1. Introduction
Periodontitis is a frequently occurring inflammation-associated condition affecting the teeth, and, when untreated, it leads to the destruction of supporting tissues surrounding the teeth [
1]. In the United States, among adults above 30 years of age, the prevalence of periodontitis is 47% [
2]. This disease is characterized by a loss of connective tissue and a loss of the periodontal ligament (PDL) with a preponderance of alveolar bone resorption [
1]. Periodontitis can manifest itself in tooth mobility, impaired masticatory ability, compromised esthetics and eventually tooth loss [
1]. Periodontitis has also been associated with increased risk of chronic diseases, including atherosclerotic cardiovascular diseases and diabetes [
3,
4].
Different non-surgical and surgical therapies are used for the treatment of periodontitis. None of these treatments are satisfactory in terms of reconstructive and disease modifying capabilities due to limited predictability and efficacy [
5,
6]. In this context, the glycoprotein sclerostin, encoded by the SOST gene, has emerged as a new research topic for periodontitis treatment. Sclerostin plays an important role in bone remodeling as an inhibitor of bone formation. Sclerostin decreases bone formation via blocking the canonical Wnt-signaling pathway by binding with the Wnt co-receptors LRP 5/6 [
7]. Co-receptor LRP4 can also bind to sclerostin and, thus, enhance the inhibition of bone formation [
8]. Sclerostin is mainly expressed by osteocytes, cementocytes, and hypertrophic chondrocytes [
9]. Next to these cells, sclerostin has also been identified in PDL fibroblasts [
10,
11,
12,
13].
The absence of sclerostin causes sclerosing bone disorders such as sclerosteosis or Van Buchem Disease. These diseases are characterized by generalized bone overgrowth due to an increased osteoblastic activity that is not counterbalanced by increased osteoclastic activity [
9]. The recognition of the clinical consequences of sclerostin absence stimulated the development of sclerostin inhibitors as a potential therapy for bone disorders characterized by excessive bone resorption, such as osteoporosis [
14]. As a result, romosozomab, a monoclonal antibody, was developed, which binds and inhibits sclerostin, leading to an increase in bone formation and a decrease in bone resorption [
15]. In 2019, romosozumab received its first global approval by the Food and Drug Administration for treating osteoporosis in patients with a high risk of fracture [
16], which was followed by its approval for use in the European Union [
17].
In recent years, the monoclonal sclerostin antibody has also been investigated in pre-clinical studies in the dental field, for example, as a promising therapy in augmenting alveolar bone healing and stimulating bone regeneration in rats with a surgically generated bone defect [
18]. Taut et al. concluded that the monoclonal sclerostin antibody stimulated bone regeneration in rats following experimental periodontitis [
18]. Moreover, Chen et al. proved that a monoclonal sclerostin antibody treatment stimulated alveolar crest height and bone mass in ovariectomized rats with localized periodontitis [
19]. Moreover, Liu et al. reported that sclerostin inhibition in rats with alveolar bone loss resulted in the additional stimulation of alveolar bone volume [
20]. Similarly, Yao et al. evaluated the effect of sclerostin antibodies on osseous defects around teeth [
21]. They showed that the systemic administration of sclerostin antibodies enhanced bone regeneration; however, locally applied sclerostin failed to improve bone repair [
21].
Studies on sclerostin in dentistry have mainly focused on the effect of sclerostin and sclerostin blocking antibodies in the osteocytes of the alveolar bone. Although sclerostin is mainly produced by osteocytes, several studies suggest a role for PDL fibroblasts in sclerostin signaling. Jäger et al. discovered that increasing levels of SOST mRNA and protein were identified in PDL fibroblast cultures that were induced to form minerals [
12]. In addition, different studies have demonstrated that PDL fibroblasts respond to mechanical force by increasing the expression of SOST, both at gene and protein levels [
22,
23].
Nevertheless, the regulatory mechanisms and effects of sclerostin and monoclonal sclerostin antibodies on biological parameters such as the osteogenesis and osteoclastogenesis of PDL fibroblasts are still unknown. These mechanisms and effects are potentially of value for the development of regenerative therapies in periodontitis patients. The purpose of this study is to explore the effect of sclerostin and a monoclonal sclerostin antibody, more specifically romosozumab, on osteoclastogenesis and osteogenesis in PDL fibroblast cultures.
PDL fibroblast cultures have been widely studied in bone remodeling research in dentistry, as PDL fibroblasts play an important role in both osteogenesis and osteoclastogenesis [
24]. PDL fibroblasts have the capacity to contribute both to mineralization, due to their high alkaline phosphatase (ALP) activity, and to osteoclast formation in co-cultures with peripheral blood mononuclear cells (PBMCs) [
24,
25]. The osteogenic capacity of PDL fibroblast cultures can be investigated by stimulating the cells with a mineralization medium consisting of ascorbic acid and β-glycerophosphate [
12,
26]. PDL fibroblasts, in a co-culture with PBMCs, can provide the signals that stimulate the monocytes within the PBMCs to differentiate into osteoclasts [
27].
The hypothesis is that the addition of sclerostin to PDL fibroblasts results in decreased mineralization and osteoblast activity and the addition of a blocking monoclonal antibody against sclerostin will result in increased mineralization and osteoblast activity. This study further hypothesized that the addition of sclerostin to a co-culture of PDL fibroblasts and human PBMCs results in enhanced osteoclastogenesis, whereas the addition of a monoclonal sclerostin antibody, romosozumab, will lead to decreased osteoclastogenesis.
3. Discussion
The present research was the first to assess the effects of both sclerostin antibodies and a monoclonal sclerostin antibody (romosozumab) on osteoclastogenesis and osteogenesis in PDL cultures. The PDL fibroblasts uniquely contribute to both osteoclastogenesis and osteogenesis [
28] and, therefore, both processes could be studied in the same experimental approach. Although both reagents were added until high dosages, there were limited effects on both osteogenesis and osteoclastogenesis. The low expression of sclerostin and its receptors could be the cause for this.
Regarding osteoclastogenesis, no studies have previously found that sclerostin could influence osteoclast formation in PDL-PBMC co-cultures. Here, our results suggest that the addition of a high concentration of romosozumab slightly decreases the osteoclastogenic capacity of PDL-PBMC co-cultures.
In this study, we found no effect of sclerostin on osteoclast formation in a co-culture of PDL fibroblasts and PBMCs. A possible explanation could be that osteocytes, in particular, could be receptive to sclerostin and, hence, influence osteoclast formation. Osteocytes have previously been shown to be key expressors of RANKL [
29]. It has been reported that sclerostin affects osteoclasts by upregulating the RANKL/OPG ratio [
30]. Osteoclast precursor cells express receptor RANK on their cell surface [
31]. This receptor is able to bind to its ligand RANKL, which is highly expressed by osteocytes [
31]. Binding RANK to RANKL stimulates osteoclast precursors to differentiate into multinucleated osteoclasts [
31]. Osteocytes also produce osteoprotegerin (OPG), which inhibit osteoclast differentiation by competing with RANKL for its receptor RANK on osteoclast precursor cells [
32]. In addition to osteocytes, PDL fibroblasts also express RANKL and OPG mRNA [
28,
32], although the expression of OPG is at least 100-fold higher than RANKL [
28]. It was hypothesized that sclerostin might stimulate osteoclast formation indirectly via PDL by stimulating RANKL expression. However, it could also be that sclerostin only affects osteoclast formation indirectly via RANKL produced by osteocytes and not in PDL fibroblasts, which may express lower levels of receptors for sclerostin. This thought is consistent with the findings of Wijanayaka et al., who showed that sclerostin dose-dependently upregulated the expression of RANKL mRNA and downregulated OPG mRNA in a PBMC-osteocyte co-culture [
33]. However, this idea is not supported by the research of Odagaki et al. [
34]. They found no effect in RANKL or OPG expression in osteocytes in their PDL-osteocyte co-culture system and they attributed an increased bone resorption to the RANKL/OPG signaling of the PDL fibroblasts [
34].
This study also showed that romosozumab causes a slight but significant decrease in the formation of TRAcP-positive cells at high concentrations of romosozumab. Previous research showed that sclerostin induces the RANKL/OPG ratio and romosozumab reduces this ratio [
35]. The current study showed that romosozumab decreases the number of TRAcP-positive cells, indicating that osteoclastogenesis is inhibited, possibly by decreasing the RANKL/OPG ratio of PDL fibroblasts. Thus, this result is in line with romosozumab’s alleged effect on the RANKL/OPG ratio, which is also suggested by Odagaki et al. [
34], albeit this ratio is below 1/100 in most studies analyzed by Sokos et al. [
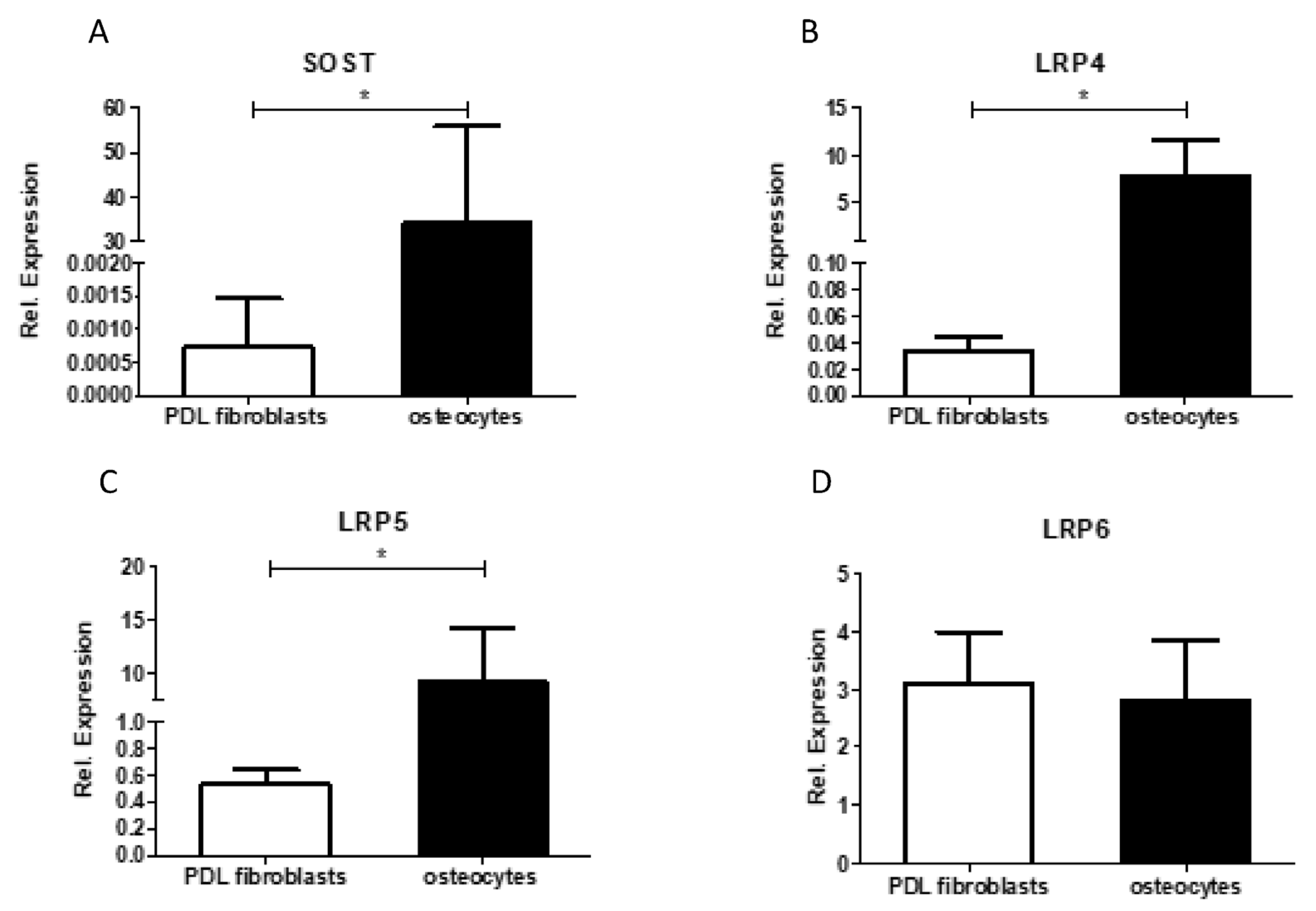
28]. Future research should focus on which cell type(s) sclerostin influences RANKL/OPG expression. The lack or low expression of sclerostin receptors LRP-4 and LRP-5, as shown in
Figure 6, make it unlikely that added or endogenous sclerostin will influence RANKL-expression in PDL fibroblasts.
Moreover, this study also revealed that neither sclerostin nor romosozumab influenced the spontaneous osteoclast formation of PBMCs. These results substantiate the hypothesis that sclerostin depends on other cell types to have an effect on osteoclast formation. In addition, thus far, it has not been reported that PBMCs express either sclerostin or its receptors [
36]. These results are in line with the data from Wijenayaka et al., who also found no effect of sclerostin on TRAcP-positive cell formation in the monocultures of PBMCs, albeit these cultures were supplemented with macrophage colony-forming factor M-CSF [
33].
It is important to note that, in the current study, osteoclastogenesis was determined according to the number of TRAcP-positive multinucleated cells. We have previously shown that PDL fibroblasts do provide the signals for the generation of multinucleated TRAcP-positive cells but that this does not lead to resorbing osteoclasts, for which extra RANKL is needed [
27]. It should be acknowledged that the resorptive activity of osteoclasts was, therefore, out of scope. Wijenayaka et al. reported that in a co-culture of PBMCs and osteocytes (MLO-Y4 cells) sclerostin can specifically result in increased resorbing activity without increasing the number of TRAcP-positive cells [
33].
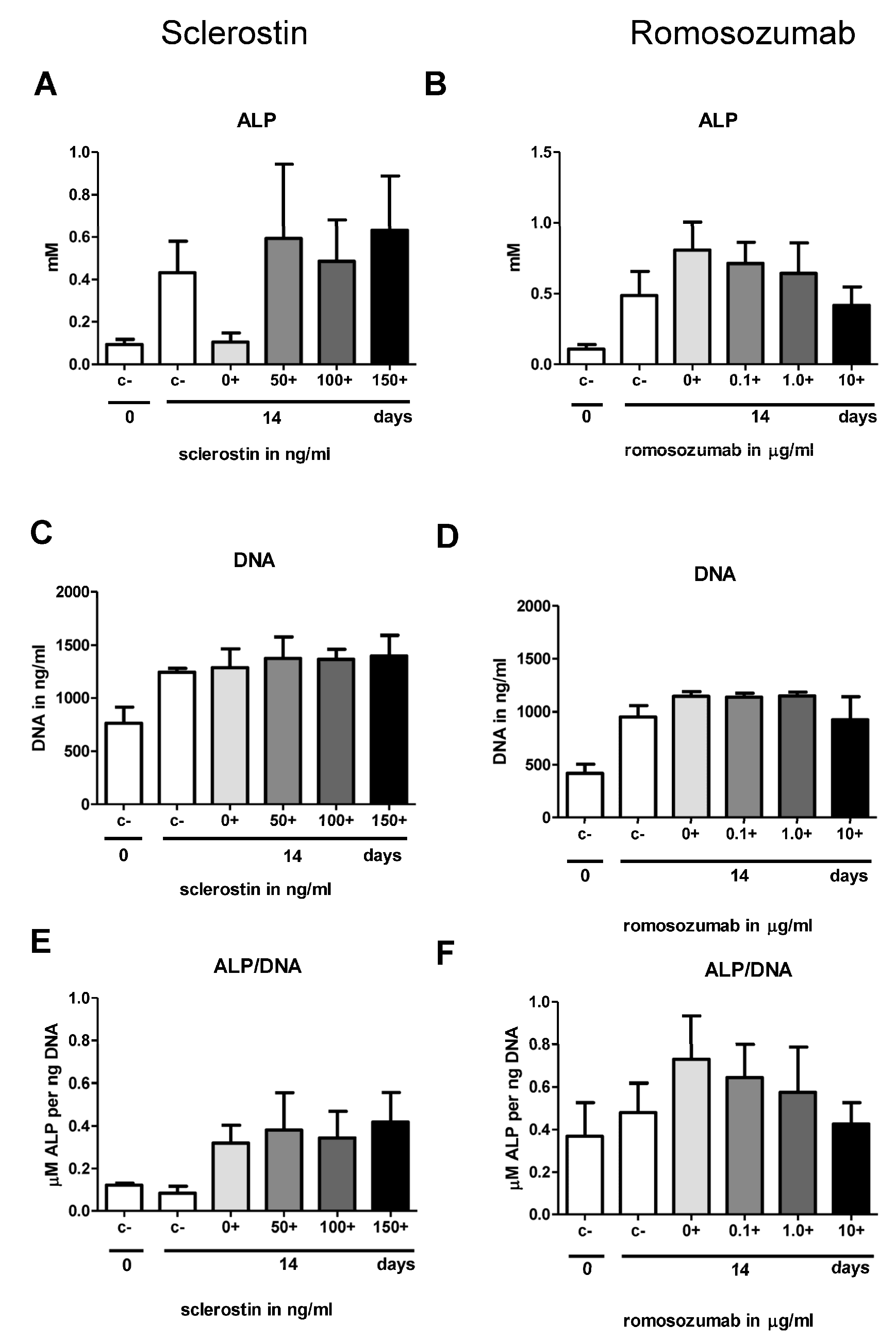
In the second part of this study, we investigated osteogenesis, the biological process most widely studied in the context of sclerostin. Contrary to expectations, this study showed that neither sclerostin nor romosozumab affected the osteogenic potential of the PDL fibroblasts. Firstly, the addition of both sclerostin and romosozumab did not show any significant increase or decrease in ALP activity. Secondly, mineral deposition by PDL fibroblasts did not seem to be affected by the addition of higher sclerostin or romosozumab levels. The present study did not show any significant increase or decrease in ALP activity by the addition of both sclerostin and romosozumab. Bezooijen et al. studied the effect of osteoblastic (KS483) cell cultures with added sclerostin [
37]. They observed that sclerostin inhibited APL activity only at the highest concentration of 2.5 μg/mL [
37]. They suggest that sclerostin only inhibits the later stages of bone formation and, therefore, has no effect on an early osteoblast differentiation marker such as ALP activity [
37]. This suggestion could also apply to the results of the present study.
For osteogenesis assays, cells were seeded and cultured in the mineralization medium with β-glycerol phosphate and ascorbic acid to stimulate osteogenic differentiation. No staining was observed in cultures with the control medium (without β-glycerol phosphate and ascorbic acid). In contrast, deposits of alizarin red were observed in cultures that contained the mineralization medium from day 14. At day 21, mineralization was visualized with alizarin red staining for six out of eight donors. These results reflect those of Knaup et al., who also found mineralization by PDL fibroblasts under mineralizing conditions from day 13 [
11]. Since we hypothesized that romosozumab would initiate bone mineral deposition, alizarin red staining was performed not only at day 21 but also at day 14. However, an initiation of bone mineral deposition with increasing levels of romosozumab could not be determined. Although only a qualitative analysis was performed, no differences were observed for any concentration of sclerostin or romosozumab. A more quantitative approach, by dissolving the red stain, could have enforced these findings. Moreover, it was noticed that there was a great level of heterogeneity in the level of mineralization between the different donors. This finding aligns with Knaup et al., who demonstrated differences between donor cells regarding their mineralization potential to form mineralized deposits of alizarin red and subsequent osteogenic differentiation potentials [
11].
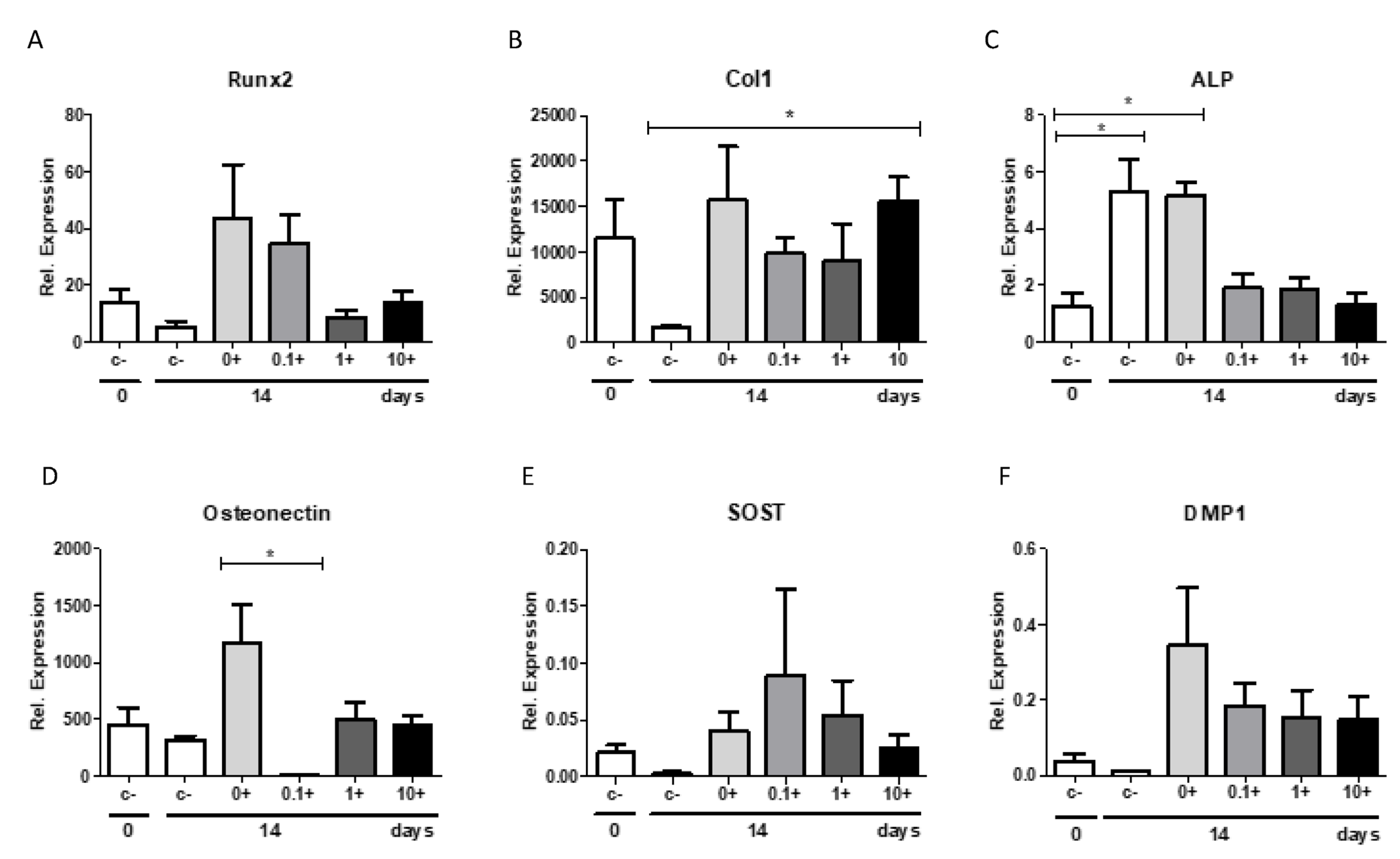
In the present study, a qPCR with different osteoblast-related genes was performed. When comparing the relative expression with and without the mineralization medium, all studied bone markers showed a strong increase in relative expression after the addition of the mineralization medium, resulting in a high anabolic potential of the PDL fibroblasts. Romosozumab did not induce the expression of the following bone-formation markers: ALP, osteonectin, Runx2, Col1, and DMP1.
It was shown in the first report of PDL cell cultures [
38], and shown in subsequent studies [
24,
25,
27], that the cultures vary largely, especially in the expression of alkaline phosphatase and in mineralization, as assessed by alizarin red. In the present study, a large variation in these parameters was also observed, as shown in the alizarin red staining in
Figure 4B,C, the alkaline phosphatase staining in
Figure 3A,B, and the sclerostin expression in
Figure 6A. Despite this variation, the results could be analyzed by repeated measures: each donor was exposed to the varying concentrations of sclerostin or romosozumab.
It should be acknowledged that the relative expression of SOST mRNA was relatively low in this study in comparison to the other osteoblastic markers. The SOST mRNA expression was between 70,000-fold and 633,000-fold lower than the expression of Col1, between 148-fold and 1333-fold lower than the expression of RUNX2, between 30-fold and 60,000-fold lower than the expression of osteonectin, and between 15-fold and 1083-fold lower than the expression of ALP. The low level of SOST mRNA in the cell cultures suggests that SOST mRNA is primarily restricted to a few cell types, and most likely osteocytes. Indeed, we also confirmed in the present study that the expression of SOST and LRP4 and LRP5 was much higher in osteocytes than in PDL fibroblasts. Similarly, in previous research, Bezooijen et al. suggested that SOST mRNA expression might be restricted to osteocytes [
37].
Another possible explanation for the low relative expression of SOST mRNA may be the differentiation-dependent expression of SOST mRNA, as also suggested by Jäger et al. [
12]. Bezooijen et al. investigated the onset of SOST mRNA expression in cultured human and mouse MSCs and KS483 cells under osteogenic conditions by adding the mineralization medium [
37]. They reported that SOST mRNA expression could not be detected in undifferentiated cells but was always detectable in late differentiated cells [
37]. Furthermore, SOST mRNA expression was limited to the mineralization phase of osteoblastic cell cultures [
37]. Thus, they concluded that the onset of SOST mRNA depends on the differentiation stage of the cells. In addition, some researchers indicate that PDL fibroblasts grown in cultures represent an immature form of PDL fibroblasts [
39]. The possible immature form of the PDL fibroblasts in the studied cultures could explain why low SOST mRNA expression was found.
Alternatively, it could be that PDL fibroblasts hardly express SOST at the protein level; hence, romosozumab would not have any effect on the expression of the studied bone formation markers. Similarly, the absence of an effect of sclerostin could be due to the relatively low expression of receptors for sclerostin (LRP4, 5, or 6) in PDL fibroblasts, resulting in an absent SOST signaling.
In line with the low expression of SOST mRNA was the low expression of DMP1, which is also typically associated with osteocyte expression. DMP1 is known as an osteocyte-specific gene, as it plays an important role in osteocyte maturation [
31]. Thus, the results indicate that differentiation as induced by osteogenic conditions did not lead to differentiation in the osteocyte-like phenotype.
The limited effect of both sclerostin and romosozumab on osteogenesis and osteoclastogenesis observed in the current study might be caused by the function of the PDL itself. An important biological function of PDL fibroblasts is the maintenance of PDL homeostasis by not only preventing osteoclast formation at the surface of the root [
28] but also preventing too much bone formation by releasing locally acting regulators to avoid tooth ankylosis [
24]. This protective function of the PDL may allow little or no interference of a negative bone regulator of bone formation such as sclerostin. In this context, Knaup et al. assumed that sclerostin might be part of a negative feedback loop in PDL fibroblasts in which an enhanced level of sclerostin could inhibit mineralization formation during osteogenic differentiation to prevent extreme mineralization [
11]. They examined that PDL fibroblasts with low baseline SOST levels demonstrate earlier mineralization than those with higher initial SOST levels [
11]. Contrarily, high levels of SOST resulted in a smaller decrease in the formation of mineralization by PDL fibroblasts and decreased osteogenic differentiation [
11]. In addition, Manokawinchoke et al. suggested a role for sclerostin in PDL homeostasis [
22]. They proposed that mechanical forces induce the expression of sclerostin and thereby prevent bone deposition in the PDL space [
22].
One of the limitations of this study is the heterogeneity between the different PDL fibroblast cultures from the different donors. Marchesan et al. recommended a large donor base for PDL cultures to normalize for cellular heterogeneity and various factors that can influence the cultured cells [
40].
Moreover, in the current study, romosozumab only showed a small effect on the expression of osteogenic markers. Sclerostin reduces osteoblastic bone formation by antagonizing the canonical Wnt/β-catenin signaling pathway by binding with the Wnt co-receptors, LRP 5/6 [
7]. LRP 4 was also discovered as a sclerostin receptor [
8]. Furthermore, sclerostin also has inhibitory effects on the bone morphogenetic protein signaling pathway [
41]. In addition to these two pathways, other pathways have been suggested [
42,
43]. Research should show which receptors for sclerostin contribute to the effects seen in this study and on which pathway(s) these effects depend. Further studies should include LRP receptors, as research suggests that LRP5 depletion in mice affects the structure of the PDL fibroblasts and reduces osteoclastogenesis activity in the PDL [
44].
In conclusion, this study showed that both sclerostin and romosozumab, individually, had very limited effects on osteoclastogenesis and osteogenesis in PDL fibroblast cultures. PDL fibroblasts likely do not express enough sclerostin or sclerostin receptors to induce any effect. The limited interaction of sclerostin with PDL fibroblasts may be explained by the intrinsic properties of the PDL fibroblasts themselves, as they must resist excessive bone formation and bone degradation under physiological circumstances.
4. Materials and Methods
4.1. Sclerostin and Monoclonal Sclerostin Antibody
Recombinant human sclerostin was acquired from R&D Systems (Minneapolis, MN, USA) and dissolved in water. Biological mode of action was tested at R&D in a cell line, MC3T3-E1, that responds to Wnt-3a, in which sclerostin-inhibited alkaline phosphatase activity in the range of concentrations that we have used takes place. Monoclonal sclerostin antibody (Amgen®, Thousand Oaks, CA, USA, romosozumab, EU/1/19/1411/001, CNK: 4230-116) was purchased as Evinity (trademark), hereinafter referred to as romosozumab. It was collected from one of the pens that are used for treating osteoporosis patients. All romosozumab for clinical use was subjected to stringent quality control. Romosozumab was further diluted 9 times in water, with a stock solution of 10 mg/mL, which was further diluted 1000× for the highest concentration used in the assays.
As an additional control, containing the same solvent, monoclonal sclerostin antibody (HI romosozumab) was inactivated by heating the romosozumab for 10 min at 90 °C. HI romosozumab was used as a control in some of the experiments.
4.2. Isolation and Culturing of PDL Fibroblasts
PDL fibroblasts were isolated from roots of the third molars of eight healthy individuals between 18 and 25 years of age. All individuals underwent extraction of the third molar at Amsterdam UMC (location VUmc, Amsterdam, The Netherlands), no inflammation was visible at the site of extraction. All samples were retrieved with written informed consent and approved by the medical ethical committee of Vumc (2016.105) and ACTA-ETC (2021-55908). No signs of caries, gingivitis, or periodontitis were present (pockets ≤ 3 mm without bleeding). For all experiments, researchers were not aware of the identity of the PDL donors. Cells were stored in liquid nitrogen. PDL was harvested by scraping the cells off the third molar. Additionally, cells were seeded in a culture medium consisting of 90% Dulbecco’s minimal essential medium (DMEM, Thermo Fischer Scientific, Waltham, MA, USA), 10% fetal clone I (FCI, HyClone, Logan, UT, USA), and 1% antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin, and 250 ng/mL Amphotericin B; Sigma-Aldrich, St. Louis, MO, USA) and maintained in a humified atmosphere of 5% CO2 in air at 37 °C. All assays were conducted with 4th or 5th passage cells.
4.3. Isolation of Osteocyte Enriched mRNA
Fibular bone was collected from 4 donors (age: 32–79, 1 female, 3 male). The donors presented no medical history of skeletal pathology or trauma. All bone tissue was collected as surgical waste during mandible reconstruction surgery and obtained with donors’ consent. All protocols were approved by the local Medical Ethical Committee of the Amsterdam University Medical Centers (2016.105). Fibular bone explants were washed in Hanks’ balanced salt solution (HBSS; Thermo Fisher Scientific, Waltham, MA, USA). They were incubated in minimal essential medium (MEM; Thermo Fisher Scientific) containing 2 mg/mL type II collagenase (Worthington Biochemical, Lakewood, CA, USA) for 2 h in a shaking water bath at 37 °C and washed again twice with HBSS. Then, soft tissue was removed by scraping. Cleaned cortical bone explants were cut into small explants measuring 8.0 × 3.0 × 1.5 mm (l × w × h) using a diamond disc H-345-220 (Horico, Berlin, Germany), a handpiece (KaVo, Biberach an der Riss, Germany), and a foot control (KaVo), as described earlier [
45]. Explants were cooled during cutting in ice-cold HBSS. They were pre-cultured for 1 or 2 days in 6-well plates (Merck KGaA, Darmstadt, Germany) containing MEM supplemented with 5% fetal bovine serum (FBS; Lonza BioWhittaker, Basel, Switzerland), 5% bovine calf serum (BCS; Thermo Fisher Scientific), 1% penicillin-streptomycin (10,000 U/mL; Thermo Fisher Scientific), and 0.5% amphotericin B solution (Merck KGaA, Darmstadt, Germany) at 37 °C.
Bone explants were pulverized using a 6775 Freezer/Mill cryogenic grinder (SPEX SamplePrep, Metuchen, NJ, USA; rate: 10 cycles/s; 20 impacts/s) for 2 min in liquid nitrogen. After pulverization, the TRIzol/bone powder mixture was incubated for 1 h in a shaking water bath at 37 °C. Immediately thereafter, RNA was either isolated, or the TRIzol/bone powder mixture was stored at −80 °C before RNA isolation. Two hundred microliter chloroform was added per ml of TRIzol/bone powder mixture, followed by 15 min of centrifugation at 12,000 rpm at 4 °C. Isolation and purification of RNA was performed using RNeasy Midi columns (Qiagen, Hilden, Germany) or Zymo-Spin IIICG columns (Direct-zol RNA MiniPrep Plus, Zymo Research, Irvine, CA, USA). The supernatants were collected, mixed with an equal volume of 70% ethanol, and transferred to RNeasy Midi columns or were mixed with an equal volume of 100% ethanol and transferred to Zymo-Spin IIICG columns. RNA isolation and DNase I digestion were performed following the manufacturer’s instructions. The RNA concentration was determined by Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) and Qubit RNA HS assay kits (Thermo Fisher Scientific).
4.4. Isolation of PBMCs
PBMCs from a buffy coat (Sanquin, Amsterdam, The Netherlands) of a healthy donor were isolated. Firstly, the buffy coat was diluted 1:1 with 1% PBS-citrate. The diluted buffy coat was carefully layered on Lymphoprep (Axisshield Po CAS, Oslo, Norway) and centrifuged for 30 min at 800 G without brakes. Next, the interphase with PBMCs was taken off, washed with 1% PBS-Citrate solution, and centrifuged for 10 min at 400 G. This was repeated three times. Finally, the supernatant was removed, and the pellet was resuspended in basal culture medium before culturing. Cells were counted with Muse® Cell Analyzer.
4.5. Osteoclastogenesis Assays
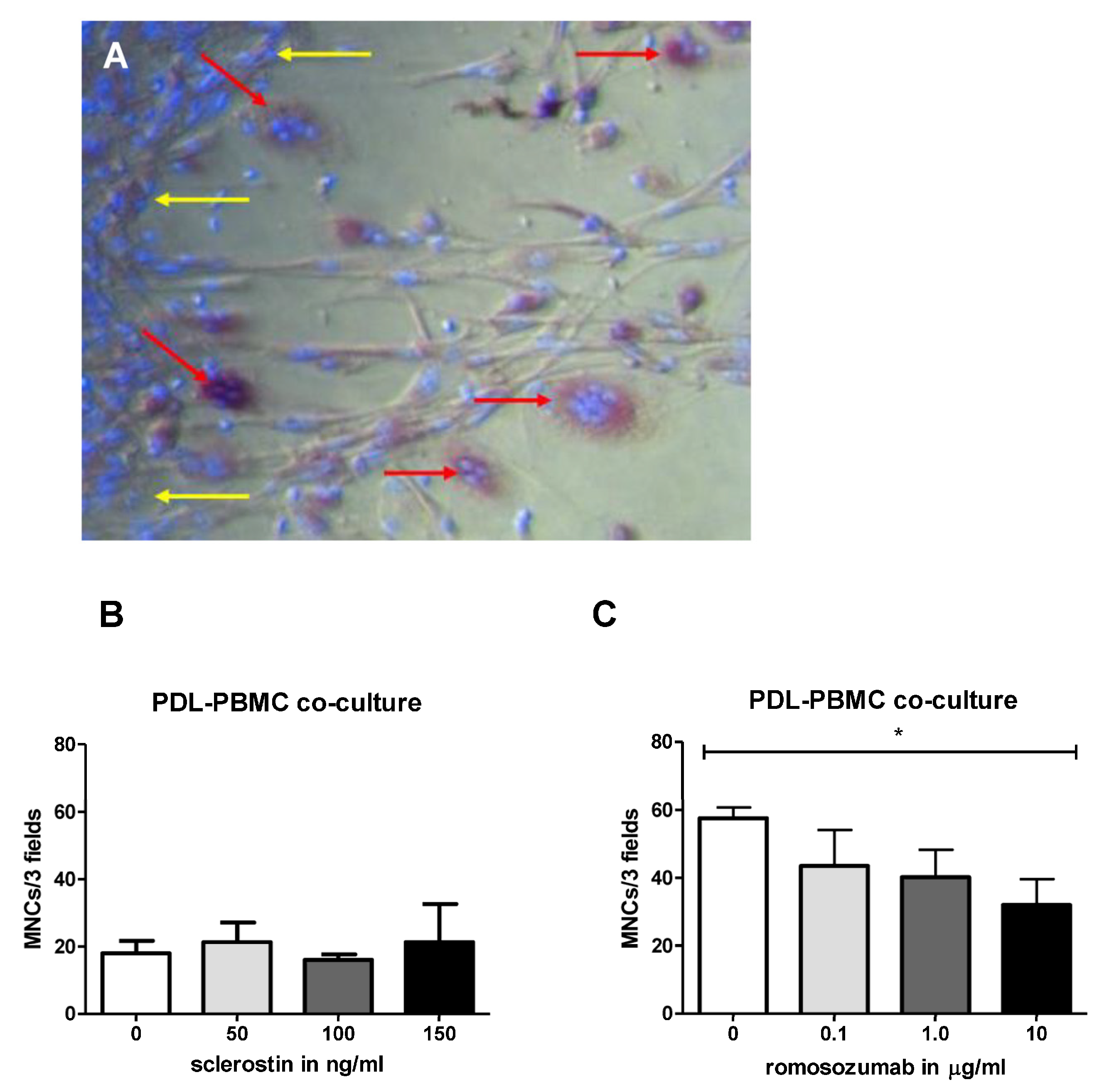
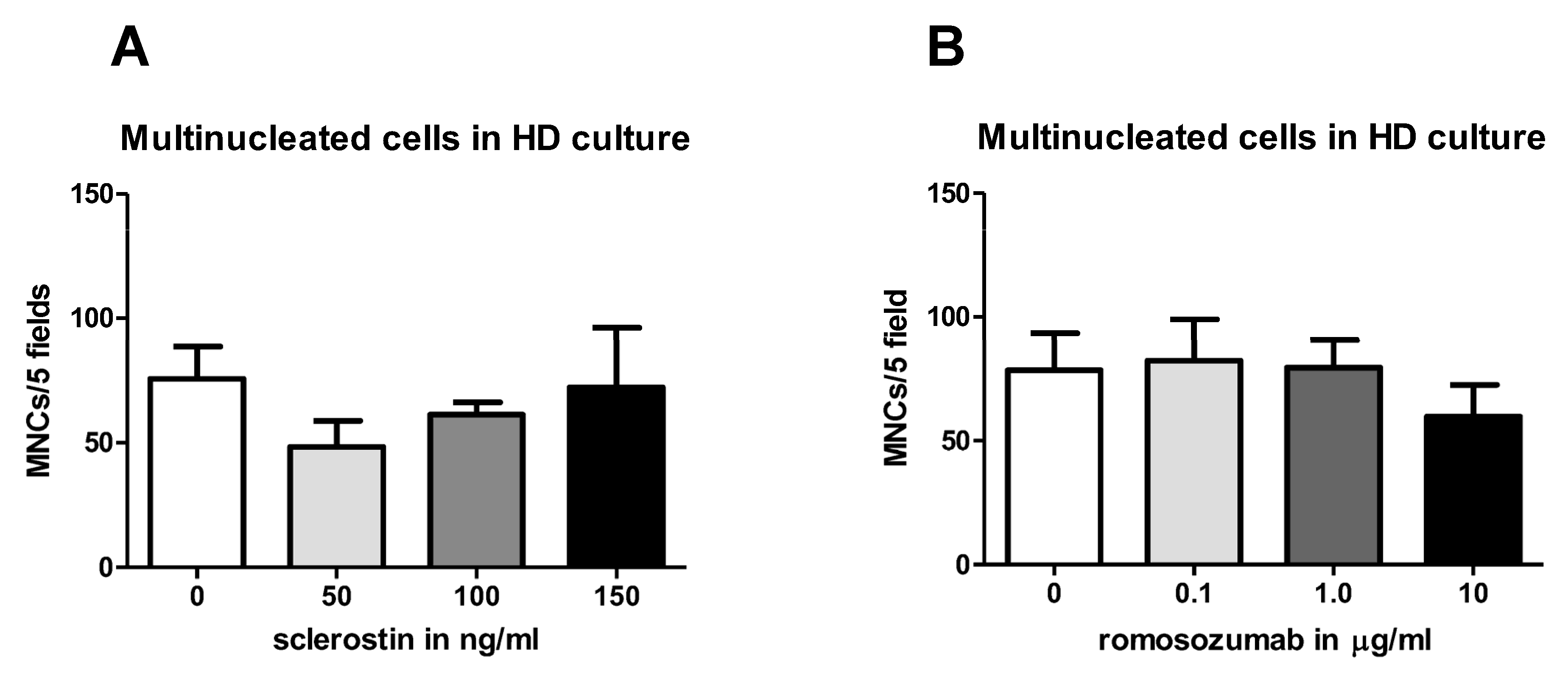
Different osteoclastogenesis assays were performed. A high density (HD) monoculture was plated with 0.5 × 106 PBMCs four times in 96-well plates, cultured in DMEM, and supplemented with 10% FCI, 1% PSF, and an antibiotics cocktail containing penicillium, streptomycin, and fungisone. Sclerostin was added in concentrations of 50, 100, and 150 ng/mL. These relatively high concentrations were chosen based on a previous experiment (not described here) in which concentrations up to 50 ng/mL did not result in a significant effect.
For the co-culture, PDL at a density of 1.5 × 104 cells per well were seeded in duplicate in a 48-well plate. The next day, 0.5 × 106 PBMCs were added to each well. The test setups were made with the following co-culture: (1) Culture medium with solvent of sclerostin (water). (2) Culture medium + 50 ng/mL sclerostin. (3) Culture medium + 100 ng/mL sclerostin. (4) Culture medium + 150 ng/mL sclerostin at t = 0. The same experiments were performed with romosozumab with concentrations up to 10 µg/mL (in excess of 100,000 of the literature outcomes). For co-culture experiments, PDL were co-cultured with PBMCs in the presence of culture medium with (1) 10 µg/mL heat-inactivated romosozumab as a control; (2) 0.1 µg/mL romosozumab; (3) 1 µg/mL romosozumab; and (4) 10 µg/mL romosozumab.
Culture medium of all osteoclastogenesis assays was refreshed every 3 or 4 days. All plates were incubated at 37 °C and 5% CO2. Cells of osteoclastogenesis assays were harvested at t = 0 and t = 21 for tartrate-acid phosphatase (TRAcP) staining.
4.6. TRAcP Staining
After 21 days of culturing, cells of the osteoclastogenesis assays were washed with PBS, fixed in 4% formaldehyde for 10 min, and washed again with PBS. In accordance with the protocol of the manufacturer Sigma-Aldrich, the TRAcP staining solution was prepared. An amount of 300 µL of TRAcP staining solution was added to each well and the wells were incubated for 10 min in a dark place at room temperature. Then, nuclei were counterstained with 4′,6-diamidino-2-fenylindole (DAPI) for 5 min and washed with PBS. TRAcP-positive cells with three or more nuclei were labelled as osteoclasts. TRAcP-positive cells with three nuclei or more were counted using micrographs taken from a light microscope with a fluorescence device. Five (PBMCs HD culture) or three standardized (co-culture PDL-PBMC) areas were selected per well (magnification 20×) and photographed to determine the number of nuclei of TRAcP-positive multinucleated cells (MNCs).
4.7. Osteogenesis Assays
4.7.1. Sclerostin
PDL fibroblasts of three different donors were seeded in duplicate in 48-well plates (3.0 × 10
4 cells/well). Mineralization medium was used to induce and stimulate mineralization by the PDL fibroblasts [
12]. The following test conditions were used: (1) culture medium; (2) mineralization medium, comprising culture medium + 50 mM β-glycerophosphate (Sigma-Aldrich, St. Louis, MO, USA) + 50 mM ascorbic acid (Sigma-Aldrich); (3) mineralization medium + 50 ng/mL sclerostin; (4) mineralization medium + 100 ng/mL sclerostin; (5) mineralization medium + 150 ng/mL sclerostin. The concentrations of sclerostin were determined based on comparable previously conducted studies with osteocytes or PDL fibroblasts [
33]. Cells were harvested for ALP activity assay at t = 0 and t = 14, and alizarin red staining was performed at t = 21.
4.7.2. Romosozumab
PDL fibroblasts of five different donors were seeded and cultured in the same way as described for sclerostin osteogenesis assay. Romosozumab was used in concentrations of 0.1, 1.0, and 10 µg/mL. HI romosozumab was added to the control group with mineralization medium. Cells were harvested for ALP activity assay at t = 0 and t = 14, alizarin red staining at t = 14 and t = 21, and qPCR analysis at t = 0 and t = 14 for the examination of osteogenic markers.
Culture medium of all osteogenesis assays was replaced twice a week. All plates were incubated during the experiments at 37 °C and 5% CO2.
4.8. Alkaline Phosphatase Activity and DNA Concentration
At day 0 and day 14 cells from the osteogenic assays were harvested for measurements of alkaline phosphatase (ALP) activity and DNA concentration. Cells lysates were achieved by washing the cells first with PBS, adding 200 µL Milli-Q water to each well, and then freezing the cells at −20 °C. Subsequently, after three cycles of freeze-thaw, adherent cells were scraped off the well with a plastic cell scraper. ALP was determined using 4-nitrophenyl phosphate disodium salt at pH 10.3. After incubating the plate for one hour at 37 °C, the reaction process was blocked with NaOH. With a Synergy HT spectrophotometer (BioTek, Instruments, Inc. Winooski, VT, USA), absorbance was measured at 405 nm.
Using the CyQuant Cell Proliferation Assay Kit (Molecular Probes (C7026), Leiden, The Netherlands), DNA concentrations were measured from the same cell lysates that were used for the ALP. For the calculation of DNA concentration, fluorescence was measured according to the protocol using the Synergy HT spectrophotometric microplate reader.
4.9. Alizarin Red Staining
After 14 days and 21 days of culturing, mineral deposition was inspected in duplicate or single wells per donor. Cells were washed with PBS, fixed for 10 min in 4% PBS buffered formaldehyde and then washed with Milli-Q water. For the staining, 300 µL of 1% Alizarin Red staining solution at pH 4.3 (Sigma-Aldrich, St. Louis, MO, USA) was added to each well and cells were incubated for 10 min at room temperature and rinsed with Milli-Q water until the background was clear. Mineral deposition was visible via the formation of red precipitate, which was often in the form of nodules.
4.10. Quantitative Polymerase Chain Reaction (qPCR)
At day 0 and day 14 RNA was isolated from cultured cells with different concentrations of romosozumab. Firstly, for adherent cells the supernatant was removed and lysis buffer containing β- Mercaptoethanol (150 µL t = 0 and 200 µL t = 14) was added. Samples were stored at −80 °C. The RNA from the different samples was extracted according to the manufacturer’s protocol of the RNeasy kit (Qiagen, Düsseldorf, Germany). Finally, RNA concentration was measured with a Synergy HT spectrophotometer.
For the synthesis of first strand cDNA from the RNA samples, a Fermentas kit (First strand cDNA synthesis kit Fermentas #K1612) was used. For one reaction, 9 µL RNA was mixed together with 2 µL primer mix consisting of Oligo (dT18) and D(N)6 primers, incubated at 65 °C for 5 min, and then put on ice. An amount of 9 µL master mix, consisting of buffer (4 µL), dNTP’s (2 µL), RNAse Inhibitor (1 µL) and Reverse Transcriptase (2 µL) was added. The mix was successively incubated at 25 °C for 5 min, at 37 °C for 1 h, and at 70 °C for 5 min. cDNA was diluted to 1 ng/µL and samples were stored at −20 °C. First strand cDNA samples were used as a template in the qPCR.
PCR primers of osteoblast-related genes were designed (
Table 1). An amount of 1 µM of each PCR primer was added to the cDNA samples as described in the previous section. qPCR was performed on the LightCycler
® (Roche). qPCR started with an initial activation step for 10 min at 94 °C, followed by a run of 40 cycles. This cycle run comprised a template denaturation step at 94 °C for 30 s and the primer annealing and primer extension step combined at 60 °C for 1 min.
Low expression housekeeping gene Porphobilinogen deaminase (PBGD), a gene without pseudogenes, was used to normalize the samples. The expression of PGBD was not influenced by experimental factors. By calculating ΔCT, samples were normalized for the expression of PGBD. Relative expression of the different genes is expressed (2−(ΔCT)).
4.11. Statistical Analysis
For analyzing the data sets, Graphpad Prism 5 software was used. Comparisons between the different groups in the number of osteoclasts and the number of nuclei per cell were analyzed using one-way ANOVA followed by a Kruskal–Wallis post-test. For analyzing ALP activity, DNA levels, and relative expression of different osteogenic markers, a Friedman test was used, followed by Dunn’s multiple comparison test. All the data are shown as mean ± SEM. p values of p < 0.05 were considered to indicate statistical significance. Mineral deposition by PDL fibroblasts (alizarin red staining) was detected visually. All statistical tests were repeated measurement tests, where n = 3 or n = 5 were used for comparison per donor.