Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases
Abstract
1. Introduction
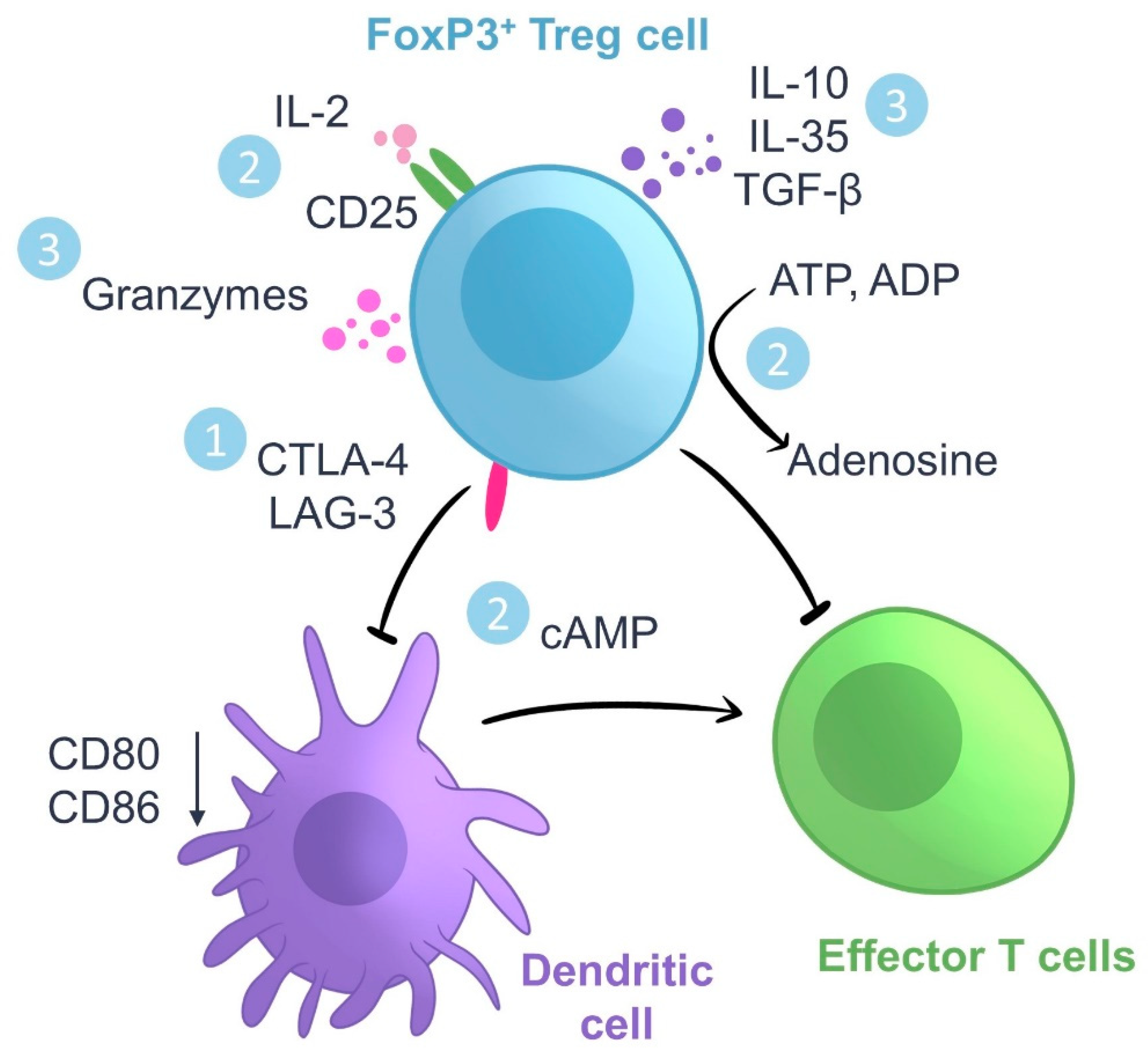
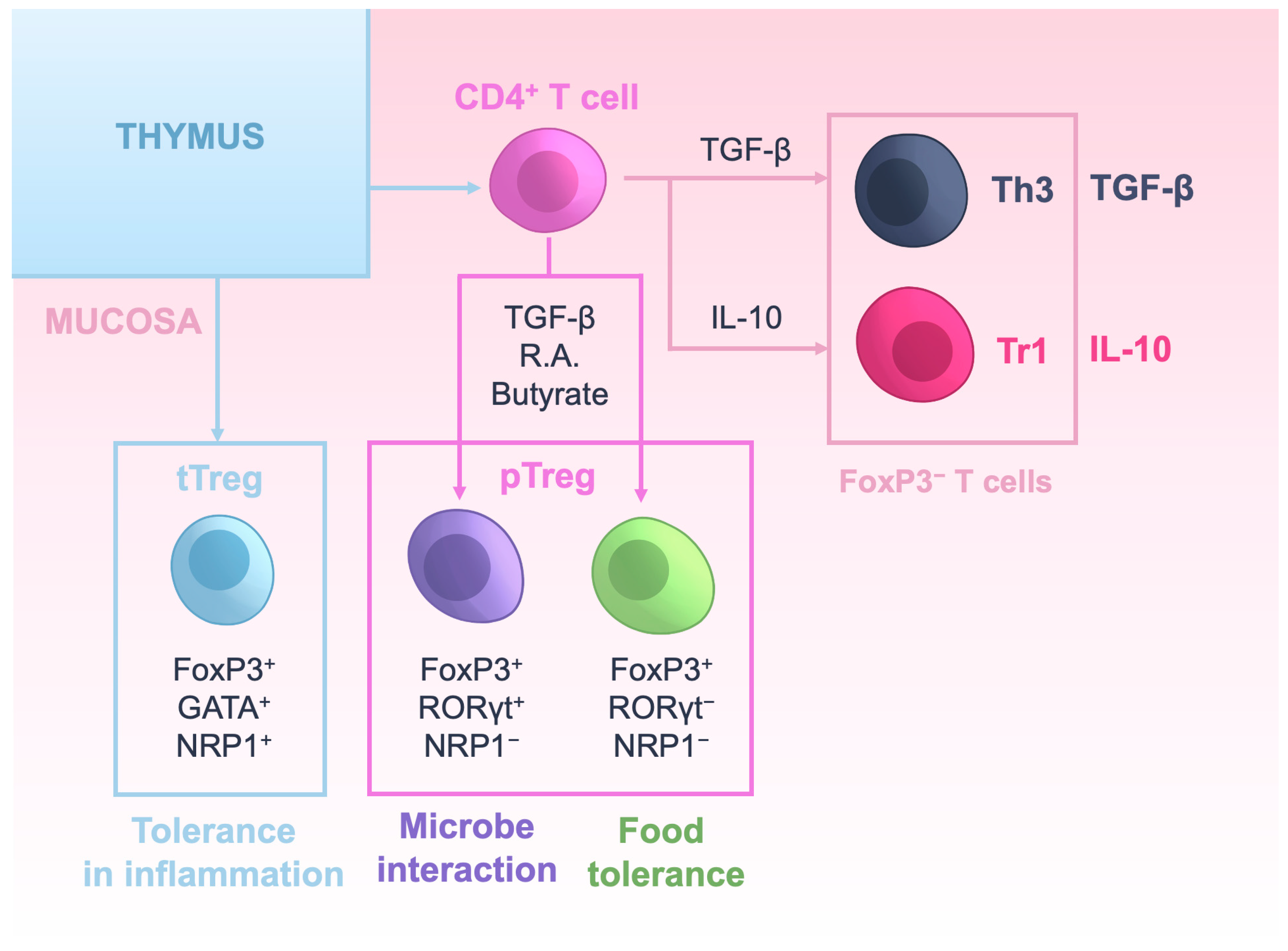
2. Regulatory T Cells: Ontogeny and Mechanisms of Action
3. Tregs in Mucosal Tissues
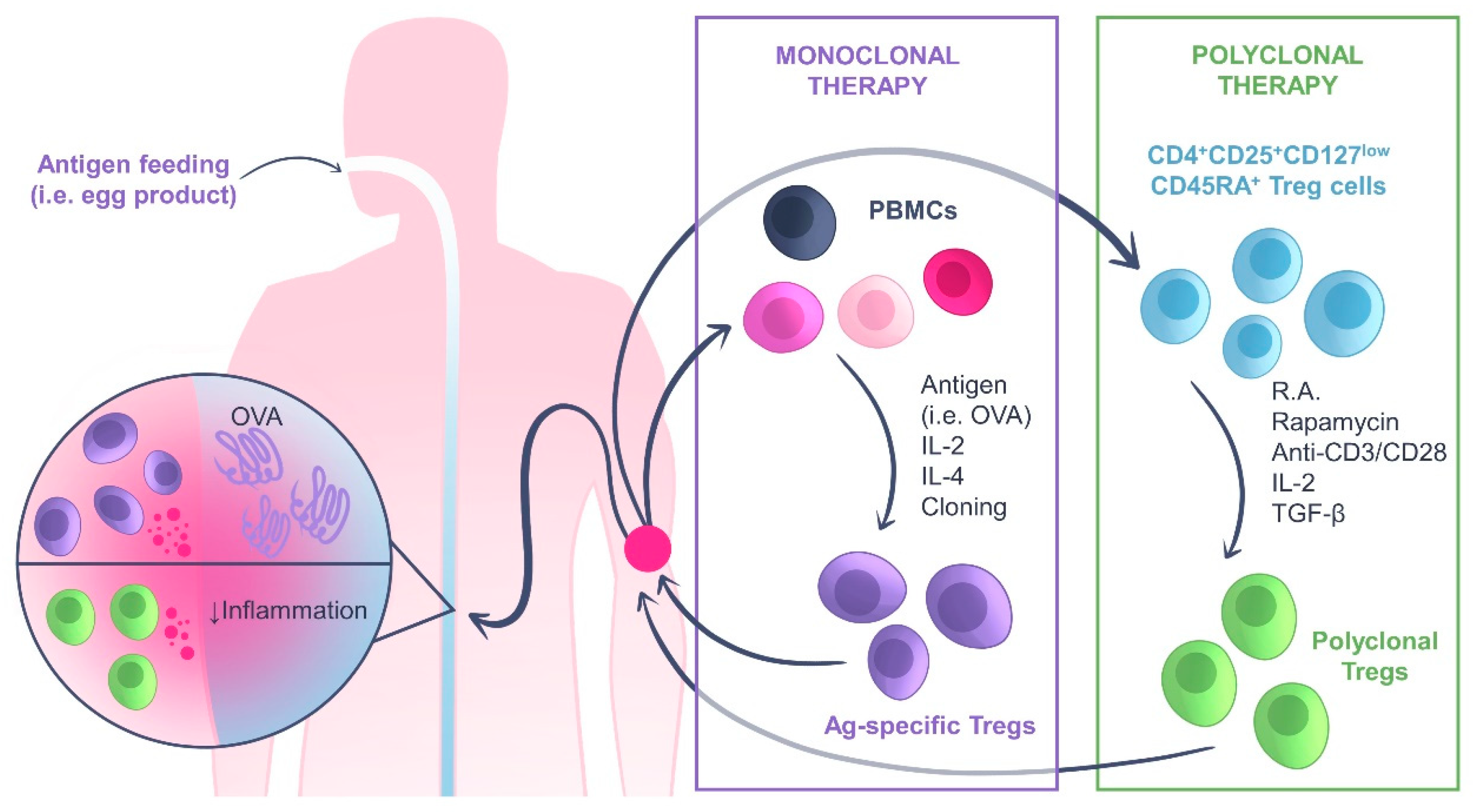
4. Adoptive Treg Cell Transfer Therapy: An Overview
Adoptive Treg Cell Transfer Therapy for Inflammatory Bowel Disease
5. Prompting the Expansion of Tregs In Vivo for Therapeutic and Prophylactic Purposes
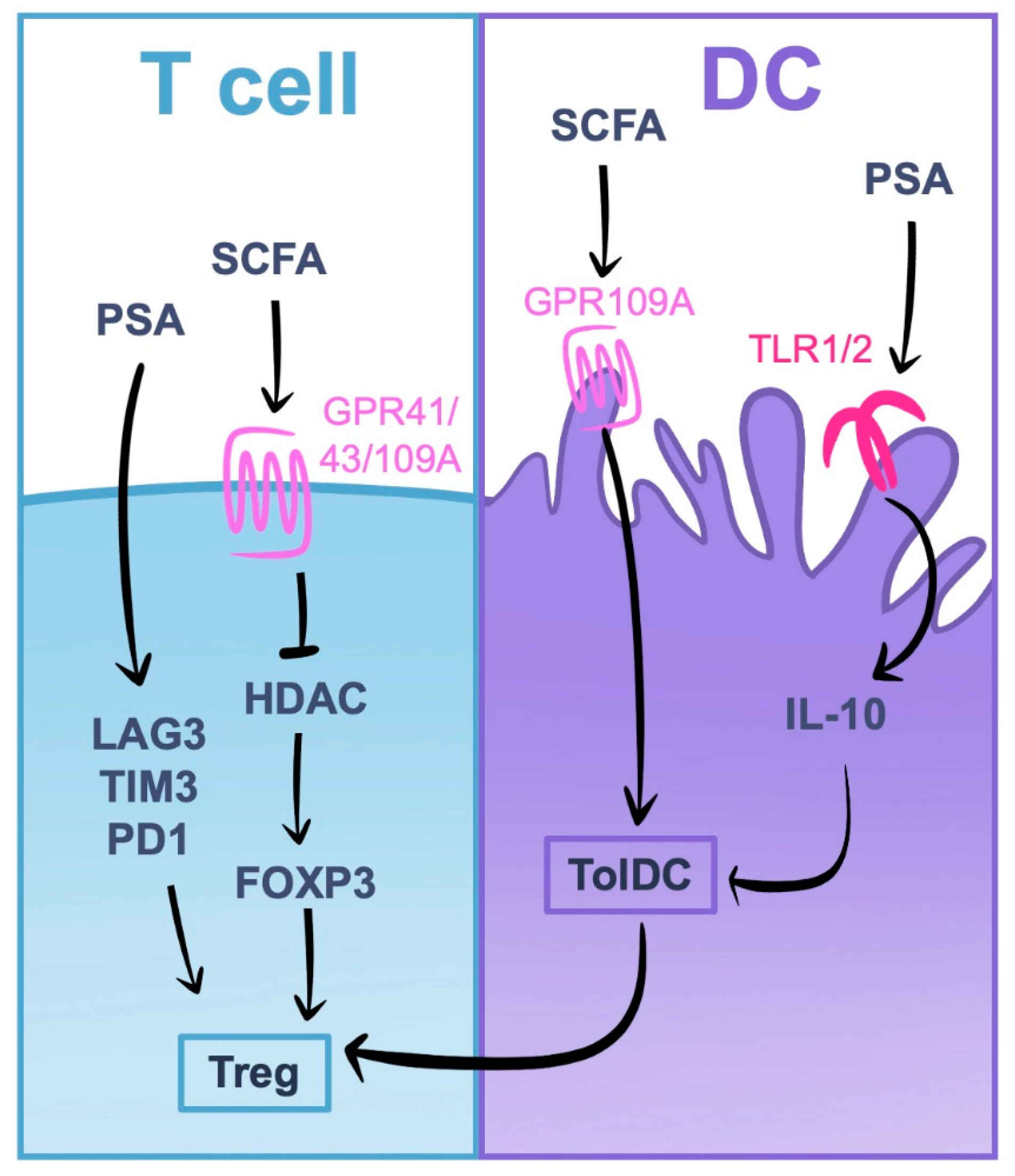
5.1. Induction of Tregs by Gut Microbiota
5.2. Induction of Tregs with Micronutrients
5.3. Inducing Tregs with Low Dose IL-2 Therapy
| Study ID | Disease | Treatment | Status | Ref. | Outcomes/Objective | |
|---|---|---|---|---|---|---|
| Probiotics | NCT01500941 | Atopic Dermatitis | Lactobacillus salivarius and Bifidobacterium breve (daily) | C | [138] | Clinical parameters improved. Th17/Treg ratio progressively decreased in the probiotic group. Effect remains 2 months after the discontinuation of treatment. |
| NCT02349711 | Allergic Rhinitis | L. gasseri, B. bifidum, and B. longum) (twice a day) | C | [139] | Symptoms of rhino-conjunctivitis improved. Treg cells increased during the treatment. | |
| NCT05208528 | Allergic Rhinitis | Bifidobacterium longum ES1 (daily) | R | - | Determine whether the treatment reduces the symptoms associated with allergic rhinitis by modulating the gut microbiota and enhancing Tregs. | |
| SCFA/Fiber | NCT05152615 | Rheumatoid Arthritis | SCFA Dietary Supplement (3 times daily) and methotrexate | R | - | Study whether oral butyrate modifies the gut microbiome and Treg cells. |
| NCT05576597 | Rheumatoid Arthritis | Sodium Butyrate (daily) | R | - | Evaluate the effects of sodium butyrate supplementation on intestinal inflammation and the changes of percentage of T-cell subtypes, especially Treg numbers. | |
| NCT04574024 | MS | High-Fiber Supplement (daily) | E | - | Analyze the effect of a high-fiber supplement on the growth of SCFA-producing intestinal bacteria and development of Tregs. | |
| Vitamin A | NCT01225289 | MS | Vitamin A (daily) | C | [140] | Vitamin A supplementation increased TGF-β and FoxP3 gene expression and may modify the skewing of naive CD4+ T towards a Th2/Treg response. |
| NCT01668615 | Chronic immune thrombocytopenia | All-trans-retinoid acid (3 times a day) | C | [141] | Therapy enhanced the percentage of Treg cells, Foxp3 expression and IL-10 production. | |
| Vitamin D | NCT04472481 | Active Rheumatoid Arthritis | Ergocalciferol (Vitamin D2) (weekly) | C | [142] | The percentage of Treg cells increased and was associated with a reduction in the DAS-28 score. |
| NCT01390480 | T1D | Cholecalciferol (Vitamin D3)(Daily) | C | [143] | Suppressive function of Tregs in patients with T1D improved. The cholecalciferol group required less insulin doses. | |
| NCT00940719 | MS | Vitamin D3 (daily) | C | [144] | Vitamin D3 increases the proportion of IL-10+CD4+ T cells and decreases the ratio of Th1/Th2 cells. | |
| IRCT2015010910891N2 | MS | High-dose vitamin D3 (every 5 days) | C | [145] | Serum IL-10 levels increased significantly while calcium levels remained stable. | |
| - | SLE | Vitamin D (daily) | C | [146] | Treg/Th17 ratio increased and decreased SLE DAI scores. | |
| Low dose IL-2 (Ld-IL2) | NCT01353833 | T1D | Ld-IL2 (Aldes-leukin) (Daily) | C | [131] | Increase the proportion of Treg cells. |
| NCT01988506 | Autoimmune inflammatory diseases | Ld-IL2 (daily for 5 days, fortnightly for 6 months) | C | [135] | The Ld-IL2 treatment selectively activated and expanded Tregs and is safe in different diseases and its associated treatments. | |
| NCT00539695 | GVHD | Ld-IL2 (three times weekly) | C | [147] | Ld-IL2 treatment increased Treg cell numbers and may be associated with a lower incidence of viral infections and GVHD. | |
| NCT02467504 | Rheumatoid Arthritis | Ld-IL2 (3 cycles of 14 days, every other day) and Methotrexate | C | [133] | The ratio of Treg/Th17 cells increased in the Ld-IL2 plus Methotrexate group. Inflammatory cytokines were reduced. |
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhangnia, P.; Akbarpour, M. Immunological Tolerance. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 206–220. [Google Scholar]
- Pelaez-Prestel, H.F.; Sanchez-Trincado, J.L.; Lafuente, E.M.; Reche, P.A. Immune Tolerance in the Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 2149. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, R.; Kohler, G.; Lamers, M.C. Transitional B cells are the target of negative selection in the B cell compartment. J. Exp. Med. 1995, 181, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Hogquist, K.A. T-cell tolerance: Central and peripheral. Cold Spring Harb. Perspect. Biol. 2012, 4, a006957. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R.; Frelinger, J. Mechanisms of unresponsiveness: T- and B-cell mediated mechanisms of anergy. Adv. Exp. Med. Biol. 2001, 489, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The development and function of regulatory T cells. Cell. Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef]
- Dikiy, S.; Rudensky, A.Y. Principles of regulatory T cell function. Immunity 2023, 56, 240–255. [Google Scholar] [CrossRef]
- Catalan, D.; Mansilla, M.A.; Ferrier, A.; Soto, L.; Oleinika, K.; Aguillon, J.C.; Aravena, O. Immunosuppressive Mechanisms of Regulatory B Cells. Front. Immunol. 2021, 12, 611795. [Google Scholar] [CrossRef]
- Schuster, I.S.; Coudert, J.D.; Andoniou, C.E.; Degli-Esposti, M.A. “Natural Regulators”: NK Cells as Modulators of T Cell Immunity. Front. Immunol. 2016, 7, 235. [Google Scholar] [CrossRef]
- Bourque, J.; Hawiger, D. Life and death of tolerogenic dendritic cells. Trends Immunol. 2023, 44, 110–118. [Google Scholar] [CrossRef]
- Lopes, N.; Correia, V.G.; Palma, A.S.; Brito, C. Cracking the Breast Cancer Glyco-Code through Glycan-Lectin Interactions: Targeting Immunosuppressive Macrophages. Int. J. Mol. Sci. 2021, 22, 1972. [Google Scholar] [CrossRef]
- Molero-Abraham, M.; Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Torres-Gomez, A.; Subiza, J.L.; Lafuente, E.M.; Reche, P.A. Human Oral Epithelial Cells Impair Bacteria-Mediated Maturation of Dendritic Cells and Render T Cells Unresponsive to Stimulation. Front. Immunol. 2019, 10, 1434. [Google Scholar] [CrossRef]
- Sanchez-Trincado, J.L.; Pelaez-Prestel, H.F.; Lafuente, E.M.; Reche, P.A. Human Oral Epithelial Cells Suppress T Cell Function via Prostaglandin E2 Secretion. Front. Immunol. 2022, 12, 740613. [Google Scholar] [CrossRef] [PubMed]
- Amersfoort, J.; Eelen, G.; Carmeliet, P. Immunomodulation by endothelial cells—Partnering up with the immune system? Nat. Rev. Immunol. 2022, 22, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg Enhancing Therapies to Treat Autoimmune Diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Vojdani, A.; Erde, J. Regulatory T cells, a potent immunoregulatory target for CAM researchers: Modulating tumor immunity, autoimmunity and alloreactive immunity (III). Evid.-Based Complement. Alternat. Med. 2006, 3, 309–316. [Google Scholar] [CrossRef]
- Tough, D.F.; Lombardi, G. Therapeutic opportunities for regulatory T-cell enhancing approaches. Clin. Exp. Immunol. 2023, 211, 93–95. [Google Scholar] [CrossRef]
- McCallion, O.; Bilici, M.; Hester, J.; Issa, F. Regulatory T-cell therapy approaches. Clin. Exp. Immunol. 2023, 211, 96–107. [Google Scholar] [CrossRef]
- Traxinger, B.R.; Richert-Spuhler, L.E.; Lund, J.M. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2022, 15, 398–407. [Google Scholar] [CrossRef]
- Germain, R.N. Special regulatory T-cell review: A rose by any other name: From suppressor T cells to Tregs, approbation to unbridled enthusiasm. Immunology 2008, 123, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.; Kumar, V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008, 29, 337–342. [Google Scholar] [CrossRef]
- Tang, Q.; Bluestone, J.A. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat. Immunol. 2008, 9, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2019, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Wang, Y.H.; Lee, H.K.; Ito, T.; Wang, Y.H.; Cao, W.; Liu, Y.J. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005, 436, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Yadav, M.; Stephan, S.; Bluestone, J.A. Peripherally induced tregs—Role in immune homeostasis and autoimmunity. Front. Immunol. 2013, 4, 232. [Google Scholar] [CrossRef]
- Paiva, R.S.; Lino, A.C.; Bergman, M.L.; Caramalho, I.; Sousa, A.E.; Zelenay, S.; Demengeot, J. Recent thymic emigrants are the preferential precursors of regulatory T cells differentiated in the periphery. Proc. Natl. Acad. Sci. USA 2013, 110, 6494–6499. [Google Scholar] [CrossRef]
- Lin, X.; Chen, M.; Liu, Y.; Guo, Z.; He, X.; Brand, D.; Zheng, S.G. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int. J. Clin. Exp. Pathol. 2013, 6, 116–123. [Google Scholar]
- Lee, W.; Lee, G.R. Transcriptional regulation and development of regulatory T cells. Exp. Mol. Med. 2018, 50, e456. [Google Scholar] [CrossRef]
- Huang, H.; Ma, Y.; Dawicki, W.; Zhang, X.; Gordon, J.R. Comparison of induced versus natural regulatory T cells of the same TCR specificity for induction of tolerance to an environmental antigen. J. Immunol. 2013, 191, 1136–1143. [Google Scholar] [CrossRef]
- Kanamori, M.; Nakatsukasa, H.; Okada, M.; Lu, Q.; Yoshimura, A. Induced Regulatory T Cells: Their Development, Stability, and Applications. Trends Immunol. 2016, 37, 803–811. [Google Scholar] [CrossRef]
- Shevach, E.M.; Thornton, A.M. tTregs, pTregs, and iTregs: Similarities and differences. Immunol. Rev. 2014, 259, 88–102. [Google Scholar] [CrossRef]
- Masteller, E.L.; Tang, Q.; Bluestone, J.A. Antigen-specific regulatory T cells—Ex vivo expansion and therapeutic potential. Semin. Immunol. 2006, 18, 103–110. [Google Scholar] [CrossRef]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, I.K.; Park, Y.J.; Kim, Y.S.; Kim, Y.J.; Chang, W.S.; Lee, Y.S.; Kweon, M.N.; Chung, Y.; Kang, C.Y. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 8742–8747. [Google Scholar] [CrossRef]
- Ye, J.; Su, X.; Hsueh, E.C.; Zhang, Y.; Koenig, J.M.; Hoft, D.F.; Peng, G. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma+ and FOXP3+ T cells with potent suppressive function. Eur. J. Immunol. 2011, 41, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Boeld, T.J.; Eder, R.; Huehn, J.; Floess, S.; Wieczorek, G.; Olek, S.; Dietmaier, W.; Andreesen, R.; Edinger, M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009, 39, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, V.; Duchez, S.; Branchtein, M.; How-Kit, A.; Cassius, C.; Daunay, A.; Shen, Y.; Dubanchet, S.; Colisson, R.; Vanneaux, V.; et al. Microenvironment tailors nTreg structure and function. Proc. Natl. Acad. Sci. USA 2019, 116, 6298–6307. [Google Scholar] [CrossRef]
- Malviya, V.; Yshii, L.; Junius, S.; Garg, A.D.; Humblet-Baron, S.; Schlenner, S.M. Regulatory T-cell stability and functional plasticity in health and disease. Immunol. Cell. Biol. 2023, 101, 112–129. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Chen, L.; Fang, L. Tr1 Cells as a Key Regulator for Maintaining Immune Homeostasis in Transplantation. Front. Immunol. 2021, 12, 671579. [Google Scholar] [CrossRef]
- Weiner, H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001, 182, 207–214. [Google Scholar] [CrossRef]
- Weiner, H.L. Oral tolerance: Immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001, 3, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Sakaguchi, S. Regulatory T cells: Roles of T cell receptor for their development and function. Semin. Immunopathol. 2010, 32, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Y.; Low, J.S.; Tanimine, N.; Finn, K.K.; Priyadharshini, B.; Germana, S.K.; Kaech, S.M.; Turka, L.A. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 2018, 25, 1204–1213.e4. [Google Scholar] [CrossRef]
- Jurewicz, M.; Nanaware, P.; Stern, L. MHC class II peptide loading regulates selection and function of regulatory T cells. Mol. Immunol. 2022, 150, 3. [Google Scholar] [CrossRef]
- Iikuni, N.; Lourenco, E.V.; Hahn, B.H.; La Cava, A. Cutting edge: Regulatory T cells directly suppress B cells in systemic lupus erythematosus. J. Immunol. 2009, 183, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef]
- Thornton, A.M.; Shevach, E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000, 164, 183–190. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Extracellular adenosine-mediated modulation of regulatory T cells. Front. Immunol. 2014, 5, 304. [Google Scholar] [CrossRef]
- McNally, A.; Hill, G.R.; Sparwasser, T.; Thomas, R.; Steptoe, R.J. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc. Natl. Acad. Sci. USA 2011, 108, 7529–7534. [Google Scholar] [CrossRef]
- Wahl, S.M.; Swisher, J.; McCartney-Francis, N.; Chen, W. TGF-beta: The perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 2004, 76, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Arce-Sillas, A.; Alvarez-Luquin, D.D.; Tamaya-Dominguez, B.; Gomez-Fuentes, S.; Trejo-Garcia, A.; Melo-Salas, M.; Cardenas, G.; Rodriguez-Ramirez, J.; Adalid-Peralta, L. Regulatory T Cells: Molecular Actions on Effector Cells in Immune Regulation. J. Immunol. Res. 2016, 2016, 1720827. [Google Scholar] [CrossRef] [PubMed]
- Gravano, D.M.; Vignali, D.A. The battle against immunopathology: Infectious tolerance mediated by regulatory T cells. Cell. Mol. Life Sci. 2012, 69, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.M.; Hall, J.A.; Blank, R.B.; Bouladoux, N.; Oukka, M.; Mora, J.R.; Belkaid, Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007, 204, 1775–1785. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Grainger, J.R.; Bouladoux, N.; Konkel, J.E.; Oldenhove, G.; Ribeiro, C.H.; Hall, J.A.; Yagi, R.; Naik, S.; Bhairavabhotla, R.; et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J. Clin. Investig. 2011, 121, 4503–4515. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.W.; Han, D.; Yi, J.; Jung, J.; Yang, B.G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Geva-Zatorsky, N.; Oh, S.; Konnikova, L.; Zemmour, D.; McGuire, A.M.; Burzyn, D.; Ortiz-Lopez, A.; Lobera, M.; Yang, J.; et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015, 349, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Hanna, B.S.; Wang, G.; Galvan-Pena, S.; Mann, A.O.; Ramirez, R.N.; Munoz-Rojas, A.R.; Smith, K.; Wan, M.; Benoist, C.; Mathis, D. The gut microbiota promotes distal tissue regeneration via RORgamma(+) regulatory T cell emissaries. Immunity 2023, 56, 829–846.e8. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, R.; Hu, J. Role of dendritic cells in the induction of regulatory T cells. Cell. Biosci. 2011, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Quintana, F.J.; da Cunha, A.P.; Dake, B.T.; Koeglsperger, T.; Starossom, S.C.; Weiner, H.L. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS ONE 2011, 6, e23618. [Google Scholar] [CrossRef]
- Akbari, O.; DeKruyff, R.H.; Umetsu, D.T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001, 2, 725–731. [Google Scholar] [CrossRef]
- Esensten, J.H.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J. Allergy Clin. Immunol. 2018, 142, 1710–1718. [Google Scholar] [CrossRef]
- Amini, L.; Kaeda, J.; Fritsche, E.; Roemhild, A.; Kaiser, D.; Reinke, P. Clinical adoptive regulatory T Cell therapy: State of the art, challenges, and prospective. Front. Cell. Dev. Biol. 2022, 10, 1081644. [Google Scholar] [CrossRef]
- Hoffmann, P.; Ermann, J.; Edinger, M.; Fathman, C.G.; Strober, S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002, 196, 389–399. [Google Scholar] [CrossRef]
- Cohen, J.L.; Trenado, A.; Vasey, D.; Klatzmann, D.; Salomon, B.L. CD4+CD25+ immunoregulatory T Cells: New therapeutics for graft-versus-host disease. J. Exp. Med. 2002, 196, 401–406. [Google Scholar] [CrossRef]
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef]
- Scalapino, K.J.; Tang, Q.; Bluestone, J.A.; Bonyhadi, M.L.; Daikh, D.I. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 2006, 177, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Weigert, O.; von Spee, C.; Undeutsch, R.; Kloke, L.; Humrich, J.Y.; Riemekasten, G. CD4+Foxp3+ regulatory T cells prolong drug-induced disease remission in (NZBxNZW) F1 lupus mice. Arthritis Res. Ther. 2013, 15, R35. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; Carpentier, P.A.; Anger, H.A.; Miller, S.D. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002, 169, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Canavan, J.B.; Scotta, C.; Vossenkamper, A.; Goldberg, R.; Elder, M.J.; Shoval, I.; Marks, E.; Stolarczyk, E.; Lo, J.W.; Powell, N.; et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 2016, 65, 584–594. [Google Scholar] [CrossRef]
- Baron, K.J.; Turnquist, H.R. Clinical Manufacturing of Regulatory T Cell Products For Adoptive Cell Therapy and Strategies to Improve Therapeutic Efficacy. Organogenesis 2023, 19, 2164159. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front. Immunol. 2019, 10, 43. [Google Scholar] [CrossRef]
- Scotta, C.; Esposito, M.; Fazekasova, H.; Fanelli, G.; Edozie, F.C.; Ali, N.; Xiao, F.; Peakman, M.; Afzali, B.; Sagoo, P.; et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4+CD25+FOXP3+ T regulatory cell subpopulations. Haematologica 2013, 98, 1291–1299. [Google Scholar] [CrossRef]
- Afzali, B.; Edozie, F.C.; Fazekasova, H.; Scotta, C.; Mitchell, P.J.; Canavan, J.B.; Kordasti, S.Y.; Chana, P.S.; Ellis, R.; Lord, G.M.; et al. Comparison of regulatory T cells in hemodialysis patients and healthy controls: Implications for cell therapy in transplantation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1396–1405. [Google Scholar] [CrossRef]
- Sun, G.; Hou, Y.; Gong, W.; Liu, S.; Li, J.; Yuan, Y.; Zhang, D.; Chen, Q.; Yan, X. Adoptive Induced Antigen-Specific Treg Cells Reverse Inflammation in Collagen-Induced Arthritis Mouse Model. Inflammation 2018, 41, 485–495. [Google Scholar] [CrossRef]
- Selck, C.; Dominguez-Villar, M. Antigen-Specific Regulatory T Cell Therapy in Autoimmune Diseases and Transplantation. Front. Immunol. 2021, 12, 661875. [Google Scholar] [CrossRef] [PubMed]
- Trenado, A.; Charlotte, F.; Fisson, S.; Yagello, M.; Klatzmann, D.; Salomon, B.L.; Cohen, J.L. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Investig. 2003, 112, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008, 8, 458–466. [Google Scholar] [CrossRef]
- Pedros, C.; Duguet, F.; Saoudi, A.; Chabod, M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 974–995. [Google Scholar] [CrossRef]
- Maul, J.; Loddenkemper, C.; Mundt, P.; Berg, E.; Giese, T.; Stallmach, A.; Zeitz, M.; Duchmann, R. Peripheral and intestinal regulatory CD4+CD25high T cells in inflammatory bowel disease. Gastroenterology 2005, 128, 1868–1878. [Google Scholar] [CrossRef]
- Marsal, J.; Barreiro-de Acosta, M.; Blumenstein, I.; Cappello, M.; Bazin, T.; Sebastian, S. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front. Med. 2022, 9, 897936. [Google Scholar] [CrossRef]
- Mottet, C.; Uhlig, H.H.; Powrie, F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 2003, 170, 3939–3943. [Google Scholar] [CrossRef]
- Desreumaux, P.; Foussat, A.; Allez, M.; Beaugerie, L.; Hebuterne, X.; Bouhnik, Y.; Nachury, M.; Brun, V.; Bastian, H.; Belmonte, N.; et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 2012, 143, 1207–1217.e2. [Google Scholar] [CrossRef]
- Brun, V.; Bastian, H.; Neveu, V.; Foussat, A. Clinical grade production of IL-10 producing regulatory Tr1 lymphocytes for cell therapy of chronic inflammatory diseases. Int. Immunopharmacol. 2009, 9, 609–613. [Google Scholar] [CrossRef]
- Voskens, C.J.; Stoica, D.; Roessner, S.; Vitali, F.; Zundler, S.; Rosenberg, M.; Wiesinger, M.; Wunder, J.; Siegmund, B.; Schuler-Thurner, B.; et al. Safety and tolerability of a single infusion of autologous ex vivo expanded regulatory T cells in adults with ulcerative colitis (ER-TREG 01): Protocol of a phase 1, open-label, fast-track dose-escalation clinical trial. BMJ Open 2021, 11, e049208. [Google Scholar] [CrossRef]
- Voskens, C.; Stoica, D.; Rosenberg, M.; Vitali, F.; Zundler, S.; Ganslmayer, M.; Knott, H.; Wiesinger, M.; Wunder, J.; Kummer, M.; et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut 2023, 72, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Geem, D.; Harusato, A.; Flannigan, K.; Denning, T.L. Harnessing regulatory T cells for the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Taitz, J.; Sun, S.M.; Langford, L.; Ni, D.; Macia, L. Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development. Front. Nutr. 2022, 9, 878382. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; de Zoeten, E.F.; Ozkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar] [CrossRef]
- Kamada, N.; Nunez, G. Role of the gut microbiota in the development and function of lymphoid cells. J. Immunol. 2013, 190, 1389–1395. [Google Scholar] [CrossRef]
- Narushima, S.; Sugiura, Y.; Oshima, K.; Atarashi, K.; Hattori, M.; Suematsu, M.; Honda, K. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes. 2014, 5, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, Y.; Xia, X.; Liang, J.; Liu, F.; Dou, H.; Hou, Y. Bacteroides fragilis alleviates the symptoms of lupus nephritis via regulating CD1d and CD86 expressions in B cells. Eur. J. Pharmacol. 2020, 884, 173421. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mehrabian, P.; Boyajian, S.; Wu, W.L.; Selicha, J.; Vonderfecht, S.; Mazmanian, S.K. The Protective Role of Bacteroides fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere 2018, 3, e00587-18. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.A.; Jones, M.B.; Hambor, J.; Cobb, B.A. Characterization of Polysaccharide A Response Reveals Interferon Responsive Gene Signature and Immunomodulatory Marker Expression. Front. Immunol. 2020, 11, 556813. [Google Scholar] [CrossRef]
- Barletta, B.; Rossi, G.; Schiavi, E.; Butteroni, C.; Corinti, S.; Boirivant, M.; Di Felice, G. Probiotic VSL#3-induced TGF-Œ≤ ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol. Nutr. Food Res. 2013, 57, 2233–2244. [Google Scholar] [CrossRef]
- Jia, H.; Ren, S.; Wang, X. Heat-killed probiotic regulates the body’s regulatory immunity to attenuate subsequent experimental autoimmune arthritis. Immunol. Lett. 2019, 216, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Donaldson, G.P.; Mikulski, Z.; Boyajian, S.; Ley, K.; Mazmanian, S.K. Bacterial colonization factors control specificity and stability of the gut microbiota. Nature. 2013, 501, 426–429. [Google Scholar] [CrossRef]
- Shepherd, E.S.; DeLoache, W.C.; Pruss, K.M.; Whitaker, W.R.; Sonnenburg, J.L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. Nature 2018, 557, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Issazadeh-Navikas, S.; Teimer, R.; Bockermann, R. Influence of dietary components on regulatory T cells. Mol. Med. 2012, 18, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Hall, J.A.; Sun, C.M.; Cai, Q.; Ghyselinck, N.; Chambon, P.; Belkaid, Y.; Mathis, D.; Benoist, C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity 2008, 29, 758–770. [Google Scholar] [CrossRef]
- Mucida, D.; Pino-Lagos, K.; Kim, G.; Nowak, E.; Benson, M.J.; Kronenberg, M.; Noelle, R.J.; Cheroutre, H. Retinoic acid can directly promote TGF-beta-mediated Foxp3+ Treg cell conversion of naive T cells. Immunity 2009, 30, 471–472. [Google Scholar] [CrossRef]
- Povoleri, G.A.M.; Nova-Lamperti, E.; Scotta, C.; Fanelli, G.; Chen, Y.C.; Becker, P.D.; Boardman, D.; Costantini, B.; Romano, M.; Pavlidis, P.; et al. Human retinoic acid-regulated CD161+ regulatory T cells support wound repair in intestinal mucosa. Nat. Immunol. 2018, 19, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.U.; Kang, I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhuang, Q.S.; Ji, H.F. Assessment of vitamin D levels in type 1 and type 2 diabetes patients: Results from metaanalysis. Mol. Nutr. Food Res. 2016, 60, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Lv, Z.; Fan, X.; Wang, L.; Han, F.; Wang, H.; Bi, S. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci. Lett. 2014, 570, 108–113. [Google Scholar] [CrossRef]
- Guan, S.Y.; Cai, H.Y.; Wang, P.; Lv, T.T.; Liu, L.N.; Mao, Y.M.; Zhao, C.N.; Wu, Q.; Dan, Y.L.; Sam, N.B.; et al. Association between circulating 25-hydroxyvitamin D and systemic lupus erythematosus: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2019, 22, 1803–1813. [Google Scholar] [CrossRef]
- Hafkamp, F.M.J.; Groot Kormelink, T.; de Jong, E.C. Targeting DCs for Tolerance Induction: Don’t Lose Sight of the Neutrophils. Front. Immunol. 2021, 12, 732992. [Google Scholar] [CrossRef]
- Hafkamp, F.M.J.; Taanman-Kueter, E.W.M.; van Capel, T.M.M.; Kormelink, T.G.; de Jong, E.C. Vitamin D3 Priming of Dendritic Cells Shifts Human Neutrophil-Dependent Th17 Cell Development to Regulatory T Cells. Front. Immunol. 2022, 13, 872665. [Google Scholar] [CrossRef]
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415. [Google Scholar] [CrossRef]
- Martinez-Blanco, M.; Perez-Rodriguez, L.; Lozano-Ojalvo, D.; Molina, E.; Lopez-Fandino, R. Ovalbumin-Derived Peptides Activate Retinoic Acid Signalling Pathways and Induce Regulatory Responses Through Toll-Like Receptor Interactions. Nutrients 2020, 12, 831. [Google Scholar] [CrossRef]
- Hourihane, J.O.B.; Beyer, K.; Abbas, A.; Fernandez-Rivas, M.; Turner, P.J.; Blumchen, K.; Nilsson, C.; Ibanez, M.D.; Deschildre, A.; Muraro, A.; et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): A multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child. Adolesc. Health 2020, 4, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Bar-Or, A.; Oger, J.; Traboulsee, A.; Patry, D.; Young, C.; Olsson, T.; Li, D.; Hartung, H.P.; Krantz, M.; et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology 2011, 77, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.; Whittall, T.; Bergmeier, L.A.; Lindblad, M.; Lundin, S.; Shinnick, T.; Mizushima, Y.; Holmgren, J.; Lehner, T. Oral tolerization with peptide 336-351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clin. Exp. Immunol. 2004, 137, 201–208. [Google Scholar] [CrossRef]
- Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group; Krischer, J.P.; Schatz, D.A.; Bundy, B.; Skyler, J.S.; Greenbaum, C.J. Effect of Oral Insulin on Prevention of Diabetes in Relatives of Patients With Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 1891–1902. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Rasmussen, J.P.; Gavin, M.A.; Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005, 6, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Malek, T.R.; Bayer, A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004, 4, 665–674. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Matsuoka, K.; Koreth, J.; Kim, H.T.; Bascug, G.; McDonough, S.; Kawano, Y.; Murase, K.; Cutler, C.; Ho, V.T.; Alyea, E.P.; et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci. Transl. Med. 2013, 5, 179ra143. [Google Scholar] [CrossRef]
- Letourneau, S.; Krieg, C.; Pantaleo, G.; Boyman, O. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J. Allergy Clin. Immunol. 2009, 123, 758–762. [Google Scholar] [CrossRef]
- Hartemann, A.; Bensimon, G.; Payan, C.A.; Jacqueminet, S.; Bourron, O.; Nicolas, N.; Fonfrede, M.; Rosenzwajg, M.; Bernard, C.; Klatzmann, D. Low-dose interleukin 2 in patients with type 1 diabetes: A phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013, 1, 295–305. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Wei, Y.; Sun, X.; Chen, Y.; Deng, J.; Jin, Y.; Gan, Y.; Hu, X.; Jia, R.; et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 2016, 22, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Miao, M.; Zhang, R.; Liu, X.; Zhao, X.; Shao, M.; Liu, T.; Jin, Y.; Chen, J.; Liu, H.; et al. Efficacy and safety of low-dose interleukin-2 in combination with methotrexate in patients with active rheumatoid arthritis: A randomized, double-blind, placebo-controlled phase 2 trial. Signal. Transduct. Target. Ther. 2022, 7, 67. [Google Scholar] [CrossRef]
- Koreth, J.; Kim, H.T.; Jones, K.T.; Lange, P.B.; Reynolds, C.G.; Chammas, M.J.; Dusenbury, K.; Whangbo, J.; Nikiforow, S.; Alyea, E.P., 3rd; et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood 2016, 128, 130–137. [Google Scholar] [CrossRef]
- Rosenzwajg, M.; Lorenzon, R.; Cacoub, P.; Pham, H.P.; Pitoiset, F.; El Soufi, K.; Ribet, C.; Bernard, C.; Aractingi, S.; Banneville, B.; et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 2019, 78, 209–217. [Google Scholar] [CrossRef]
- Tahvildari, M.; Dana, R. Low-Dose IL-2 Therapy in Transplantation, Autoimmunity, and Inflammatory Diseases. J. Immunol. 2019, 203, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nguyen, T.T.; Impellizzieri, D.; Mineura, K.; Shibuya, R.; Gomariz, A.; Haberecker, M.; Nilsson, J.; Nombela-Arrieta, C.; Jungraithmayr, W.; et al. Biased IL-2 signals induce Foxp3-rich pulmonary lymphoid structures and facilitate long-term lung allograft acceptance in mice. Nat. Commun. 2023, 14, 1383. [Google Scholar] [CrossRef]
- Iemoli, E.; Trabattoni, D.; Parisotto, S.; Borgonovo, L.; Toscano, M.; Rizzardini, G.; Clerici, M.; Ricci, E.; Fusi, A.; De Vecchi, E.; et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J. Clin. Gastroenterol. 2012, 46, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Dennis-Wall, J.C.; Culpepper, T.; Nieves, C., Jr.; Rowe, C.C.; Burns, A.M.; Rusch, C.T.; Federico, A.; Ukhanova, M.; Waugh, S.; Mai, V.; et al. Probiotics (Lactobacillus gasseri KS-13, Bifidobacterium bifidum G9-1, and Bifidobacterium longum MM-2) improve rhinoconjunctivitis-specific quality of life in individuals with seasonal allergies: A double-blind, placebo-controlled, randomized trial. Am. J. Clin. Nutr. 2017, 105, 758–767. [Google Scholar] [CrossRef]
- Saboor-Yaraghi, A.A.; Harirchian, M.H.; Mohammadzadeh Honarvar, N.; Bitarafan, S.; Abdolahi, M.; Siassi, F.; Salehi, E.; Sahraian, M.A.; Eshraghian, M.R.; Roostaei, T.; et al. The Effect of Vitamin A Supplementation on FoxP3 and TGF-beta Gene Expression in Avonex-Treated Multiple Sclerosis Patients. J. Mol. Neurosci. 2015, 56, 608–612. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, R.; Wang, Z.; He, Y.; Bai, X.; Zhu, M.; Yu, Z.; Ruan, C.G. Efficacy of immunomodulatory therapy with all-trans retinoid acid in adult patients with chronic immune thrombocytopenia. Thromb. Res. 2016, 140, 73–80. [Google Scholar] [CrossRef]
- El-Banna, H.S.; Gado, S.E. Vitamin D: Does it help Tregs in active rheumatoid arthritis patients. Expert. Rev. Clin. Immunol. 2020, 16, 847–853. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Smolders, J.; Peelen, E.; Thewissen, M.; Cohen Tervaert, J.W.; Menheere, P.; Hupperts, R.; Damoiseaux, J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef]
- Floris, G.; Giartosio, A.; Rinaldi, A. Essential sulfhydryl groups in diamine oxidase from Euphorbia characias latex. Arch. Biochem. Biophys. 1983, 220, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Carvalho, C.; Boleixa, D.; Bettencourt, A.; Leal, B.; Guimaraes, J.; Neves, E.; Oliveira, J.C.; Almeida, I.; Farinha, F.; et al. Vitamin D supplementation effects on FoxP3 expression in T cells and FoxP3+/IL-17A ratio and clinical course in systemic lupus erythematosus patients: A study in a Portuguese cohort. Immunol. Res. 2017, 65, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Nasser, A.A.; Ku, S.; Castillo-Caro, P.; Hazrat, Y.; Wu, M.F.; Liu, H.; Melenhorst, J.; Barrett, A.J.; Ito, S.; Foster, A.; et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin. Cancer Res. 2014, 20, 2215–2225. [Google Scholar] [CrossRef]
- Ferreira, L.M.R.; Muller, Y.D.; Bluestone, J.A.; Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 2019, 10, 749–769. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.; Chen, X.; Finn, P.B.; Qi, L.S. Advances in CRISPR therapeutics. Nat. Rev. Nephrol. 2023, 19, 9–22. [Google Scholar] [CrossRef]
- Fransson, M.; Piras, E.; Burman, J.; Nilsson, B.; Essand, M.; Lu, B.; Harris, R.A.; Magnusson, P.U.; Brittebo, E.; Loskog, A.S. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflamm. 2012, 9, 112. [Google Scholar] [CrossRef]
- Kim, Y.C.; Zhang, A.H.; Yoon, J.; Culp, W.E.; Lees, J.R.; Wucherpfennig, K.W.; Scott, D.W. Engineered MBP-specific human Tregs ameliorate MOG-induced EAE through IL-2-triggered inhibition of effector T cells. J. Autoimmun. 2018, 92, 77–86. [Google Scholar] [CrossRef]
- Elinav, E.; Waks, T.; Eshhar, Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology 2008, 134, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.P.; Notley, C.A.; Xue, S.-A.; Bendle, G.M.; Holler, A.; Schumacher, T.N.; Ehrenstein, M.R.; Stauss, H.J. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 19078–19083. [Google Scholar] [CrossRef] [PubMed]
- Velasco-de Andres, M.; Munoz-Sanchez, G.; Carrillo-Serradell, L.; Gutierrez-Hernandez, M.D.M.; Catala, C.; Isamat, M.; Lozano, F. Chimeric antigen receptor-based therapies beyond cancer. Eur. J. Immunol. 2023, 53, e2250184. [Google Scholar] [CrossRef] [PubMed]
| IBD | Phase | Source of the Cells | Study ID | Status |
|---|---|---|---|---|
| CD | I/IIa | Antigen-specific autologous expanded Ova-Tregs | Eudract, Number: 2006-004712-44 | Completed |
| IIb | Antigen-specific autologous expanded Ova-Tregs (Ovasave) | NCT02327221 | Terminated | |
| I/II | Expanded autologous CD4+CD25+CD127lowCD45RA+ Tregs (TR004 drug) | NCT03185000 | Recruiting | |
| UC | I | Autologous ex vivo expanded CD25+ Tregs expanded in the presence of rapamycin, IL-2 and CD3/CD28 beads | NCT04691232 | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiyouzi, T.; Pelaez-Prestel, H.F.; Reyes-Manzanas, R.; Lafuente, E.M.; Reche, P.A. Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 7797. https://doi.org/10.3390/ijms24097797
Fiyouzi T, Pelaez-Prestel HF, Reyes-Manzanas R, Lafuente EM, Reche PA. Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. International Journal of Molecular Sciences. 2023; 24(9):7797. https://doi.org/10.3390/ijms24097797
Chicago/Turabian StyleFiyouzi, Tara, Hector F. Pelaez-Prestel, Raquel Reyes-Manzanas, Esther M. Lafuente, and Pedro A. Reche. 2023. "Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases" International Journal of Molecular Sciences 24, no. 9: 7797. https://doi.org/10.3390/ijms24097797
APA StyleFiyouzi, T., Pelaez-Prestel, H. F., Reyes-Manzanas, R., Lafuente, E. M., & Reche, P. A. (2023). Enhancing Regulatory T Cells to Treat Inflammatory and Autoimmune Diseases. International Journal of Molecular Sciences, 24(9), 7797. https://doi.org/10.3390/ijms24097797







