Abstract
Both progression from the early pathogenic events to clinically manifest cardiovascular diseases (CVD) and cancer impact the integrity of the vascular system. Pathological vascular modifications are affected by interplay between endothelial cells and their microenvironment. Soluble factors, extracellular matrix molecules and extracellular vesicles (EVs) are emerging determinants of this network that trigger specific signals in target cells. EVs have gained attention as package of molecules with epigenetic reversible activity causing functional vascular changes, but their mechanisms are not well understood. Valuable insights have been provided by recent clinical studies, including the investigation of EVs as potential biomarkers of these diseases. In this paper, we review the role and the mechanism of exosomal epigenetic molecules during the vascular remodeling in coronary heart disease as well as in cancer-associated neoangiogenesis.
1. Introduction
The vasculature controls homeostasis and the functional integration of tissues and organ systems [1,2]. Hemodynamic and bioactive stimuli stimulate the vasculature, which responds with multiple structural and phenotypic changes that are well described during the onset and progression both of cardiovascular diseases (CVDs) and cancer [1,2,3]. Starting from fatty streaks, cell components of the vascular wall change their function, contributing to the progression of atheromatous plaques, chronic inflammation and clinical manifestations of coronary heart disease (CHD) [4,5,6,7,8,9]. Endothelial adaptative remodeling also occurs during cancer growth, representing a key event during the transition from local to systemic and metastasis dissemination [10]. Different metabolic and epigenetic signaling between endothelial cells and the microenvironment are crucial to the initiation and progression of pathological vascular modifications [1,2,3,11]. Therefore, investigation of the pathophysiology of the vascular remodeling mechanism both in CVDs and cancer is of great value for finding new disease targets and biomarkers. A central role in vasculature modifications is the crosstalk between several cell types necessary to transfer information from one site to another. An emerging body of evidence implies that extracellular vesicles (EVs), which circulate in biological fluids, are vehicles of a variety of molecules to recipient endothelial cells during CVD and cancer growth [12,13,14,15,16,17,18]. However, the role of EVs and their epigenetic influences in the pathogenesis of vascular damage are poorly explored. Here, we discuss the epigenetic effects exerted by EVs on the vasculature both in coronary heart disease (CHD) and cancer.
2. EVs Biology and Function
According to the International Society for Extracellular Vesicles (ISEV) (https://www.isev.org, accessed on 15 April 2023), vesicles include both small and medium/large particles, also known as exosomes (Exo) and microvesicles (MVs), which differ by size and effect on biogenesis [19,20]. The literature reports that EVs can be isolated from biological fluids, such as serum, plasma, urine and amniotic fluid, as well as from the conditioned medium of cells cultured and treated in vitro [12,13,16]. EVs can provide “circulating information” to promote intercellular communication [20]. However, since their number and molecular composition can differ considerably, depending on the micro-environmental and pathophysiological status of their cellular origin, their purity and the method of preparation are very important [21,22]. The EVs’ cargo is known to include proteins and nucleic acids such as DNA, mRNA and a variety of non-coding RNAs (ncRNAs) that, when released in target cells, differentially regulate gene expression [23,24,25]. Exosomes not only regulate physiological states, such as tissue regeneration, immune surveillance and stem cell plasticity [26,27,28,29], but also participate in the pathophysiology of CVD, neurodegenerative diseases and malignant tumors [30,31,32] and have potential value for precise diagnosis, prognosis assessment and disease treatment. Moreover, given their biological role, EVs can affect the pathogenesis of CVD and tumor progression [33,34,35]. Specific EVs containing microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circRNAs, may recruit DNA methylase (DNAm) or histone deacetylase enzymes to induce epigenetic changes in target cells [5]. Growing evidence indicates that epigenetic mechanisms are closely related to cardiovascular disease development and cancer neoangiogenesis [36,37,38]. Herein, we review recent findings on the interplay between the molecular cargo of EVs that induces epigenetic modifications in vessel during both CVD and cancer vascularization.
3. The Epigenetic Role of EVs in Cardiovascular Damage
3.1. EVs and Peripheral Endothelial Dysfunction
Epigenetic changes contribute to the development of CVDs and begin with progressive alterations of the endothelial tissue [3,7]. Endothelial dysfunction is characterized by impaired vascular function, loss of antithrombotic function, excessive proliferation of vascular smooth muscle cells (VSMCs), platelet activation and macrophage migration [9,39]. In endothelial dysfunctional cells, intercellular and vascular cell adhesion molecule-1 (ICAM-1 and VCAM-1) are overexpressed and promote the leukocytes’ adhesion [40]. Several aberrant epigenetic modifiers, mainly miRNAs and lncRNAs, are transported by EVs on dysfunctional endothelial cells (ECs) that modify gene expression and contribute to the acceleration of vascular disease to advanced atherosclerosis [41,42] (Figure 1).
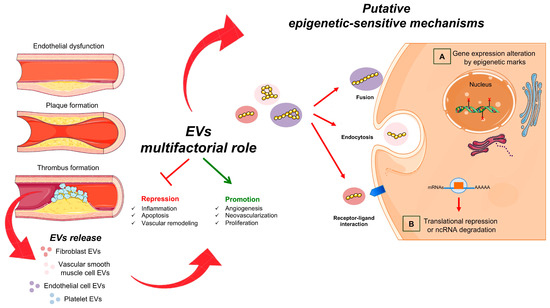
Figure 1.
Extracellular vesicles and vascular damage. Schematic representation of atherosclerotic EVs. They are produced by the different cell types involved in the mechanism of plaque formation. Harmful EVs can promote atherogenesis by altering vascular integrity, increasing inflammatory response and promoting thrombus formation. On the other hand, beneficial EVs, containing specific ncRNAs, can play athero-protective roles by promoting the repair of damaged endothelium and repressing the activation of inflammatory cells. The EVs interact with target cells via direct fusion, endocytosis or the binding of surface proteins. After this invagination, cytosolic proteins or RNA content-EVs transfer to the extracellular space. In the target cells, ncRNAs (such as lncRNAs, miRNAs, circRNAs) can (A) control nuclear gene expression via epigenetic changes and/or (B) affect protein function.
Ding Yu Lee and colleagues demonstrated that the downregulation of miR-10a in both the circulation (serum) and aortic endothelium is highly associated with atherogenesis. miR-10a reduced leukocyte infiltration through GATA6/VCAM-1 signaling pathway, thereby decreasing plaque formation. Thus, this study showed that miR-10a downregulation is associated with atherosclerosis and its induction could prove to be a novel therapeutic target for this disease [43]. Evidence also demonstrated that several miRNAs regulate the leukocytes’ infiltration into the endothelium vessel. One example is miR-155-EVs from VSMCs that target ECs down-regulate integrin proteins and tight junctions. These EVs generate leaky membranes, favor leukocytes and other immune cells migration into sub-endothelium where macrophages can maturate in the foam cells [3,7,40,44]. Moreover, data showed that miRNA-155- and miRNA-223-EVs may also target mononuclear cells, favoring LDL accumulation and their transformation in damaged endothelium [45,46]. Hall and colleagues outlined the importance of an EV-derived circRNA, named Circ-Lpr6, for vascular pathogenesis in both animal and human models. This novel circRNA acts as a sponge for miR-145, thereby regulating its downstream targets. Molecular studies demonstrated that Circ-Lpr6 impairs miR-145-mediated regulation of VSMCs differentiation, migration and proliferation. Furthermore, its overexpression resulted in a decreased intimal area in mouse aortas. Therefore, Circ-Lrp6 could be required to counterbalance miR-145 into VSMCs, providing further evidence that ncRNAs are an important target in this disease, as the ratio between Circ-Lpr6 bound to miR-145 versus unbound plays a role in vascular pathogenesis [47]. Another feature of endothelial dysfunctional vessels is the alteration of the coagulation pathway due to abnormal platelets activation. EVs released following this stimulus are found in chemokine RANTES (normal activation-regulated, expressed, and presumably secreted T cell), thus promoting the neutrophils’ adhesion and migration, starting inflammation [48].
3.2. EVs in Inflammation and Calcification
Cellular damage plays a critical role in the inflammatory response, delivering a high amount of EVs containing pro- and anti-inflammatory signals to target cells [3,7,43]. Although ncRNAs are not classically considered as direct epigenetic modificators, they might have a role in such events. Protective mechanisms are activated by miRNA-126, miRNA-210 and miRNA-214 promoting angiogenesis and vasculogenesis [48,49,50]. In particular, miRNA126 increased the expression of chemokine 12 ligand (CCL12), reduced macrophage entry into the vascular wall and protected the vascular endothelium [51]. A supportive role for this action mechanism derived from the observation that the silencing of miRNA-126 caused leaky vessels, hemorrhage of blood from arterial vessels and partial embryonic lethality. Silencing also induced a loss of vascular integrity and defects in ECs proliferation and angiogenesis [52]. Although miRNA-214 targeting damaged ECs, it also promoted angiogenesis and thus favored the restoration of vessel integrity [50]. The contribution of EVs to endothelial inflammation has been reported to be due to DC-derived EVs that activated the TNF-α/NF-κβ pathway, contributing to the progression of atherosclerosis in animal model [53]. In response to inflammatory stimuli, ECs transferred EV-miR-92a-3p to VSMC, resulting in cell proliferation, while EV miR-146a inhibited their migration, further exacerbating the inflammatory response [54,55,56]. The RNCR3 ncRNA released from ECs in aortic atherosclerotic lesions showed a protective role, regulating miR-185-5p in VSMC target cells. Specifically, miR-185-5p favored the interaction between RNCR3 and Klf2, promoting ECs vasoprotection [57]. In vivo knockdown of RNCR3 worsens atherosclerosis, hypercholesterolemia and inflammation [57]. Thus, EV-transported ncRNAs have a key role in the onset of CVDs. EVs are also involved in the progression of vascular calcification [58]. Analysis of murine and human vessels showed that macrophage-derived EVs promoted intimal microcalcification [59]. Furthermore, the enrichment of these EVs with the calcium-binding protein S100A9 facilitated the calcification progression together with VSMC-derived EVs [59]. In response to this stimulus, miRNA150-EVs promoted vascular maintenance by activating the endothelial vascular endothelial growth factor (VEGF)-A/VEGFR/PI3K/Akt pathway [60]. Although little data are available on EVs regulating apoptosis during endothelial damage, it was demonstrated that HUVECs treated with macrophage-derived EVs activate NF-κB and TLR4 signaling pathways, causing ECs apoptosis [53].
3.3. EVs and CHD
Inflammation and calcification have interconnected roles in the pathophysiology of CHD and acute coronary syndrome (ACS) [3,7,11]. For the first time, the transcriptomic analysis of EVs from ACS patients revealed the presence of mRNA coding for epigenetic enzymes, such as DNA methyltransferases (DNMTs) [61]. Notably, ACS-derived EVs absorbed on PBMCs from healthy subjects modulated the expression of several genes, presumably via epigenetic mechanism in agreement with previously reported [62,63,64,65,66]. In particular, EVs induced methylome changes promoting a significant activation of Synaptosin (SYP), Cell Adhesion Molecule L1 Like (CHL1) and SH2 Domain Containing Adaptor Protein B (SHB) genes [61]. These findings underlined the epigenetic contribution in the role of EVs in ACS.
In the clinical practice, CHD diagnosis is made by coronary CT scan [7,67] and coronary angiography [7,68]. However, it is still unclear whether EVs could be a tool to improve CHD risk estimation future investigations should be conducted to combine EVs and clinical parameters. Shi and colleagues demonstrated that human blood samples from healthy subjects have 86% more EVs compared to CHD patients [69,70]. Therefore, both EV count and content are of potential interest as disease-specific biomarkers [71,72,73,74,75].
Nowadays, it is known that, in the cardiovascular system, EVs also play important epigenetic-indirect roles in the acute post-myocardial infarction (MI) damage and repair mechanisms inducing myocardial remodeling and cardiac regeneration [76]. Recently, echocardiographic experiments had shown that exosomes derived from the mesenchymal stem cell of adipose tissue (ADSC) could improve left ventricular ejection fraction, whereas their administration could significantly alleviate MI-induced cardiac fibrosis [77]. Additionally, ADSC-exosomes treatment has been shown to reduce cardiomyocyte apoptosis, increasing angiogenesis. Molecular experiments demonstrated that ADSCs-derived exosomes can promote microvascular ECs proliferation and migration and inhibit cardiomyocytes apoptosis through miRNA-205. This assumption was confirmed via the transfection of ADSC-derived exosomes into MI-induced mice and observing a decrease in cardiac fibrosis, an increase in angiogenesis, and an improvement of cardiac function [77]. Cardiac fibroblast-derived EVs modulate cardiac remodeling, favoring intercellular communication between cardiomyocytes (CM) and cardiac fibroblasts [78,79]. Consistently, exosomal miRNA-21 CM-derived, reduced the expression of sarcoplasmic protein sorbin 2 containing SH3 domain (SORBS2) and PDZ and LIM domain 5 (PDLIM5), producing an increase in cell size and stimulating cardiomyocyte hypertrophy [80,81]. Moreover, miRNA-21 promoted the cardiac fibrosis inducing the endothelial-to-mesenchymal cell transition [82] and altered expression of superoxide dismutase (SOD) in HF patients [83,84]. Increasing research has attracted attention to the roles of EVs-ncRNAs as potential diagnostic biomarkers. Other than miRNAs, several studies have indicated that lncRNAs significantly regulate fibrosis, thereby having a direct effect on ECM gene expression, the TGF-β signaling pathway and the proliferation of fibroblasts or transition to myofibroblast [23,85,86,87]. These effects are also proposed to be mediated by paracrine communication of EVs between donor and recipient cells, especially in cardiomyocytes and fibroblasts [23]. Exosomes-containing lncRNA ZFAS1 could induce cardiac fibrosis via the Wnt4/β-catenin signal pathway by sponging miR-4711-5p in cardiac fibroblasts [85]. LncRNA MIAT is up-regulated in serum-derived EVs from atrial fibrillation (AF) patients. MIAT aggravated the atrial remodeling and promoted AF by binding with miR-485-5p [86]. Neat1 is obviously up-regulated by P53 and HIF2A in cardiomyocytes in response to hypoxia and is enriched in cardiomyocyte-derived exosomes. Neat1 is essential for cell survival and fibroblast functions. This finding was confirmed via genetic knockout of Neat1 that impaired cardiac function during MI [87]. Although currently studies aim at the analysis of EVs from human samples in AF, HF and MI repair, large studies need to be performed to determine their utility in the clinical routine [88]. In addition to all the findings on basic mechanisms involving EVs, various clinical studies have been initiated to date. Some of these are still at the recruitment stage, and the few completed studies have not yet published their first results (NCT03660683, NCT04142138, NCT03034265, NCT03984006, NCT02822131, NCT00331331, NCT03837470 and NCT04266639). All studies listed in Table 1 will investigate the general cargo of EVs from different biological fluids and tissues, but none have provided help to investigating specific molecules. Trials are evaluating the role of EVs as carriers of anti-inflammatory signal or drug delivery systems. However, these are still few, and their results are not fully available (Table 1).

Table 1.
Clinical studies of EVs’ evaluation as diagnostic biomarker for CVD treatment.
4. Basic Mechanisms and Epigenetic Role of EVs in Tumor Vascularization
4.1. EVs in Neoangiogenesis
Initiation of angiogenesis is an important early event in premalignant lesions to supply nutrients and oxygen [1,9] (Figure 2).
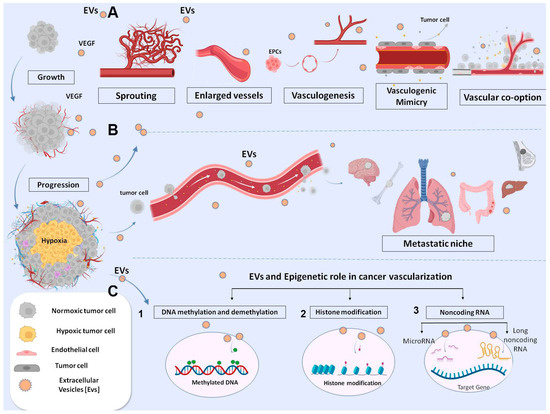
Figure 2.
Schematic representation of EVs involved in different mechanisms of tumor angiogenesis. Tumor cell-derived exosomes carrying biological molecules are involved in various angiogenic mechanisms: (A) sprouting angiogenesis, vessel enlargement, arteriogenesis, vascular mimicry and vessel-cooption favoring tumor growth and pre-metastasis niche formation; (B) Exosomes from malignant cells released locally or at distant sites can induce vessel modifications, with endothelial damage favoring intravasation. Triggering endothelial-mesenchymal transition, which favors endothelial migration at the distal site. Stimulation of extravasation at distant pre-metastatic niche; and (C) Schematic representation of epigenetic mechanisms: 1 DNA methylation; 2 Histone modifications; 3 non-coding RNA targets intracellular mRNA.
EVs such as extracellular organelles elicit signaling between tumor and vascular cells carrying functional and epigenetic modifications to adapt the microenvironment to tumor growth conditions (Figure 2). Tumor hypoxia is the most potent stimulus of EVs biogenesis [89]. EV content may activate the proliferative and migratory status of ECs, favoring neo-vessel formation via different mechanisms, including epigenetic ones [90] (Figure 2). The canonical VEGF (or Notch signaling pathway) was described as being activated by EVs from different tumor types because ECs promoted sprouting and proliferation [91,92,93,94] (Figure 2). Other metabolic programs have also been proven to influence the migration of ECs [16]. We reported that EVs containing EDIL3 activate a mitochondrial and vesicular trafficking program through the purinergic receptor 4 in normal quiescent ECs. This stimulus promotes endothelial cell survival, proliferation, motility and organization into capillary-like structures in vitro and in vivo [16].
EVs may also regulate endothelial function via epigenetic mechanisms. In preclinical and clinical settings, a variety of ncRNAs and long noncoding RNA (lncRNA) transcripts packaged in EVs have been shown to be capable of modifying gene expression without altering the DNA sequence [95]. Generally, EVs-miRNAs are transferred into ECs, where they are post-transcriptionally downregulating antiangiogenic genes, promoting growth and vessel sprouting. However, the role of individual exosomal miRNAs is dependent on tumor histology and stage. In squamous cell carcinoma (ESCC), miR-181b-5p predicts a poorer overall survival. It was also shown to be involved in vessel formation by promoting endothelial growth via AKT targeting endothelial transcript for PTEN and PHLPP2 [96]. In multiple myeloma, the number of EVs carrying miR-135b is significantly increased compared to normal individuals. Transfer of this miRNA in ECs downregulates the inhibitor of hypoxia-inducible factor 1 (HIF-1), resulting in the formation of endothelial tubes through the HIF signaling pathway [97]. MiR-9-EVs from glioma play a pivotal role in both pathogenesis and vascularization. When miR-9 is absorbed by ECs, the increased neoangiogenesis activates multiple pathways, among them COL18A1, Thrombospondin-2 (THBS2), Protein patched homolog 1 (PTCH1) and prolyl hydroxylase 3 (PHD3) via MYC and OCT4 [98]. One of the most common miRNAs associated with poor prognosis of several cancer types is miR-210 [99,100]. Exosomal miR-210 uptake from ECs promotes angiogenesis by targeting specific processes depending on the tumor microenvironment [100]. The chromatin epigenetic regulation of proangiogenic genes has also been attributed to different lncRNA packaged in EVs, although the mechanism is not fully elucidated [101,102,103] (Figure 2). LncRNAs can work as molecular scaffold, assembling several chromatin regulator proteins and reshaping the DNA structure in the nucleus and thus favoring the transcription or silencing of target genes [104]. In other cases, lncRNAs can act as ceRNAs or sponges, sequestering endogenous miRNAs and inhibiting their action on target. For example, gastric cancer exosomal lncRNA PVT1 was reported to have the capacity to activate proliferation of ECs by forming a complex with polycomb repressive complex 2 (PRC2), the major histone methyltransferase on the STAT3 promoter, activating the transcription of the VEGFAs [105]. Several exosomal lncRNAs and proteins have been identified that originate from non-small cells lung cancer (NSCLC), some of which have a role in neoangiogenesis, e.g., lncRNA-p21 and Ubiquitin-fold modifier conjugating enzyme 1 (UFC1) protein [105,106,107]. The mechanism identified for UFC1 revealed the binding to the enhancer of Zeste 2 (EZH2) protein in the polycomb repressor complex 2 (PRC2) and their accumulation at the promoter region of the PTEN gene. This complex catalyzes the trimethylation of H3K27, the inhibition of PTEN expression and the activation of angiogenesis via AKT. UFC1 knockdown inhibited NSCLC growth in a mouse xenograft tumor model [107]. Another pro-angiogenic mechanism is downstream Angiopoietin 2/Tyrosin kinase endothelial receptor 2 (Ang2/Tie2). This pathway is activated by lncRNA EPIC1 in NSCLC and promotes channel formation and the proliferation of ECs [108]. Furthermore, the switch of ECs towards the proliferative phenotype was attributed to exosomal MANTIS. This lncRNA influenced the epigenetic regulation of angiogenic sprouting and alignment of ECs, modifying the chromatin complex, SWI/SNF (Switch/Sucrose non-fermentable), to facilitate the access to RNA polymerase II machinery and gene transcription [109].
Different mechanisms have been attributed to metastasis-associated lung adenocarcinoma transcript 1 (MALAT-1), which may promote angiogenesis in endothelial ovarian carcinoma and in lung cancer [110,111]. Studies have demonstrated the influence of MALAT-1 and other lncRNAs in the complex interplay among lncRNA-miRNA-mRNA governing the sequestration and inactivation of miRNAs. For example, lncNKX2–1 from gastric cancer was reported to increase tumor growth and angiogenesis in both in vitro and in vivo models to direct sponge miR-145–5p, which upregulates Serpin Family E member 1 (SERPINE1) and vascular endothelial growth factor receptor (VEGFR) 2 signaling pathways [112]. A similar mechanism was also defined for receptor activity-modifying protein 2 (RAMP2-AS1) from chondrosarcoma, Urothelial cancer associated (UCA1) for pancreatic cancer, Small nucleolar RNA host gene 1 (SNHG1) from breast [112,113,114], RNA CCAT2 in exosomes from gliomas [115,116] and LINC00707 from cervical or bladder cancer [117,118]. Taken together, these data indicated that EVs have a role in tumor neoangiogenesis through epigenetic mechanisms. Emerging research is evaluating exosomes and their content as potential cancer biomarkers in clinical setting (Table 2). A survey on ClinicalTrials.gov (https://clinicaltrials.gov/, accessed on 15 April 2023) shows a total of 88 trials, of which 60 (68%) EVs’ cargo from different source are used as biomarkers of disease or response to therapy applications. In the case of exosome therapy, as a drug-delivery system, seven trials (8%) have been registered and one clinical trial (1.72%) is related to exosome as vaccine study. Although no definitive data are available, a manufacturing practice (GMP) as exosome production method and purification methods is present in the current trial. Furthermore, the European Medicines Agency (https://www.ema.europa.eu/en, accessed on 15 April 2023) has released scientific recommendations on classification of advanced therapy medicinal products. This nascent field of biomedicine offers new opportunities for the treatment of diseases and dysfunctions of the human body. In addition, it provides guidelines, recommendations, for researchers. However, the transition of EVs from basic research to clinical application is still premature.

Table 2.
Clinical studies of EV evaluation as diagnostic biomarkers for cancer treatment.
4.2. EVs and Vascular Alterations in Tumor Progression
The disruption of vascular integrity and the consequent increase in vascular permeability allows tumor cells to penetrate the vasculature and metastasize. EVs secreted by cancer cells during tumor growth play an important role in vascular leakage (Figure 2). Studies have demonstrated that EVs from metastatic cancer cells may modify ECs’ permeability by altering cytoskeletal-associated proteins or downregulating the RhoA/ROCK pathway [119]. EVs containing miRNAs (including miR-200c, miR-141 and miR-429), which correlated with a poor prognosis of colon cancer, were demonstrated to downregulate ZEB protein and modify the membrane permeability of ECs [120]. Similarly, EVs carrying miR-25-3p, more frequent in patients with metastasis than in those without, were demonstrated to induce vascular leakage and enhance colon cancer metastasis [121]. The same is true for EVs miR-105 in breast cancer [122]. Furthermore, EVs miR-939 correlated with poor prognosis of gastric cancer and increased the permeability of ECs by downregulating the transcription of VE-cadherin [123]. A similar mechanism was reported for LncX26 in gastric cancer [124]. Additionally, the loosening of endothelial integrity and ultimately inducing colon cancer metastasis were attributed to post-transcriptional down regulation of p120-catenin (p120) mediated by transferring of exosomal miR-27b-3p from colon cancer [125] or miR-638 from HCC [126]. In NSCL cancer patients, exosomal miR-375-3p positively correlated with patient TNM stages. In vivo, miR-375-3p-enriched exosomes destroyed the endothelial structure of lung, liver and brain tissues of mice, leading to increased blood vessel permeability. Exosomal miR-375-3p internalized by breaking the tight junction of ECs through negative regulation of claudin-1 expression [127]. Another important pathway of EVs systemic activity is the degradation of extracellular matrix (ECM), favoring tumor growth and preparation of the metastasis niche. In particular, MMP-1 in EVs derived from cancer cells is a representative indicator of ECM degradation [128] (Figure 2). Evidence shows that the pharmacological reduction of EVs decreases the secretion of MMPs, resulting in an inhibition of tumor growth and metastasis [128,129]. This pathway is epigenetically regulated by exosomal lnc-MMP2-2 released by NSLC. The lncRNA internalized into ECs competed with miR-1207-5p to downregulate EPB41L5 and increased vascular permeability, promoting brain metastasis [130]. These data indicate a key role of EVs in damaging the endothelium and promoting intravasation of tumor cells and extravasation at distal sites (Figure 2). Moreover, considering the epigenetic roles of some ncRNAs and their capacity to control gene expression, the development of epigenetic-related drugs with higher specificity and fewer side effects would be a possible future approach.
4.3. EVs in Metastatic Niche: And Other Types of Vessels
Tumor vasculatures increase the tumor capability to metastasize by acquiring mesenchymal characteristics [131,132]. Both normal and tumor endothelial ECs are capable of acquiring a mesenchymal phenotype, increasing the tumor’s ability to metastasize. TECs comparing to ECs exhibiting cytogenetic abnormalities, such as aneuploid chromosomes, are differ metabolically, more resistant to starvation and release more angiocrine molecules and EVs [133,134]. The presence of TEC is associated with conversion of indolent tumor to more aggressive phenotype, and their presence was correlated with reactivation of dormant CD44v6+ tumor stem cells [135,136]. ECs and TECs within the vasculature gain a mesenchymal phenotype (vimentin alfa-smooth muscle actin positive), in some tumor types, shed from the endothelial lining layer of neoplastic vasculature into peripheral blood to the colonizing distal organ [135,136,137,138]. The mechanism is not fully understood. Exosomal Annexin A1 is one of the triggers inducing endothelial-to-mesenchymal transition (EndEMT) and activating RAC1/PAK2 in the gastric cancer model, whereas TGF-β on the surface of EVs triggers the same phenotypic switch in melanoma [139,140,141]. Moreover, data from EVs containing miR-92a-3p, derived from colon cancer cells, showed upregulation of mesenchymal markers, such as snail and vimentin, and downregulated the tight junction marker ZO-1 in HUVECs referred as “partial-EndoMT” [142]. EVs released from tumor cells during hypoxia participate in the EndMT switch of TECs, particularly EVs loaded with lysyl oxidase-like 2 protein (LOXL2) [143]. LOXL2 is a member of the LOX family and regulates extracellular matrix (ECM) remodeling, angiogenesis and premetastatic niche formation. LOXL2 acts as deaminase enzyme into the nucleus contributing to transcription, alternative splicing and miRNA regulation. All these pathways contribute to EndoEMT transition, proliferation and migration of ECs and TECs to distant organs and vasculogenic mimicry mechanism (Figure 2) [143]. Exosomal lncRNA antisense transcript of GATA6 (GATA6-AS) has also been reported to bind LOXL2, which regulates endothelial gene expression underlying EndoEMT transition [144]. The authors demonstrated that GATA6-AS associated with LOXL2 activates H3K4me3 marks and positively regulates the angiogenesis-related genes periostin and cyclooxygenase-1 in ECs. [144]. According to recent studies a multitude of other forms of neovascularization have been observed in cancer settings, including vascular looping and splitting (intussusception), vasculogenic mimicry and remodeling of larger ‘feeding’ vessels (arterio/venogenesis). An epigenetic mechanism mediated by EVs was also involved in vascular mimicry (Figure 2). Here, tumor cells undergoing epithelial-mesenchymal transition (EMT) form microvascular channels contributing to tumor vascularization [145]. These vessel types are negative to CD31 antigen and insensitive to anti angiogenic therapy. The mechanism involved the transferring of EVs containing lncRNAs into target cells, where they act as sponge of selective miRNAs or as scaffold for chromatin remodeling [145,146,147,148,149,150,151]. These mechanisms have been described for HULC in glioblastoma MALAT-1 in gastric cancer and in lung cancer Linc0155 [152,153,154]. Vasculogenic cooption (VCO) is another way by which tumor cells incorporate vessels from normal tissue to gain access to blood supply and growth locally (Figure 2). It is reported that tumor cells can migrate along blood vessels beyond the advancing front of the tumor and may spread to distant metastases [143]. Vessel cooption was initially described in gliomas and lung metastasis [155,156]. Then, VCO was reported in breast cancer metastasis and gastric metastasis from colon [143]. The mechanism of VCO is mediated by VEGF, but it is reasonable to think that EVs rich in VEGF are also involved [155].
5. Conclusions and Perspectives
Some pathogenic aspects of cancer and CHD are defined by the pathophysiology of vascular damage induced by key events in which EVs might play a role. EV counts, types and contents tend to vary, depending on collection methods. By analyzing literature data, we found that only three ncRNAs, specifically, miR-92a-3p, miR-145 and miR-210, were involved in vascular damage, both in the context of CVDs and in tumor neoangiogenesis, although with different mechanisms, underling the possible common pathway. Epigenetic tissue-specific reversible changes induced by exosomal cargo could play a role in the initiation and progression of disease complications. In terms of biomarkers, exosomal ncRNAs could be superior compared to non-exosomal ncRNAs for their specify in methods preparation, the protection from degradation and stability. Moreover, exosomal ncRNAs exist in considerable amounts in different body fluids that can be non-invasively sampled. Considering the epigenetic reversible roles of some ncRNAs, the establishment of novel approaches for modulating their expression or counteract their action could be a future goal [18]. To this end, in the cardiovascular field, 28 clinical studies were started which include exosome analyses (Table 1). In the field of oncology, 88 trials have begun in order to investigate the potential prognostic role of EVs. The most promising application appears to be biomarkers for gastrointestinal cancers (Table 2). However, larger, randomized, prospectively enrolling multicenter trials are necessary to identify EVs as potential clinical biomarkers to better guide clinical treatments.
Author Contributions
Conceptualization, C.N., F.d.N., C.S. and C.B.; resources, C.S., C.B. and F.d.N.; writing-original draft preparation, C.S., C.B. and F.d.N.; supervision and final approval of the intellectual content, C.N.; funding acquisition, C.N., C.S., C.B. and F.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by: PRIN2017F8ZB89 from the Italian Ministry of University and Research (MIUR) (Claudio Napoli) and Ricerca Corrente (RC) 2019 from the Italian Ministry of Health (Claudio Napoli); Internal University Research Grant “VALERE: Vanvitelli Project 2020” (Concetta Schiano) from the Italian Ministry of University and Research (MUR); Internal competitive Research Grant “V:ALERE 2020” (Filomena de Nigris) from the Italian Ministry of University and Research (MUR); Swiss Heart Foundation no. FF21017 (Carolina Balbi).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef]
- Napoli, C.; Crudele, V.; Soricelli, A.; Al-Omran, M.; Vitale, N.; Infante, T.; Mancini, F.P. Primary prevention of atherosclerosis: A clinical challenge for the reversal of epigenetic mechanisms? Circulation 2012, 125, 2363–2373. [Google Scholar] [CrossRef]
- Napoli, C.; D’Armiento, F.P.; Mancini, F.P.; Postiglione, A.; Witztum, J.L.; Palumbo, G.; Palinski, W. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J. Clin. Investig. 1997, 100, 2680–2690. [Google Scholar] [CrossRef]
- Napoli, C.; Glass, C.K.; Witztum, J.L.; Deutsch, R.; D’Armiento, F.P.; Palinski, W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of early lesions in children (FELIC) study. Lancet 1999, 354, 1234–1241. [Google Scholar] [CrossRef]
- Stojanović, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-induced inflammation: An important player and key therapeutic target in atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lerman, L.O.; de Nigris, F.; Gossl, M.; Balestrieri, M.L.; Lerman, A. Rethinking primary prevention of atherosclerosis-related diseases. Circulation 2006, 114, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Martin-Padura, I.; de Nigris, F.; Migliaccio, E.; Mansueto, G.; Minardi, S.; Rienzo, M.; Lerman, L.O.; Stendardo, M.; Giorgio, M.; De Rosa, G.; et al. p66Shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein E knockout mice. Endothelium 2008, 15, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Santi, A.; Zanivan, S. Extracellular vesicles as central regulators of blood vessel function in cancer. Sci. Signal. 2022, 15, eaaz4742. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Palinski, W.; Monti, M.; Camerlingo, R.; Iacobucci, I.; Bocella, S.; Pinto, F.; Iannuzzi, C.; Mansueto, G.; Pignatiello, S.; Fazioli, F.; et al. Lysosome purinergic receptor P2X4 regulates neoangiogenesis induced by microvesicles from sarcoma patients. Cell Death Dis. 2021, 12, 797. [Google Scholar] [CrossRef]
- Benincasa, G.; Mansueto, G.; Napoli, C. Fluid-based assays and precision medicine of cardiovascular diseases: The ‘hope’ for Pandora’s box? J. Clin. Pathol. 2019, 72, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Sarno, F.; Benincasa, G.; List, M.; Barabasi, A.L.; Baumbach, J.; Ciardiello, F.; Filetti, S.; Glass, K.; Loscalzo, J.; Marchese, C.; et al. Clinical epigenetics settings for cancer and cardiovascular diseases: Real-life applications of network medicine at the bedside. Clin. Epigenet. 2021, 13, 66. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Khan, K.; Kim, J.H. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of mxtracellular vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Fatmous, M.; Claridge, B.; Poh, Q.H.; Simpson, R.J.; Greening, D.W. A Protocol for isolation, purification, characterization, and functional dissection of exosomes. Methods Mol. Biol. 2021, 2261, 105–149. [Google Scholar]
- Barile, L.; Cervio, E.; Lionetti, V.; Milano, G.; Ciullo, A.; Biemmi, V.; Bolis, S.; Altomare, C.; Matteucci, M.; Di Silvestre, D.; et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: Role of pregnan-cy-associated plasma protein-A. Cardiovasc. Res. 2018, 114, 992–1005. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, T.; Chen, Y.; Wang, X.; Wu, T.; Xie, Z.; Luo, J.; Yu, Y.; Yu, H. Circulating Exosomal miRNAs as Novel Biomarkers for Stable Coronary Artery Disease. Biomed Res. Int. 2020, 2020, 3593962. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Arcucci, V.; Stacker, S.A.; Achen, M.G. Control of Gene Expression by Exosome-Derived Non-Coding RNAs in Cancer Angiogenesis and Lymphangiogenesis. Biomolecules 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From biomarkers to mediators of physiology and disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Bjørge, I.M.; Kim, S.Y.; Mano, J.F.; Kalioni, S.B.; Chrzanowski, W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—A new paradigm for tissue repair. Biomater. Sci. 2017, 6, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem cell-derived extracellular vsicles and immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef]
- Aguiar Koga, B.A.; Fernandes, L.A.; Fratini, P.; Sogayar, M.C.; Carreira, A.C.O. Role of MSC-derived small extracellular vesicles in tissue repair and regeneration. Front. Cell Dev. Biol. 2023, 10, 1047094. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Qin, H.; Yan, Y.; Wu, W.; Gong, S.; Wang, L.; Jiang, R.; Zhao, Q.; Sun, Y.; Wang, Q.; et al. Exosomal circular RNAs: Biogenesis, effect, and application in cardiovascular diseases. Front. Cell Dev. Biol. 2022, 10, 948256. [Google Scholar] [CrossRef]
- Polanco, J.C.; Li, C.; Durisic, N.; Sullivan, R.; Götz, J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 2018, 6, 10. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Huang, G. Small extracellular vesicles in metabolic remodeling of tumor cells: Cargos and translational application. Front. Pharmacol. 2022, 13, 1009952. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; D’Alessio, A.; Tamagnone, L. Message in a bottle: Endothelial cell regulation by extracellular vesicles. Cancers 2022, 14, 1969. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Coscioni, E.; de Nigris, F.; Donatelli, F. Emergent expansion of clinical epigenetics in patients with cardiovascular diseases. Curr. Opin. Cardiol. 2021, 36, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Rosa-Garrido, M.; Chapski, D.J.; Vondriska, T.M. Epigenomes in cardiovascular disease. Circ. Res. 2018, 122, 1586–1607. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Peng, M.; Sun, R.; Hong, Y.; Wang, J.; Xie, Y.; Zhang, X.; Li, J.; Guo, H.; Xu, P.; Li, Y.; et al. Extracellular vesicles carrying proinflammatory factors may spread atherosclerosis to remote locations. Cell. Mol. Life Sci. 2022, 79, 430. [Google Scholar] [CrossRef]
- Costantino, S.; Libby, P.; Kishore, R.; Tardif, J.C.; El-Osta, A.; Paneni, F. Epigenetics and precision medicine in cardiovascular patients: From basic concepts to the clinical arena. Eur. Heart J. 2018, 39, 4150–4158. [Google Scholar] [CrossRef]
- Shu, Z.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles containing microRNAs in vascular endothelial dysfunction. J. Cell Mol. Med. 2019, 23, 7933–7945. [Google Scholar] [CrossRef]
- Oggero, S.; de Gaetano, M.; Marcone, S.; Fitzsimons, S.; Pinto, A.L.; Ikramova, D.; Barry, M.; Burke, D.; Montero-Melendez, T.; Cooper, D.; et al. Extracellular vesicles from monocyte/platelet aggregates modulate human atherosclerotic plaque reactivity. J. Extracell. Vesicles 2021, 10, 12084. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yang, T.L.; Huang, Y.H.; Lee, C.I.; Chen, L.J.; Shih, Y.T.; Wei, S.Y.; Wang, W.L.; Wu, C.C.; Chiu, J.J. Induction of microRNA-10a using retinoic acid receptor-α and retinoid x receptor-α agonists inhibits atherosclerotic lesion formation. Atherosclerosis 2018, 271, 36–44. [Google Scholar] [CrossRef]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.F.; Climent, M.; Quintavalle, M.; Farina, F.M.; Schorn, T.; Zani, S.; Carullo, P.; Kunderfranco, P.; Civilini, E.; Condorelli, G.; et al. Circ_Lrp6, a Circular RNA Enriched in Vascular Smooth Muscle Cells, Acts as a Sponge Regulating miRNA-145 Function. Circ. Res. 2019, 124, 498–510. [Google Scholar] [CrossRef]
- Kuravi, S.J.; Harrison, P.; Rainger, G.E.; Nash, G.B. Ability of Platelet-Derived Extracellular Vesicles to Promote Neutrophil- Endothelial Cell Interactions. Inflammation 2019, 42, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Eyyupkoca, F.; Ercan, K.; Kiziltunc, E.; Ugurlu, I.B.; Kocak, A.; Eyerci, N. Determination of microRNAs associated with adverse left ventricular remodeling after myocardial infarction. Mol. Cell. Biochem. 2022, 30, 55–63. [Google Scholar] [CrossRef]
- Liu, L.; Koike, H.; Ono, T.; Hayashi, S.; Kudo, F.; Kaneda, A.; Kagechika, H.; Manabe, I.; Nakashima, T.; Oishi, Y. Identification of a KLF5-dependent program and drug development for skeletal muscle atrophy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102895118. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, X.; Jiang, L.; Li, Y.; Zheng, Q. Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling. Cell Commun. Signal. 2022, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Sun, B.; Gupta, A.; Rempel, H.; Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 2016, 30, 3097–3106. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Karunakaran, D.; Geoffrion, M.; Cheng, H.; Tandoc, K.; Perisic Matic, L.; Hedin, U.; Maegdefessel, L.; Fish, J.E.; Rayner, K.J. Extracellular vesicles secreted by atherogenic macrophages transfer MicroRNA to inhibit cell migration. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J. Am. Heart Assoc. 2016, 5, e004099. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Jiang, Q.; Wang, X.Q.; Wang, Y.N.; Yang, H.; Yao, M.D.; Liu, C.; Li, X.M.; Yao, J.; Liu, B.; et al. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016, 7, e2248. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic witching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Z.; Li, L.; Yan, J.; Shao, C.; Bao, Z.; Jing, L.; Pang, Q.; Geng, Y.; Zhang, L. RAGE/galectin-3 yields intraplaque calcification transformation via sortilin. Acta Diabetol. 2019, 56, 457–472. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Luo, P.; Gao, Y.; Yang, J.; Lao, K.H.; Wang, G.; Cockerill, G.; Hu, Y.; Xu, Q.; et al. XBP1 splicing triggers miR-150 transfer from smooth muscle cells to endothelial cells via extracellular vesicles. Sci. Rep. 2016, 6, 28627. [Google Scholar] [CrossRef]
- Bian, Y.; Cai, W.; Lu, H.; Tang, S.; Yang, K.; Tan, Y. miR-150-5p affects AS plaque with ASMC proliferation and migration by STAT. Open Med. 2021, 1, 1642–1652. [Google Scholar] [CrossRef]
- Schiano, C.; Balbi, C.; Burrello, J.; Ruocco, A.; Infante, T.; Fiorito, C.; Panella, S.; Barile, L.; Mauro, C.; Vassalli, G.; et al. De novo DNA methylation induced by circulating extracellular vesicles from acute coronary syndrome patients. Atherosclerosis 2022, 354, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Zou, Y.; Mao, C.; Li, D.; Wang, C.; Zhang, Z. MicroRNA-221 inhibits the transition of endothelial progenitor cells to mesenchymal cells via the PTEN/FoxO3a signaling pathway. Adv. Clin. Exp. Med. 2021, 30, 1263–1270. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Altamirano, F.; Kim, S.Y.; Tong, D.; Ferdous, A.; Piristine, H.; Dasgupta, S.; Wang, X.; French, K.M.; Villalobos, E.; et al. Xbp1s-FoxO1 axis governs lipid accumulation and contractile performance in heart failure with preserved ejection fraction. Nat. Commun. 2021, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, K.; Shi, M.; Chen, P.I.; Hennigs, J.K.; Zhao, Z.; Wang, M.; Li, C.G.; Saito, T.; Taylor, S.; Sa, S.; et al. Smooth muscle contact drives endothelial regeneration by BMPR2-Notch1-mediated metabolic and epigenetic changes. Circ. Res. 2019, 124, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Armache, A.; Yang, S.; Martínez de Paz, A.; Robbins, L.E.; Durmaz, C.; Cheong, J.Q.; Ravishankar, A.; Daman, A.W.; Ahimovic, D.J.; Klevorn, T.; et al. Histone H3.3 phosphorylation amplifies stimulation-induced transcription. Nature 2020, 583, 852–857. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Moreno-Indias, I.; Sanchez-Alcoholado, L.; Ramos-Molina, B.; Alcaide-Torres, J.; Morcillo, S.; Ocaña-Wilhelmi, L.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Altered adipose tissue DNA methylation status in metabolic syndrome: Relationships between global DNA methylation and specific methylation at adipogenic, lipid metabolism and inflammatory candidate genes and metabolic variables. J. Clin. Med. 2019, 13, 87. [Google Scholar] [CrossRef]
- Infante, T.; Forte, E.; Schiano, C.; Punzo, B.; Cademartiri, F.; Cavaliere, C.; Salvatore, M.; Napoli, C. Evidence of association of circulating epigenetic-sensitive biomarkers with suspected coronary heart disease evaluated by Cardiac Computed Tomography. PLoS ONE 2019, 14, e0210909. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Del Viscovo, L.; De Rimini, M.L.; Padula, S.; Caso, P.; Napoli, C. Network Medicine: A clinical approach for precision medicine and personalized therapy in coronary Heart Disease. J. Atheroscler. Thromb. 2020, 27, 279–302. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, X.; Hao, S.; He, Q.; Shen, Z. Plasma levels of miR-143 and miR-145 are associated with coronary in-stent restenosis within 1 year of follow-up after drug-eluting stent implantation. Ann. Transl. Med. 2020, 8, 756. [Google Scholar] [CrossRef]
- Shi, C.; Alvarez-Olmedo, D.; Zhang, Y.; Pattar, B.S.B.; O’Brien, E.R. The Heat shock protein 27 immune complex enhances exosomal cholesterol efflux. Biomedicines 2020, 8, 290. [Google Scholar] [CrossRef]
- Mathiesen, A.; Hamilton, T.; Carter, N.; Brown, M.; McPheat, W.; Dobrian, A. Endothelial extracellular vesicles: From keepers of health to messengers of disease. Int. J. Mol. Sci. 2021, 22, 4640. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Burrello, J.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Pianezzi, E.; Burrello, A.; Caporali, E.; Grazioli, L.G.; Martinetti, G.; et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine 2021, 67, 103369. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Bolis, S.; Vassalli, G.; Barile, L. Flow cytometric analysis of extracellular vesicles from cell-conditioned media. J. Vis. Exp. 2019, 12, 144. [Google Scholar]
- Amabile, N.; Rautou, P.E.; Tedgui, A.; Boulanger, C.M. Microparticles: Key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 2010, 36, 907–916. [Google Scholar] [CrossRef]
- Mause, S.F.; Weber, C. Microparticles: Protagonists of a novel communication network for intercellular information exchange. Circ. Res 2010, 107, 1047–1057. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, L.; Huang, L.; Cheng, H.; Wang, L.; Xu, L.; Hu, D.; He, C.; Fu, C.; Wei, Q. CD44 promotes angiogenesis in myocardial infarction through regulating plasma exosome uptake and further enhancing FGFR2 signaling transduction. Mol. Med. 2022, 328, 145–147. [Google Scholar] [CrossRef]
- Wang, T.; Li, T.; Niu, X.; Hu, L.; Cheng, J.; Guo, D.; Ren, H.; Zhao, R.; Ji, Z.; Liu, P.; et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol. Direct. 2023, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Chen, C.; Wen, Z.; Wang, D.W. The roles of cardiac fibroblasts and endothelial cells in myocarditis. Front. Cardiovasc. Med. 2022, 9, 882027. [Google Scholar] [CrossRef]
- Su, X.L.; Wang, S.H.; Komal, S.; Cui, L.G.; Ni, R.C.; Zhang, L.R.; Han, S.N. The caspase-1 inhibitor VX765 upregulates connexin 43 expression and improves cell-cell communication after myocardial infarction via suppressing the IL-1β/p38 MAPK pathway. Acta Pharmacol. Sin. 2022, 43, 2289–2301. [Google Scholar] [CrossRef]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Investig. 2014, 124, 2136–2146. [Google Scholar] [CrossRef]
- Di, J.; Yang, M.; Zhou, H.; Li, M.; Zhao, J. MicroRNA-21-containing microvesicles from tubular epithelial cells promote cardiomyocyte hypertrophy. Ren. Fail. 2021, 1, 391–400. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Y.; Shi, S.; Zhou, M.; Zhou, Y.; Wang, M.; Chiu, J.J.; Huang, Z.; Zhang, W.; Liu, M.; et al. Inhibition of miR-21 alleviated cardiac perivascular fibrosis via repressing EndMT in T1DM. J. Cell. Mol. Med. 2020, 1, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Jazbutyte, V.; Fiedler, J.; Gupta, S.K.; Yin, X.; Xu, Q.; Galuppo, P.; Kneitz, S.; Mayr, M.; Ertl, G.; et al. Short communication: Asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circ. Res. 2010, 107, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Yamada, K.A.; Patel, A.Y.; Topkara, V.K.; George, I.; Cheema, F.H.; Ewald, G.A.; Mann, D.L.; Nerbonne, J.M. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodelling with mechanical circulatory support. Circulation 2014, 129, 1009–1021. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J. Bone marrow mesenchymal stem cell-derived exosomes carrying long noncoding RNA ZFAS1 alleviate oxidative stress and inflammation in ischemic stroke by inhibiting microRNA-15a-5p. Metab. Brain Dis. 2022, 37, 2545–2557. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, H.; Li, Y.; Cheng, D.; Tang, Y.; Sang, H. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin. Transl. Med. 2021, 11, e482. [Google Scholar] [CrossRef]
- Kenneweg, F.; Bang, C.; Xiao, K.; Boulanger, C.M.; Loyer, X.; Mazlan, S.; Schroen, B.; Hermans-Beijnsberger, S.; Foinquinos, A.; Hirt, M.N.; et al. Long noncoding RNA-enriched vesicles secreted by hypoxic cardiomyocytes drive cardiac fibrosis. Mol. Ther. Nucleic Acids 2019, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zeyidan, J.; Chengyu, L.; Xinyu, Y.; Lingfei, S.; Emeli, C.; Lingying, Z.; Ji, L.; Guoping, L. Extracellular non-coding RNAs in cardiovascular diseases. Pharmaceutics 2023, 15, 155. [Google Scholar]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of hypoxia-induced exosomes in tumor biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef]
- Adnani, L.; Spinelli, C.; Tawil, N.; Rak, J. Role of extracellular vesicles in cancer-specific interactions between tumour cells and the vasculature. Semin. Cancer Biol. 2022, 87, 196–213. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, C.; Lum, D.; Druso, J.E.; Blank, B.; Wilson, K.F.; Welm, A.; Antonyak, M.A.; Cerione, R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017, 8, 14450. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Liu, S.; Wang, T.; Ianni, A.; Bober, E.; Braun, T.; Xiang, R.; Yue, S. Exosomal tetraspanins mediate cancer metastasis by altering host microenvironment. Oncotarget 2017, 8, 62803–62815. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M. The role of Notch ligand, Delta-like ligand 4 (DLL4), in cancer angiogenesis—Implications for therapy. Eur. Surg. 2021, 53, 274–280. [Google Scholar] [CrossRef]
- Sheldon, H.; Heikamp, E.; Turley, H.; Dragovic, R.; Thomas, P.; Oon, C.E.; Leek, R.; Edelmann, M.; Kessler, B.; Sainson, R.C.; et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010, 116, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, J.; Chen, L.; Bian, H.; Hu, J.; Li, D.; Xia, C.; Xu, H. Tumor-Derived EV-Encapsulated miR-181b-5p induces angiogenesis to foster tumorigenesis and metastasis of ESCC. Mol. Ther. Nucleic Acids 2020, 20, 421–437. [Google Scholar] [CrossRef]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Hagiwara, K.; Yoshioka, Y.; Takeshita, F.; Ochiya, T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013, 288, 10849–10859. [Google Scholar] [CrossRef]
- Camps, C.; Buffa, F.M.; Colella, S.; Moore, J.; Sotiriou, C.; Sheldon, H.; Harris, A.L.; Gleadle, J.M.; Ragoussis, J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin. Cancer Res. 2008, 14, 1340–1348. [Google Scholar] [CrossRef]
- Li, B.; Hong, J.; Hong, M.; Wang, Y.; Yu, T.; Zang, S.; Wu, Q. piRNA-823 delivered by multiple myeloma-derived extracellular vesicles promoted tumorigenesis through re-educating endothelial cells in the tumor environment. Oncogene 2019, 38, 5227–5238. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.; Ma, H.; Lin, S.; Zhang, D.; Wu, W.; Liao, Z.; Chen, M.; Ye, H.; Li, Q.; et al. LncRNA CCAT2, involving miR-34a/TGF-β1/Smad4 signaling, regulate hepatic stellate cells proliferation. Sci. Rep. 2022, 12, 21199. [Google Scholar] [CrossRef] [PubMed]
- de Nigris, F.; Mancini, F.P.; Schiano, C.; Infante, T.; Zullo, A.; Minucci, P.B.; Al-Omran, M.; Giordano, A.; Napoli, C. Osteosarcoma cells induce endothelial cell proliferation during neo-angiogenesis. J. Cell. Physiol. 2013, 228, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Yang, D.; Xie, F.; Wang, Z. The role of long non-coding RNAs in angiogenesis and anti-angiogenic therapy resistance in cancer. Mol. Ther. Nucleic Acids 2022, 28, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, P.; Cui, P.; Qin, Y.; Hu, C.; Wu, J.; Zhou, Z.; Zhang, W.; Qin, L.; Huang, G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 2018, 37, 4094–4109. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.J.; Marrades, R.M.; Molins, L.; Viñolas, N.; Moises, J.; Canals, J.; Han, B.; Li, Y.; Martinez, D.; Monzó, M.; et al. Extracellular vesicle lincRNA-p21 expression in tumor-draining pulmonary vein defines prognosis in NSCLC and modulates endothelial cell behavior. Cancers 2020, 12, 734. [Google Scholar] [CrossRef]
- Zang, X.; Gu, J.; Zhang, J.; Shi, H.; Hou, S.; Xu, X.; Yan, C.; Yu, Z.; Fei, M.; Hui, Q.; et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020, 11, 215. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, H.; Cao, Y.; Zhang, S.; Zhang, X.; Wei, P.; Jun, X.; Wen, D.; Bao, W. LncRNA EPIC1 promotes tumor angiogenesis via activating the Ang2/Tie2 axis in non-small cell lung cancer. Life Sci. 2021, 267, 118933. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Günther, S.; et al. Long Noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation 2017, 136, 65–79. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Lin, Y.Y.; Hua, K.Q. Sci Exosomal metastasis-associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. 2018, 14, 1960–1973. [Google Scholar] [CrossRef]
- Poulet, C.; Njock, M.S.; Moermans, C.; Louis, E.; Louis, R.; Malaise, M.; Guiot. Exosomal long non-coding RNAs in lung diseases. Int. J. Mol. Sci. 2020, 21, 3580. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Z.; Cheng, F.; Shao, Z. Exosomal NA RAMP2-AS1 derived from chondrosarcoma cells promotes angiogenesis through miR-2355-5p/VEGFR2 Axis. OncoTargets Ther. 2020, 13, 3291–3301. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Yang, Y.; Chen, W.; Zhang, K.; Teng, B.; Huang, C.; Zhao, Q.; Qiu, Z. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Mol. Ther. Nucleic Acids 2020, 22, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Yang, Y.; Liu, S.; Liu, H. Hypoxic breast cancer cell-derived exosomal SNHG1 promotes breast cancer growth and angiogenesis via regulating miR-216b-5p/JAK2 Axis. Cancer Manag. Res. 2022, 14, 123–133. [Google Scholar] [CrossRef]
- Lang, H.L.; Hu, G.W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017, 38, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.L.; Shu, Y.G.; Tao, M.Y. LncRNA CCAT2 promotes angiogenesis in glioma through activation of VEGFA signalling by sponging miR-424. Mol. Cell. Biochem. 2020, 468, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, J.; Fan, F.; Zhou, P. LINC00707 regulates miR-382-5p/VEGFA pathway to enhance cervical cancer progression. J. Immunol. Res. 2021, 29, 5524632. [Google Scholar] [CrossRef]
- Gao, T.; Ji, Y. Long Noncoding RNA LINC00707 accelerates tumorigenesis and progression of bladder cancer via targeting miR-145/CDCA3 regulatory loop. Urol. Int. 2021, 105, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Fontana, S.; Monteleone, F.; Taverna, S.; Di Bella, M.A.; Di Vizio, D.; Alessandro, R. Exosomes from metastatic cancer cells transfer amoeboid phenotype to non-metastatic cells and increase endothelial permeability: Their emerging role in tumor heterogeneity. Sci. Rep. 2017, 7, 4711. [Google Scholar] [CrossRef]
- Holzner, S.; Senfter, D.; Stadler, S.; Staribacher, A.; Nguyen, C.H.; Gaggl, A.; Geleff, S.; Huttary, N.; Krieger, S.; Jager, W.; et al. Colorectal cancer cell-derived microRNA200 modulates the resistance of adjacent blood endothelial barriers in vitro. Oncol. Rep. 2016, 36, 3065–3071. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast cancer-secreted miR-939 downregulates VE-cadherin and destroys the barrier function of endothelial monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, S.; Du, K.; Zheng, N.; Liu, Y.; Chen, H.; Xie, G.; Ma, Y.; Zhou, Y.; Zheng, Y.; et al. Gastric cancer–secreted exosomal X26nt increases angiogenesis and vascular permeability by targeting VE-cadherin. Cancer Sci. 2020, 112, 1839–1852. [Google Scholar] [CrossRef]
- Dou, R.; Liu, K.; Yang, C.; Zheng, J.; Shi, D.; Lin, X.; Wei, C.; Zhang, C.; Fang, Y.; Huang, S.; et al. EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clin. Transl. Med. 2021, 11, e595. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Noda, T.; Okumura, Y.; Kobayashi, S.; Iwagami, Y.; Yamada, D.; Tomimaru, Y.; Akita, H.; Gotoh, K.; Takeda, Y.; et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021, 112, 1275–1288. [Google Scholar] [CrossRef]
- Shuangshuang, M.; Sufei, Z.; Zhiliang, L.; Xinfeng, W.; Yan, W.; Guochao, Z.; Haiyan, X.; Jianbing, H.; Yuanyuan, L.; Chengming, L.; et al. Exosomal miR-375-3p breaks vascular barrier and promotes small cell lung cancer metastasis by targeting claudin-1. Transl. Lung Cancer 2021, 10, 3155–3172. [Google Scholar]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.I.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef] [PubMed]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Thery, C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef]
- Dongming, W.; Shihua, D.; Li, L.; Teng, L.; Ting, Z.; Jing, L.; Ye, Y.; Ying, X. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood-brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death 2021, 20, 721. [Google Scholar]
- Gasparics, A.; Rosivall, L.; Krizbai, I.A.; Sebe, A. When the endothelium scores an own goal: Endothelial cells actively augment metastatic extravasation through endothelial-mesenchymal transition. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H1055–H1063. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Akino, T.; Hida, K.; Hida, Y.; Tsuchiya, K.; Freedman, D.; Muraki, C.; Ohga, N.; Matsuda, K.; Akiyama, K.; Harabayashi, T.; et al. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am. J. Pathol. 2009, 175, 2657–2667. [Google Scholar] [CrossRef]
- Ohga, N.; Ishikawa, S.; Maishi, N.; Akiyama, K.; Hida, Y.; Kawamoto, T.; Sadamoto, Y.; Osawa, T.; Yamamoto, K.; Kondoh, M.; et al. Heterogeneity of tumor endothelial cells: Comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 2012, 180, 1294–1307. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, A.R.; Nam, J.K.; Kim, J.M.; Kim, J.Y.; Seo, H.R.; Lee, H.J.; Cho, J.; Lee, Y.J. Tumour vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6+cancer cell and macrophage polarization. Nat. Commun. 2018, 9, 5108. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Lyros, O.; Medda, R.; Jovanovic, N.; Schmidt, J.L.; Otterson, M.F.; Johnson, C.P.; Behmaram, B.; Shaker, R.; Rafiee, P. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: Role of IL-1beta and TGF-beta. Am. J. Physiol.-Cell Physiol. 2014, 307, C859–C877. [Google Scholar] [CrossRef]
- Beerepoot, L.V.; Mehra, N.; Vermaat, J.S.; Zonnenberg, B.A.; Gebbink, M.F.; Voest, E.E. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann. Oncol. 2004, 15, 139–145. [Google Scholar] [CrossRef]
- Gopal, S.K.; Greening, D.W.; Hanssen, E.G.; Zhu, H.J.; Simpson, R.J.; Mathias, R.A. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget 2016, 7, 19709–19722. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Yeon, J.H.; Jeong, H.E.; Seo, H.; Cho, S.; Kim, K.; Na, D.; Chung, S.; Park, J.; Choi, N.; Kang, J.Y. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018, 76, 146–153. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhang, Z.J.; Shang, L.R.; Luo, Y.W.; Lin, Y.F.; Yuan, Y.; Zhuang, S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018, 68, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- de Jong, O.G.; van Balkom, B.W.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like. J. Cell. Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Khanh Truong, A.C.; Becker, L.M.; Saavedra-García, P.; Carmeliet, P. Tumor vessel co-option: The past & the future. Front. Oncol. 2022, 12, 965277. [Google Scholar]
- Neumann, P.; Jaé, N.; Knau, A.; Glaser, S.F.; Fouani, Y.; Rossbach, O.; Krüger, M.; John, D.; Bindereif, A.; Grote, P.; et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL. Nat. Commun. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Zeng, Y.; Yao, X.; Liu, X.; He, X.; Li, L.; Liu, X.; Yan, Z.; Wu, J.; Fu, B.M. Antiangiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J. Extracell. Vesicles 2019, 8, 1629865. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Yang, J.P.; Liao, Y.D.; Mai, D.M.; Xie, P.; Qiang, Y.Y.; Zheng, L.S.; Wang, M.Y.; Mei, Y.; Meng, D.F.; Xu, L.; et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: A meta-analysis. Angiogenesis 2016, 19, 191–200. [Google Scholar] [CrossRef]
- Xiang, T.; Lin, Y.X.; Ma, W.; Zhang, H.J.; Chen, K.M.; He, G.P.; Zhang, X.; Xu, M.; Feng, Q.S.; Chen, M.Y.; et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies. Nat. Commun. 2018, 9, 5009. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, W.; Cai, Z.; Wang, Q.; Gao, J.; Ci, H.; Feng, Z.; Ma, L. The Biomarker like the correlation between vasculogenic mimicry, vascular endothelial cadherin, sex-determining region on Y-Box transcription factor 17, and Cyclin D1 in oesophageal squamous cell carcinoma. J. Oncol. 2022, 29, 8915503. [Google Scholar]
- Luo, Q.; Wang, J.; Zhao, W.; Peng, Z.; Liu, X.; Li, B.; Zhang, H.; Shan, B.; Zhang, C.; Duan, C. Vasculogenic mimicry in carcinogenesis and clinical applications. J. Hematol. Oncol. 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Tiantian, Y.; Jing, W.; Yuchen, H.; Min, Z.; Jie, H. Long non-coding RNA HULC stimulates the epithelial–mesenchymal transition process and vasculogenic mimicry in human glioblastoma. Cancer Med. 2021, 10, 5270–5282. [Google Scholar]
- Li, Y.; Wu, Z.; Yuan, J.; Sun, L.; Lin, L.; Huang, N.; Bin, J.; Liao, Y.; Liao, W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017, 1, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shen, Y.; Ren, H.; Wang, L.; Yang, J.; Wang, Y. Repression of linc01555 up-regulates angiomotin-p130 via the microRNA-122-5p/clic1 axis to impact vasculogenic mimicry-mediated chemotherapy resistance in small cell lung cancer. Cell Cycle 2023, 22, 255–268. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, J.; Li, Z.; Long, Q. Mathematical modelling of a brain tumour initiation and early development: A coupled model of glioblastoma growth, pre-existing vessel co-option, angiogenesis and blood perfusion. PLoS ONE 2016, 11, e0150296. [Google Scholar] [CrossRef] [PubMed]
- Krusche, B.; Ottone, C.; Clements, M.P.; Johnstone, E.R.; Goetsch, K.; Lieven, H.; Mota, S.G.; Singh, P.; Khadayate, S.; Ashraf, A.; et al. EphrinB2 drives perivascular invasion and proliferation of glioblastoma stem-like cells. eLife 2016, 5, e14845. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).