Nanoscale Structural Comparison of Fibrillin-1 Microfibrils Isolated from Marfan and Non-Marfan Syndrome Human Aorta
Abstract
1. Introduction
2. Results
2.1. Population Overview and Genetic Background
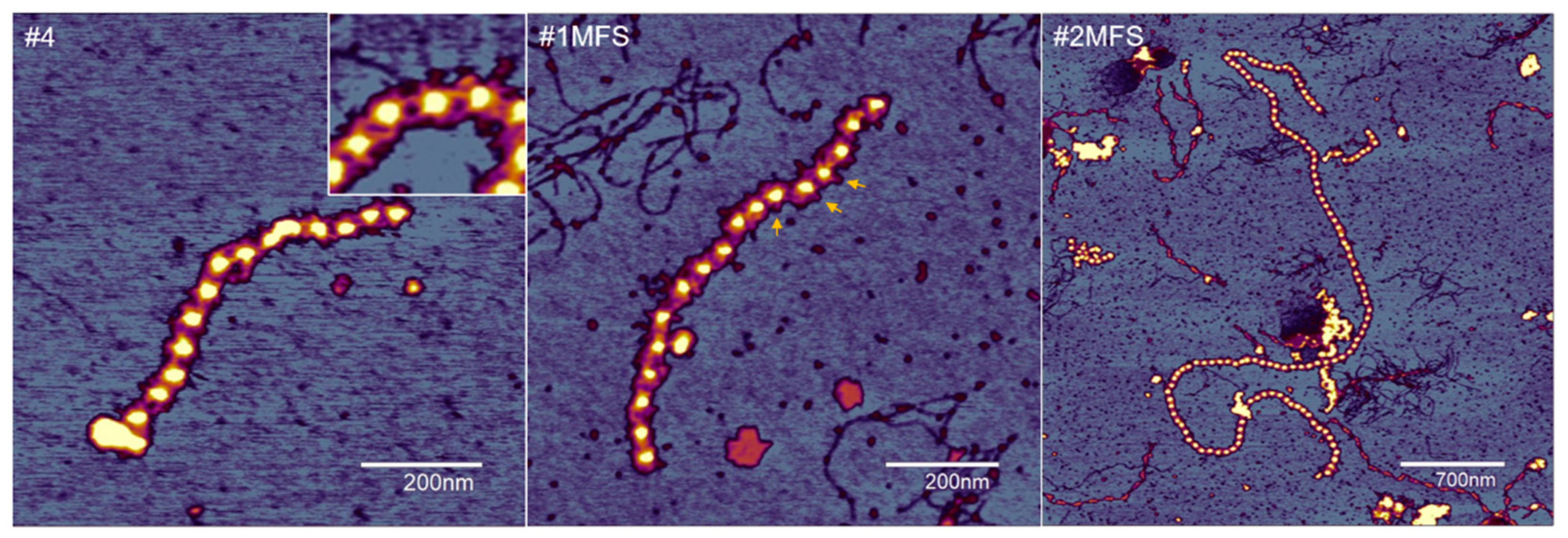
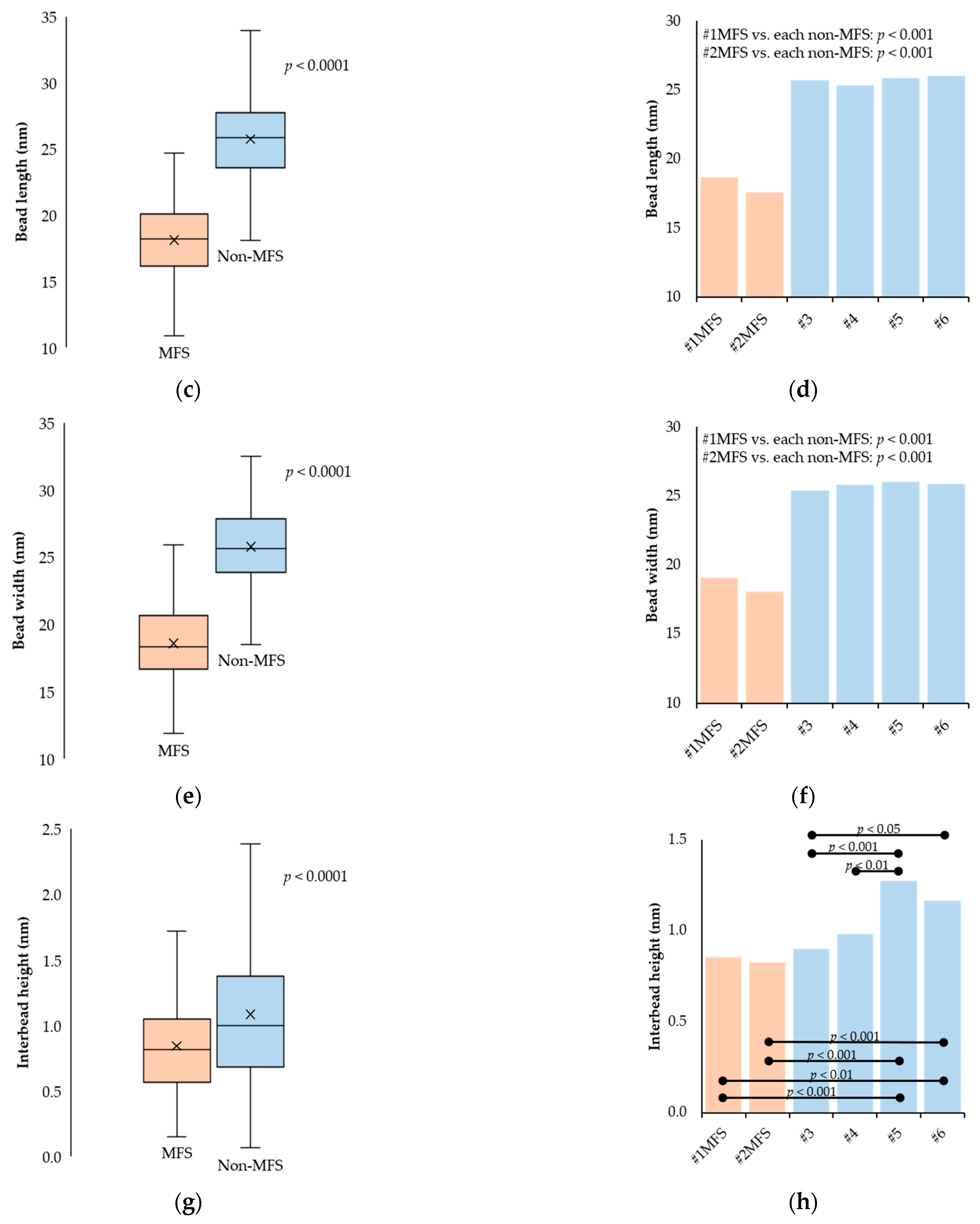
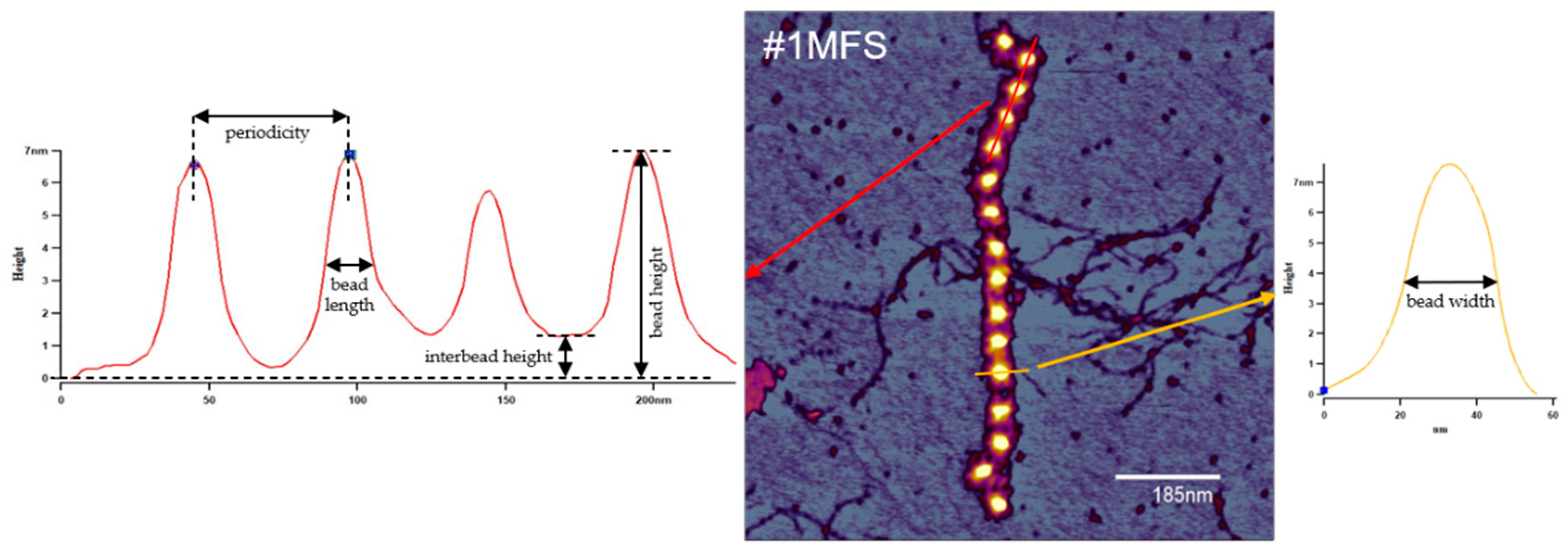
2.2. Fibrillin Microfibril Bead and Interbead Ultrastructure
2.3. Fibrillin Microfibril Periodicity
3. Discussion
4. Materials and Methods
4.1. Aortic Tissue Samples
4.2. Fibrillin-1 Microfibril Isolation
4.3. Atomic Force Microscopy
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, I.; Jensen, S.; Handford, P. TB domain proteins: Evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem. J. 2011, 433, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Handford, P.A.; Mayhew, M.; Brownlee, G.G. Calcium binding to fibrillin? Nature 1991, 353, 395. [Google Scholar] [CrossRef] [PubMed]
- Knott, V.; Downing, A.K.; Cardy, C.M.; Handford, P. Calcium binding properties of an epidermal growth factor-like domain pair from human fibrillin-1. J. Mol. Biol. 1996, 255, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Downing, A.K.; Knott, V.; Werner, J.M.; Cardy, C.M.; Campbell, I.D.; Handford, P.A. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: Implications for the Marfan syndrome and other genetic disorders. Cell 1996, 85, 597–605. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Mechling, D.E.; Boswell, B.A.; Keene, D.R.; Sakai, L.Y.; Bachinger, H.P. Calcium determines the shape of fibrillin. J. Biol. Chem. 1997, 272, 7368–7373. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Ono, R.N.; Sakai, L.Y. Calcium stabilizes fibrillin-1 against proteolytic degradation. J. Biol. Chem. 1997, 272, 1231–1236. [Google Scholar] [CrossRef]
- Jovanovic, J.; Takagi, J.; Choulier, L.; Abrescia, N.G.; Stuart, D.I.; van der Merwe, P.A.; Mardon, H.J.; Handford, P.A. αvβ6 is a novel receptor for human fibrillin-1. Comparative studies of molecular determinants underlying integrin-RGD affinity and specificity. J. Biol. Chem. 2007, 282, 6743–6751. [Google Scholar] [CrossRef]
- Thomson, J.; Singh, M.; Eckersley, A.; Cain, S.A.; Sherratt, M.J.; Baldock, C. Fibrillin microfibrils and elastic fibre proteins: Functional interactions and extracellular regulation of growth factors. Semin. Cell Dev. Biol. 2019, 89, 109–117. [Google Scholar] [CrossRef]
- Ramirez, F.; Sakai, L.Y. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010, 339, 71–82. [Google Scholar] [CrossRef]
- Kielty, C.M. Fell-Muir Lecture: Fibrillin microfibrils: Structural tensometers of elastic tissues? Int. J. Exp. Pathol. 2017, 98, 172–190. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Ramirez, F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J. Cell Biol. 1995, 129, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Cantalupo, A.; Sedes, L.; Ramirez, F. The Multiple Functions of Fibrillin-1 Microfibrils in Organismal Physiology. Int. J. Mol. Sci. 2022, 23, 1892. [Google Scholar] [CrossRef] [PubMed]
- Sakai, L.Y.; Keene, D.R.; Engvall, E. Fibrillin, a new 350-kD glycoprotein is a component of extracellular microfbrils. J. Cell Biol. 1986, 103, 2499–2509. [Google Scholar] [CrossRef]
- Godwin, A.R.F.; Singh, M.; Lockhart-Cairns, M.P.; Alanazi, Y.F.; Cain, S.A.; Baldock, C. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix Biol. 2019, 84, 17–30. [Google Scholar] [CrossRef]
- Gibson, M.A.; Cleary, E.G. The immunohistochemical localisation of microfibril-associated glycoprotein (MAGP) in elastic and non-elastic tissues. Immunol. Cell Biol. 1987, 65, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Whittaker, S.P.; Shuttleworth, C.A. Fibrillin: Evidence that chondroitin sulphate proteoglycans are components of microfibrils and associate with newly synthesized monomers. FEBS Lett. 1996, 386, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.A.; Morgan, A.; Sherratt, M.J.; Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Proteomic analysis of fibrillin-rich microfibrils. Proteomics 2006, 6, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Yadin, D.; Robertson, I.; Handford, P. Evolutionary Insights into Fibrillin Structure and Function in the Extracellular Matrix. In Evolution of Extracellular Matrix. Biology of Extracellular Matrix; Keeley, F., Mecham, R., Eds.; Springer: Heidelberg, Germany, 2013; pp. 121–162. [Google Scholar]
- Takeda, N.; Inuzuka, R.; Maemura, S.; Morita, H.; Nawata, K.; Fujita, D.; Taniguchi, Y.; Yamauchi, H.; Yagi, H.; Kato, M.; et al. Impact of Pathogenic FBN1 Variant Types on the Progression of Aortic Disease in Patients With Marfan Syndrome. Circ. Genom. Precis. Med. 2018, 11, e002058. [Google Scholar] [CrossRef]
- Franken, R.; el Morabit, A.; de Waard, V.; Timmermans, J.; Scholte, A.J.; Berg, M.P.V.D.; Marquering, H.; Planken, N.R.; Zwinderman, A.H.; Mulder, B.J.; et al. Increased aortic tortuosity indicates a more severe aortic phenotype in adults with Marfan syndrome. Int. J. Cardiol. 2015, 194, 7–12. [Google Scholar] [CrossRef]
- Ágg, B.; Szilveszter, B.; Daradics, N.; Benke, K.; Stengl, R.; Kolossváry, M.; Pólos, M.; Radovits, T.; Ferdinandy, P.; Merkely, B.; et al. Increased visceral arterial tortuosity in Marfan syndrome. Orphanet. J. Rare Dis. 2020, 15, 91. [Google Scholar] [CrossRef]
- Stengl, R.; Agg, B.; Szilveszter, B.; Benke, K.; Daradics, N.; Rusko, B.; Vattay, B.; Merkely, B.; Polos, M.; Szabolcs, Z. Case Report: Morphological Characterization and Long-Term Observation of Bilateral Sequential Internal Mammary Artery Aneurysms in a Patient With Confirmed FBN1 Mutation. Front. Cardiovasc. Med. 2021, 8, 697591. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- de Beaufort, H.W.L.; Trimarchi, S.; Korach, A.; Di Eusanio, M.; Gilon, D.; Montgomery, D.G.; Evangelista, A.; Braverman, A.C.; Chen, E.P.; Isselbacher, E.M.; et al. Aortic dissection in patients with Marfan syndrome based on the IRAD data. Ann. Cardiothorac. Surg. 2017, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Evangelista, A.; Serrano-Fiz, S.; Villar, S.; Ospina, V.; Martínez, D.; De Villarreal, J.; Sanchez, V.; Moñivas, V.; Mingo, S.; et al. Aortic Complications in Marfan Syndrome: Should We Anticipate Preventive Aortic Root Surgery? Ann. Thorac. Surg. 2020, 109, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Pólos, M.; Stengl, R.; Şulea, C.-M.; Benke, K.; Bartha, E.; Ágg, B.; Koppányi, Á.; Hartyánszky, I.; Székely, A.; Németh, E.; et al. Changing strategies in aortic root reconstruction in Marfan syndrome. Orv. Hetil. 2021, 162, 696–704. [Google Scholar] [CrossRef]
- Stengl, R.; Agg, B.; Polos, M.; Matyas, G.; Szabo, G.; Merkely, B.; Radovits, T.; Szabolcs, Z.; Benke, K. Potential predictors of severe cardiovascular involvement in Marfan syndrome: The emphasized role of genotype-phenotype correlations in improving risk stratification-a literature review. Orphanet. J. Rare Dis. 2021, 16, 245. [Google Scholar] [CrossRef]
- Wang, W.-J.; Han, P.; Zheng, J.; Hu, F.-Y.; Zhu, Y.; Xie, J.-S.; Guo, J.; Zhang, Z.; Dong, J.; Zheng, G.-Y.; et al. Exon 47 skipping of fibrillin-1 leads preferentially to cardiovascular defects in patients with thoracic aortic aneurysms and dissections. J. Mol. Med. 2013, 91, 37–47. [Google Scholar] [CrossRef]
- Pees, C.; Michel-Behnke, I.; Hagl, M.; Laccone, F. Detection of 15 novel mutations in 52 children from 40 families with the Marfan or Loeys-Dietz syndrome and phenotype-genotype correlations. Clin. Genet. 2014, 86, 552–557. [Google Scholar] [CrossRef]
- Baudhuin, L.M.; Kotzer, K.E.; Lagerstedt, S.A. Increased frequency of FBN1 truncating and splicing variants in Marfan syndrome patients with aortic events. Genet. Med. 2015, 17, 177–187. [Google Scholar] [CrossRef]
- Franken, R.; Groenink, M.; de Waard, V.; Feenstra, H.M.; Scholte, A.J.; van den Berg, M.P.; Pals, G.; Zwinderman, A.H.; Timmermans, J.; Mulder, B.J. Genotype impacts survival in Marfan syndrome. Eur. Heart J. 2016, 37, 3285–3290. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Stengl, R.; Bors, A.; Ágg, B.; Pólos, M.; Matyas, G.; Molnár, M.J.; Fekete, B.; Csabán, D.; Andrikovics, H.; Merkely, B.; et al. Optimising the mutation screening strategy in Marfan syndrome and identifying genotypes with more severe aortic involvement. Orphanet. J. Rare Dis. 2020, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Benke, K.; Agg, B.; Szilveszter, B.; Tarr, F.; Nagy, Z.B.; Polos, M.; Daroczi, L.; Merkely, B.; Szabolcs, Z. The role of transforming growth factor-beta in Marfan syndrome. Cardiol. J. 2013, 20, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFbeta signaling and cell stemness. Matrix Biol. 2018, 71–72, 82–89. [Google Scholar] [CrossRef]
- Franken, R.; Hartog, A.W.D.; Radonic, T.; Micha, D.; Maugeri, A.; van Dijk, F.S.; Meijers-Heijboer, H.E.; Timmermans, J.; Scholte, A.J.; Berg, M.P.V.D.; et al. Beneficial Outcome of Losartan Therapy Depends on Type of FBN1 Mutation in Marfan Syndrome. Circ. Cardiovasc. Genet. 2015, 8, 383–388. [Google Scholar] [CrossRef]
- Franken, R.; Heesterbeek, T.J.; De Waard, V.; Zwinderman, A.H.; Pals, G.; Mulder, B.J.; Groenink, M. Diagnosis and genetics of Marfan syndrome. Expert. Opin. Orphan. Drugs 2014, 2, 1049–1062. [Google Scholar] [CrossRef]
- Isogai, Z.; Aspberg, A.; Keene, D.R.; Ono, R.N.; Reinhardt, D.P.; Sakai, L.Y. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J. Biol. Chem. 2002, 277, 4565–4572. [Google Scholar] [CrossRef]
- Isogai, Z.; Ono, R.N.; Ushiro, S.; Keene, D.R.; Chen, Y.; Mazzieri, R.; Charbonneau, N.L.; Reinhardt, D.P.; Rifkin, D.B.; Sakai, L.Y. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003, 278, 2750–2757. [Google Scholar] [CrossRef]
- Henderson, M.; Polewski, R.; Fanning, J.C.; Gibson, M.A. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J. Histochem. Cytochem. 1996, 44, 1389–1397. [Google Scholar] [CrossRef]
- Hanssen, E.; Franc, S.; Garrone, R. Atomic force microscopy and modeling of natural elastic fibrillin polymers. Biol. Cell 1998, 90, 223–228. [Google Scholar] [CrossRef]
- Kielty, C.M.; Sheratt, M.J.; Marson, A.; Baldock, C. Fibrillin microfibrils. Adv. Protein. Chem. 2005, 70, 405–436. [Google Scholar] [CrossRef] [PubMed]
- Eckersley, A.; Mellody, K.T.; Pilkington, S.; Griffiths, C.E.M.; Watson, R.E.B.; O’Cualain, R.; Baldock, C.; Knight, D.; Sherratt, M.J. Structural and compositional diversity of fibrillin microfibrils in human tissues. J. Biol. Chem. 2018, 293, 5117–5133. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, F.; Trotter, J. Morphology and biomechanics of the microfibrillar network of sea cucumber dermis. J. Exp. Biol. 1996, 199, 1817–1828. [Google Scholar] [CrossRef]
- Akhtar, R.; Cruickshank, J.K.; Zhao, X.; Walton, L.A.; Gardiner, N.J.; Barrett, S.D.; Graham, H.K.; Derby, B.; Sherratt, M.J. Localized micro- and nano-scale remodelling in the diabetic aorta. Acta Biomater. 2014, 10, 4843–4851. [Google Scholar] [CrossRef]
- Kielty, C.M.; Rantamaki, T.; Child, A.H.; Shuttleworth, C.A.; Peltonen, L. Cysteine-to-arginine point mutation in a ‘hybrid’ eight-cysteine domain of FBN1: Consequences for fibrillin aggregation and microfibril assembly. J. Cell Sci. 1995, 108, 1317–1323. [Google Scholar] [CrossRef]
- Hibbert, S.A.; Watson, R.E.B.; Griffiths, C.E.M.; Gibbs, N.K.; Sherratt, M.J. Selective proteolysis by matrix metalloproteinases of photo-oxidised dermal extracellular matrix proteins. Cell. Signal. 2019, 54, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.; Comerford, E.J.; Wegg, M.; Mills, A.; Barrett, S.D.; Smith, K.D.; Sherratt, M.J.; Akhtar, R. Investigation of fibrillin microfibrils in the canine cruciate ligament in dogs with different predispositions to ligament rupture. Res. Vet. Sci. 2020, 133, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, M.J.; Holmes, D.F.; Shuttleworth, C.A.; Kielty, C.M. Scanning transmission electron microscopy mass analysis of fibrillin-containing microfibrils from foetal elastic tissues. Int. J. Biochem. Cell. Biol. 1997, 29, 1063–1070. [Google Scholar] [CrossRef]
- Wright, D.W.; Mayne, R. Vitreous humor of chicken contains two fibrillar systems: An analysis of their structure. J. Ultrastruct. Mol. Struct. Res. 1988, 100, 224–234. [Google Scholar] [CrossRef]
- Wallace, R.N.; Streeten, B.W.; Hanna, R.B. Rotary shadowing of elastic system microfibrils in the ocular zonule, vitreous, and ligamentum nuchae. Curr. Eye Res. 1991, 10, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; Koster, A.J.; Ziese, U.; Rock, M.J.; Sherratt, M.J.; Kadler, K.E.; Shuttleworth, A.; Kielty, C.M. The supramolecular organization of fibrillin-rich microfibrils. J. Cell Biol. 2001, 152, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, M.J.; Baldock, C.; Haston, J.L.; Holmes, D.F.; Jones, C.J.P.; Shuttleworth, C.A.; Wess, T.J.; Kielty, C.M. Fibrillin Microfibrils are Stiff Reinforcing Fibres in Compliant Tissues. J. Mol. Biol. 2003, 332, 183–193. [Google Scholar] [CrossRef]
- Matt, P.; Schoenhoff, F.; Habashi, J.; Holm, T.; Van Erp, C.; Loch, D.; Carlson, O.D.; Griswold, B.F.; Fu, Q.; De Backer, J.; et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation 2009, 120, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Imai, Y.; Nishimura, H.; Kato, M.; Takeda, N.; Nawata, K.; Taketani, T.; Morota, T.; Takamoto, S.; Nagai, R.; et al. Circulating transforming growth factor beta-1 level in Japanese patients with Marfan syndrome. Int. Heart J. 2013, 54, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Agg, B.; Benke, K.; Szilveszter, B.; Polos, M.; Daroczi, L.; Odler, B.; Nagy, Z.B.; Tarr, F.; Merkely, B.; Szabolcs, Z. Possible extracardiac predictors of aortic dissection in Marfan syndrome. BMC Cardiovasc. Disord. 2014, 14, 47. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Pyeritz, R.E.; Crawford, E.S.; Byers, P.H. Marfan syndrome: Defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J. Clin. Investig. 1992, 89, 79–86. [Google Scholar] [CrossRef]
- Hutchinson, S.; Furger, A.; Halliday, D.; Judge, D.P.; Jefferson, A.; Dietz, H.C.; Firth, H.; Handford, P.A. Allelic variation in normal human FBN1 expression in a family with Marfan syndrome: A potential modifier of phenotype? Hum. Mol. Genet. 2003, 12, 2269–2276. [Google Scholar] [CrossRef]
- Kielty, C.M.; Cummings, C.; Whittaker, S.P.; Shuttleworth, C.A.; Grant, M.E. Isolation and ultrastructural analysis of microfibrillar structures from foetal bovine elastic tissues. Relative abundance and supramolecular architecture of type VI collagen assemblies and fibrillin. J. Cell Sci. 1991, 99, 797–807. [Google Scholar] [CrossRef]



| MFS | Non-MFS | |||||
|---|---|---|---|---|---|---|
| Sample/Patient | #1 | #2 | #3 | #4 | #5 | #6 |
| Sex | F | M | M | F | M | M |
| Age | 37 | 17 | 66 | 55 | 34 | 72 |
| Family history | Positive | Negative | ||||
| Aortic involvement | Present | |||||
| Ectopia lentis | Absent | |||||
| FBN1 mutation | Present | |||||
| Systemic features * | Wrist and thumb signs | |||||
| Pectus excavatum | Pectus carinatum | |||||
| Pes planus | ||||||
| Reduced US/LS ** + increased arm/height + no severe scoliosis | Scoliosis | |||||
| Skin striae | - | |||||
| Myopia ≥ 3 diopters | - | |||||
| Mitral valve prolapse | ||||||
| Systemic score | 9 | 8 | ||||
| Periodicity | ||||
|---|---|---|---|---|
| Number of Values Registered (n) | Mean (nm) | Minimum (nm) | Maximum (nm) | |
| MFS group | ||||
| #1MFS | 276 | 51.57 ± 6.32 | 31.22 | 72.86 |
| #2MFS | 564 | 50.57 ± 6.03 | 28.46 | 69.36 |
| Non-MFS group | ||||
| #3 | 298 | 52.17 ± 7.29 | 22.69 | 69.69 |
| #4 | 281 | 50.97 ± 6.27 | 29.59 | 68.85 |
| #5 | 618 | 52.27 ± 6.68 | 31.71 | 71.37 |
| #6 | 355 | 51.43 ± 7.11 | 31.74 | 95.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șulea, C.M.; Mártonfalvi, Z.; Csányi, C.; Haluszka, D.; Pólos, M.; Ágg, B.; Stengl, R.; Benke, K.; Szabolcs, Z.; Kellermayer, M.S.Z. Nanoscale Structural Comparison of Fibrillin-1 Microfibrils Isolated from Marfan and Non-Marfan Syndrome Human Aorta. Int. J. Mol. Sci. 2023, 24, 7561. https://doi.org/10.3390/ijms24087561
Șulea CM, Mártonfalvi Z, Csányi C, Haluszka D, Pólos M, Ágg B, Stengl R, Benke K, Szabolcs Z, Kellermayer MSZ. Nanoscale Structural Comparison of Fibrillin-1 Microfibrils Isolated from Marfan and Non-Marfan Syndrome Human Aorta. International Journal of Molecular Sciences. 2023; 24(8):7561. https://doi.org/10.3390/ijms24087561
Chicago/Turabian StyleȘulea, Cristina M., Zsolt Mártonfalvi, Csilla Csányi, Dóra Haluszka, Miklós Pólos, Bence Ágg, Roland Stengl, Kálmán Benke, Zoltán Szabolcs, and Miklós S. Z. Kellermayer. 2023. "Nanoscale Structural Comparison of Fibrillin-1 Microfibrils Isolated from Marfan and Non-Marfan Syndrome Human Aorta" International Journal of Molecular Sciences 24, no. 8: 7561. https://doi.org/10.3390/ijms24087561
APA StyleȘulea, C. M., Mártonfalvi, Z., Csányi, C., Haluszka, D., Pólos, M., Ágg, B., Stengl, R., Benke, K., Szabolcs, Z., & Kellermayer, M. S. Z. (2023). Nanoscale Structural Comparison of Fibrillin-1 Microfibrils Isolated from Marfan and Non-Marfan Syndrome Human Aorta. International Journal of Molecular Sciences, 24(8), 7561. https://doi.org/10.3390/ijms24087561






