Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
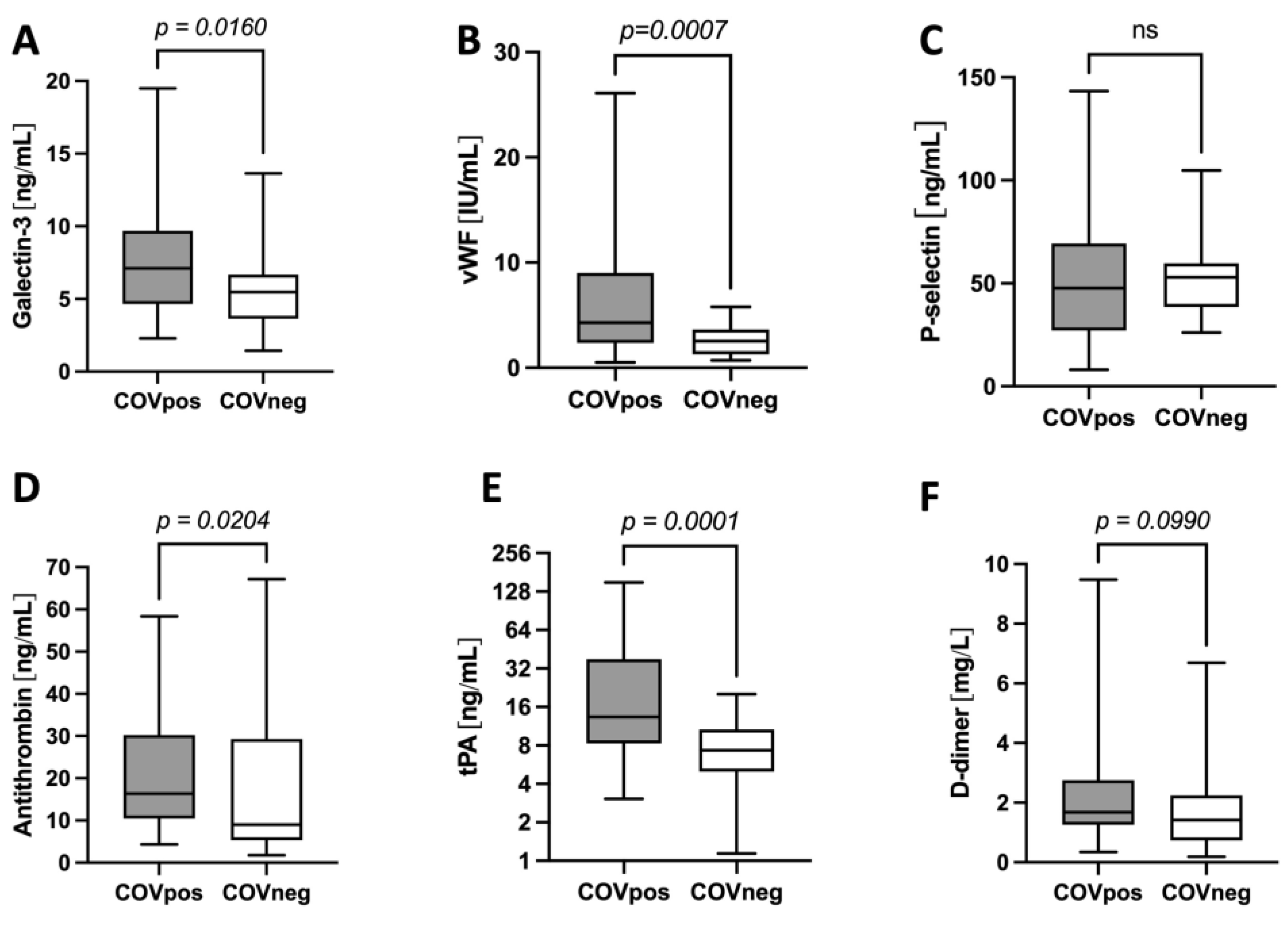
2.2. Galectin-3 Levels Are Higher and Correlate with Markers of Increased Thrombogenicity in Patients with COVID-19
2.3. Levels of Markers Associated with Treg Are Increased in COVID-19 and Correlate with Galectin-3 Levels
2.4. Galectin-3 Levels Relate to Clinical Severity
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Study Population
4.2. Data Collection
4.3. Enzyme-Linked Immunosorbend Assay
4.4. Multiplex Bead-Based Arrays
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Weekly Epidemiological Update on COVID-19—15 February 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---15-february-2023 (accessed on 22 February 2023).
- Del Valle, D.M.; Kim-Schulze, S.; Hsin-Hui, H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature helps predict COVID-19 severity and death. medRxiv 2020. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Ortega-Paz, L.; Capodanno, D.; Montalescot, G.; Angiolillo, D.J. Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management. J. Am. Heart Assoc. 2021, 10, e019650. [Google Scholar] [CrossRef]
- Jakobs, K.; Reinshagen, L.; Puccini, M.; Friebel, J.; Wilde, A.B.; Alsheik, A.; Rroku, A.; Landmesser, U.; Haghikia, A.; Krankel, N.; et al. Disease Severity in Moderate-to-Severe COVID-19 Is Associated With Platelet Hyperreactivity and Innate Immune Activation. Front. Immunol. 2022, 13, 844701. [Google Scholar] [CrossRef]
- Doevelaar, A.A.N.; Bachmann, M.; Holzer, B.; Seibert, F.S.; Rohn, B.J.; Bauer, F.; Witzke, O.; Dittmer, U.; Bachmann, M.; Yilmaz, S.; et al. von Willebrand Factor Multimer Formation Contributes to Immunothrombosis in Coronavirus Disease 2019. Crit. Care Med. 2021, 49, e512–e520. [Google Scholar] [CrossRef]
- Agrati, C.; Sacchi, A.; Tartaglia, E.; Vergori, A.; Gagliardini, R.; Scarabello, A.; Bibas, M. The Role of P-Selectin in COVID-19 Coagulopathy: An Updated Review. Int. J. Mol. Sci. 2021, 22, 7942. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Rezaie, A.R.; Giri, H. Anticoagulant and signaling functions of antithrombin. J. Thromb. Haemost. 2020, 18, 3142–3153. [Google Scholar] [CrossRef]
- Roemisch, J.; Gray, E.; Hoffmann, J.N.; Wiedermann, C.J. Antithrombin: A new look at the actions of a serine protease inhibitor. Blood Coagul. Fibrinolysis 2002, 13, 657–670. [Google Scholar] [CrossRef]
- Matsuo, T.; Kobayashi, H.; Kario, K.; Suzuki, S. Fibrin D-dimer in thrombogenic disorders. Semin. Thromb. Hemost. 2000, 26, 101–107. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Urano, T.; Castellino, F.J.; Suzuki, Y. Regulation of plasminogen activation on cell surfaces and fibrin. J. Thromb. Haemost. 2018, 16, 1487–1497. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef]
- Blanda, V.; Bracale, U.M.; Di Taranto, M.D.; Fortunato, G. Galectin-3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 9232. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Henderson, N.C.; Sethi, T. The regulation of inflammation by galectin-3. Immunol. Rev. 2009, 230, 160–171. [Google Scholar] [CrossRef]
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in heart failure: The past, current and future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Tang, J.K.K. The utility of growth differentiation factor-15, galectin-3, and sST2 as biomarkers for the diagnosis of heart failure with preserved ejection fraction and compared to heart failure with reduced ejection fraction: A systematic review. Heart Fail. Rev. 2021, 26, 799–812. [Google Scholar] [CrossRef]
- Cervantes-Alvarez, E.; la Rosa, N.L.; la Mora, M.S.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Kusnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembinski, M.; Kapusta, M.; Bociaga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study. Biomolecules 2021, 11, 1136. [Google Scholar] [CrossRef] [PubMed]
- Karsli, E.; Anabarli Metin, D.; Canacik, O.; Sabirli, R.; Kaymaz, B.; Kurt, O.; Koseler, A. Galectin-3 as a Potential Prognostic Biomarker for COVID-19 Disease: A Case-Control Study. Cureus 2022, 14, e28805. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069. [Google Scholar] [CrossRef]
- Ho, J.E.; Gao, W.; Levy, D.; Santhanakrishnan, R.; Araki, T.; Rosas, I.O.; Hatabu, H.; Latourelle, J.C.; Nishino, M.; Dupuis, J.; et al. Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am. J. Respir. Crit. Care Med. 2016, 194, 77–83. [Google Scholar] [CrossRef]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of galectin-3 in human pulmonary fibrosis. Allergol. Int. 2007, 56, 57–65. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Zheng, Y.; Yang, J.; Liu, Y.; Qi, Z.; Wu, M.; Fan, Z.; Yin, K.; Chen, Y.; et al. Galectin 3 enhances platelet aggregation and thrombosis via Dectin-1 activation: A translational study. Eur. Heart J. 2022, 43, 3556–3574. [Google Scholar] [CrossRef]
- Jakobs, K.; Rauch, U. Galectin-3 inhibitors as novel antithrombotic drugs with almost no bleeding risk: Wishful thinking or a realistic vision? Eur. Heart J. 2022, 43, 3575–3577. [Google Scholar] [CrossRef]
- Shahneh, F.; Grill, A.; Klein, M.; Frauhammer, F.; Bopp, T.; Schafer, K.; Raker, V.K.; Becker, C. Specialized regulatory T cells control venous blood clot resolution through SPARC. Blood 2021, 137, 1517–1526. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T Cells: Regulation of Identity and Function. Front. Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef]
- Dhar, S.K.; Vishnupriyan, K.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef]
- Sharma, A.; Rudra, D. Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front. Immunol. 2018, 9, 883. [Google Scholar] [CrossRef]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in T(reg) cell function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhu, Y.; Mao, J.; Du, R.C. T cell immunobiology and cytokine storm of COVID-19. Scand. J. Immunol. 2021, 93, e12989. [Google Scholar] [CrossRef]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Arrieta, V.; Martinez-Martinez, E.; Ibarrola, J.; Alvarez, V.; Sadaba, R.; Garcia-Pena, A.; Fernandez-Celis, A.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. A role for galectin-3 in the development of early molecular alterations in short-term aortic stenosis. Clin. Sci. 2017, 131, 935–949. [Google Scholar] [CrossRef]
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, S.; Yilmaz, F.M.; Bastug, A.; Ozbay, B.O.; Aydos, O.; Yucel, C.; Bodur, H.; Yilmaz, G. Assessment of Galectin-1, Galectin-3, and Prostaglandin E2 Levels in Patients with COVID-19. Jpn. J. Infect. Dis. 2021, 74, 530–536. [Google Scholar] [CrossRef]
- Berber, N.K.; Geckil, A.A.; Altan, N.O.; Kiran, T.R.; Otlu, O.; Erdem, M.; In, E. Efficacy of Serum Apelin and Galectin-3 as Potential Predictors of Mortality in Severe COVID-19 Patients. J. Med. Virol. 2023, 95, e28494. [Google Scholar] [CrossRef] [PubMed]
- Aleksova, A.; Sinagra, G.; Beltrami, A.P.; Pierri, A.; Ferro, F.; Janjusevic, M.; Gagno, G. Biomarkers in the management of acute heart failure: State of the art and role in COVID-19 era. ESC Heart Fail. 2021, 8, 4465–4483. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Sexton, T.; Smyth, S.; International, C.-T.B.C.I. COVID-19 and biomarkers of thrombosis: Focus on von Willebrand factor and extracellular vesicles. J. Thromb. Thrombolysis 2021, 52, 1010–1019. [Google Scholar] [CrossRef]
- Rostami, M.; Mansouritorghabeh, H.; Parsa-Kondelaji, M. High levels of Von Willebrand factor markers in COVID-19: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 22, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Dolgushina, N.; Gorodnova, E.; Beznoshenco, O.; Romanov, A.; Menzhinskaya, I.; Krechetova, L.; Sukhikh, G. Von Willebrand Factor and ADAMTS-13 Are Associated with the Severity of COVID-19 Disease. J. Clin. Med. 2022, 11, 4006. [Google Scholar] [CrossRef]
- Bertolin, A.J.; Dalcoquio, T.F.; Salsoso, R.; de, M.F.R.H.; Kalil-Filho, R.; Hajjar, L.A.; Siciliano, R.F.; Kallas, E.G.; Baracioli, L.M.; Lima, F.G.; et al. Platelet Reactivity and Coagulation Markers in Patients with COVID-19. Adv. Ther. 2021, 38, 3911–3923. [Google Scholar] [CrossRef]
- Clark, C.C.; Jukema, B.N.; Barendrecht, A.D.; Spanjaard, J.S.; Jorritsma, N.K.N.; Smits, S.; de Maat, S.; Seinen, C.W.; Verhoef, S.; Parr, N.M.J.; et al. Thrombotic Events in COVID-19 Are Associated With a Lower Use of Prophylactic Anticoagulation Before Hospitalization and Followed by Decreases in Platelet Reactivity. Front. Med. 2021, 8, 650129. [Google Scholar] [CrossRef]
- Cani, E.; Dwivedi, D.J.; Liaw, K.L.; Fraser, D.D.; Yeh, C.H.; Martin, C.; Slessarev, M.; Cerroni, S.E.; Fox-Robichaud, A.A.; Weitz, J.I.; et al. Immunothrombosis Biomarkers for Distinguishing Coronavirus Disease 2019 Patients From Noncoronavirus Disease Septic Patients With Pneumonia and for Predicting ICU Mortality. Crit. Care Explor. 2021, 3, e0588. [Google Scholar] [CrossRef]
- Heinz, C.; Miesbach, W.; Herrmann, E.; Sonntagbauer, M.; Raimann, F.J.; Zacharowski, K.; Weber, C.F.; Adam, E.H. Greater Fibrinolysis Resistance but No Greater Platelet Aggregation in Critically Ill COVID-19 Patients. Anesthesiology 2021, 134, 457–467. [Google Scholar] [CrossRef]
- Rahaghi, F.N.; Pistenmaa, C.L. Hypercoagulation in COPD: The clot thickens. ERJ Open. Res. 2021, 7, 00534–02021. [Google Scholar] [CrossRef]
- Liu, M.; Hu, R.; Jiang, X.; Mei, X. Coagulation dysfunction in patients with AECOPD and its relation to infection and hypercapnia. J. Clin. Lab. Anal. 2021, 35, e23733. [Google Scholar] [CrossRef]
- Gaughan, E.E.; Quinn, T.M.; Mills, A.; Bruce, A.M.; Antonelli, J.; MacKinnon, A.C.; Aslanis, V.; Li, F.; O’Connor, R.; Boz, C.; et al. An Inhaled Galectin-3 Inhibitor in COVID-19 Pneumonitis: A Phase Ib/IIa Randomized Controlled Clinical Trial (DEFINE). Am. J. Respir. Crit. Care Med. 2023, 207, 138–149. [Google Scholar] [CrossRef]
- Haunhorst, S.; Bloch, W.; Javelle, F.; Kruger, K.; Baumgart, S.; Drube, S.; Lemhofer, C.; Reuken, P.; Stallmach, A.; Muller, M.; et al. A scoping review of regulatory T cell dynamics in convalescent COVID-19 patients—Indications for their potential involvement in the development of Long COVID? Front. Immunol. 2022, 13, 1070994. [Google Scholar] [CrossRef]


| Total Population (n = 90) | COVpos (n = 55) | COVneg (n = 35) | p Value | |
|---|---|---|---|---|
| Age | 70 (56.5–79.25) | 69 (55–76) | 73 (58–81) | 0.146 |
| Gender, male | 58 (64.4%) | 38 (69.1%) | 20 (57.1%) | 0.267 |
| BMI (kg/m2) * | 26.33 (24.58–30.33) | 26.70 (24.69–30.70) | 25.11 (22.92–28-50) | 0.135 |
| On ICU | 26 (28.9%) | 20 (36.4%) | 6 (17.1%) | 0.059 |
| Died | 10 (11.1%) | 10 (18.2%) | 0 (0%) | 0.006 |
| Pre-existing conditions | ||||
| Heart failure | 9 (10%) | 2 (3.6%) | 7 (20%) | 0.025 |
| Coronary artery disease | 19 (21.1%) | 7 (12.7%) | 12 (34.3%) | 0.019 |
| Arterial hypertension | 58 (64.4%) | 34 (61.8%) | 24 (68.6%) | 0.652 |
| Diabetes mellitus | 22 (24.4%) | 14 (25.5%) | 8 (22.9%) | 1.0 |
| Peripheral artery disease | 14 (15.6%) | 2 (3.6%) | 12 (34.3%) | <0.001 |
| Hypercholesterinemia | 25 (27.8%) | 15 (27.3%) | 10 (28.6%) | 1.0 |
| COPD | 17 (18.9%) | 4 (7.3%) | 13 (37.1%) | <0.001 |
| Medication | ||||
| Prophylactic anticoagulation | 56 (62.2%) | 34 (61.8%) | 22 (62.9%) | 1.0 |
| Therapeutic anticoagulation | 34 (37.8%) | 21 (38.2%) | 13 (37.1%) | 1.0 |
| ASS | 35 (38.9%) | 23 (41.8%) | 12 (34.3%) | 0.513 |
| ADP Receptor Antagonist | 2 (2.2%) | 2 (3.6%) | 0 (0%) | 0.519 |
| Betablocker | 32 (35.6%) | 15 (27.3%) | 17 (48.6%) | 0.046 |
| RAAS-blockage | 35 (38.9%) | 17 (30.9%) | 18 (51.4%) | 0.076 |
| Diuretics | 36 (40%) | 19 (34.5%) | 17 (48.6) | 0.195 |
| Statins | 23 (25.6%) | 14 (25.5%) | 9 (25.7%) | 1.0 |
| Glucocorticoids | 39 (43.3%) | 32 (58.2%) | 7 (20%) | <0.001 |
| Remdesivir | 1 (1.1%) | 1 (1.8%) | 0 (0%) | 1.0 |
| Tocilizumab | 1 (1.1%) | 1 (1.8%) | 0 (0%) | 1.0 |
| Inhalative Therapy | 63 (70%) | 41 (74.5%) | 22 (62.9%) | 0.250 |
| Laboratory values | ||||
| Creatinine (mg/dL) * | 0.92 (0.76–1.19) | 0.92 (0.7–1.16) | 0.94 (0.78–1.23) | 0.649 |
| BUN (mg/dL) * | 41.5 (27–59) | 48 (27–63) | 34 (25–52) | 0.105 |
| NT-proBNP (ng/L) * | 488 (173–1695.25) | 359 (136–1616) | 930 (322–2318) | 0.079 |
| CRP (mg/dL) * | 65.3 (25.6–105.05) | 69 (18.9–125) | 62.1 (38.78–102.15) | 0.807 |
| Hemoglobin (g/dL) * | 11.5 (9.78–12.8) | 11.4 (9.4–12.6) | 11.8 (10.1 -13.4) | 0.204 |
| Leukocytes (n/nL) * | 8.77 (6.72–12.16) | 8.51 (6.77–12.37) | 8.83 (6.67–11.16) | 0.878 |
| Thrombocytes (n/pL) * | 281.5 (229.25–371.25) | 297 (234–397) | 279 (209–322) | 0.111 |
| MPV (fL) * | 10.4 (9.7–11.23) | 10.4 (9.68–11.53) | 10.35 (9.7–10.98) | 0.374 |
| Galectin-3 (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Total Population | COVpos | COVneg | ||||
| vWF (IU/mL) | r = 0.380 | p < 0.001 | r = 0.428 | p = 0.002 | r = 0.122 | p = 0.485 |
| MPV (fL) | r = 0.212 | p = 0.054 | r = 0.346 | p = 0.011 | r = −0.057 | p = 0.757 |
| P-selectin (ng/mL) | r = −0.129 | p = 0.241 | r = 0.000 | p = 1.000 | r = −0.241 | p = 0.162 |
| Antithrombin (ng/mL) | r = 0.432 | p < 0.001 | r = 0.339 | p = 0.014 | r = 0.430 | p = 0.010 |
| tPA (ng/mL) | r = 0.015 | p = 0.896 | r = 0.028 | p = 0.846 | r = −0.294 | p = 0.114 |
| D-dimer (mg/mL) | r = 0.385 | p < 0.001 | r = 0.462 | p < 0.001 | r = 0.196 | p = 0.267 |
| Galectin-3 (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Total Population | COVpos | COVneg | ||||
| IL-2 (pg/mL) | r = 0.181 | p = 0.105 | r = 0.308 | p = 0.037 | r = −0.032 | p = 0.854 |
| IL-10 (pg/mL) | r = 0.258 | p = 0.016 | r = 0.336 | p = 0.015 | r = −0.093 | p = 0.594 |
| sCD25 (pg/mL) | r = 0.346 | p = 0.001 | r = 0.392 | p = 0.004 | r = 0.183 | p = 0.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccini, M.; Jakobs, K.; Reinshagen, L.; Friebel, J.; Schencke, P.-A.; Ghanbari, E.; Landmesser, U.; Haghikia, A.; Kränkel, N.; Rauch, U. Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. Int. J. Mol. Sci. 2023, 24, 7683. https://doi.org/10.3390/ijms24097683
Puccini M, Jakobs K, Reinshagen L, Friebel J, Schencke P-A, Ghanbari E, Landmesser U, Haghikia A, Kränkel N, Rauch U. Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. International Journal of Molecular Sciences. 2023; 24(9):7683. https://doi.org/10.3390/ijms24097683
Chicago/Turabian StylePuccini, Marianna, Kai Jakobs, Leander Reinshagen, Julian Friebel, Philipp-Alexander Schencke, Emily Ghanbari, Ulf Landmesser, Arash Haghikia, Nicolle Kränkel, and Ursula Rauch. 2023. "Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19" International Journal of Molecular Sciences 24, no. 9: 7683. https://doi.org/10.3390/ijms24097683
APA StylePuccini, M., Jakobs, K., Reinshagen, L., Friebel, J., Schencke, P.-A., Ghanbari, E., Landmesser, U., Haghikia, A., Kränkel, N., & Rauch, U. (2023). Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. International Journal of Molecular Sciences, 24(9), 7683. https://doi.org/10.3390/ijms24097683





