Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function
Abstract
1. Introduction
2. Results
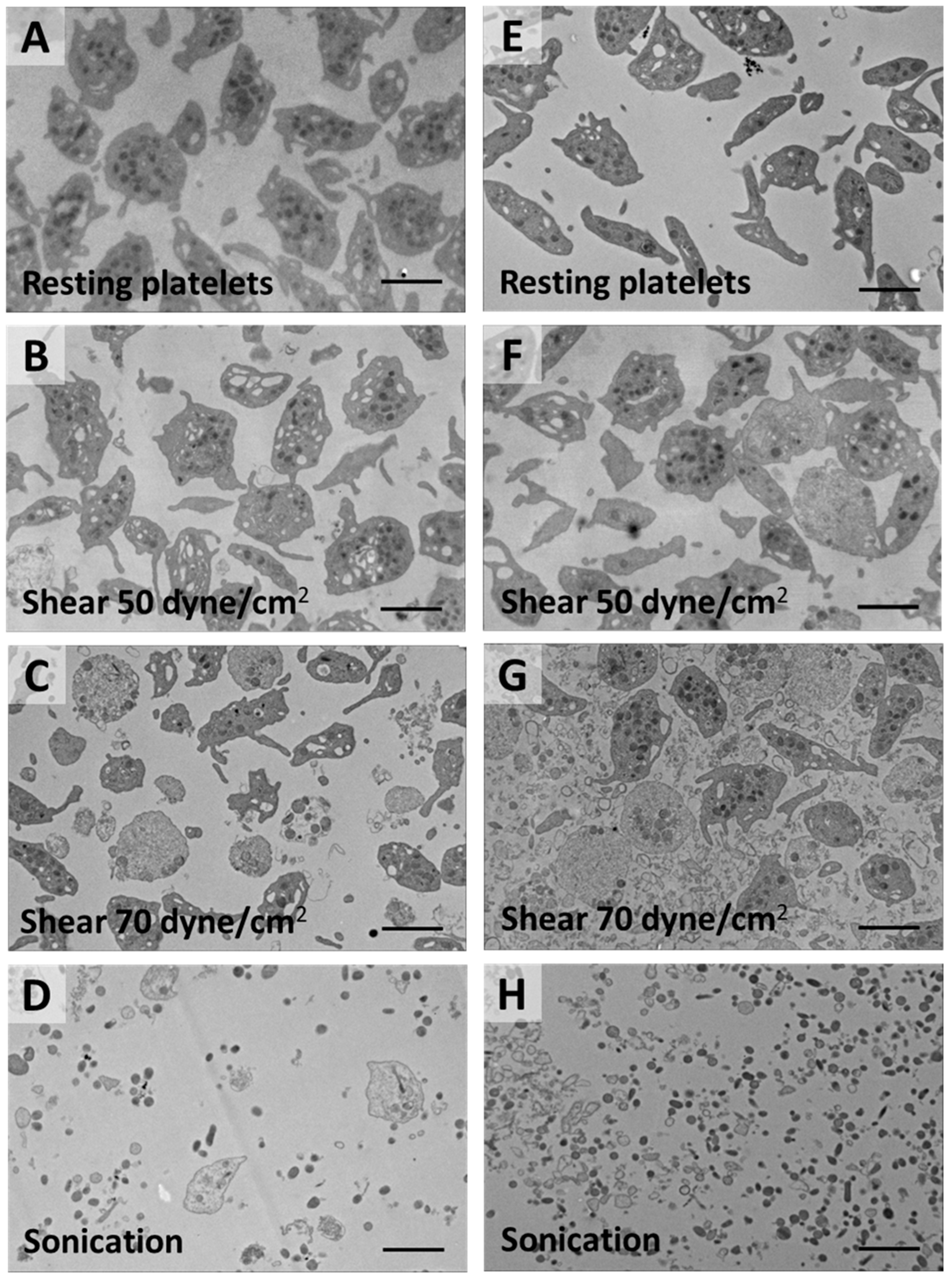
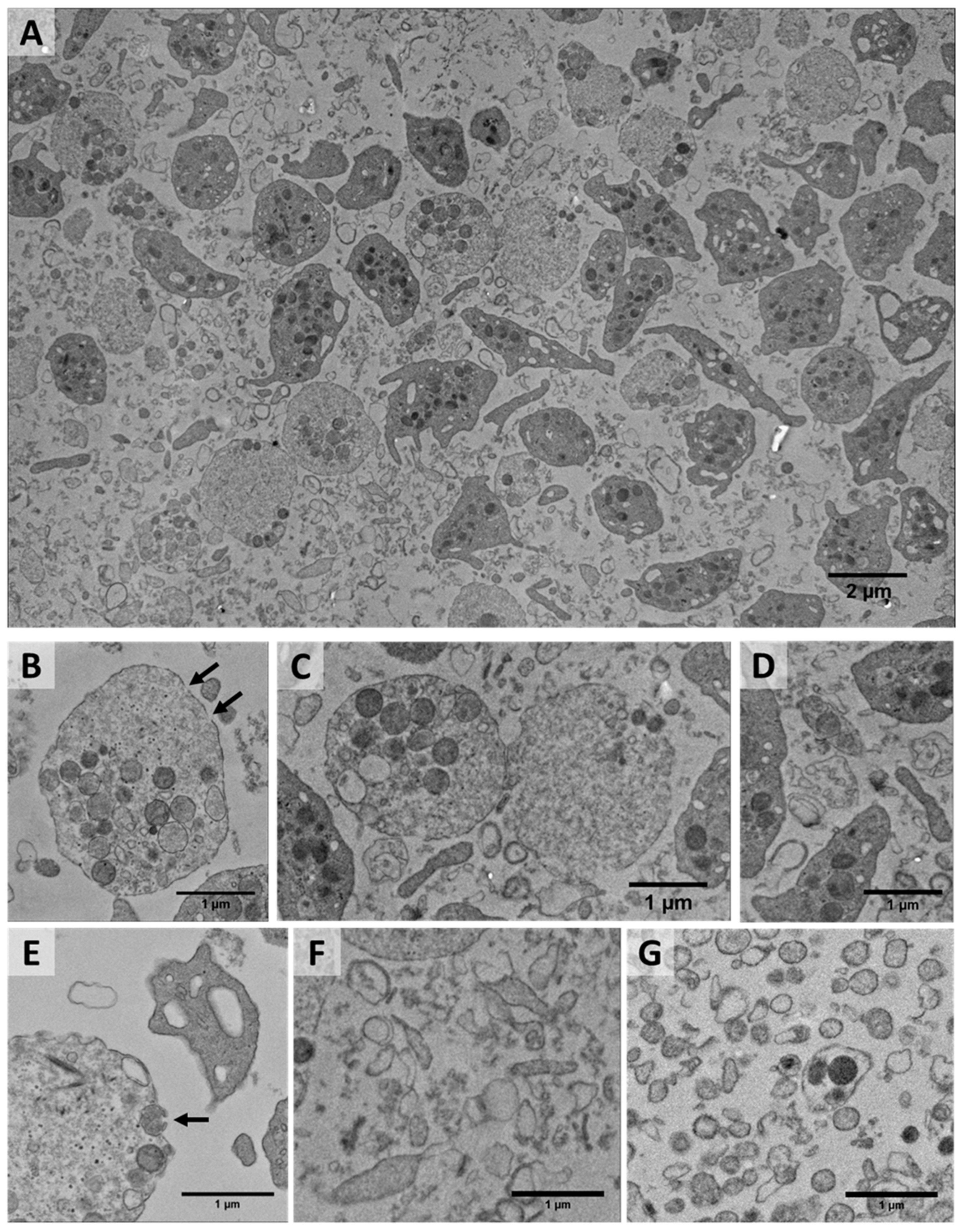
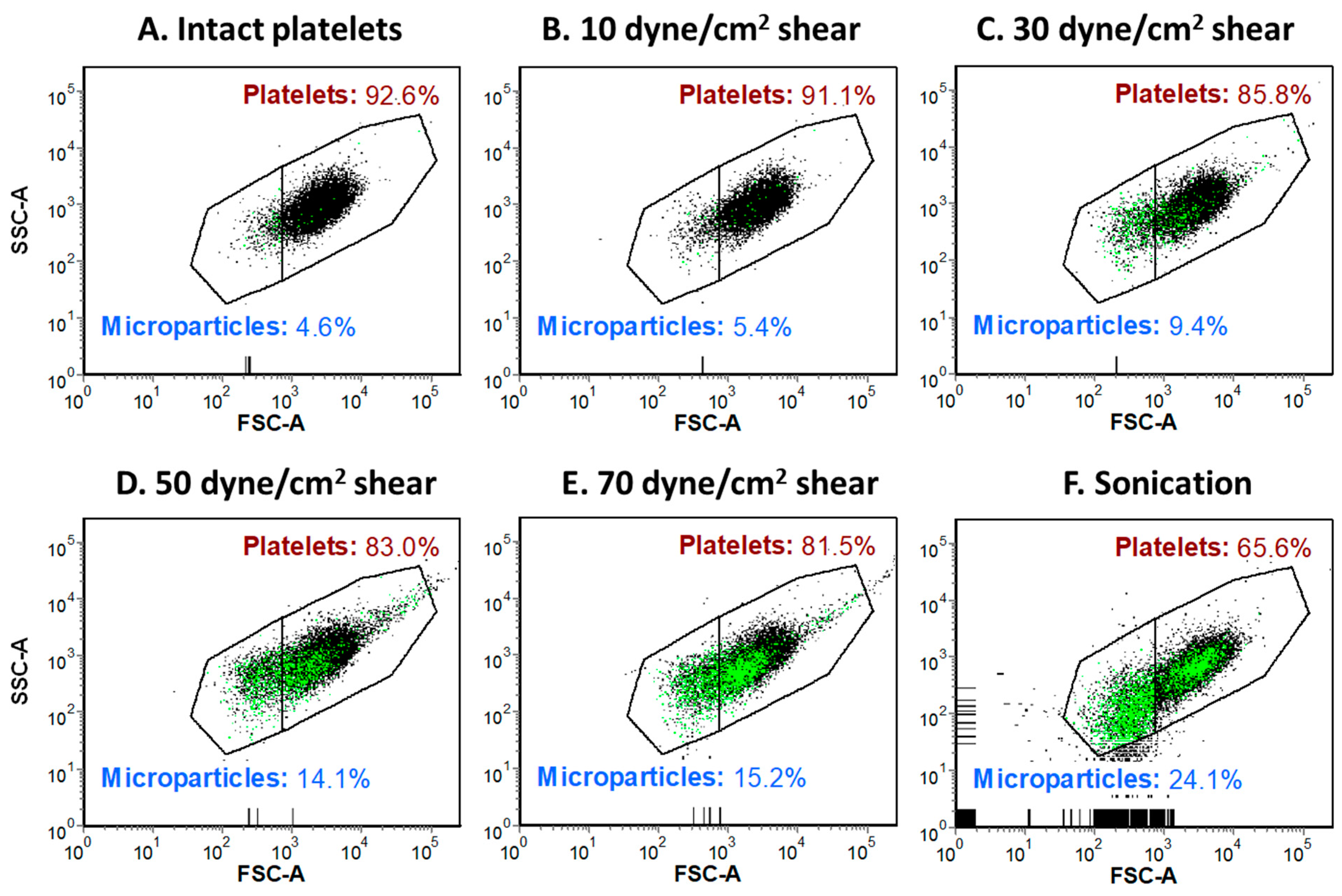
2.1. Shear-Induced Alterations of Platelet Morphology and Microparticle Generation
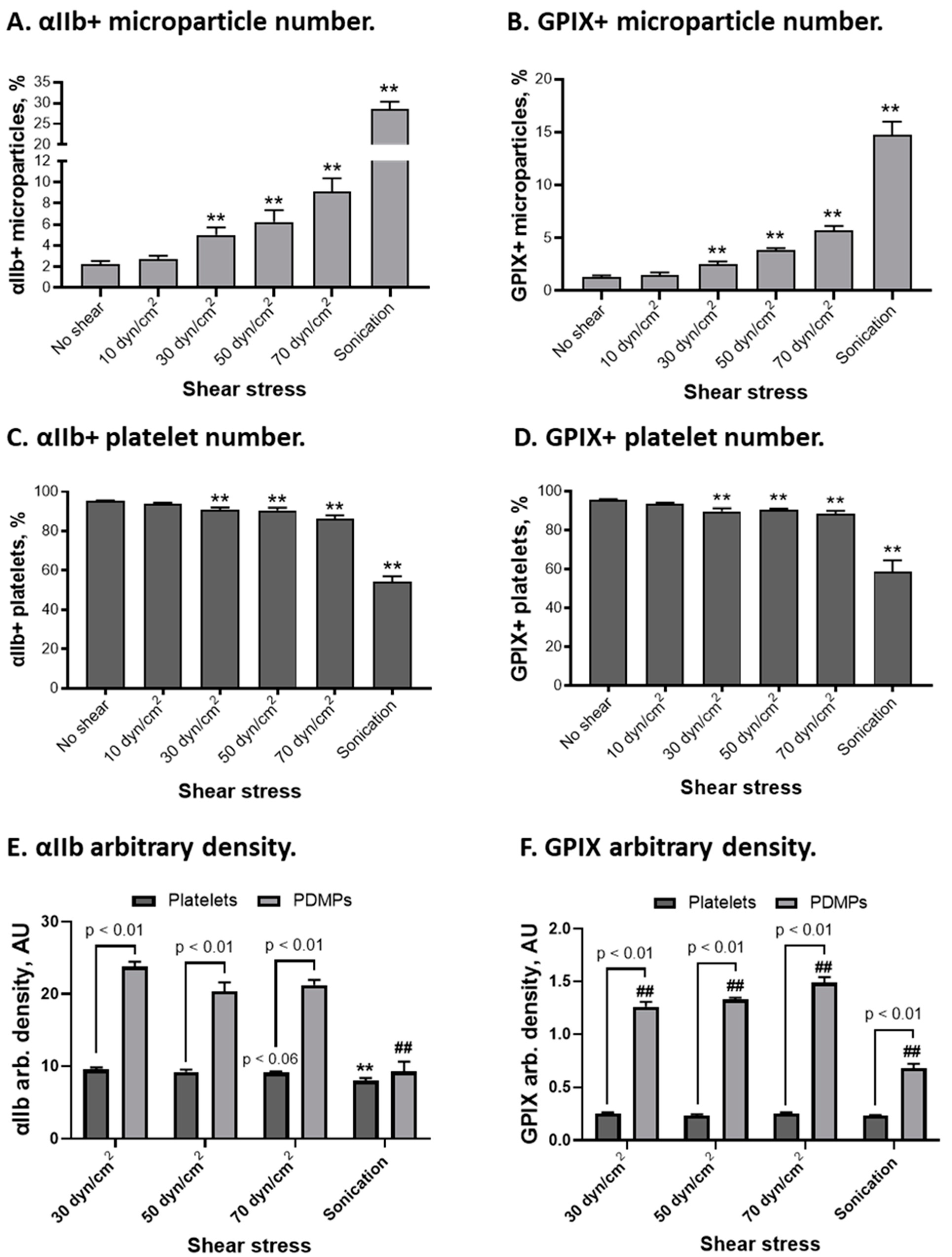
2.2. αIIbβ3 and GPIX Distribution on Platelets and Platelet-Derived Microparticles
2.3. GPVI and PECAM-1 Distribution on Platelets and Platelet-Derived Microparticles
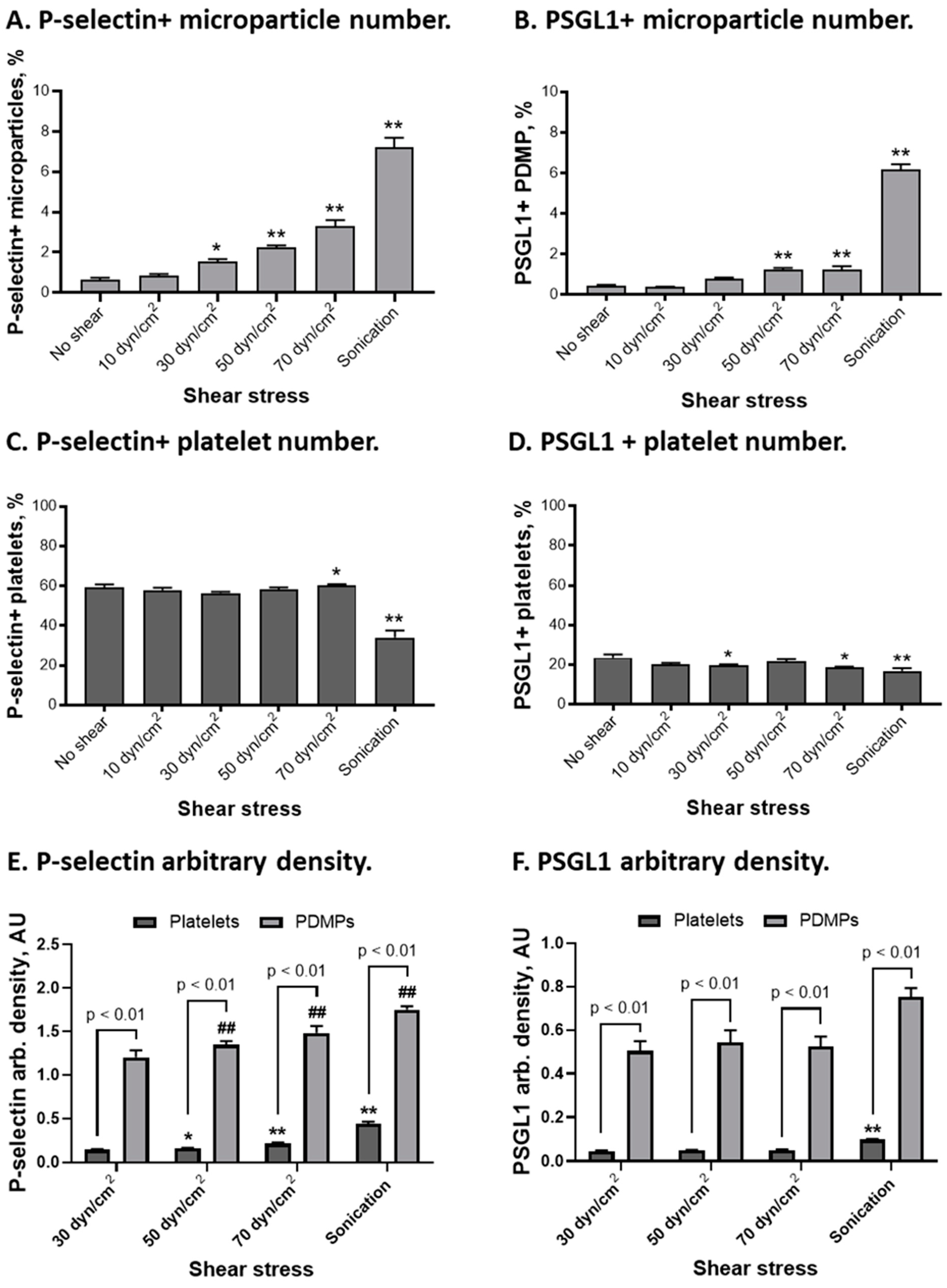
2.4. P-Selectin and PSGL1 Distribution on Platelets and Platelet-Derived Microparticles
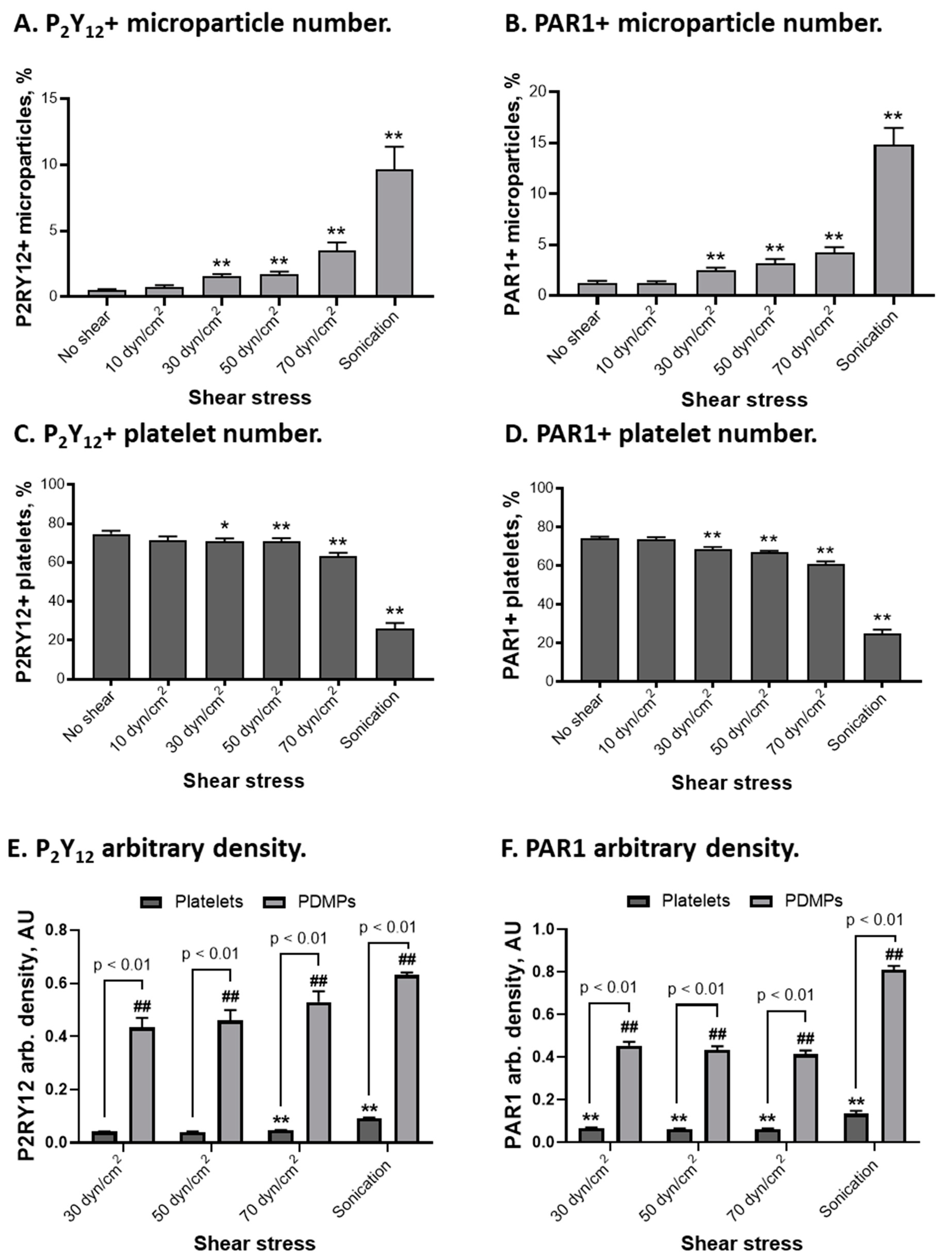
2.5. P2Y12 and PAR1 Distribution on Platelets and Platelet-Derived Microparticles
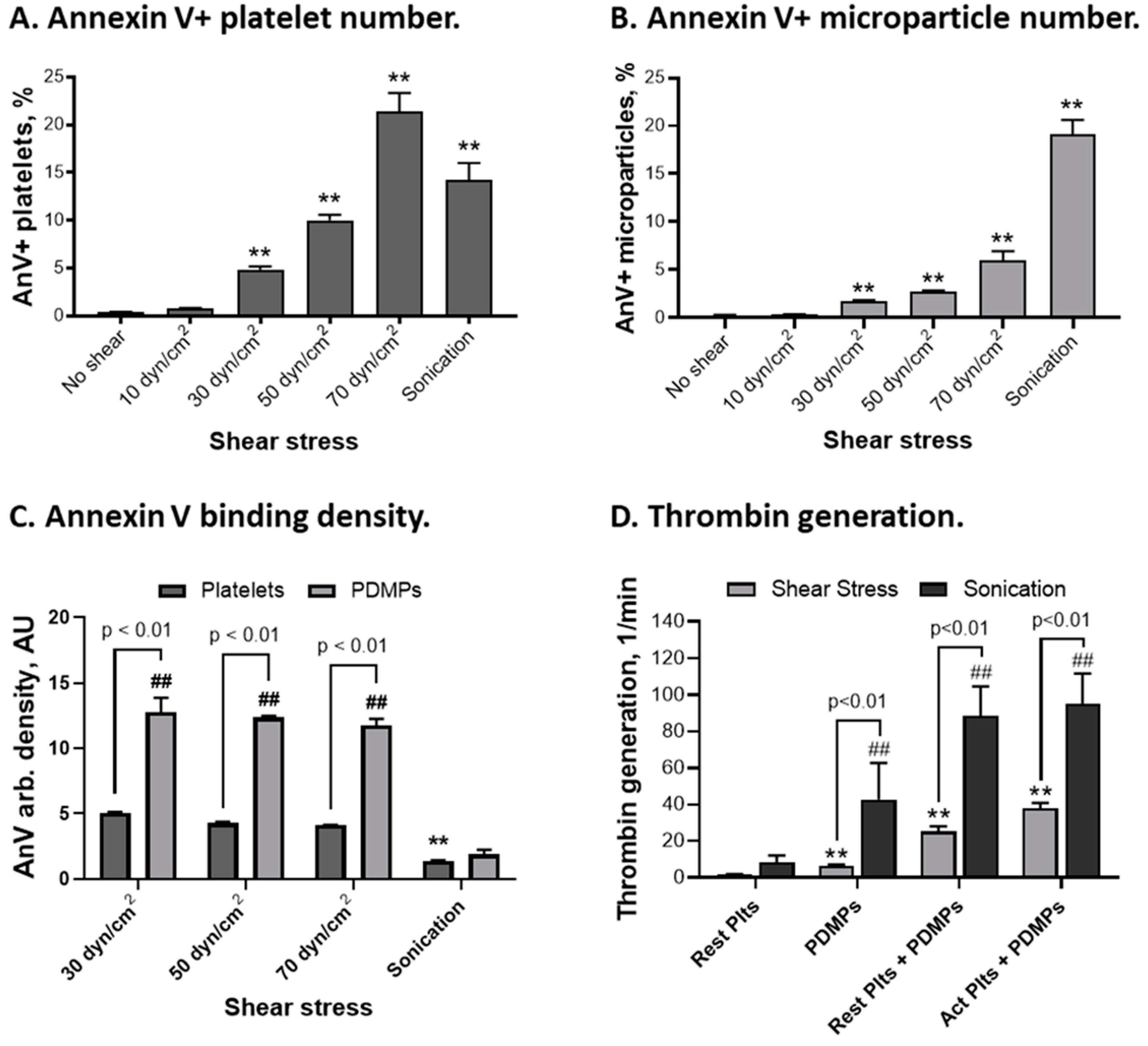
2.6. Procoagulant Activity of Platelets and Platelet-Derived Microparticles
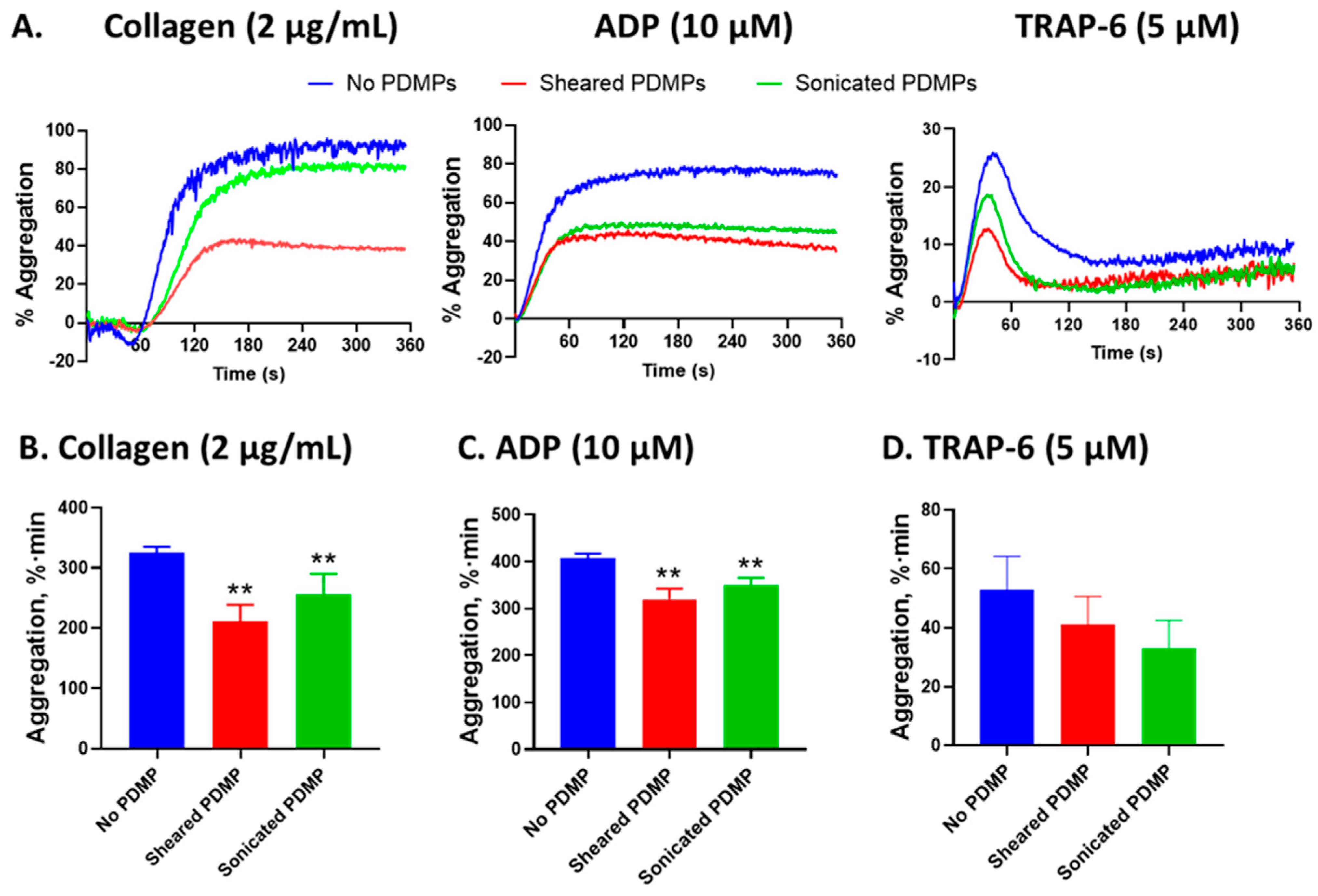
2.7. The Effect of Platelet-Derived Microparticles on Platelet Aggregation
3. Discussion
4. Methods
4.1. Blood Collection and Platelet Isolation
4.2. Platelet Exposure to Shear Stress and Sonication
4.3. Platelet and Microparticle Imaging via Transmission Electron Microscopy
4.4. Surface Expression of Platelet Receptors on Platelets and Microparticles
4.5. Thrombin Generation on Platelets and Microparticles
4.6. Platelet Aggregation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kokkinidis, D.G.; Armstrong, E.J. Current developments in endovascular therapy of peripheral vascular disease. J. Thorac. Dis. 2020, 12, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Rwin, J.P.; Gentile, F.; Jneid, H.; Krieger, R.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar] [PubMed]
- Potapov, E.V.; Ignatenko, S.; Nasseri, B.A.; Loebe, M.; Harke, C.; Bettmann, M.; Doller, A.; Regitz-Zagrosek, V.; Hetzer, R.; Debakey, M.E. Clinical Significance of PlA Polymorphism of Platelet GP IIb/IIIa Receptors During Long-Term VAD Support. Ann. Thorac. Surg. 2004, 77, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.E. Improving the Integration of Palliative Care in Heart Failure—It’s Hard to Hit a Moving Target. JAMA Intern. Med. 2020, 180, 1213–1214. [Google Scholar] [CrossRef]
- Koliopoulou, A.; McKellar, S.H.; Rondina, M.; Selzman, C.H. Bleeding and thrombosis in chronic ventricular assist device therapy. Curr. Opin. Cardiol. 2016, 31, 299–307. [Google Scholar] [CrossRef]
- Tscharre, M.; Michelson, A.D.; Gremmel, T. Novel Antiplatelet Agents in Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 191–200. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, Y.; Kim, S.H.; Kim, S.; Kim, J.Y.; Kim, T.S.; Hwang, Y.; Kim, J.H.; Jang, S.W.; Lee, M.Y.; et al. Single direct oral anticoagulant therapy in stable patients with atrial fibrillation beyond 1 year after coronary stent implantation. Heart 2022, 108, 285–291. [Google Scholar] [CrossRef]
- Guedeney, P.; Mehran, R.; Collet, J.P.; Claessen, B.E.; Ten Berg, J.; Dangas, G.D. Antithrombotic Therapy After Transcatheter Aortic Valve Replacement. Circ. Cardiovasc. Interv. 2019, 12, e007411. [Google Scholar] [CrossRef]
- Morici, N.; Varrenti, M.; Brunelli, D.; Perna, E.; Cipriani, M.; Ammirati, E.; Frigerio, M.; Cattaneo, M.; Oliva, F. Antithrombotic therapy in ventricular assist device (VAD) management: From ancient beliefs to updated evidence. A narrative review. Int. J. Cardiol. Hear. Vasc. 2018, 20, 20–26. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Cleveland, J.C.; Cowger, J.A.; Hall, S.; Salerno, C.T.; Naka, Y.; Horstmanshof, D.; Chuang, J.; Wang, A.; et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022, 328, 1233–1242. [Google Scholar] [CrossRef]
- Slepian, M.J.; Italiano, J.; Bluestein, D.; Sheriff, J.; Roka-Moiia, Y. Evolving perspectives on mechanical circulatory support biocompatibility and interfaces. Ann. Cardiothorac. Surg. 2021, 10, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1729–1737. [Google Scholar] [CrossRef]
- Girdhar, G.; Xenos, M.; Alemu, Y.; Chiu, W.-C.; Lynch, B.E.; Jesty, J.; Einav, S.; Slepian, M.J.; Bluestein, D. Device Thrombogenicity Emulation: A Novel Method for Optimizing Mechanical Circulatory Support Device Thromboresistance. PLoS ONE 2012, 7, e32463. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.-C.; Girdhar, G.; Xenos, M.; Alemu, Y.; Soares, J.S.; Einav, S.; Slepian, M.; Bluestein, D. Thromboresistance comparison of the HeartMate II ventricular assist device with the device thrombogenicity emulation- optimized HeartAssist 5 VAD. J. Biomech. Eng. 2014, 136, 021014. [Google Scholar] [CrossRef]
- Slepian, M.J.; Sheriff, J.; Hutchinson, M.; Tran, P.; Bajaj, N.; Garcia, J.G.N.; Scott Saavedra, S.; Bluestein, D. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J. Biomech. 2017, 50, 20–25. [Google Scholar] [CrossRef]
- Roka-Moiia, Y.; Walk, R.; Palomares, D.E.; Ammann, K.R.; Dimasi, A.; Italiano, J.E.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Platelet Activation via Shear Stress Exposure Induces a Differing Pattern of Biomarkers of Activation versus Biochemical Agonists. Thromb. Haemost. 2020, 120, 776–792. [Google Scholar] [CrossRef]
- Muslem, R.; Caliskan, K.; Leebeek, F.W.G. Acquired coagulopathy in patients with left ventricular assist devices. J. Thromb. Haemost. 2018, 16, 429–440. [Google Scholar] [CrossRef]
- Chen, K.-D.; Li, Y.-S.; Kim, M.; Li, S.; Yuan, S.; Chien, S.; Shyy, J.Y.-J. Mechanotransduction in Response to Shear Stress. J. Biol. Chem. 1999, 274, 18393–18400. [Google Scholar] [CrossRef]
- Hansen, C.E.; Qiu, Y.; McCarty, O.J.T.; Lam, W.A. Platelet Mechanotransduction. Annu. Rev. Biomed. Eng. 2018, 20, 253–275. [Google Scholar] [CrossRef]
- Roka-Moiia, Y.; Ammann, K.R.; Miller-Gutierrez, S.; Sweedo, A.; Palomares, D.; Italiano, J.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Shear-mediated platelet activation in the free flow II: Evolving mechanobiological mechanisms reveal an identifiable signature of activation and a bi-directional platelet dyscrasia with thrombotic and bleeding features. J. Biomech. 2021, 123, 110415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Kareem, K.; Tran, D.; Conway, R.G.; Arias, K.; Griffith, B.P.; Wu, Z.J. Device-induced platelet dysfunction in mechanically assisted circulation increases the risks of thrombosis and bleeding. Artif. Organs 2019, 43, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Roka-Moiia, Y.; Miller-Gutierrez, S.; Palomares, D.E.; Italiano, J.E.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Platelet Dysfunction During Mechanical Circulatory Support: Elevated Shear Stress Promotes Downregulation of αIIbβ3 and GPIb via Microparticle Shedding Decreasing Platelet Aggregability. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Lozano, M.L.; Navarro-Núñez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.J.; Swieringa, F.; Misztal, T.; Mastenbroek, T.G.; Feijge, M.A.H.; Bock, P.E.; Donners, M.M.P.C.; Collins, P.W.; Li, R.; van der Meijden, P.E.J.; et al. Platelet heterogeneity in activation-induced glycoprotein shedding: Functional effects. Blood Adv. 2018, 2, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Gardiner, E.E. Basic mechanisms of platelet receptor shedding. Platelets 2017, 28, 319–324. [Google Scholar] [CrossRef]
- Naganuma, Y.; Satoh, K.; Yi, Q.; Asazuma, N.; Yatomi, Y.; Ozaki, Y. Cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1) in platelets exposed to high shear stress. J. Thromb. Haemost. 2004, 2, 1998–2008. [Google Scholar] [CrossRef]
- Hosseini, E.; Mohtashami, M.; Ghasemzadeh, M. Down-regulation of platelet adhesion receptors is a controlling mechanism of thrombosis, while also affecting post-transfusion efficacy of stored platelets. Thromb. J. 2019, 17, 20. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Mairuhu, A.; Italiano, J. Platelet- and Megakaryocyte-Derived Microparticles. Semin. Thromb. Hemost. 2010, 36, 881–887. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liu, L.; Zaske, A.M.; Zhou, Z.; Fu, Y.; Yang, X.; Conyers, J.L.; Li, M.; Dong, J.F.; et al. Contact-and agonist-regulated microvesiculation of human platelets. Thromb. Haemost. 2013, 110, 331–339. [Google Scholar]
- Gyulkhandanyan, A.V.; Mutlu, A.; Allen, D.J.; Freedman, J.; Leytin, V. BH3-mimetic ABT-737 induces strong mitochondrial membrane depolarization in platelets but only weakly stimulates apoptotic morphological changes, platelet shrinkage and microparticle formation. Thromb. Res. 2014, 133, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Jesel, L.; Freyssinet, J.M.; Toti, F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Aatonen, M.T.; Öhman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Leytin, V.; Allen, D.J.; Mykhaylov, S.; Mis, L.; Lyubimov, E.V.; Garvey, B.; Freedman, J. Pathologic high shear stress induces apoptosis events in human platelets. Biochem. Biophys. Res. Commun. 2004, 320, 303–310. [Google Scholar] [CrossRef]
- Diehl, P.; Aleker, M.; Helbing, T.; Sossong, V.; Beyersdorf, F.; Olschewski, M.; Bode, C.; Moser, M. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact. Cardiovasc. Thorac. Surg. 2010, 11, 133–137. [Google Scholar] [CrossRef]
- Arias, K.; Sun, W.; Wang, S.; Sorensen, E.N.; Feller, E.; Kaczorowski, D.; Griffith, B.; Wu, Z.J. Acquired platelet defects are responsible for nonsurgical bleeding in left ventricular assist device recipients. Artif. Organs 2022, 46, 2244–2256. [Google Scholar] [CrossRef]
- Koganti, S.; Eleftheriou, D.; Gurung, R.; Hong, Y.; Brogan, P.; Rakhit, R.D. Persistent circulating platelet and endothelial derived microparticle signature may explain on-going pro-thrombogenicity after acute coronary syndrome. Thromb. Res. 2021, 206, 60–65. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Ding, J.; Gao, J.; Griffith, B.P.; Wu, Z.J. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: Glycoprotein Ibα and glycoprotein VI. Thromb. Res. 2015, 135, 692–698. [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Wu, Z.J. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif. Organs 2016, 40, 659–668. [Google Scholar] [CrossRef]
- Neumüller, J.; Ellinger, A.; Wagner, T. Transmission Electron Microscopy of Platelets FROM Apheresis and Buffy-Coat-Derived Platelet Concentrates. In The Transmission Electron Microscope—Theory and Applications; InTech: Tokyo, Japan, 2015. [Google Scholar]
- Roka-Moiia, Y.; Walawalkar, V.; Liu, Y.; Italiano, J.E.; Slepian, M.J.; Taylor, R.E. DNA Origami-Platelet Adducts: Nanoconstruct Binding without Platelet Activation. Bioconjugate Chem. 2022, 33, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, A.A.; Nevzorova, T.A.; Mordakhanova, E.R.; Andrianova, I.A.; Litvinov, R.I. Structural characterization of platelets and platelet microvesicles. Cell Tissue Biol. 2016, 10, 217–226. [Google Scholar] [CrossRef]
- Gyulkhandanyan, A.V.; Mutlu, A.; Freedman, J.; Leytin, V. Selective triggering of platelet apoptosis, platelet activation or both. Br. J. Haematol. 2013, 161, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wei, H. Mecanisms of Microparticle Release from Human Platelets; University of Cambridge: Cambridge, UK, 2019. [Google Scholar]
- Reininger, A.J.; Heijnen, H.F.G.G.; Schumann, H.; Specht, H.M.; Schramm, W.; Ruggeri, Z.M. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 2006, 107, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, S.; Chen, Z.; Zhang, J.; Li, T.; Arias, K.; Griffith, B.P.; Wu, Z.J. Impact of high mechanical shear stress and oxygenator membrane surface on blood damage relevant to thrombosis and bleeding in a pediatric ECMO circuit. Artif. Organs 2020, 44, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sheriff, J.; Soares, J.S.; Gao, C.; Pothapragada, S.; Zhang, N.; Deng, Y.; Bluestein, D. Multiscale Modeling of Flow Induced Thrombogenicity Using Dissipative Particle Dynamics and Coarse Grained Molecular Dynamics. Am. Soc. Mech. Eng. 2014, 55614, V01BT36A002. [Google Scholar]
- Pothapragada, S.; Zhang, P.; Sheriff, J.; Livelli, M.; Slepian, M.J.; Deng, Y.; Bluestein, D. A phenomenological particle-based platelet model for simulating filopodia formation during early activation. Int. J. Numer. Methods Biomed. Eng. 2015, 31, e02702. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Zhang, W.; Lee-Sundlov, M.M.; Casari, C.; Berndt, M.C.; Lanza, F.; Bergmeier, W.; Hoffmeister, K.M.; Frank Zhang, X.; et al. Desialylation of O-glycans on glycoprotein Ibα drives receptor signaling and platelet clearance. Haematologica 2020, 105, 220. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Slepian, M.J.; Deng, Y.; Bluestein, D. A multiscale biomechanical model of platelets: Correlating with in-vitro results. J. Biomech. 2017, 50, 26–33. [Google Scholar] [CrossRef]
- Roka-Moiia, Y.; Li, M.; Ivich, A.; Muslmani, S.; Kern, K.B.; Slepian, M.J. Impella 5.5 Versus Centrimag: A Head-to-Head Comparison of Device Hemocompatibility. ASAIO J. 2020, 66, 1142–1151. [Google Scholar] [CrossRef]
- Michelson, A.D.; Benoit, S.E.; Furman, M.I.; Barnard, M.R.; Nurden, P.; Nurden, A.T. The platelet surface expression of glycoprotein V is regulated by two independent mechanisms: Proteolysis and a reversible cytoskeletal-mediated redistribution to the surface-connected canalicular system. Blood 1996, 87, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Massé, J.M.; Cramer, E.M. Alpha-Granule Membrane Mirrors the Platelet Plasma Membrane and Contains the Glycoproteins Ib, IX, and V. Blood 1996, 87, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, T.; Massé, J.M.; Rendu, F.; Guichard, J.; Cramer, E.M. Platelet and Megakaryocyte Dense Granules Contain Glycoproteins Ib and IIb-IIIa. Blood 1997, 89, 4047–4057. [Google Scholar] [CrossRef]
- Keuren, J.F.W.; Wielders, S.J.H.; Ulrichts, H.; Hackeng, T.; Heemskerk, J.W.M.; Deckmyn, H.; Bevers, E.M.; Lindhout, T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Asazuma, N.; Satoh, K.; Yatomi, Y.; Ozaki, Y. Collagen-induced generation of platelet-derived microparticles in whole blood is dependent on ADP released from red blood cells and calcium ions. Platelets 2009, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.X.; Harbour, S.N.; Wee, J.L.; Lau, L.M.; Andrews, R.K.; Jackson, D.E. Proteolytic cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) is regulated by a calmodulin-binding motif. FEBS Lett. 2004, 568, 70–78. [Google Scholar] [CrossRef]
- Vandendries, E.R.; Furie, B.C.; Furie, B. Role of P-selectin and PSGL-1 in coagulation and thrombosis. Thromb. Haemost. 2004, 92, 459–466. [Google Scholar] [CrossRef]
- Frenette, P.S.; Denis, C.V.; Weiss, L.; Jurk, K.; Subbarao, S.; Kehrel, B.; Hartwig, J.H.; Vestweber, D.; Wagner, D.D. P-Selectin glycoprotein ligand 1 (PSGL-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J. Exp. Med. 2000, 191, 1413–1422. [Google Scholar] [CrossRef]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived From Exocytosis of Multivesicular Bodies and α-Granules. Blood J. 2015, 94, 3791–3799. [Google Scholar] [CrossRef]
- Molino, M.; Bainton, D.F.; Hoxie, J.A.; Coughlin, S.R.; Brass, L.F. Thrombin Receptors on Human Platelets Initial localization and subsequent redistribution during platelet activation. J. Biol. Chem. 1997, 272, 6011–6017. [Google Scholar] [CrossRef]
- Ramström, S.; Öberg, K.V.; Åkerström, F.; Enström, C.; Lindahl, T.L. Platelet PAR1 receptor density-Correlation to platelet activation response and changes in exposure after platelet activation. Thromb. Res. 2008, 121, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Szabo, K.; Haft, C.R.; Trejo, J.A. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol. Biol. Cell 2002, 13, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padró, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell. Mol. Life Sci. 2022, 79, 190. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, J.; Bluestein, D.; Girdhar, G.; Jesty, J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann. Biomed. Eng. 2010, 38, 1442–1450. [Google Scholar] [CrossRef]
- Kim, H.-H.; Shin, J.-H.; Kim, J.-H.; Youn, Y.-N. Outcome of Extracorporeal Ventricular Assist Device for Cardiogenic Shock as a Bridge to Transplantation. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Opfermann, P.; Felli, A.; Schlömmer, C.; Dworschak, M.; Bevilacqua, M.; Mouhieddine, M.; Zimpfer, D.; Zuckermann, A.; Steinlechner, B. A Prospective Observational Study on Multiplate®-, ROTEM®- and Thrombin Generation Examinations Before and Early After Implantation of a Left Ventricular Assist Device (LVAD). Front. Med. 2022, 9, 760816. [Google Scholar] [CrossRef]
- Shah, M.D.; Bergeron, A.L.; Dong, J.F.; López, J.A. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets 2008, 19, 365–372. [Google Scholar] [CrossRef]
- Nobili, M.; Sheriff, J.; Morbiducci, U.; Redaelli, A.; Bluestein, D. Platelet activation due to hemodynamic shear stresses: Damage accumulation model and comparison to in vitro measurements. ASAIO J. (American Soc. Artif. Intern. Organs) 2008, 54, 64–72. [Google Scholar] [CrossRef]
- Sheriff, J.; Tran, P.L.; Hutchinson, M.; DeCook, T.; Slepian, M.J.; Bluestein, D.; Jesty, J. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artif. Organs 2016, 40, 586–595. [Google Scholar] [CrossRef]
- Chandler, W.L. Measurement of microvesicle levels in human blood using flow cytometry. Cytom. Part B Clin. Cytom. 2016, 90, 326–336. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Sunita, R.P.H.; Battinelli, E.; Klement, G.L.; Martha, S.V.; Italiano, J.E. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Rothe, G.; Ruf, A.; Barlage, S.; Tschöpe, D.; Clemetson, K.J.; Goodall, A.H.; Michelson, A.D.; Nurden, A.T.; Shankey, T.V. European Working Group on Clinical Cell Analysis: Consensus protocol for the flow cytometric characterisation of platelet function. Thromb. Haemost. 1998, 79, 885–896. [Google Scholar] [CrossRef]
- Araki, R.; Nishimura, R.; Kuroda, R.; Fujiki, T.; Mase, S.; Noguchi, K.; Ikawa, Y.; Maeba, H.; Yachie, A. A characteristic flow cytometric pattern with broad forward scatter and narrowed side scatter helps diagnose immune thrombocytopenia (ITP). Int. J. Hematol. 2018, 108, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Jesty, J.; Bluestein, D. Acetylated Prothrombin as a Substrate in the Measurement of the Procoagulant Activity of Platelets: Elimination of the Feedback Activation of Platelets by Thrombin. Anal. Biochem. 1999, 272, 64–70. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roka-Moiia, Y.; Ammann, K.R.; Miller-Gutierrez, S.; Sheriff, J.; Bluestein, D.; Italiano, J.E.; Flaumenhaft, R.C.; Slepian, M.J. Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function. Int. J. Mol. Sci. 2023, 24, 7386. https://doi.org/10.3390/ijms24087386
Roka-Moiia Y, Ammann KR, Miller-Gutierrez S, Sheriff J, Bluestein D, Italiano JE, Flaumenhaft RC, Slepian MJ. Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function. International Journal of Molecular Sciences. 2023; 24(8):7386. https://doi.org/10.3390/ijms24087386
Chicago/Turabian StyleRoka-Moiia, Yana, Kaitlyn R. Ammann, Samuel Miller-Gutierrez, Jawaad Sheriff, Danny Bluestein, Joseph E. Italiano, Robert C. Flaumenhaft, and Marvin J. Slepian. 2023. "Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function" International Journal of Molecular Sciences 24, no. 8: 7386. https://doi.org/10.3390/ijms24087386
APA StyleRoka-Moiia, Y., Ammann, K. R., Miller-Gutierrez, S., Sheriff, J., Bluestein, D., Italiano, J. E., Flaumenhaft, R. C., & Slepian, M. J. (2023). Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function. International Journal of Molecular Sciences, 24(8), 7386. https://doi.org/10.3390/ijms24087386







