Prenatal and Neonatal Pulmonary Thrombosis as a Potential Complication of SARS-CoV-2 Infection in Late Pregnancy
Abstract
1. Introduction
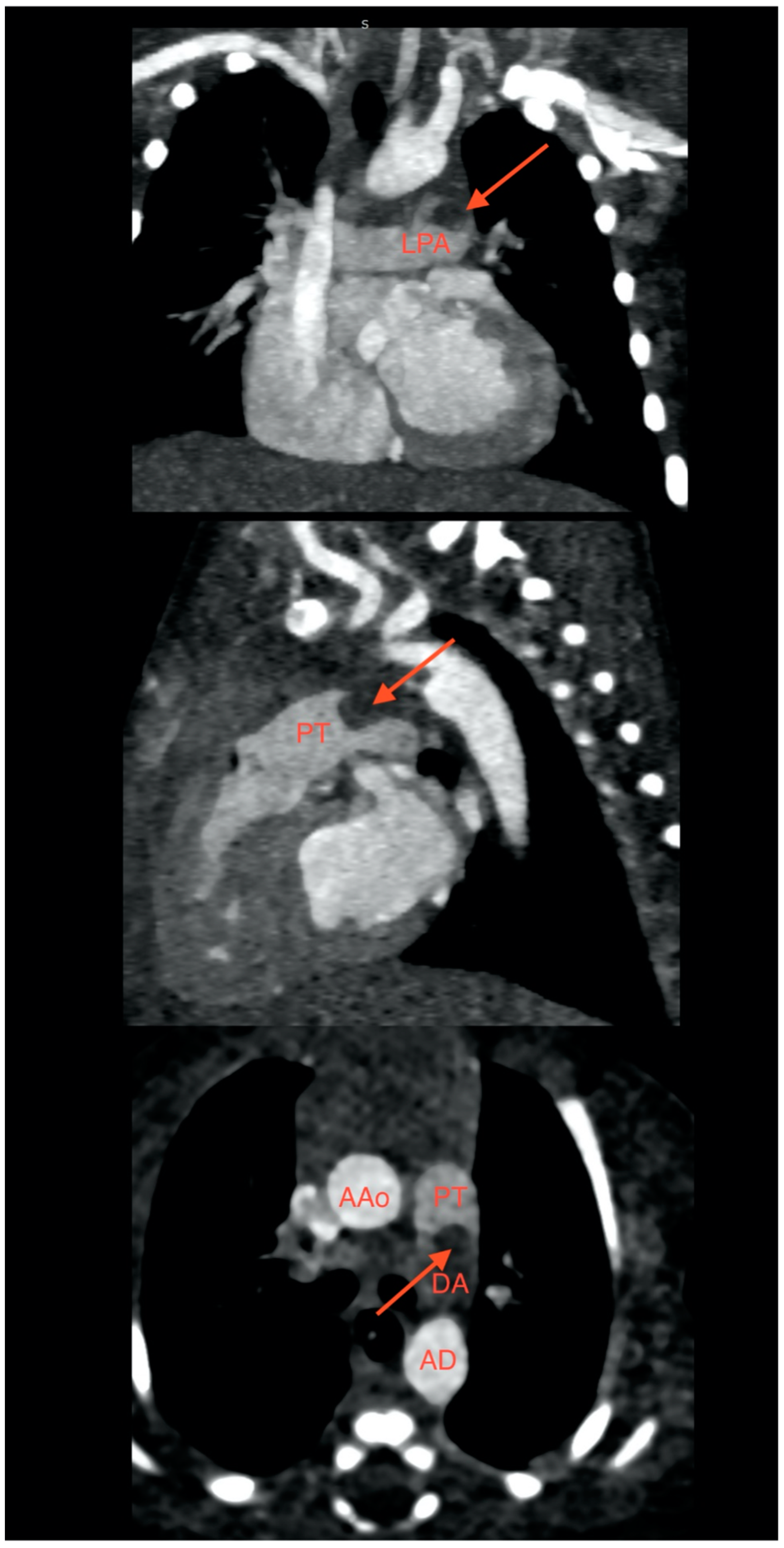
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Makatsariya, A.; Bitsadze, V.; Khizroeva, J.; Vorobev, A.; Makatsariya, N.; Egorova, E.; Mischenko, A.; Mashkova, T.; Antonova, A. Neonatal thrombosis. J. Matern.-Fetal Neonatal Med. 2020, 35, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, E.K.; Atchison, C.M.; Arlikar, S.; Ayala, I.; Barrett, L.; Branchford, B.R.; Streiff, M.; Takemoto, C.; Goldenberg, N.A. Risk factors for hospital-sssociated venous thromboembolism in the neonatal intensive care unit. Thromb. Res. 2014, 134, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Easterlin, M.C.; Li, Y.; Yieh, L.; Gong, C.L.; Jaffray, J.; Hall, M.; Friedlich, P.S.; Lakshmanan, A. Predictors of venous thromboembolism among infants in children’s hospitals in the United States: A retrospective Pediatric Health Information Study. J. Perinatol. 2022, 42, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Grosse, S.D.; Thornburg, C.D.; Yusuf, H.; Tsai, J.; Hooper, W.C. Trends in Venous Thromboembolism-Related Hospitalizations, 1994–2009. Pediatrics 2012, 130, e812–e820. [Google Scholar] [CrossRef] [PubMed]
- Branchford, B.R.; Mourani, P.; Bajaj, L.; Manco-Johnson, M.; Wang, M.; Goldenberg, N.A. Risk factors for in-hospital venous thromboembolism in children: A case-control study employing diagnostic validation. Haematologica 2012, 97, 509–515. [Google Scholar] [CrossRef]
- Nowak-Göttl, U.; Dübbers, A.; Kececioglu, D.; Koch, H.G.; Kotthoff, S.; Runde, J.; Vielhaber, H. Factor V Leiden, protein C, and lipoprotein (a) in catheter-related thrombosis in childhood: A prospective study. J. Pediatr. 1997, 131, 608–612. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Delli Muti, N.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: A systematic review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef]
- Dale, L. Neurological Complications of COVID-19: A Review of the Literature. Cureus 2022, 14, e27633. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Whitworth, H.; Sartain, S.E.; Kumar, R.; Armstrong, K.; Ballester, L.; Betensky, M.; Cohen, C.T.; Diaz, R.; Diorio, C.; Goldenberg, N.A.; et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood 2021, 138, 190–198. [Google Scholar] [CrossRef]
- Campi, F.; Longo, D.; Bersani, I.; Savarese, I.; Lucignani, G.; Haass, C.; Paolino, M.C.; Vadalà, S.; De Liso, P.; Di Capua, M.; et al. Neonatal Cerebral Venous Thrombosis following Maternal SARS-CoV-2 Infection in Pregnancy. Neonatology 2022, 119, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.J.; Vauloup-Fellous, C.; Prevot, S.; Zupan, V.; Suffee, C.; Do Cao, J.; Benachi, A.; De Luca, D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020, 11, 3572. [Google Scholar] [CrossRef]
- Lima, A.R.O.; Cardoso, C.C.; Bentim, P.R.B.; Voloch, C.M.; Rossi, Á.D.; da Costa, R.M.M.S.C.; da Paz, J.A.S.; Agostinho, R.F.; Figueiredo, V.R.F.S.; Júnior, J.S.S.; et al. Maternal SARS-CoV-2 Infection Associated to Systemic Inflammatory Response and Pericardial Effusion in the Newborn: A Case Report. J. Pediatr. Infect. Dis. Soc. 2020, 10, 536–539. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; Dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Ribes, A.; Vardon-Bounes, F.; Mémier, V.; Poette, M.; Au-Duong, J.; Garcia, C.; Minville, V.; Sié, P.; Bura-Rivière, A.; Voisin, S.; et al. Thromboembolic events and COVID-19. Adv. Biol. Regul. 2020, 77, 100735. [Google Scholar] [CrossRef]
- Smadja, D.M.; Guerin, C.L.; Chocron, R.; Yatim, N.; Boussier, J.; Gendron, N.; Khider, L.; Hadjadj, J.; Goudot, G.; DeBuc, B.; et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020, 23, 611–620. [Google Scholar]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nature reviews. Immunology 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Eketunde, A.O.; Mellacheruvu, S.P.; Oreoluwa, P. A review of postmortem findings in patients with COVID-19. Cureus 2020, 12, e9438. [Google Scholar]
- Blondon, M.; Cereghetti, S.; Pugin, J.; Marti, C.; Darbellay Farhoumand, P.; Reny, J.; Calmy, A.; Combescure, C.; Mazzolai, L.; Pantet, O.; et al. Therapeutic anticoagulation to prevent thrombosis, coagulopathy, and mortality in severe COVID-19: The Swiss COVID-HEP randomized clinical trial. Res. Pract. Thromb. Haemost. 2022, 6, e12712. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lapić, I.; Radić Antolic, M.; Horvat, I.; Premužić, V.; Palić, J.; Rogić, D.; Zadro, R. Association of polymorphisms in genes encoding prothrombotic and cardiovascular risk factors with disease severity in COVID-19 patients: A pilot study. J. Med. Virol. 2022, 94, 3669–3675. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Alonso, D.; Murias, S.; Martínez-de-Azagra Garde, A.; Soriano-Arandes, A.; Pareja, M.; Otheo, E.; Moraleda, C.; Tagarro, A.; Calvo, C. Prevalence of thrombotic complications in children with SARS-CoV-2. Arch. Dis. Child. 2021, 106, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, E.A. Epidemiology of venous thromboembolism in neonates and children. Thromb. Res. 2006, 118, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Schiappacasse, C.; Alarcón-Andrade, G.; Valenzuela, G.; Ferreiro, M.; Cavagnaro, A.; García-Salum, T.; Gutiérrez, M.; Medina, R.A. Pulmonary Artery Thrombosis in a Newborn With Severe Coronavirus Disease 2019. Pediatr. Infect. Dis. J. 2021, 40, e252–e254. [Google Scholar] [CrossRef]
- More, K.; Aiyer, S.; Goti, A.; Parikh, M.; Sheikh, S.; Patel, G.; Kallem, V.; Soni, R.; Kumar, P. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: A case series. Eur. J. Pediatr. 2022, 181, 1883–1898. [Google Scholar] [CrossRef]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef]
- Rey-Jurado, E.; Espinosa, Y.; Astudillo, C.; Jimena Cortés, L.; Hormazabal, J.; Noguera, L.P.; Cofré, F.; Piñera, C.; González, R.; Bataszew, A.; et al. Deep immunophenotyping reveals biomarkers of multisystemic inflammatory syndrome in children in a Latin American cohort. J. Allergy Clin. Immunol. 2022, 150, 1074–1085.e11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Rostad, C.A.; Jaggi, P.; Villacis Nunez, D.S.; Prince, C.; Lu, A.; Hussaini, L.; Nguyen, T.H.; Malik, S.; Ponder, L.A.; et al. Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis (HLH). J. Allergy Clin. Immunol. 2022, 149, 1592–1606.e16. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef]
- Esteve-Sole, A.; Anton, J.; Pino-Ramirez, R.M.; Sanchez-Manubens, J.; Fumadó, V.; Fortuny, C.; Rios-Barnes, M.; Sanchez-de-Toledo, J.; Girona-Alarcón, M.; Mosquera, J.M.; et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021, 131, e144554. [Google Scholar] [CrossRef]
- Gurlevik, S.L.; Ozsurekci, Y.; Sağ, E.; Derin Oygar, P.; Kesici, S.; Akca, Ü.K.; Cuceoglu, M.K.; Basaran, O.; Göncü, S.; Karakaya, J.; et al. The difference of the inflammatory milieu in MIS-C and severe COVID-19. Pediatr. Res. 2022, 92, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Castagnoli, R.; Vakkilainen, S.; Liu, C.; Delmonte, O.M.; Oguz, C.; Kaplan, I.M.; Alehashemi, S.; Burbelo, P.D.; Bhuyan, F.; et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 2022, 28, 1050–1062. [Google Scholar] [CrossRef]
- Caldarale, F.; Giacomelli, M.; Garrafa, E.; Tamassia, N.; Morreale, A.; Poli, P.; Timpano, S.; Baresi, G.; Zunica, F.; Cattalini, M.; et al. Plasmacytoid Dendritic Cells Depletion and Elevation of IFN-γ Dependent Chemokines CXCL9 and CXCL10 in Children with Multisystem Inflammatory Syndrome. Front. Immunol. 2021, 12, 654587. [Google Scholar] [CrossRef]
- Trapani, S.; Rubino, C.; Lasagni, D.; Pegoraro, F.; Resti, M.; Simonini, G.; Indolfi, G. Thromboembolic complications in children with COVID-19 and MIS-C: A narrative review. Front. Pediatr. 2022, 10, 944743. [Google Scholar] [CrossRef] [PubMed]
- Amonkar, P.S.; Gavhane, J.B.; Kharche, S.N.; Kadam, S.S.; Bhusare, D.B. Aortic thrombosis in a neonate with COVID-19-related fetal inflammatory response syndrome requiring amputation of the leg: A case report. Paediatr. Int. Child Health 2021, 41, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kubli, S.P.; Yoshinaga, S.K.; Pfeffer, K.; Mak, T.W. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020, 27, 3209–3225. [Google Scholar] [CrossRef]
- Stevens, H.; Canovas, R.; Tran, H.; Peter, K.; McFadyen, J.D. Inherited Thrombophilias Are Associated With a Higher Risk of COVID-19–Associated Venous Thromboembolism: A Prospective Population-Based Cohort Study. Circulation 2022, 145, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7, 622473. [Google Scholar] [CrossRef] [PubMed]
- Hickey, S.E.; Curry, C.J.; Toriello, H.V. ACMG Practice Guideline: Lack of evidence for MTHFR polymorphism testing. Genet. Med. 2013, 15, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Graydon, J.S.; Claudio, K.; Baker, S.; Kocherla, M.; Ferreira, M.; Roche-Lima, A.; Rodríguez-Maldonado, J.; Duconge, J.; Ruaño, G. Ethnogeographic prevalence and implications of the 677C>T and 1298A>C MTHFR polymorphisms in US primary care populations. Biomark. Med. 2019, 13, 649–661. [Google Scholar] [CrossRef] [PubMed]
- ElHassan, N.O.; Sproles, C.; Sachdeva, R.; Bhutta, S.T.; Szabo, J.S. A neonate with left pulmonary artery thrombosis and left lung hypoplasia: A case report. J. Med. Case Rep. 2010, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, T.; Antle, A.; Studer, M.; Thompson, M.; Perry, S.; Mahnke, C.B. Neonatal Pulmonary Artery Thrombosis Presenting as Persistent Pulmonary Hypertension of the Newborn. Pediatr. Cardiol. 2008, 30, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Flynn, N.R.; Barnette, D.A.; Woeltje, K.F.; Miller, G.P.; Swamidass, S.J. Machine learning liver-injuring drug interactions with non-steroidal anti-inflammatory drugs (NSAIDs) from a retrospective electronic health record (EHR) cohort. PLoS Comput. Biol. 2021, 17, e1009053. [Google Scholar] [CrossRef]
- Datta, A.; Matlock, M.K.; Le Dang, N.; Moulin, T.; Woeltje, K.F.; Yanik, E.L.; Joshua Swamidass, S. ‘Black Box’ to ‘Conversational’ Machine Learning: Ondansetron Reduces Risk of Hospital-Acquired Venous Thromboembolism. IEEE J. Biomed. Health Inform. 2021, 25, 2204–2214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulaziz-Opiela, G.; Sobieraj, A.; Sibrecht, G.; Bajdor, J.; Mroziński, B.; Kozłowska, Z.; Iciek, R.; Wróblewska-Seniuk, K.; Wender-Ożegowska, E.; Szczapa, T. Prenatal and Neonatal Pulmonary Thrombosis as a Potential Complication of SARS-CoV-2 Infection in Late Pregnancy. Int. J. Mol. Sci. 2023, 24, 7629. https://doi.org/10.3390/ijms24087629
Abdulaziz-Opiela G, Sobieraj A, Sibrecht G, Bajdor J, Mroziński B, Kozłowska Z, Iciek R, Wróblewska-Seniuk K, Wender-Ożegowska E, Szczapa T. Prenatal and Neonatal Pulmonary Thrombosis as a Potential Complication of SARS-CoV-2 Infection in Late Pregnancy. International Journal of Molecular Sciences. 2023; 24(8):7629. https://doi.org/10.3390/ijms24087629
Chicago/Turabian StyleAbdulaziz-Opiela, Gazala, Anna Sobieraj, Greta Sibrecht, Julia Bajdor, Bartłomiej Mroziński, Zuzanna Kozłowska, Rafał Iciek, Katarzyna Wróblewska-Seniuk, Ewa Wender-Ożegowska, and Tomasz Szczapa. 2023. "Prenatal and Neonatal Pulmonary Thrombosis as a Potential Complication of SARS-CoV-2 Infection in Late Pregnancy" International Journal of Molecular Sciences 24, no. 8: 7629. https://doi.org/10.3390/ijms24087629
APA StyleAbdulaziz-Opiela, G., Sobieraj, A., Sibrecht, G., Bajdor, J., Mroziński, B., Kozłowska, Z., Iciek, R., Wróblewska-Seniuk, K., Wender-Ożegowska, E., & Szczapa, T. (2023). Prenatal and Neonatal Pulmonary Thrombosis as a Potential Complication of SARS-CoV-2 Infection in Late Pregnancy. International Journal of Molecular Sciences, 24(8), 7629. https://doi.org/10.3390/ijms24087629




