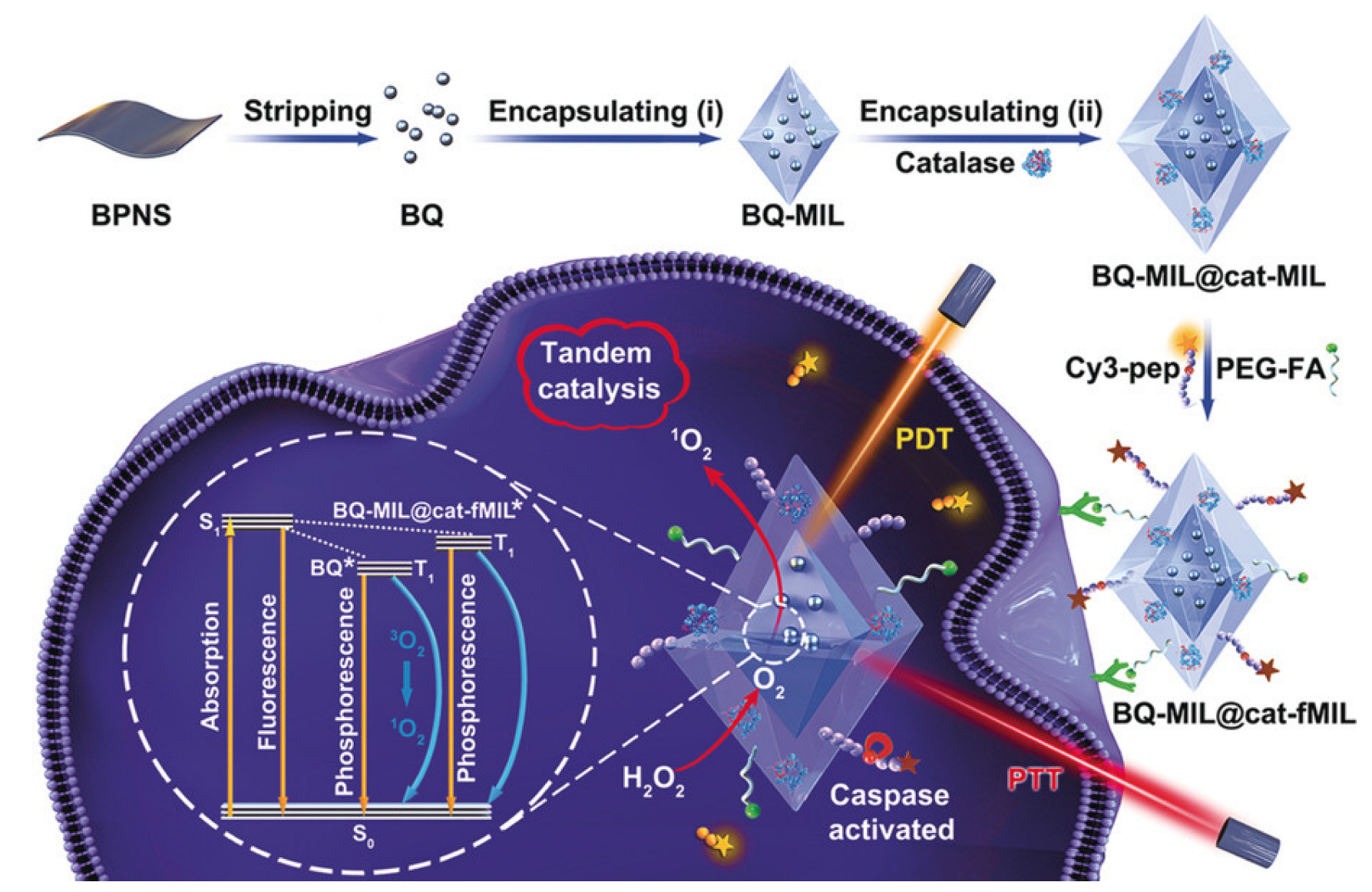
- Liu, J.; Liu, T.; Du, P.; Zhang, L.; Lei, J. Metal–Organic Framework (MOF) Hybrid as a Tandem Catalyst for Enhanced Therapy against Hypoxic Tumor Cells. Angew. Chem. 2019, 131, 7890–7894. [Google Scholar] [CrossRef]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.-P.; Zhou, H.-C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chem. 2018, 130, 5827–5832. [Google Scholar] [CrossRef]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An Ultrasound Activated Vesicle of Janus Au-MnO Nanoparticles for Promoted Tumor Penetration and Sono-Chemodynamic Therapy of Orthotopic Liver Cancer. Angew. Chem. Int. Ed. 2020, 59, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
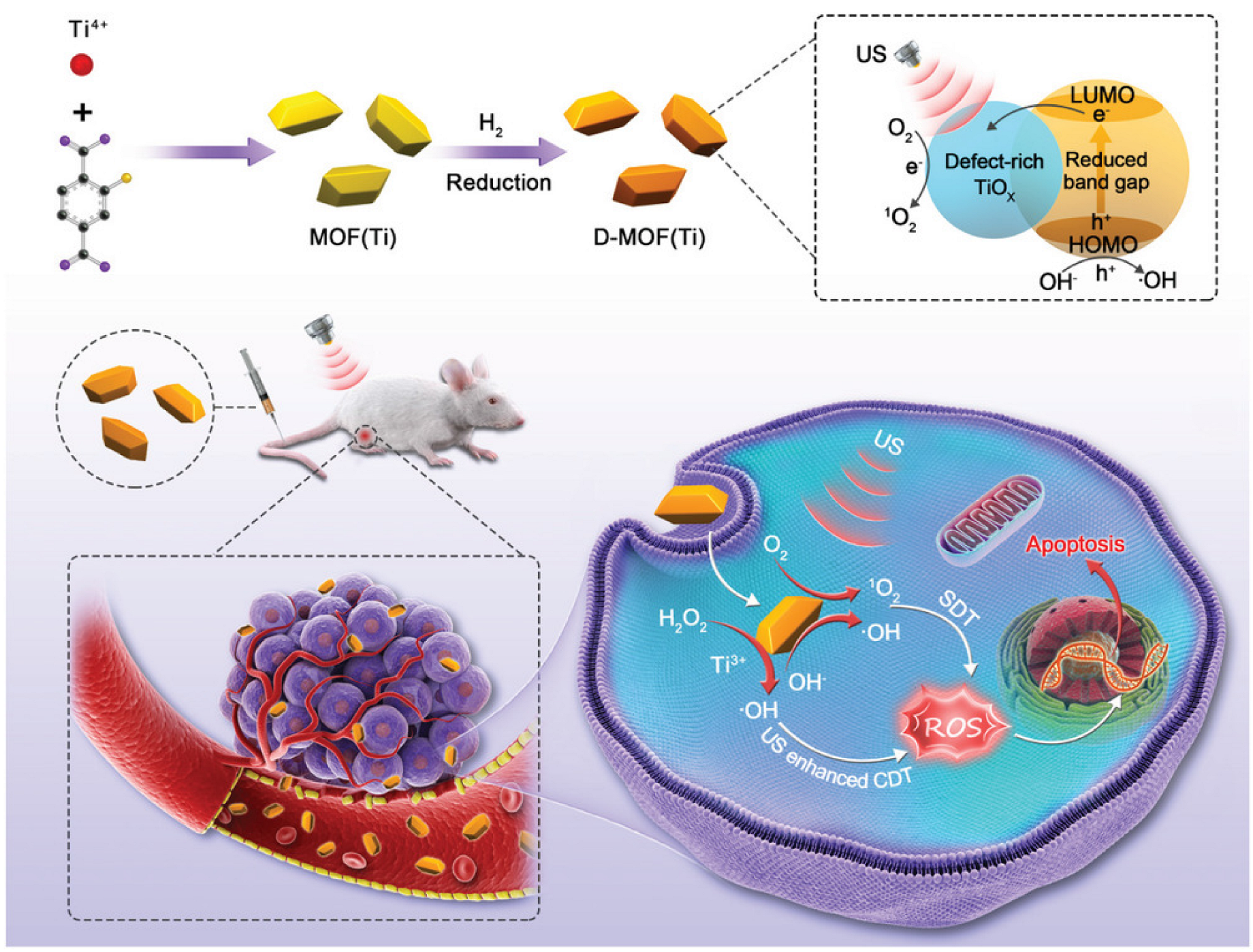
- Liang, S.; Xiao, X.; Bai, L.; Liu, B.; Yuan, M.; Ma, P.; Pang, M.; Cheng, Z.; Lin, J. Conferring Ti-Based MOFs with Defects for Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2021, 33, 2100333. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Wells, C.J.R.; Forgan, R.S. Multivariate Modulation of the Zr MOF UiO-66 for Defect-Controlled Combination Anticancer Drug Delivery. Angew. Chem. 2020, 132, 5249–5255. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Shi, Z.; Yang, B.; Liu, H.; Ding, S.; Wang, X.; Lei, Q.; Wu, J.; Fang, W. MOF Nanoparticles with Encapsulated Autophagy Inhibitor in Controlled Drug Delivery System for Antitumor. ACS Appl. Mater. Interfaces 2018, 10, 2328–2337. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Xia, X.; Cao, Z.; Deng, Y.; Tang, B. Sr/PTA Metal Organic Framework as A Drug Delivery System for Osteoarthritis Treatment. Sci. Rep. 2019, 9, 17570. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Shi, R.; Kang, T.; Pang, G.; Wang, B.; Zhao, Y.; Zeng, X.; Zou, C.; Wu, P.; et al. Synthesis of Hollow Nanocages MOF-5 as Drug Delivery Vehicle to Solve the Load-Bearing Problem of Insoluble Antitumor Drug Oleanolic Acid (OA). Inorg. Chem. Commun. 2018, 96, 20–23. [Google Scholar] [CrossRef]
- Li, L.; Han, S.; Zhao, S.; Li, X.; Liu, B.; Liu, Y. Chitosan Modified Metal-Organic Frameworks as a Promising Carrier for Oral Drug Delivery. RSC Adv. 2020, 10, 45130–45138. [Google Scholar] [CrossRef]
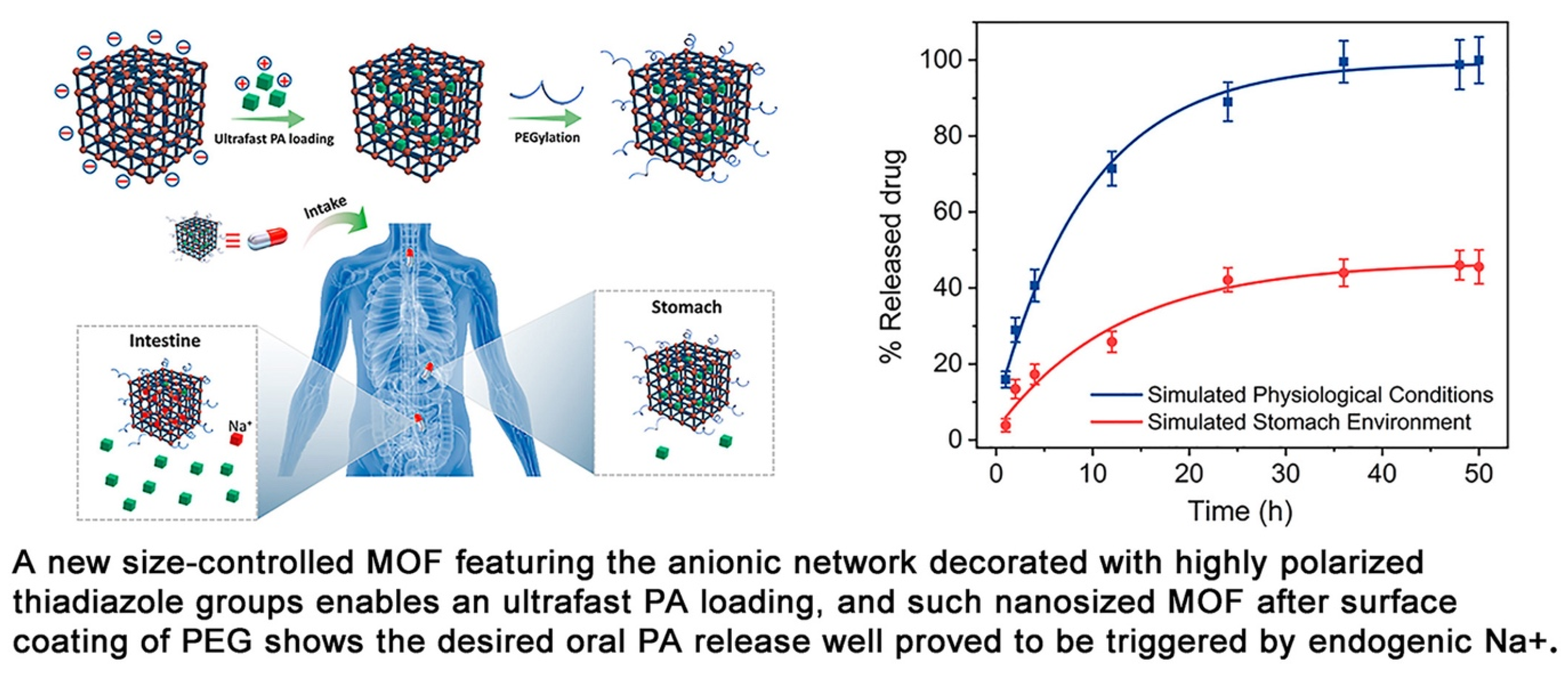
- Jiang, K.; Ni, W.; Cao, X.; Zhang, L.; Lin, S. A Nanosized Anionic MOF with Rich Thiadiazole Groups for Controlled Oral Drug Delivery. Mater. Today Bio 2022, 13, 100180. [Google Scholar] [CrossRef]
- Kim, S.-N.; Park, C.G.; Huh, B.K.; Lee, S.H.; Min, C.H.; Lee, Y.Y.; Kim, Y.K.; Park, K.H.; Choy, Y.B. Metal-Organic Frameworks, NH2-MIL-88(Fe), as Carriers for Ophthalmic Delivery of Brimonidine. Acta Biomater. 2018, 79, 344–353. [Google Scholar] [CrossRef]
- Gandara-Loe, J.; Ortuño-Lizarán, I.; Fernández-Sanchez, L.; Alió, J.L.; Cuenca, N.; Vega-Estrada, A.; Silvestre-Albero, J. Metal-Organic Frameworks as Drug Delivery Platforms for Ocular Therapeutics. ACS Appl. Mater. Interfaces 2019, 11, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Gandara-Loe, J.; Souza, B.E.; Missyul, A.; Giraldo, G.; Tan, J.-C.; Silvestre-Albero, J. MOF-Based Polymeric Nanocomposite Films as Potential Materials for Drug Delivery Devices in Ocular Therapeutics. ACS Appl. Mater. Interfaces 2020, 12, 30189–30197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, J.; Gong, M.; Li, K.; Gu, J. Specific Screening of Prostate Cancer Individuals Using an Enzyme-Assisted Substrate Sensing Platform Based on Hierarchical MOFs with Tunable Mesopore Size. J. Am. Chem. Soc. 2021, 143, 15145–15151. [Google Scholar] [CrossRef] [PubMed]
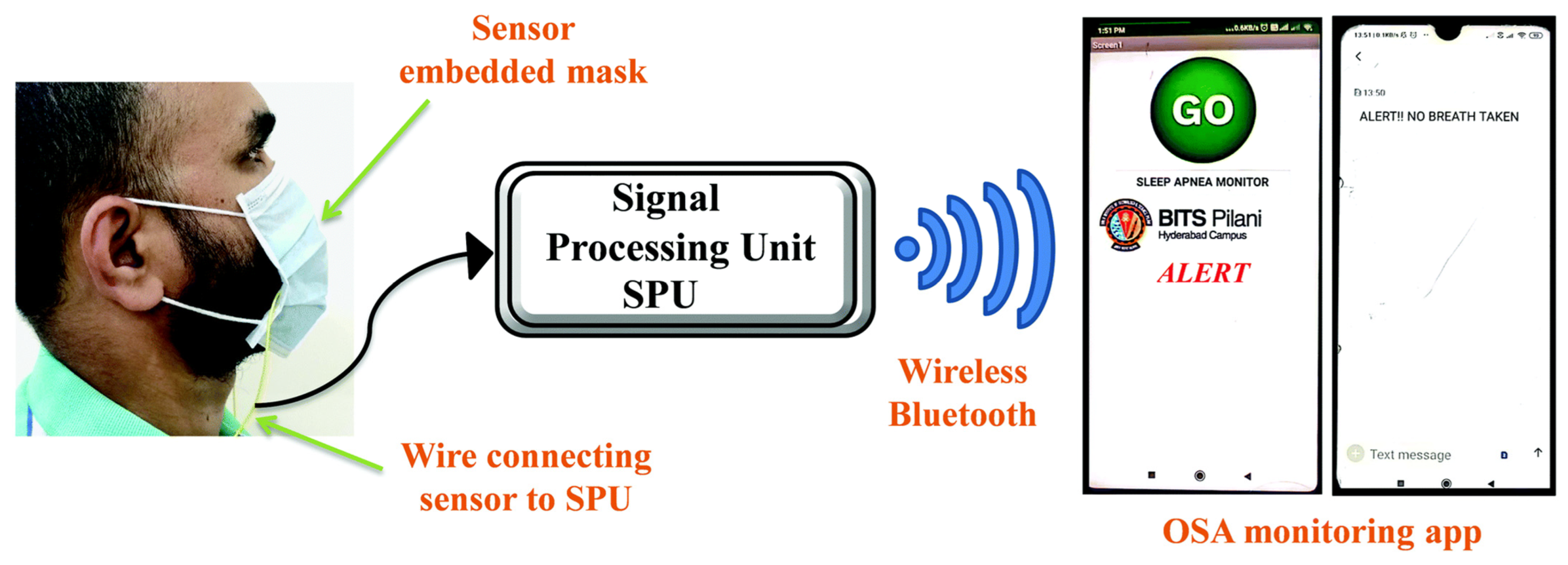
- Leelasree, T.; Selamneni, V.; Akshaya, T.; Sahatiya, P.; Aggarwal, H. MOF Based Flexible, Low-Cost Chemiresistive Device as a Respiration Sensor for Sleep Apnea Diagnosis. J. Mater. Chem. B 2020, 8, 10182–10189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jia, X.; Zhen, W.; Zhang, M.; Jiang, X. Small-Sized MOF-Constructed Multifunctional Diagnosis and Therapy Platform for Tumor. ACS Biomater. Sci. Eng. 2019, 5, 4435–4441. [Google Scholar] [CrossRef]
- Shang, W.; Zeng, C.; Du, Y.; Hui, H.; Liang, X.; Chi, C.; Wang, K.; Wang, Z.; Tian, J. Core–Shell Gold Nanorod@Metal–Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma. Adv. Mater. 2017, 29, 1604381. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Y.; Tan, R.; Li, H.; Tu, Y. Determination of β-Amyloid Oligomer Using Electrochemiluminescent Aptasensor with Signal Enhancement by AuNP/MOF Nanocomposite. Microchim. Acta 2021, 188, 53. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Mei, L.; Zhang, L.; Wang, X.; Liao, X.; Qiao, X.; Hong, C. PSA Detection Electrochemical Immunosensor Based on MOF-235 Nanomaterial Adsorption Aggregation Signal Amplification Strategy. Microchem. J. 2021, 171, 106870. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Osman, D.I.; Ali, O.I.; Shousha, W.G.; Shoeib, M.A.; Shawky, S.M.; Sheta, S.M. A Novel Ag/Zn Bimetallic MOF as a Superior Sensitive Biosensing Platform for HCV-RNA Electrochemical Detection. Appl. Surf. Sci. 2021, 562, 150202. [Google Scholar] [CrossRef]
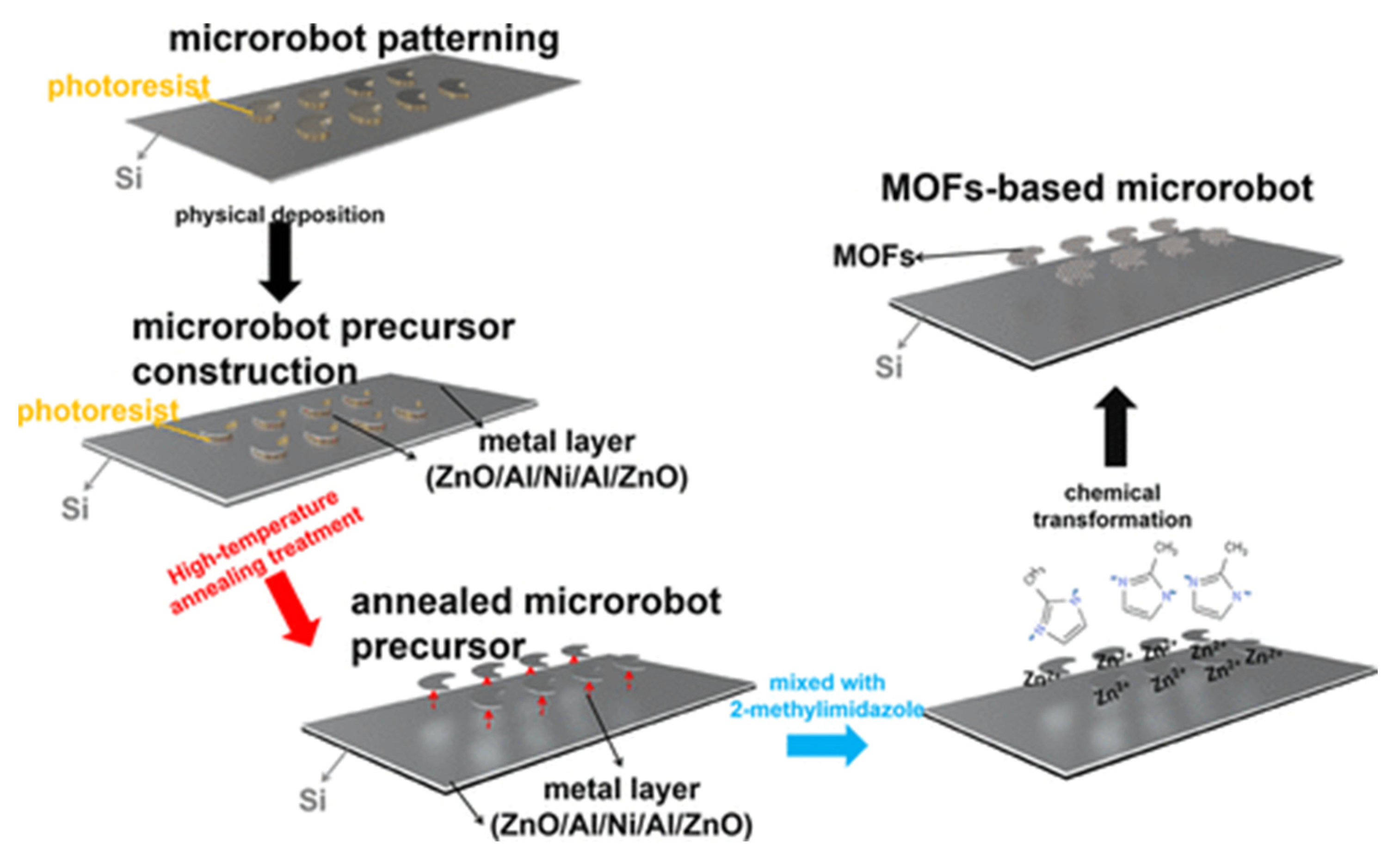
- Mu, X.; Wang, Z.; Zhong, Y.; Jiang, T.; Cheang, U.K. Development of 2D MOF-Based Microrobots under Annealing Treatment and Their Biomedical Application. Ind. Eng. Chem. Res. 2021, 60, 9465–9474. [Google Scholar] [CrossRef]
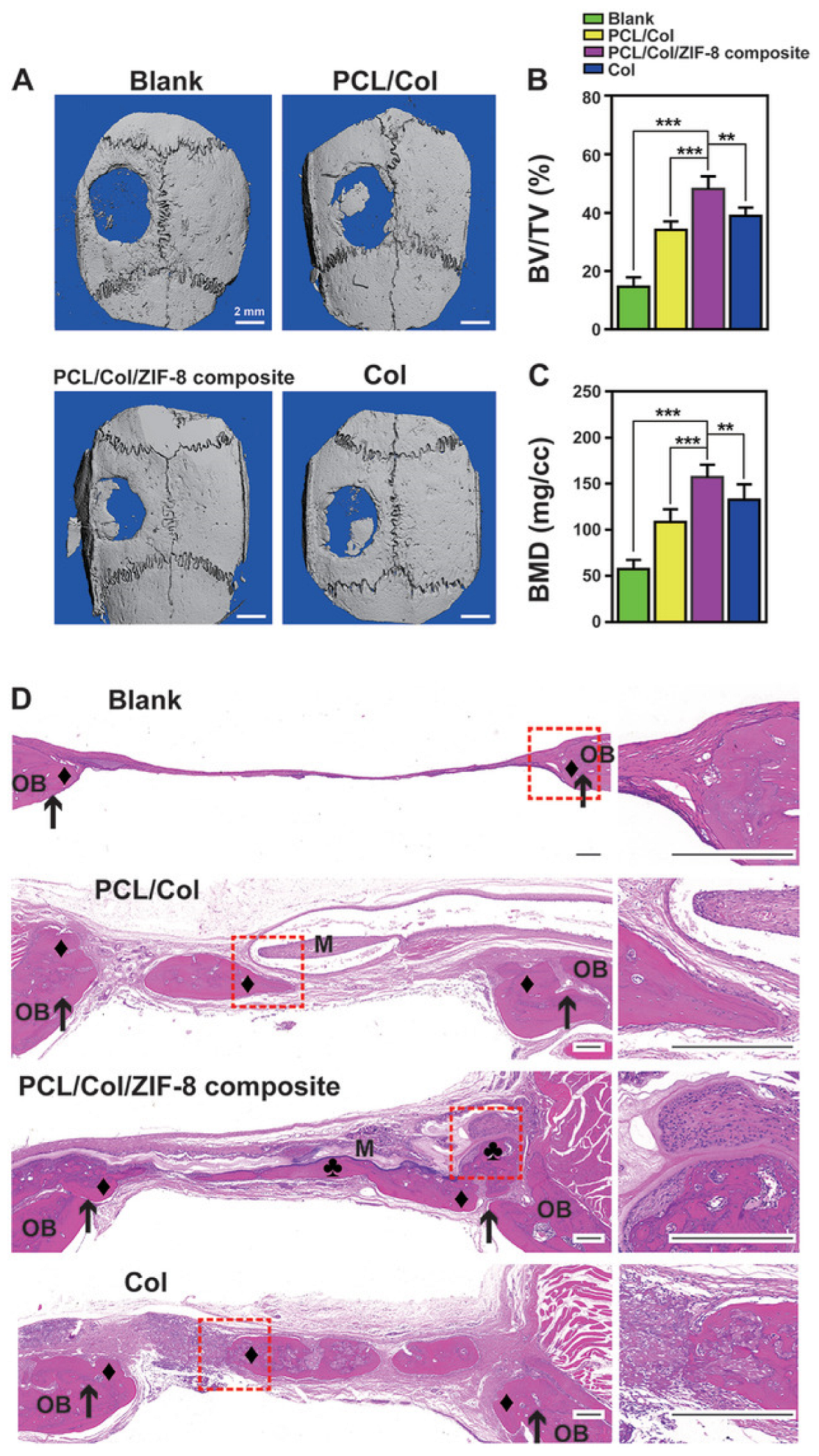
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, 2001369. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, Z.; Zhang, Z.; Zhang, Y.; Cai, G.; Li, Z. Bioactive and Anti-Corrosive Bio-MOF-1 Coating on Magnesium Alloy for Bone Repair Application. J. Alloys Compd. 2019, 788, 705–711. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, Y.; Guo, B.; Wang, Y.; Liu, W.; Sun, J.; Wang, X. Magnesium-Organic Framework-Based Stimuli-Responsive Systems That Optimize the Bone Microenvironment for Enhanced Bone Regeneration. Chem. Eng. J. 2020, 396, 125241. [Google Scholar] [CrossRef]
- Xu, C.; Kang, Y.; Dong, X.; Jiang, D.; Qi, M. Integration Exosomes with MOF-Modified Multifunctional Scaffold for Accelerating Vascularized Bone Regeneration. Chin. Chem. Lett. 2022, 34, 107528. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodyashkin, A.A.; Sergorodceva, A.V.; Kezimana, P.; Stanishevskiy, Y.M. Metal-Organic Framework (MOF)—A Universal Material for Biomedicine. Int. J. Mol. Sci. 2023, 24, 7819. https://doi.org/10.3390/ijms24097819
Vodyashkin AA, Sergorodceva AV, Kezimana P, Stanishevskiy YM. Metal-Organic Framework (MOF)—A Universal Material for Biomedicine. International Journal of Molecular Sciences. 2023; 24(9):7819. https://doi.org/10.3390/ijms24097819
Chicago/Turabian StyleVodyashkin, Andrey A., Antonina V. Sergorodceva, Parfait Kezimana, and Yaroslav M. Stanishevskiy. 2023. "Metal-Organic Framework (MOF)—A Universal Material for Biomedicine" International Journal of Molecular Sciences 24, no. 9: 7819. https://doi.org/10.3390/ijms24097819
APA StyleVodyashkin, A. A., Sergorodceva, A. V., Kezimana, P., & Stanishevskiy, Y. M. (2023). Metal-Organic Framework (MOF)—A Universal Material for Biomedicine. International Journal of Molecular Sciences, 24(9), 7819. https://doi.org/10.3390/ijms24097819





