Fatty Liver Disease, Metabolism and Alcohol Interplay: A Comprehensive Review
Abstract
1. Introduction
2. The Key Aspects in NAFLD
2.1. From NAFLD to Metabolic-Associated Fatty Liver Disease (MAFLD)
2.2. Histological Features of NASH
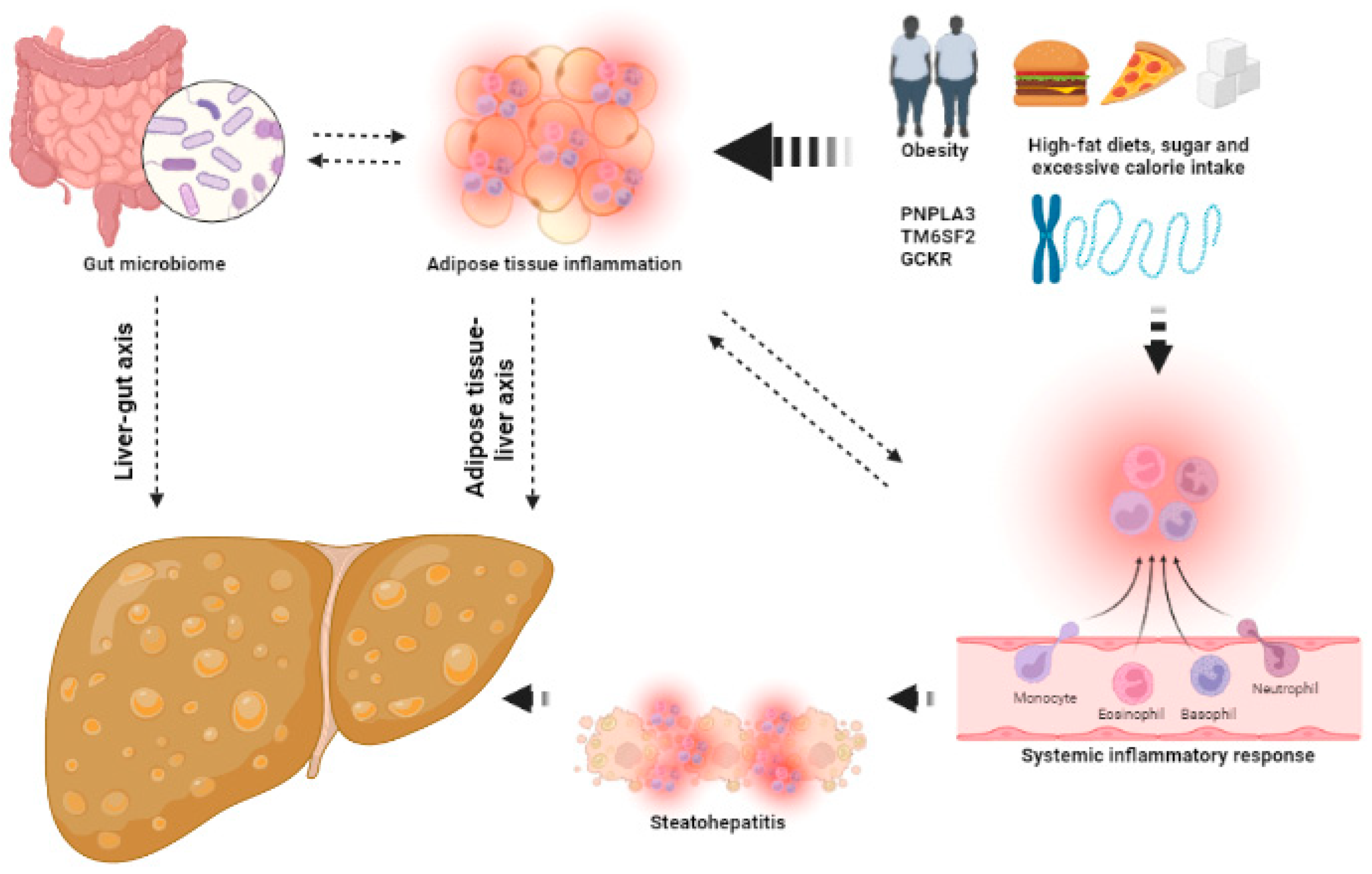
2.3. Multiple Parallel Hits in NAFLD
2.4. Adipose Tissue Inflammation
2.5. TNFα, IL-1β and IL-6
2.6. Adiponectin and Leptin
2.7. Gut Microbiome (GM)
2.8. Microbial Fermentative Pathways
2.9. Other Pathways
2.10. Genetics
2.11. Endoplasmic Reticulum (ER) Stress
2.12. Epigenetics
3. The Key Aspects in Alcohol-Related Liver Disease (ArLD)
3.1. Proposed Threshold of Alcohol Consumption for Increasing the Risk of ArLD
3.2. Histological Features of ArLD
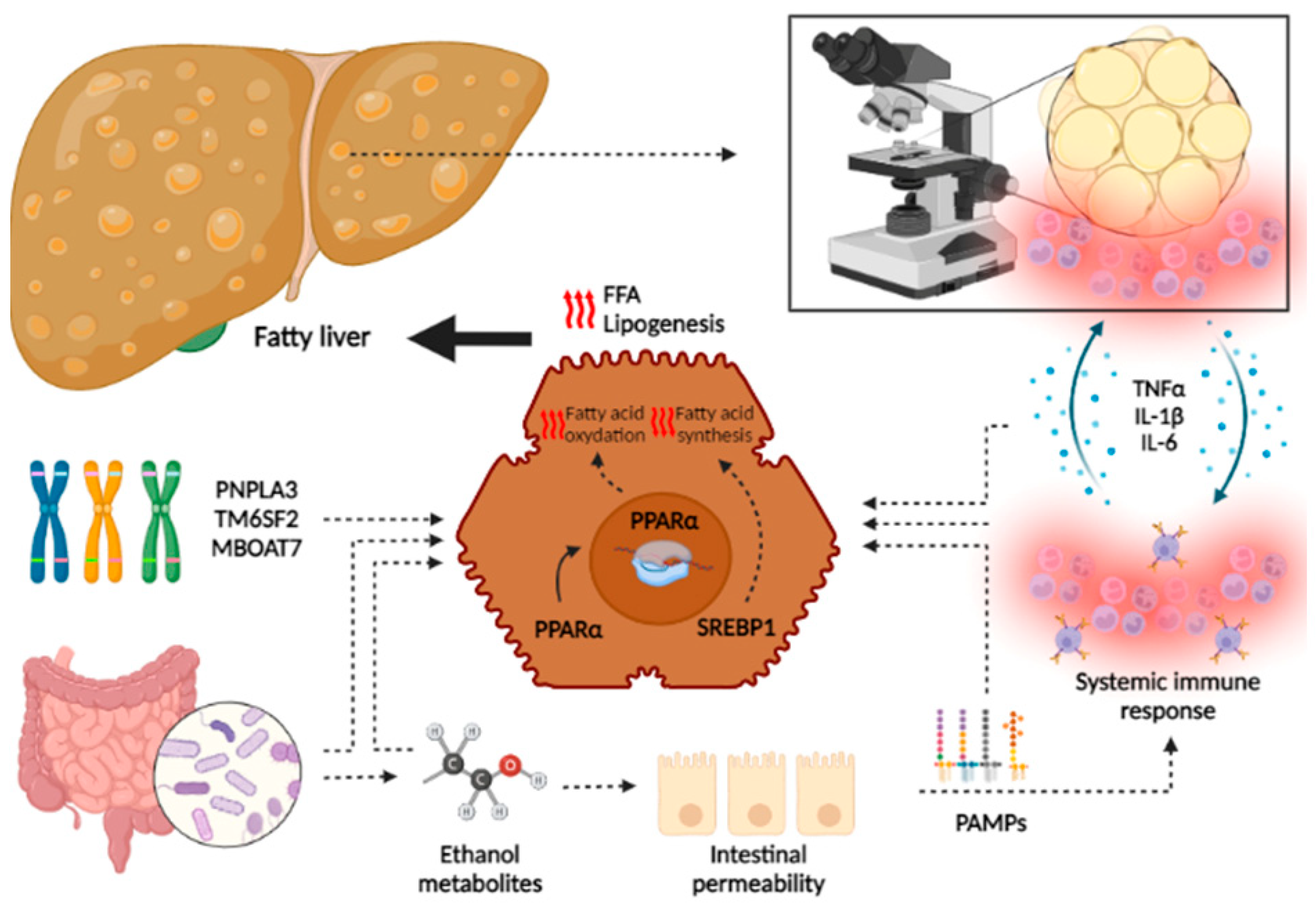
3.3. Pathophysiology of ArLD
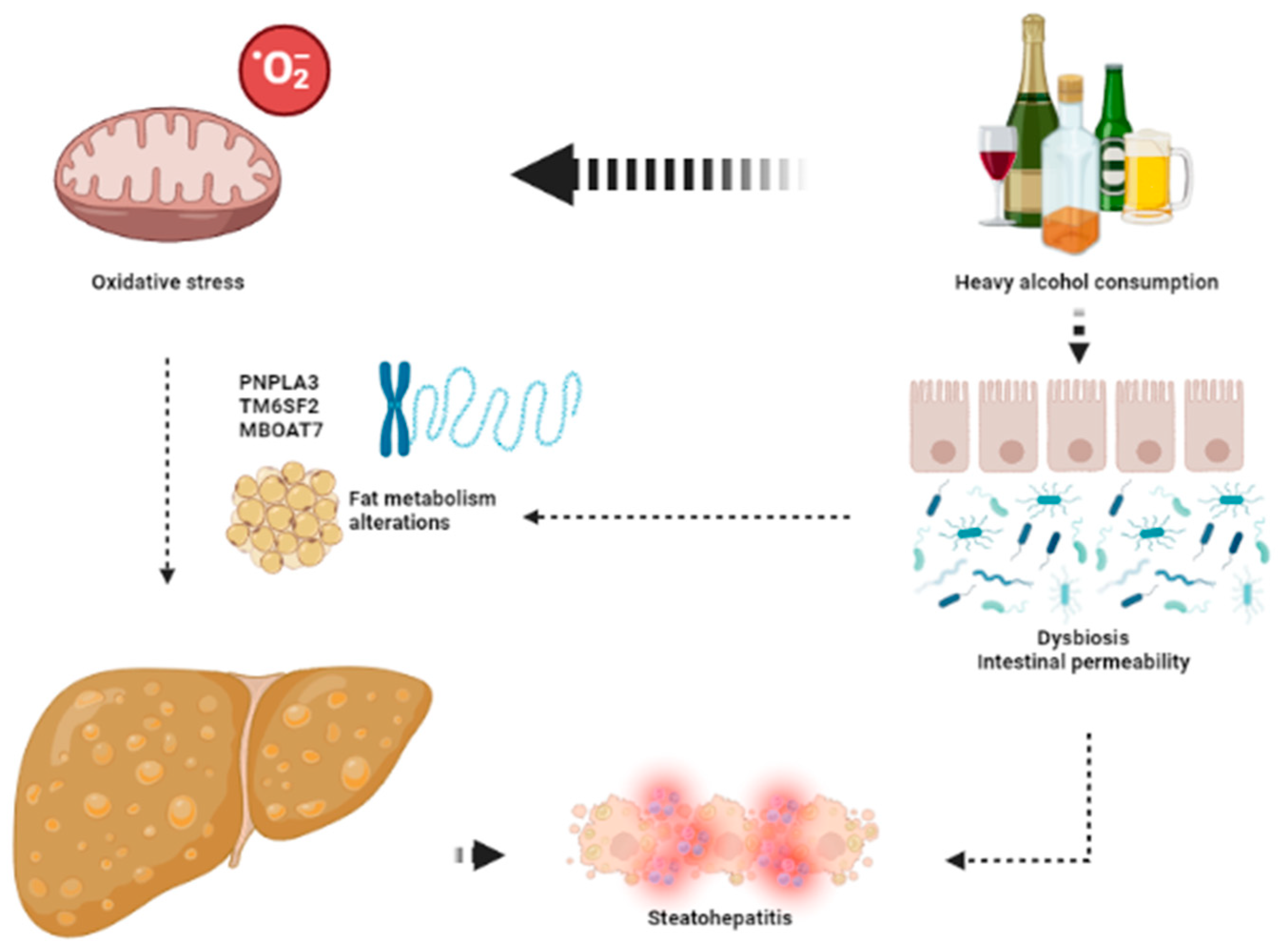
3.4. Genetics
3.5. Oxidative Stress
3.6. Epigenetics
3.7. Steatosis in the Setting of ArFL
3.8. Inflammation: From ArFL to ArSH
4. Influence of Alcohol in NAFLD
4.1. Overlap in Pathogenesis of ArLD and NAFLD
4.2. Role of Alcohol Consumption in NAFLD
4.3. Assessment of Alcohol Consumption in NAFLD Patients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Mathurin, P.; Cortez-Pinto, H.; Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: Trends, projections and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 37–49. Available online: https://pubmed.ncbi.nlm.nih.gov/36258033/ (accessed on 5 February 2023). [CrossRef] [PubMed]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global burden of alcoholic liver diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef]
- Ekstedt, M.; Nasr, P.; Kechagias, S. Natural History of NAFLD/NASH. Curr. Hepatol. Rep. 2017, 16, 391–397. [Google Scholar] [CrossRef]
- Hernandez-Tejero, M.; Clemente-Sanchez, A.; Bataller, R. Spectrum, Screening, and Diagnosis of Alcohol-related Liver Disease. J. Clin. Exp. Hepatol. 2023, 13, 75–87. Available online: http://www.jcehepatology.com/article/S0973688322004728/fulltext (accessed on 30 January 2023). [CrossRef]
- Bataller, R.; Arab, J.P.; Shah, V.H. Alcohol-Associated Hepatitis. N. Engl. J. Med. 2022, 387, 2436–2448. Available online: https://pubmed.ncbi.nlm.nih.gov/36577100/ (accessed on 30 January 2023). [CrossRef]
- Seitz, H.K.; Neuman, M.G. The History of Alcoholic Liver Disease: From an Unrecognized Disease to One of the Most Frequent Diseases in Hepatology. J. Clin. Med. 2021, 10, 858. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2015, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wong, V.W.S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Iruzubieta, P.; González, M.; Cabezas, J.; Teresa Arias-Loste, M.; Crespo, J. Diagnosis and Characterization of Non-Alcoholic Fatty Liver Disease. In Liver Research and Clinical Management; IntechOpen: London, UK, 2018. [Google Scholar]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.S.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Charlotte, F.; Heurtier, A.; Gombert, S.; Giral, P.; Bruckert, E.; Grimaldi, A.; Capron, F.; Poynard, T.; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005, 128, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Natta, M.V.; Behling, C.E.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bedossa, P.; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathologic Patterns and Biopsy Evaluation in Clinical Research Definition and Classification of Fatty Liver Disease. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2012; Volume 32, pp. 003–013. [Google Scholar]
- Chalasani, N.; Wilson, L.; Kleiner, D.E.; Cummings, O.W.; Brunt, E.M.; Ünalp, A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 829–834. [Google Scholar] [CrossRef]
- Zimmerman, H.J.; MacMurray, F.G.; Rappaport, H.; Alpert, L.K. Studies on the liver in diabetes mellitus. II. The significance of fatty metamorphosis and its correlation with insulin sensitivity. J. Lab. Clin. Med. 1950, 36, 922–928. [Google Scholar]
- Zimmerman, H.J.; MacMurray, F.G.; Rappaport, H.; Alpert, L.K. Studies of the liver in diabetes mellitus. I. Structural and functional abnormalities. J. Lab. Clin. Med. 1950, 36, 912–921. [Google Scholar]
- Patton, H.M.; Yates, K.; Unalp-Arida, A.; Behling, C.A.; Huang, T.T.K.; Rosenthal, P.; Sanyal, A.J.; Schwimmer, J.B.; Lavine, J.E. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am. J. Gastroenterol. 2010, 105, 2093–2102. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. Metabolic interplay between white, beige, brown adipocytes and the liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Minze, L.J.; Lin, J.; Zou, J.; Liu, J.Z.; Ren, Y.; Yin, Z.; Hamilton, D.J.; Reardon, P.R.; et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013, 17, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; Ito, Y.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Shetty, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin in health and disease: Evaluation of adiponectin-targeted drug development strategies. Trends Pharmcol. Sci. 2009, 30, 234–239. [Google Scholar] [CrossRef]
- Asano, T.; Watanabe, K.; Kubota, N.; Gunji, T.; Omata, M.; Kadowaki, T.; Ohnishi, S. Adiponectin knockout mice on high fat diet develop fibrosing steatohepatitis. J. Gastroenterol. Hepatol. 2009, 24, 1669–1676. [Google Scholar] [CrossRef]
- Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br. J. Surg. 1998, 85, 1658–1662.
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Vayreda, M.; Richart, C.; Gutierrez, C.; Broch, M.; Vendrell, J.; Ricart, W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J. Clin. Endocrinol. Metab. 2001, 86, 1154–1159. [Google Scholar] [CrossRef]
- Kern, P.A.; Saghizadeh, M.; Ong, J.M.; Bosch, R.J.; Deem, R.; Simsolo, R.B. The Expression of Tumor Necrosis Factor in Human Adipose Tissue Regulation by Obesity, Weight Loss, and Relationship to Lipoprotein Lipase. J. Clin. Investig. 1995, 95, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Hwi, J.K.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Molnar, C.; Geiger, S.; Graziadei, I.; Ebenbichler, C.F.; Weiss, H.; Kaser, S.; Kaser, A.; Tilg, H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Gut 2010, 59, 1259–1264. [Google Scholar] [CrossRef]
- Senn, J.J.; Klover, P.J.; Nowak, I.A.; Zimmers, T.A.; Koniaris, L.G.; Furlanetto, R.W.; Mooney, R.A. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J. Biol. Chem. 2003, 278, 13740–13746. [Google Scholar] [CrossRef]
- Procaccini, C.; Galgani, M.; De Rosa, V.; Carbone, F.; La Rocca, C.; Ranucci, G.; Iorio, R.; Matarese, G. Leptin: The Prototypic Adipocytokine its Role in, N.A.F.L.D. Curr. Pharm. Des. 2010, 16, 1902–1912. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific Deletion of SIRT1 Alters Fatty Acid Metabolism and Results in Hepatic Steatosis and Inflammation. Cell Metab 2009, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Shimabukuro, M.; Koyama, K.; Lee, Y.; Wang, M.Y.; Trieu, F.; Newgard, C.B.; Unger, R.H. Induction by leptin of uncoupling protein-2 and enzymes of fatty acid oxidation. Proc. Natl. Acad. Sci. USA 1997, 94, 6386. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Koyama, K.; Chen, G.; Wang, M.Y.; Trieu, F.; Lee, Y.; Newgard, C.B.; Unger, R.H. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc. Natl. Acad. Sci. USA 1997, 94, 4637. [Google Scholar] [CrossRef]
- Parlesak, A.; Schäfer, C.; Schütz, T.; Bode, J.C.; Bode, C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 2000, 32, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Hines, I.N.; Son, G.; Kremer, M. Contribution of gut bacteria to liver pathobiology. Gastroenterol. Res. Pract. 2010, 2010, 453563. [Google Scholar]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Cartenì, M.; Nardone, G. Gut-liver axis: The impact of gut microbiota on non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef]
- He, X.; Ji, G.; Jia, W.; Li, H. Gut Microbiota and Nonalcoholic Fatty Liver Disease: Insights on Mechanism and Application of Metabolomics. Int. J. Mol. Sci. 2016, 17, 300. [Google Scholar] [CrossRef]
- Seki, E.; Schnabl, B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J. Physiol. 2012, 590, 447–458. [Google Scholar] [CrossRef]
- Filliol, A.; Piquet-Pellorce, C.; Raguénès-Nicol, C.; Dion, S.; Farooq, M.; Lucas-Clerc, C.; Vandenabeele, P.; Bertrand, M.J.M.; Le Seyec, J.; Samson, M. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis. J. Hepatol. 2017, 66, 1205–1213. [Google Scholar] [CrossRef]
- Slijepcevic, D.; Van De Graaf, S.F.J. Bile Acid Uptake Transporters as Targets for Therapy. Dig. Dis. 2017, 35, 251–258. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Lin, M.; Frey, M.R.; Fanter, R.; Paliy, O.; Hilbush, B.; Reo, N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miyamoto, Y.; Mazagova, M.; Lee, K.C.; Eckmann, L.; Schnabl, B. Microbiota Protects Mice against Acute Alcohol-Induced Liver Injury. Alcohol. Clin. Exp. Res. 2015, 39, 2313–2323. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol, N.A.S.H. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Mir, H.; Meena, A.S.; Chaudhry, K.K.; Shukla, P.K.; Gangwar, R.; Manda, B.; Padala, M.K.; Shen, L.; Turner, J.R.; Dietrich, P.; et al. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim. Biophys. Acta BBA-Gen. Subj. 2016, 1860, 765–774. [Google Scholar] [CrossRef]
- Chaudhry, K.K.; Shukla, P.K.; Mir, H.; Manda, B.; Gangwar, R.; Yadav, N.; Padala, M.K.; Shen, L.; Turner, J.R.; Dietrich, P.; et al. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J. Nutr. Biochem. 2016, 27, 16–26. [Google Scholar] [CrossRef]
- Baker, S.S.; Baker, R.D.; Liu, W.; Nowak, N.J.; Zhu, L. Role of alcohol metabolism in non-alcoholic steatohepatitis. PLoS ONE 2010, 5, e9570. [Google Scholar] [CrossRef]
- Zhu, R.; Baker, S.S.; Moylan, C.A.; Abdelmalek, M.F.; Guy, C.D.; Zamboni, F.; Wu, D.; Lin, W.; Liu, W.; Baker, R.D.; et al. Systematic transcriptome analysis reveals elevated expression of alcohol-metabolizing genes in NAFLD livers. J. Pathol. 2016, 238, 531–542. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7. [Google Scholar] [CrossRef] [PubMed]
- Iruzubieta, P.; Medina, J.M.; Fernández-López, R.; Crespo, J.; de la Cruz, F. A role for gut microbiome fermentative pathways in fatty liver disease progression. J. Clin. Med. 2020, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Davids, M.; Herrema, H.; Aydin, O.; Tremaroli, V.; Rios-Morales, M.; Levels, H.; Bruin, S.; de Brauw, M.; Verheij, J.; et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 2022, 28, 2100–2106. Available online: https://europepmc.org/article/med/36216942 (accessed on 5 February 2023). [CrossRef] [PubMed]
- Mehedint, M.G.; Zeisel, S.H. Choline’s role in maintaining liver function: New evidence for epigenetic mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Dzierlenga, A.L.; Lu, Z.; Billheimer, D.D.; Torabzadeh, E.; Lake, A.D.; Li, H.; Novak, P.; Shipkova, P.; Aranibar, N.; et al. Metabolomic profiling distinction of human nonalcoholic fatty liver disease progression from a common rat model. Obesity 2017, 25, 1069–1076. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461. [Google Scholar] [CrossRef]
- Kantartzis, K.; Peter, A.; Machicao, F.; Rgen Machann, J.; Wagner, S.; Königsrainer, I.; Königsrainer, A.; Schick, F.; Fritsche, A.; Häring, H.U.; et al. Dissociation Between Fatty Liver and Insulin Resistance in Humans Carrying a Variant of the Patatin-Like Phospholipase 3 Gene. Diabetes 2009, 58, 2616–2623. [Google Scholar] [CrossRef]
- Kotronen, A.; Johansson, L.E.; Johansson, L.M.; Roos, C.; Westerbacka, J.; Hamsten, A.; Bergholm, R.; Arkkila, P.; Arola, J.; Kiviluoto, T.; et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia 2009, 52, 1056–1060. [Google Scholar] [CrossRef]
- Sookoian, S.; Castaño, G.O.; Burgueño, A.L.; Gianotti, T.F.; Rosselli, M.S.; Pirola, C.J. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J. Lipid Res. 2009, 50, 2111–2116. [Google Scholar] [CrossRef]
- Valenti, L.; Al-Serri, A.; Daly, A.K.; Enrico Galmozzi Rametta, R.; Dongiovanni, P.; Nobili, V.; Mozzi, E.; Roviaro, G.; Vanni, E.; Bugianesi, E. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010, 51, 1209–1217. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Maglio, C.; Fracanzani, A.L.; Pipitone, R.; Mozzi, E.; Motta, B.M.; Kaminska, D.; Rametta, R.; Grimaudo, S.; et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 2015, 61, 506–514. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Zhou, Y.; Nidhina Haridas, P.A.; Dwivedi, O.P.; Hyötyläinen, T.; Ali, A.; Juuti, A.; Leivonen, M.; Tukiainen, T.; Ahonen, L.; et al. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J. Hepatol. 2017, 67, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Zhou, Y.; Hyötyläinen, T.; Leivonen, M.; Arola, J.; Orho-Melander, M.; Orešič, M.; Yki-Järvinen, H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J. Hepatol. 2016, 65, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef]
- Santoro, N.; Zhang, C.K.; Zhao, H.; Pakstis, A.J.; Kim, G.; Kursawe, R.; Dykas, D.J.; Bale, A.E.; Giannini, C.; Pierpont, B.; et al. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology 2011, 55, 781–789. [Google Scholar] [CrossRef]
- Petta, S.; Miele, L.; Bugianesi, E.; Cammà, C.; Rosso, C.; Boccia, S.; Cabibi, D.; Di Marco, V.; Grimaudo, S.; Grieco, A.; et al. Glucokinase Regulatory Protein Gene Polymorphism Affects Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e87523. [Google Scholar] [CrossRef]
- Beer, N.L.; Tribble, N.D.; McCulloch, L.J.; Roos, C.; Johnson, P.R.V.; Orho-Melander, M.; Gloyn, A.L. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum. Mol. Genet. 2009, 18, 4081–4088. [Google Scholar] [CrossRef]
- Todd, D.J.; Lee, A.H.; Glimcher, L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008, 8, 663–674. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Winnay, J.N.; Boucher, J.; Mori, M.A.; Ueki, K.; Kahn, C.R. A Novel Interaction Between the Regulatory Subunit of PI 3-Kinase and X-box Binding Protein-1 Modulates the Unfolded Protein Response. Nat. Med. 2010, 16, 438. [Google Scholar] [CrossRef] [PubMed]
- Sha, H.; He, Y.; Chen, H.; Wang, C.; Zenno, A.; Shi, H.; Yang, X.; Zhang, X.; Qi, L. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009, 9, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Ergin, A.S.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.G., Jr.; Ozcan, U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef]
- Bellentani, S.; Tiribelli, C. The spectrum of liver disease in the general population: Lesson from the dionysos study. J. Hepatol. 2001, 35, 531–537. [Google Scholar] [CrossRef]
- Rehm, J.; Taylor, B.; Mohapatra, S.; Irving, H.; Baliunas, D.; Patra, J.; Roerecke, M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol. Rev. 2010, 29, 437–445. [Google Scholar] [CrossRef]
- Department of Health. UK Chief Medical Officers’ Low Risk Drinking Guidelines. 2016. Available online: https://www.gov.uk/ (accessed on 30 January 2023).
- Cabezas, J.; Lucey, M.R.; Bataller, R. Biomarkers for Monitoring Alcohol Use. Available online: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/cld.571 (accessed on 30 January 2023).
- An International Group. Alcoholic liver disease: Morphological manifestations. Review by an international group. Lancet 1981, 317, 707–711. [Google Scholar] [CrossRef]
- Yip, W.W.; Burt, A.D. Alcoholic liver disease. Semin. Diagn. Pathol. 2006, 23, 149–160. [Google Scholar] [CrossRef]
- Lackner, C.; Stauber, R.E.; Davies, S.; Denk, H.; Dienes, H.P.; Gnemmi, V.; Guido, M.; Miquel, R.; Paradis, V.; Schirmacher, P.; et al. Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J. Hepatol. 2021, 75, 810–819. [Google Scholar] [CrossRef]
- Altamirano, J.; Miquel, R.; Katoonizadeh, A.; Abraldes, J.G.; Duarte-Rojo, A.; Louvet, A.; Augustin, S.; Mookerjee, R.P.; Michelena, J.; Smyrk, T.C.; et al. A Histologic Scoring System for Prognosis of Patients with Alcoholic Hepatitis. Gastroenterology 2014, 146, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Oneta, C.M.; Lieber, C.S.; Li, J.J.; Rüttimann, S.; Schmid, B.; Lattmann, J.; Rosman, A.S.; Seitz, H.K. Dynamics of cytochrome P4502E1 activity in man: Induction by ethanol and disappearance during withdrawal phase. J. Hepatol. 2002, 36, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chamulitrat, W.; Spitzer, J.J. Nitric oxide and liver injury in alcohol-fed rats after lipopolysaccharide administration. Alcohol Clin. Exp. Res. 1996, 20, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.; Stickel, F.; Trépo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Buch, S.; Lau, K.; Zu Schwabedissen, H.M.; Berg, T.; Ridinger, M.; Rietschel, M.; Schafmayer, C.; Braun, F.; Hinrichsen, H.; et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology 2011, 53, 86–95. [Google Scholar] [CrossRef]
- Salameh, H.; Raff, E.; Erwin, A.; Seth, D.; Nischalke, H.D.; Falleti, E.; Burza, M.A.; Leathert, J.; Romeo, S.; Molinaro, A.; et al. PNPLA3 gene polymorphism is associated with predisposition to and severity of alcoholic liver disease. Am. J. Gastroenterol. 2015, 110, 846–856. [Google Scholar] [CrossRef]
- Bataller, R.; North, K.E.; Brenner, D.A. Genetic polymorphisms and the progression of liver fibrosis: A critical appraisal. Hepatology 2003, 37, 493–503. [Google Scholar] [CrossRef]
- Lieber, C.S.; Rubin, E.; DeCarli, L.M. Hepatic microsomal ethanol oxidizing system (MEOS): Differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem. Biophys. Res. Commun. 1970, 40, 858–865. [Google Scholar] [CrossRef]
- Seitz, H.K.; Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer 2007, 7, 599–612. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Mueller, S.; Peccerella, T.; Qin, H.; Glassen, K.; Waldherr, R.; Flechtenmacher, C.; Straub, B.K.; Millonig, G.; Stickel, F.; Bruckner, T.; et al. Carcinogenic Etheno DNA Adducts in Alcoholic Liver Disease: Correlation with Cytochrome P-4502E1 and Fibrosis. Alcohol. Clin. Exp. Res. 2018, 42, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Millonig, G.; Nair, J.; Patsenker, E.; Stickel, F.; Mueller, S.; Bartsch, H.; Seitz, H.K. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology 2009, 50, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Linhart, K.; Bartsch, H.; Seitz, H.K. The role of reactive oxygen species (ROS) and cytochrome P-450 2E1 in the generation of carcinogenic etheno-DNA adducts. Redox. Biol. 2014, 3, 56–62. [Google Scholar] [CrossRef]
- Albano, E.; Clot, P.; Morimoto, M.; Tomasi, A.; Ingelman-Sundberg, M.; French, S.W. Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 1996, 23, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Massey, V.; Cabezas, J.; Bataller, R. Epigenetics in Liver Fibrosis. Semin. Liver. Dis. 2017, 37, 219–230. Available online: http://www.thieme-connect.com/products/ejournals/html/10.1055/s-0037-1605371 (accessed on 30 January 2023). [CrossRef] [PubMed]
- Szabo, G.; Satishchandran, A. MicroRNAs in alcoholic liver disease. Semin. Liver. Dis. 2015, 35, 36–42. [Google Scholar]
- Shen, H.; French, B.A.; Tillman, B.C.; Li, J.; French, S.W. Increased DNA methylation in the livers of patients with alcoholic hepatitis. Exp. Mol. Pathol. 2015, 99, 326–329. [Google Scholar] [CrossRef]
- You, M.; Liang, X.; Ajmo, J.M.; Ness, G.C. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, 892–898. [Google Scholar] [CrossRef]
- Lu, S.C.; Huang, Z.Z.; Yang, H.; Mato, J.M.; Avila, M.A.; Tsukamoto, H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G178–G185. [Google Scholar] [CrossRef]
- Lippai, D.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. Micro-RNA-155 Deficiency Prevents Alcohol-Induced Serum Endotoxin Increase and Small Bowel Inflammation in Mice. Alcohol. Clin. Exp. Res. 2014, 38, 2217. [Google Scholar] [CrossRef]
- Lieber, C.S. Effects of ethanol upon lipid metabolism. Lipids 1974, 9, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Gao, B.; Song, B.J. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 2009, 33, 191–205. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fischer, M.; Deeg, M.A.; Crabb, D.W. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J. Biol. Chem. 2002, 277, 29342–29347. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Pinaire, J.; Fischer, M.; Dorris, R.; Crabb, D.W. The Transcriptional and DNA Binding Activity of Peroxisome Proliferator-activated Receptor α Is Inhibited by Ethanol Metabolism. J. Biol. Chem. 2001, 276, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Kim, S.J.; Gao, B. Alcohol, adipose tissue and liver disease: Mechanistic links and clinical considerations. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 50–59. [Google Scholar] [CrossRef]
- Guo, J.; Friedman, S.L. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010, 3, 21. [Google Scholar] [CrossRef]
- Iracheta-Vellve, A.; Petrasek, J.; Satishchandran, A.; Gyongyosi, B.; Saha, B.; Kodys, K.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Szabo, G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 2015, 63, 1147–1155. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Saha, B.; Satishchandran, A.; Kodys, K.; Fitzgerald, K.A.; Evelyn, A.; Kurt-Jones, E.A.; Szabo, G. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J. Leukoc. Biol. 2015, 98, 249–256. [Google Scholar] [CrossRef]
- Iracheta-Vellve, A.; Petrasek, J.; Gyogyosi, B.; Bala, S.; Csak, T.; Kodys, K.; Szabo, G. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017, 37, 968–973. [Google Scholar] [CrossRef]
- Bala, S.; Csak, T.; Kodys, K.; Catalano, D.; Ambade, A.; Furi, I.; Lowe, P.; Cho, Y.; Iracheta-Vellve, A.; Szabo, G. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J. Leukoc. Biol. 2017, 102, 487–498. [Google Scholar] [CrossRef]
- Csak, T.; Bala, S.; Lippai, D.; Kodys, K.; Catalano, D.; Iracheta-Vellve, A.; Szabo, G. MicroRNA-155 deficiency attenuates liver steatosis and fibrosis without reducing inflammation in a mouse model of steatohepatitis. PLoS ONE 2015, 10, e0129251. [Google Scholar] [CrossRef] [PubMed]
- Blaya, D.; Aguilar-Bravo, B.; Hao, F.; Casacuberta-Serra, S.; Coll, M.; Perea, L.; Vallverdú, J.; Graupera, I.; Pose, E.; Llovet, L.; et al. Expression of microRNA-155 in inflammatory cells modulates liver injury. Hepatology 2018, 68, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Csak, T.; Saha, B.; Zatsiorsky, J.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 2016, 64, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Han, S.N.; Zhang, J.Y.; Nemeth, B.T.; Pacher, P.; Feng, D.; Bataller, R.; Cabezas, J.; Stärkel, P.; Caballeria, J.; et al. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1α Transactivation in Steatohepatitis. Cell Metab. 2018, 27, 339–350.e3. [Google Scholar] [CrossRef] [PubMed]
- French, S.W.; Bardag-Gorce, F. Ubiquitin-Proteasome Pathway in the Pathogenesis of Liver Disease. In Signaling Pathways in Liver Diseases; Springer: Berlin/Heidelberg, Germany, 2005; pp. 377–389. [Google Scholar]
- Ventura-Cots, M.; Argemi, J.; Jones, P.D.; Lackner, C.; el Hag, M.; Abraldes, J.G.; Alvarado, E.; Clemente, A.; Ravi, S.; Alves, A.; et al. Clinical, histological and molecular profiling of different stages of alcohol-related liver disease. Gut 2022, 71, 1856–1866. Available online: https://pubmed.ncbi.nlm.nih.gov/34992134/ (accessed on 1 February 2023). [CrossRef]
- Affò, S.; Morales-Ibanez, O.; Rodrigo-Torres, D.; Altamirano, J.; Blaya, D.; Dapito, D.H.; Millán, C.; Coll, M.; Caviglia, J.M.; Arroyo, V.; et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 2014, 63, 1782–1792. Available online: https://pubmed.ncbi.nlm.nih.gov/24415562/ (accessed on 1 February 2023). [CrossRef]
- Massey, V.L.; Qin, L.; Cabezas, J.; Caballeria, J.; Sancho-Bru, P.; Bataller, R.; Crews, F.T. TLR7-let-7 Signaling Contributes to Ethanol-Induced Hepatic Inflammatory Response in Mice and in Alcoholic Hepatitis. Alcohol. Clin. Exp. Res. 2018, 42, 2107–2122. [Google Scholar] [CrossRef]
- Odena, G.; Chen, J.; Lozano, J.J.; Altamirano, J.; Rodrigo-Torres, D.; Affo, S.; Morales-Ibanez, O.; Matsushita, H.; Zou, J.; Dumitru, R.; et al. LPS-TLR4 Pathway Mediates Ductular Cell Expansion in Alcoholic Hepatitis. Sci. Rep. 2016, 6, 35610. Available online: https://pubmed.ncbi.nlm.nih.gov/27752144/ (accessed on 1 February 2023). [CrossRef]
- Argemi, J.; Latasa, M.U.; Atkinson, S.R.; Blokhin, I.O.; Massey, V.; Gue, J.P.; Cabezas, J.; Lozano, J.J.; Van Booven, D.; Bell, A.; et al. Defective HNF4alpha-dependent gene expression as a driver of hepatocellular failure in alcoholic hepatitis. Nat. Commun. 2019, 10, 35610. Available online: https://pubmed.ncbi.nlm.nih.gov/31311938/ (accessed on 1 February 2023). [CrossRef]
- Brandl, K.; Hartmann, P.; Jih, L.J.; Pizzo, D.P.; Argemi, J.; Ventura-Cots, M.; Coulter, S.; Liddle, C.; Ling, L.; Rossi, S.J.; et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J. Hepatol. 2018, 69, 396–405. Available online: https://pubmed.ncbi.nlm.nih.gov/29654817/ (accessed on 1 February 2023). [CrossRef]
- Gao, B.; Emami, A.; Zhou, R.; Lang, S.; Duan, Y.; Wang, Y.; Jiang, L.; Loomba, R.; Brenner, D.A.; Stärkel, P.; et al. Functional Microbial Responses to Alcohol Abstinence in Patients with Alcohol Use Disorder. Front. Physiol. 2020, 11, 370. Available online: www.frontiersin.org (accessed on 1 February 2023). [CrossRef] [PubMed]
- Hsu, C.L.; Zhang, X.; Jiang, L.; Lang, S.; Hartmann, P.; Pride, D.; Fouts, D.E.; Stärkel, P.; Schnabl, B. Intestinal virome in patients with alcohol use disorder and after abstinence. Hepatol. Commun. 2022, 6, 2058–2069. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/hep4.1947 (accessed on 1 February 2023). [CrossRef] [PubMed]
- Chu, H.; Duan, Y.; Lang, S.; Jiang, L.; Wang, Y.; Llorente, C.; Liu, J.; Mogavero, S.; Bosques-Padilla, F.; Abraldes, J.G.; et al. The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 2020, 72, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Alin, P.; Somin, M.; Neuman, M.G. Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. Int. J. Mol. Sci. 2022, 23, 16226. Available online: https://www.mdpi.com/1422-0067/23/24/16226/htm (accessed on 5 February 2023). [CrossRef]
- Tamura, S.; Shimomura, I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1139–1142. [Google Scholar] [CrossRef]
- Spivey, J.R.; Bronk, S.F.; Gores, G.J. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J. Clin. Investig. 1993, 92, 17. [Google Scholar] [CrossRef]
- Faubion, W.A.; Guicciardi, M.E.; Miyoshi, H.; Bronk, S.F.; Roberts, P.J.; Svingen, P.A.; Kaufmann, S.H.; Gores, G.J. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J. Clin. Investig. 1999, 103, 137. [Google Scholar] [CrossRef]
- Kakiyama, G.; Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Heuman, D.M.; Kang, D.J.; Takei, H.; Nittono, H.; Ridlon, J.M.; Fuchs, M.; et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G929–G937. [Google Scholar] [CrossRef]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.R.; Barritt, A.S., 4th. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef] [PubMed]
- Iracheta-Vellve, A.; Petrasek, J.; Gyongyosi, B.; Satishchandran, A.; Lowe, P.; Kodys, K.; Catalano, D.; Calenda, C.D.; Kurt-Jones, E.A.; Fitzgerald, K.A.; et al. Endoplasmic Reticulum Stress-induced Hepatocellular Death Pathways Mediate Liver Injury and Fibrosis via Stimulator of Interferon Genes. J. Biol. Chem. 2016, 291, 26794–26805. [Google Scholar] [CrossRef]
- Wang, S.; Pacher, P.; De Lisle, R.C.; Huang, H.; Ding, W.X. A Mechanistic Review of Cell Death in Alcohol-Induced Liver Injury. Alcohol. Clin. Exp. Res. 2016, 40, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Vuppalanchi, R.; Raikwar, N.S.; Deeg, M.A. Glycosylphosphatidylinositol-specific phospholipase d in nonalcoholic Fatty liver disease: A preliminary study. J. Clin. Endocrinol. Metab. 2006, 91, 2279–2285. [Google Scholar] [CrossRef]
- Ferreira, V.S.G.; Pernambuco, R.B.; Lopes, E.P.; Morais, C.N.; Rodrigues, M.C.; Arruda, M.J.; Silva, L.M.E.; Vilar, L. Frequency and risk factors associated with non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metab. 2010, 54, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Saccoccio, G.; Masutti, F.; Crocè, L.S.; Brandi, G.; Sasso, F.; Cristanini, G.; Tiribelli, C. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann. Intern. Med. 2000, 132, 112–117. [Google Scholar] [CrossRef]
- Mahady, S.E.; Wong, G.; Craig, J.C.; George, J. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: A cost utility analysis. Hepatology 2012, 56, 2172–2179. [Google Scholar] [CrossRef]
- Ghouri, N.; Preiss, D.; Sattar, N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: A narrative review and clinical perspective of prospective data. Hepatology 2010, 52, 1156–1161. [Google Scholar] [CrossRef]
- Naveau, S.; Giraud, V.; Borotto, E.; Aubert, A.; Capron, F.; Chaput, J.C. Excess weight risk factor for alcoholic liver disease. Hepatology 1997, 25, 108–111. [Google Scholar] [CrossRef]
- Ruhl, C.E.; Everhart, J.E. Joint Effects of Body Weight and Alcohol on Elevated Serum Alanine Aminotransferase in the United States Population. Clin. Gastroenterol. Hepatol. 2005, 3, 1260–1268. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Holmqvist, M.; Bendtsen, P.; Mathiesen, U.L.; Bodemar, G.; Kechagias, S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2009, 44, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.R.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; Suzuki, Y.; Inao, M.; Mochida, S.; et al. Effects of Alcohol Consumption on Hepatocarcinogenesis in Japanese Patients With Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fujimori, N.; Sugiura, A.; Yamazaki, T.; Joshita, S.; Komatsu, M.; Umemura, T.; Matsumoto, A.; Tanaka, E. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fibrosis Retrospective Study. World J. Gastroenterol. 2018, 24, 1440–1450. [Google Scholar] [CrossRef]
- Seitz, H.K.; Mueller, S.; Hellerbrand, C.; Liangpunsakul, S. Effect of chronic alcohol consumption on the development and progression of non-alcoholic fatty liver disease (NAFLD). Hepatobiliary Surg. Nutr. 2015, 4, 147. [Google Scholar]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef]
- Blomdahl, J.; Nasr, P.; Ekstedt, M.; Kechagias, S. Moderate alcohol consumption is associated with significant fibrosis progression in NAFLD. Hepatol. Commun. 2023, 7, e0003. Available online: https://pubmed.ncbi.nlm.nih.gov/36633482/ (accessed on 5 February 2023). [CrossRef]
- Raynard, B.; Balian, A.; Fallik, D.; Capron, F.; Bedossa, P.; Chaput, J.C.; Naveau, S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology 2002, 35, 635–638. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Jula, A. Binge drinking and the risk of liver events: A population-based cohort study. Liver Int. 2017, 37, 1373–1381. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Meader, N.; Bird, V.; Rizzo, M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: Meta-analysis. Br. J. Psychiatry 2012, 201, 93–100. [Google Scholar] [CrossRef]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Grossman, D.C.; Kemper, A.R.; et al. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA 2019, 321, 580–587. [Google Scholar] [PubMed]
- Loomba, R.; Bettencourt, R.; Barrett-Connor, E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: The Rancho Bernardo Study. Aliment. Pharmacol. Ther. 2009, 30, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Alatalo, P.I.; Koivisto, H.M.; Hietala, J.P.; Puukka, K.S.; Bloigu, R.; Niemelä, O.J. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am. J. Clin. Nutr. 2008, 88, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Cotrim, H.P.; Freitas, L.A.; Alves, E.; Almeida, A.; May, D.S.; Caldwell, S. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. Eur. J. Gastroenterol. Hepatol. 2009, 21, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Sanyal, A.J.; Brunt, E.M.; Unalp-Arida, A.; Donohue, M.; McCullough, A.J.; Schwimmer, J.B. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J. Hepatol. 2012, 57, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Gunji, T.; Matsuhashi, N.; Sato, H.; Fujibayashi, K.; Okumura, M.; Sasabe, N.; Urabe, A. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am. J. Gastroenterol. 2009, 104, 2189–2195. [Google Scholar] [CrossRef]
- Yamada, T.; Fukatsu, M.; Suzuki, S.; Wada, T.; Yoshida, T.; Joh, T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J. Gastroenterol. Hepatol. 2010, 25, 352–356. [Google Scholar] [CrossRef]
- Moriya, A.; Iwasaki, Y.; Ohguchi, S.; Kayashima, E.; Mitsumune, T.; Taniguchi, H.; Ikeda, F.; Shiratori, Y.; Yamamoto, K. Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2011, 33, 378–388. [Google Scholar] [CrossRef]
- Dixon, J.B.; Dixon, M.E.; O’Brien, P.E. Alcohol consumption in the severely obese: Relationship with the metabolic syndrome. Obes. Res. 2002, 10, 245–252. [Google Scholar] [CrossRef]
- Hiramine, Y.; Imamura, Y.; Uto, H.; Koriyama, C.; Horiuchi, M.; Oketani, M.; Hosoyamada, K.; Kusano, K.; Ido, A.; Tsubouchi, H. Alcohol drinking patterns and the risk of fatty liver in Japanese men. J. Gastroenterol. 2011, 46, 519–528. [Google Scholar] [CrossRef]
- Hamaguchi, M.; Kojima, T.; Ohbora, A.; Takeda, N.; Fukui, M.; Kato, T. Protective effect of alcohol consumption for fatty liver but not metabolic syndrome. World J. Gastroenterol. 2012, 18, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Chu, W.C.; Wong, G.L.; Chan, R.S.; Chim, A.M.; Ong, A.; Yeung, D.K.; Yiu, K.K.; Chu, S.H.; Woo, J.; et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012, 61, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Greenson, J.K.; Conjeevaram, H.S. Effect of lifetime alcohol consumption on the histological severity of non-alcoholic fatty liver disease. Liver Int. 2014, 34, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Staufer, K.; Huber-Schönauer, U.; Strebinger, G.; Pimingstorfer, P.; Suesse, S.; Scherzer, T.M.; Paulweber, B.; Ferenci, P.; Stimpfl, T.; Yegles, M.; et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J. Hepatol. 2022, 77, 918–930. [Google Scholar] [CrossRef] [PubMed]
| Autor, Year | Outcomes | Design | Definition for Heavy Alcohol Consumption | Criteria for Overweight | Definition of NAFLD | Results |
|---|---|---|---|---|---|---|
| Naveau et al., 1997 [157] | Assess the impact of overweight in ALD | Retrospective cohort | >50 g/day of alcohol | BMI > 27 kg/m2 in men or >25 kg/m2 in women | Not defined | Higher risk in overweight patients of: Cirrhosis (60% vs. 35%; p = 0.001) Alcoholic hepatitis (8.2% vs. 2.6%; p = 0.05) Pure steatosis (8% vs. 2.5%; p = 0.05) |
| Bellentani et al., 2001 [90] | Characterization of liver diseases in general population | Prospective cohort | >30 g/day of alcohol | BMI > 25 kg/m2 in men or >24 kg/m2 in women | Not defined. Suspected by fatty liver in ultrasonography | Higher risk of hepatic steatosis in obese drinkers (>90%). |
| Raynard et al., 2002 [163] | Influence of alcohol in NASH patients | Prospective cohort | >50 g/day | Not defined | Liver biopsy | Association between duration of alcohol abuse and risk of significant fibrosis (p < 0.005) |
| Ruhl et al., 2005 [158] | Relationship between alcohol and overweight in patients with abnormal serum trasnaminases | Prospective cohort | >2 Drinks per day in overweight or >1 drink per day in obeses | BMI > 25 kg/m2 | Not defined | Increased aminotransferase levels in obese and overweight (12% vs. 7.3% vs. 4.4%; p = 0.001) |
| Loomba et al., 2009 [164] | Association between alcohol, BMI, and liver enzymes | Cross-sectional | >30 g/day | BMI > 25 kg/m2 | Not defined | Higher ALT and GGT levels. Increased risk of liver injury. Men OR 8.9 (95% CI, 2.4–33.1) Women OR 21-fold (95% CI, 2.6–170.1), |
| Eksted et al., 2009 [159] | Influence of alcohol intake in patients with NAFLD in terms of fibrosis stage progression | Retrospective cohort | Heavy episodic drinking (HED) (>60 g/day in male or >48 g/day in women at least 1 day in the past month) | BMI > 25 kg/m2 | Liver biopsy | More fibrosis progression in HED (47% vs. 11%; p = 0.003) |
| Aberg et al, 2017 [164] | Metabolic factors implicated in the development of complicated liver disease | Retrospective cohort | >210 g/week men or >140 g/week women | BMI > 25 kg/m2 | Metabolic syndrome | Higher incidence of complicated liver disease in drinkers OR 1.002 (1.001–1.002) |
| Author, Year | Primary Endpoint | Design | Sample Size | Criteria for NAFLD | Definition of Moderate Alcohol Use | Results | Bias |
|---|---|---|---|---|---|---|---|
| Alatalo et al., 2008 [168] | Link between alcohol consumption, BMI and liver enzymes | Retrospective cohort | 457 Overweight 67 moderate alcohol use | - | <40 g/day | Higher serum ALT and GGT levels (p < 0.05) | NAFLD not defined |
| Cotrim et al., 2009 [169] | NASH on liver biopsy | Cross-sectional | 132 NAFLD 75 moderate alcohol use | Liver biopsy | <40 g/day and <280 g/week | NASH more frequent among moderate alcohol users OR 2.69 (0.14–161.3) p = 0.41 | Bariatric surgery population |
| Ekstedt et al., 2009 [159] | Fibrosis progression Development of cirrhosis | Prospective cohort | 71 NAFLD 65 moderate alcohol use | Liver biopsy | <140 g/week | Higher risk of fibrosis progression for drinkers OR 7.11 (1.99–25.5) p = 0.003 | Lifetime use not measured |
| Dunn et al., 2009 [170] | Prevalence of suspected NAFLD | Retrospective cohort | 1031 NAFLD 523 moderate alcohol use | ALT > 43 or ALT > 30 men or ALT > 19 women | <10 g/day | Reduced prevalence of suspected NAFLD with alcohol OR 0.51 (0.33–0.79) p = 0.001 | NAFLD not proven by liver biopsy |
| Gunji et al., 2009 [171] | Presence of fatty liver | Cross-sectional | 5599 Fatty liver 2879 light to moderate alcohol use | Fatty liver on imaging test | Light alcohol use 40–140 g/week Moderate alcohol use 140–280 g/week | Light [OR 0.82 (0.63–0.94)] p = 0.044 Moderate [0.75 (0.61–0.92)] p = 0.008 | NAFLD not proven by liver biopsy |
| Yamada et al., 2010 [172] | Presence of fatty liver | Cross-sectional | 3127 fatty liver 2606 moderate alcohol use | Fatty liver on ultrasonography | <23 g/day | Daily moderate appeared protective 18.7% vs. 28.5% p = 0.05 | NAFLD not proven by liver biopsy |
| Ascha et al., 2010 [160] | HCC on imaging | Prospective cohort | 195 NAFLD 58 moderate alcohol use | Liver biopsy or cryptogenetic cirrhosis + metabolic syndrome | <2 drinks daily or 3–6 drinks daily on weekends | Higher risk of HCC for any alcohol use HR 3.8 (1.6–8.9) p < 0.002 | Cirrhotic population |
| Moriya et al., 2011 [173] | Presence of fatty liver | Cross-sectional | 2141 Fatty liver 677 moderate alcohol use | Fatty liver on imaging test | <20 g/day or <140 g/week | Low prevalence of fatty liver in moderate alcohol use OR 0.47 (0.23–0.96) p < 0.001 | NAFLD not proven by liver biopsy |
| Dixon et al., 2011 [174] | NASH on liver biopsy | Cross-sectional | 108 patients NAFLD 57 moderate alcohol use | Liver biopsy | <200 g/week | NASH less frequent among alcohol users OR 0.35 (0.12–1.0) p = 0.04 Not significant in multivariant | Morbidly obese |
| Hiramine et al., 2011 [175] | Prevalence of fatty liver | Cross-sectional | 3816 fatty liver 1389 moderate alcohol use | Fatty liver on ultrasonography | <20 g/day & >21 days per month | Decreased prevalence of fatty liver in drinkers OR 0.55 [0.45, 0.67] p < 0.001 | NAFLD not proven by liver biopsy |
| Hamaguchi et al., 2012 [176] | Presence of fatty liver and metabolic syndrome | Cross-sectional | 4335 Fatty liver 937 moderate alcohol use | Fatty liver on ultrasonography | <280 g/week | Decreased prevalence of fatty liver in moderate alcohol use Men OR 0.72 (0.63–0.83) p < 0.001 Women 0.43 (0.21–0.88) p < 0.021 | |
| Wong et al., 2012 [177] | Presence of NAFLD and fibrosis | Prospective cohort | 264 fatty liver 148 moderate alcohol use | Liver fat and fibrosis assessed by proton-magnetic resonance and transient elastography | <10 g/day | Modest alcohol consumption not associated with fatty liver OR 1.37 (0.89–2.11); p = 0.15 Nor increased liver stiffness 2.3% vs. 1.7% p = 0.54 | |
| Dunn et al., 2012 [170] | NASH progression (fibrosis stage) | Cross-sectional | 582 NAFLD 331 moderate alcohol use | Liver biopsy | <20 g/day | Higher fibrosis stage among moderate alcohol users | Lifetime use not measured |
| Kwon et al., 2014 [178] | Advanced fibrosis (stage 3–4) | Cross-sectional | 77 NAFLD 52 moderate alcohol use | Liver biopsy | <40 g/week | Less risk of advanced fibrosis among drinkers OR 0.26 (0.07–0.97) p = 0.046 | Alcohol pattern not determined |
| Your Experience Last Year | |
|---|---|
| 1 | Alcohol is often taken in largen amounts or over a longer period than intended |
| 2 | There is a persistent desire or unsuccessful efforts to cut down or control alcohol use |
| 3 | A great deal of time is spent in activities necessary to obtain alcohol, use alcohol, or recover from its effects |
| 4 | Craving, or a strong desire or urge to use alcohol |
| 5 | Recurrent alcohol use resulting in a failure to fulfill major role obligation at work, school, or home. |
| 6 | Continued alcohol use despite having persistent or recurrent social or interpesonal problems caused or exacerbated by the effects of alcohol |
| 7 | Important social, occupational, or recreational activities are given up or reduced because of alcohol use. |
| 8 | Recurrent alcohol use in situation in which it is physically hazardaous. |
| 9 | Alcohol use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by alcohol |
| 10 | Tolerance, defined as either of the following
|
| 11 | Withdrawal, as manifested by either of the following:
|
| Concept | Pattern | Sample | Window | Pros | Cons | |
|---|---|---|---|---|---|---|
| Indirect | ||||||
| >1.5–2 AST/ALT ratio | Mitochondrial damage by alcohol | Chronic heavy drinking (>40 g/day). | Serum or plasm | 2–3 weeks after consumption | Cheap Accesible | Lack of sensitivity and specificity |
| GGT | Glutation metabolism | Chronic heavy drinking (>40 g/day). | Serum or plasm | 2–3 weeks after consumption | Cheap Accesible Higher values can predict steatohepatitis | Lack of sensitivity and specificity |
| MCV | Toxic effect of acetaldehyde in morphology of red blood cells | Chronic heavy drinking (>40 g/day). | Serum or plasm | 2–8 weeks after consumption Normalization after 2–4 months of abstinence | Cheap Accessible | Altered in hematological diseases |
| CDT | Deficient binding of carbohydrates to trasnferrin in presence of alcohol | Chronic heavy drinking (>40 g/day). | Serum or plasm | 2–4 weeks after consumption Normalization within 2–4 weeks of abstinence | Best sensitivity and specificity of indirect biomarkers | Not widely available |
| Direct | ||||||
| 5-HTOL | Ehtanol adducts with hydroxytryptophol | Recent consumption (>20 g/day) | Urine | < 24 h | Quick Highly specific (99%) | Low sensitivity Not widely available Not for chronic alcohol use |
| PEth | Ethanol adducts with phospholipids | Chronic consumption (>30–40 g/day) | Dry drop | 2–4 weeks after consumption Normalization within 2 weeks of abstinence | Cheap Quick Accessible | Not widely available |
| EtG | Ethanol adducts with glucuronide | Acute or chronic consumption (>10 g/day) | Blood (<36 h), urine (<96 h) and hair (months) | High specificity and sensitivity near 100% | False positives in CKD and THC consumption | |
| FAEEs | Ethanol adducts with fatty acids | Acute or chronic consumption | Plasm (<24–96 h) and hair (months) | Highly specific | Low sensitivity Not widely available | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odriozola, A.; Santos-Laso, A.; del Barrio, M.; Cabezas, J.; Iruzubieta, P.; Arias-Loste, M.T.; Rivas, C.; Duque, J.C.R.; Antón, Á.; Fábrega, E.; et al. Fatty Liver Disease, Metabolism and Alcohol Interplay: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 7791. https://doi.org/10.3390/ijms24097791
Odriozola A, Santos-Laso A, del Barrio M, Cabezas J, Iruzubieta P, Arias-Loste MT, Rivas C, Duque JCR, Antón Á, Fábrega E, et al. Fatty Liver Disease, Metabolism and Alcohol Interplay: A Comprehensive Review. International Journal of Molecular Sciences. 2023; 24(9):7791. https://doi.org/10.3390/ijms24097791
Chicago/Turabian StyleOdriozola, Aitor, Alvaro Santos-Laso, María del Barrio, Joaquín Cabezas, Paula Iruzubieta, María Teresa Arias-Loste, Coral Rivas, Juan Carlos Rodríguez Duque, Ángela Antón, Emilio Fábrega, and et al. 2023. "Fatty Liver Disease, Metabolism and Alcohol Interplay: A Comprehensive Review" International Journal of Molecular Sciences 24, no. 9: 7791. https://doi.org/10.3390/ijms24097791
APA StyleOdriozola, A., Santos-Laso, A., del Barrio, M., Cabezas, J., Iruzubieta, P., Arias-Loste, M. T., Rivas, C., Duque, J. C. R., Antón, Á., Fábrega, E., & Crespo, J. (2023). Fatty Liver Disease, Metabolism and Alcohol Interplay: A Comprehensive Review. International Journal of Molecular Sciences, 24(9), 7791. https://doi.org/10.3390/ijms24097791






