NpPP2-B10, an F-Box-Nictaba Gene, Promotes Plant Growth and Resistance to Black Shank Disease Incited by Phytophthora nicotianae in Nicotiana tabacum
Abstract
1. Introduction
2. Results
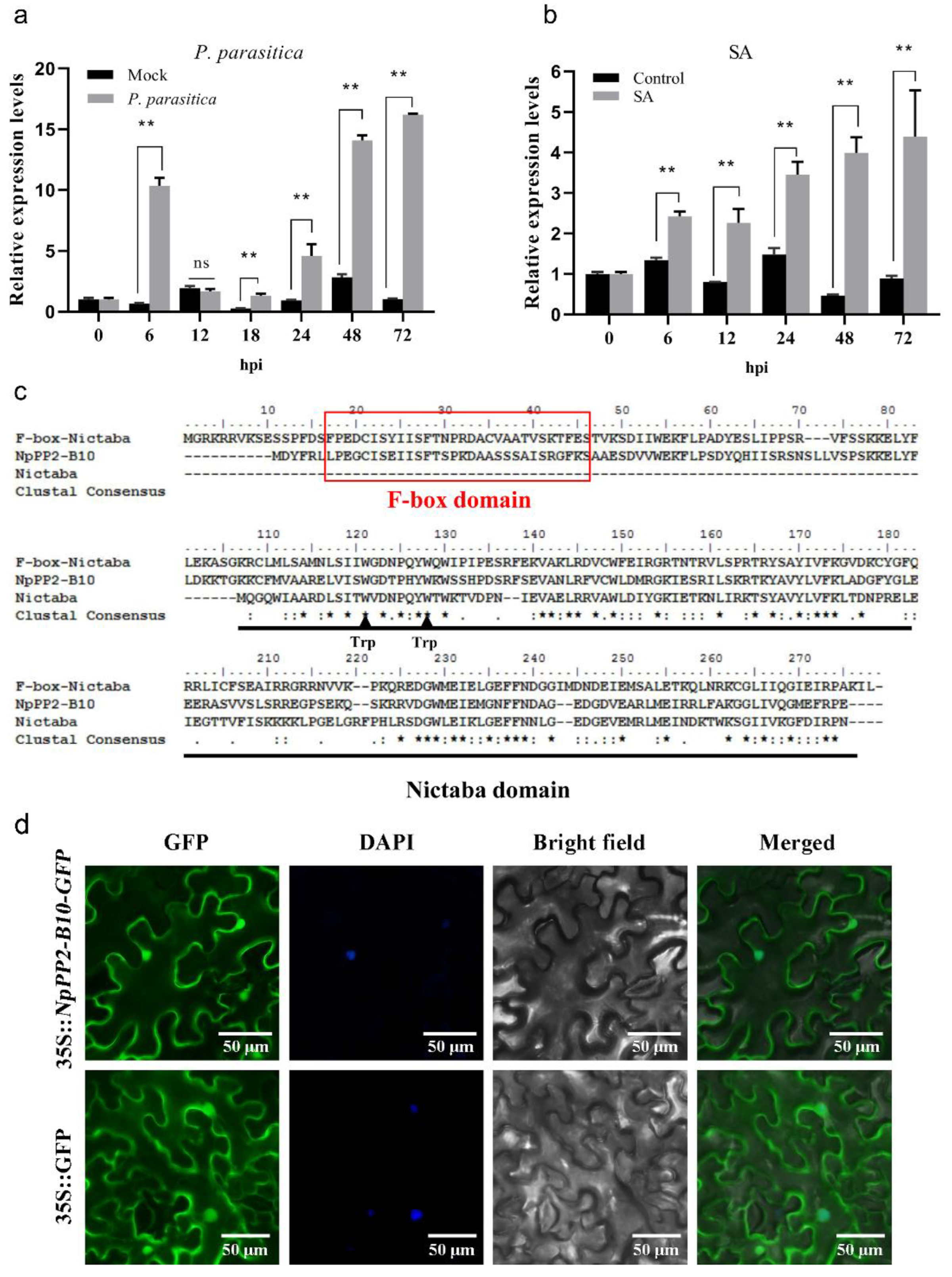
2.1. Characterization of NpPP2-B10
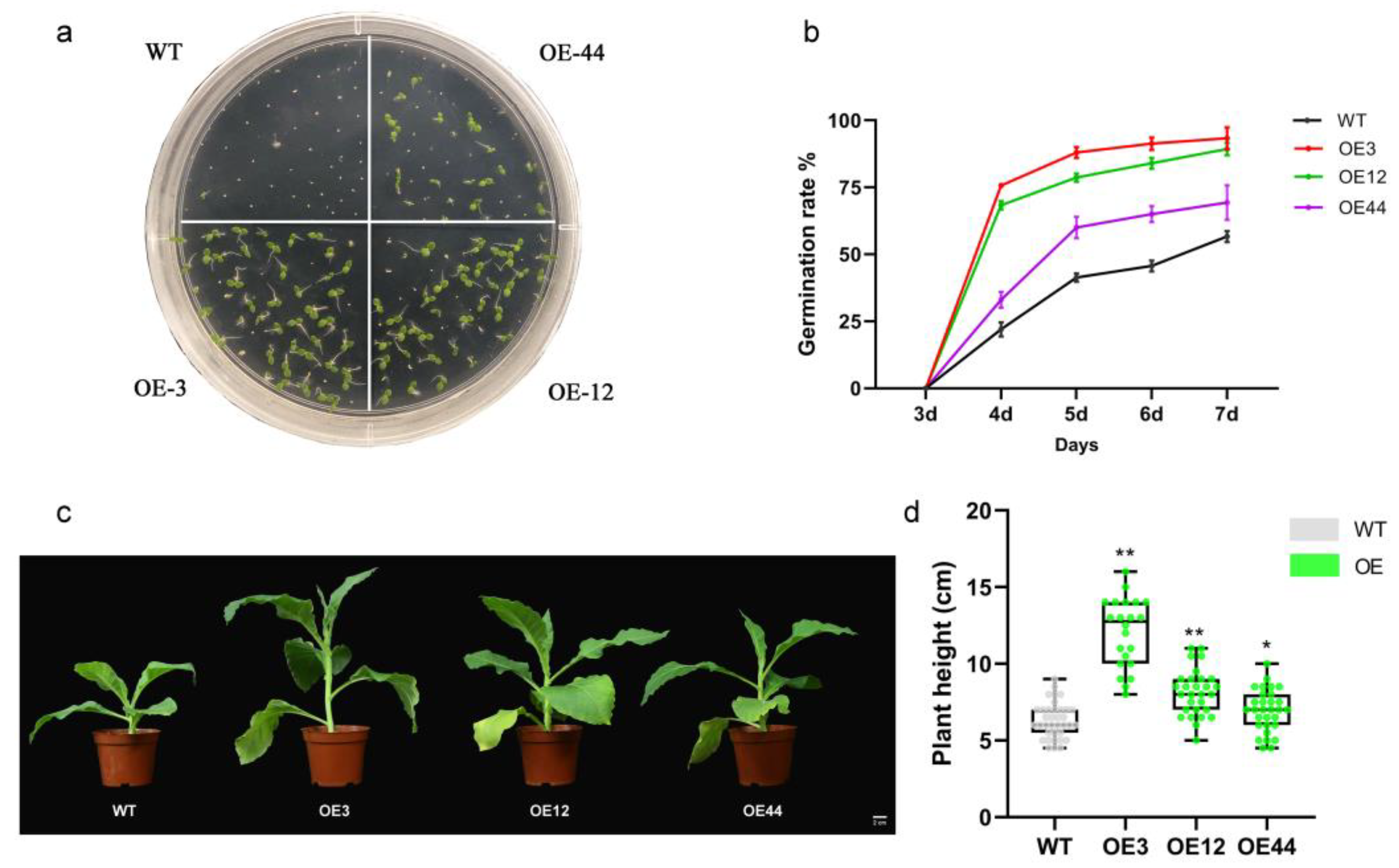
2.2. Overexpression of NpPP2-B10 Promoted Seed Germination and Plant Growth in Tobacco
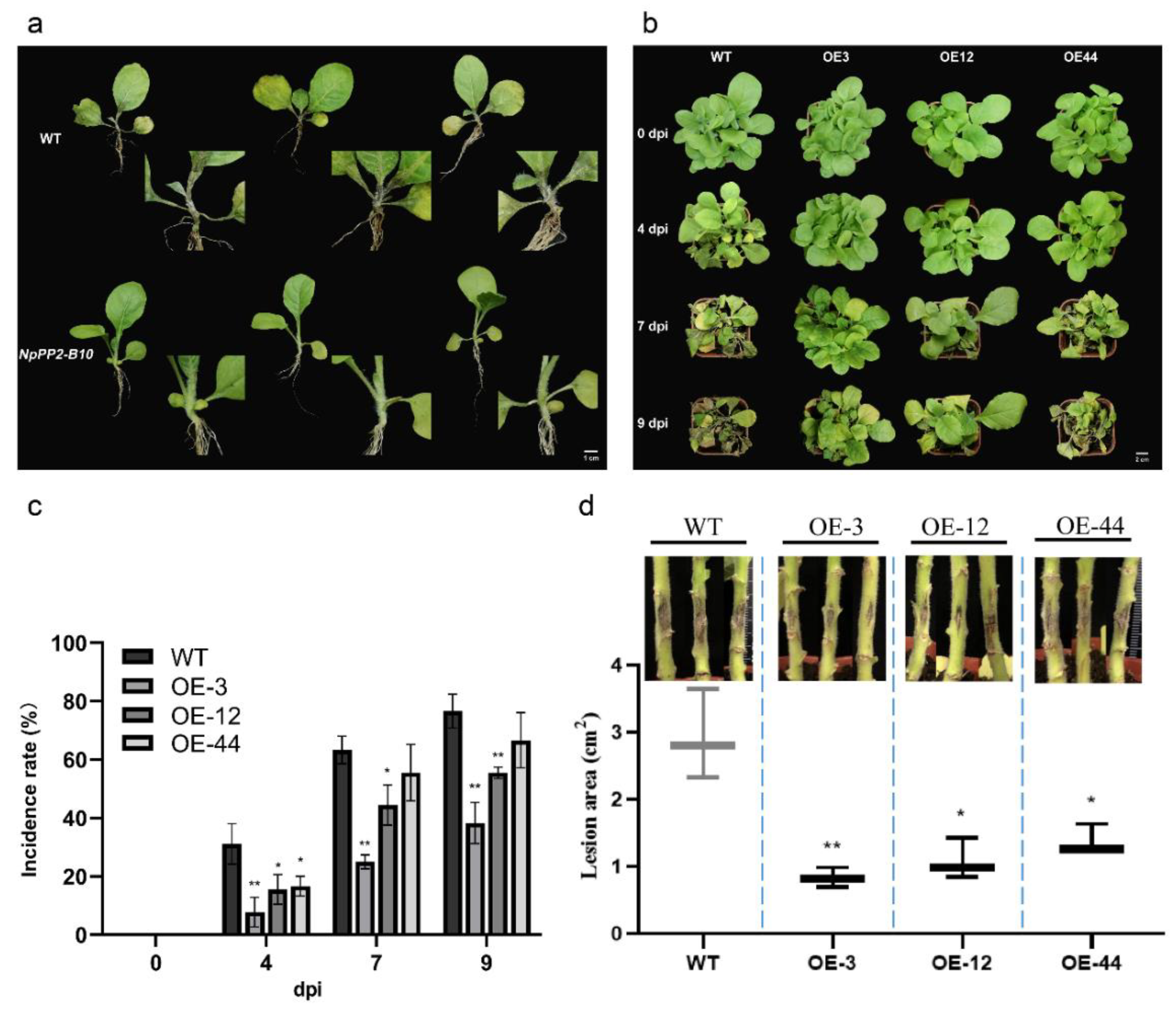
2.3. Overexpression of NpPP2-B10 Enhanced Tobacco Resistance to Black Shank Disease
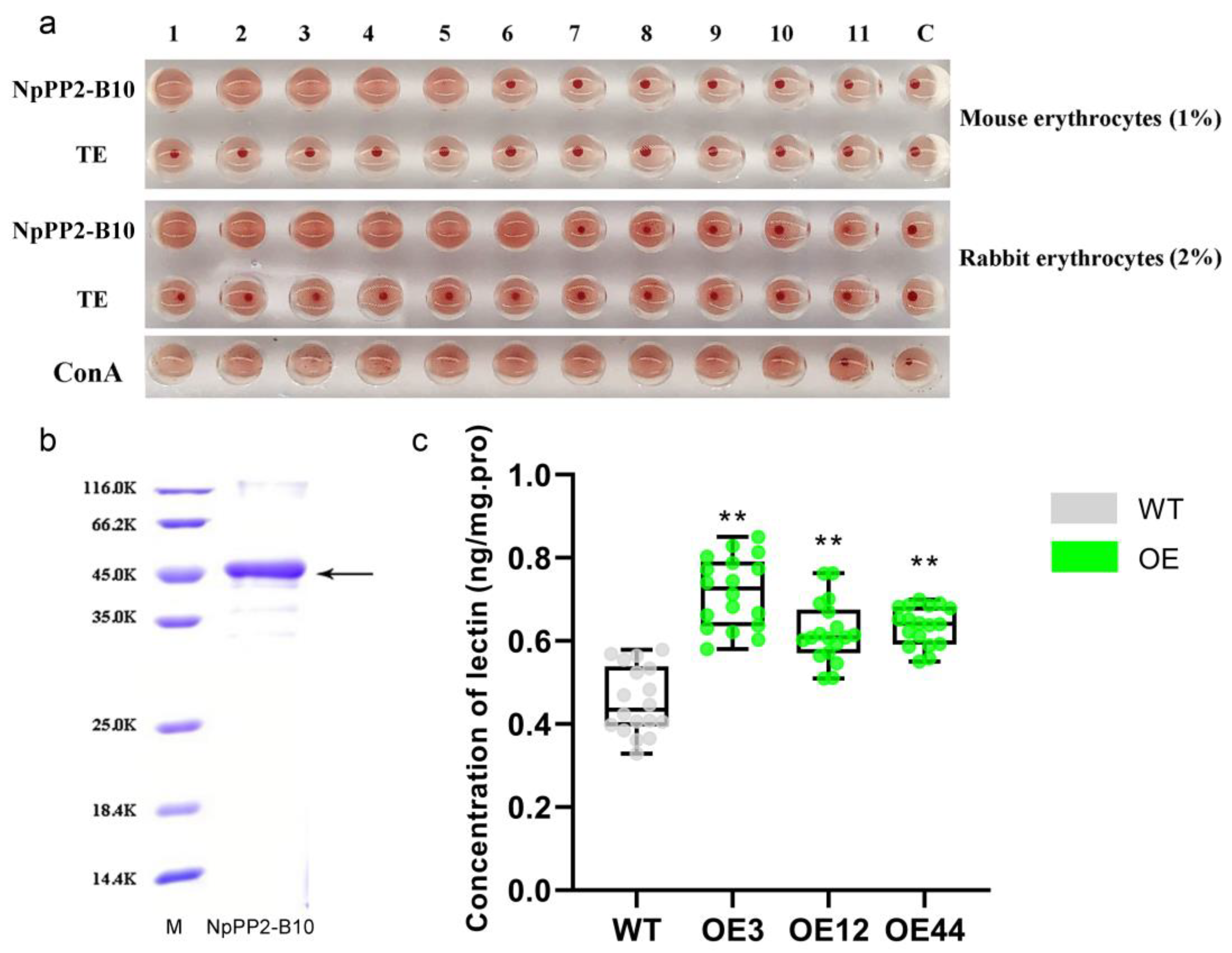
2.4. Validation of Lectin Activity of the NpPP2-B10 Protein
2.5. Interaction between NpPP2-B10 and NpSKP1-1A
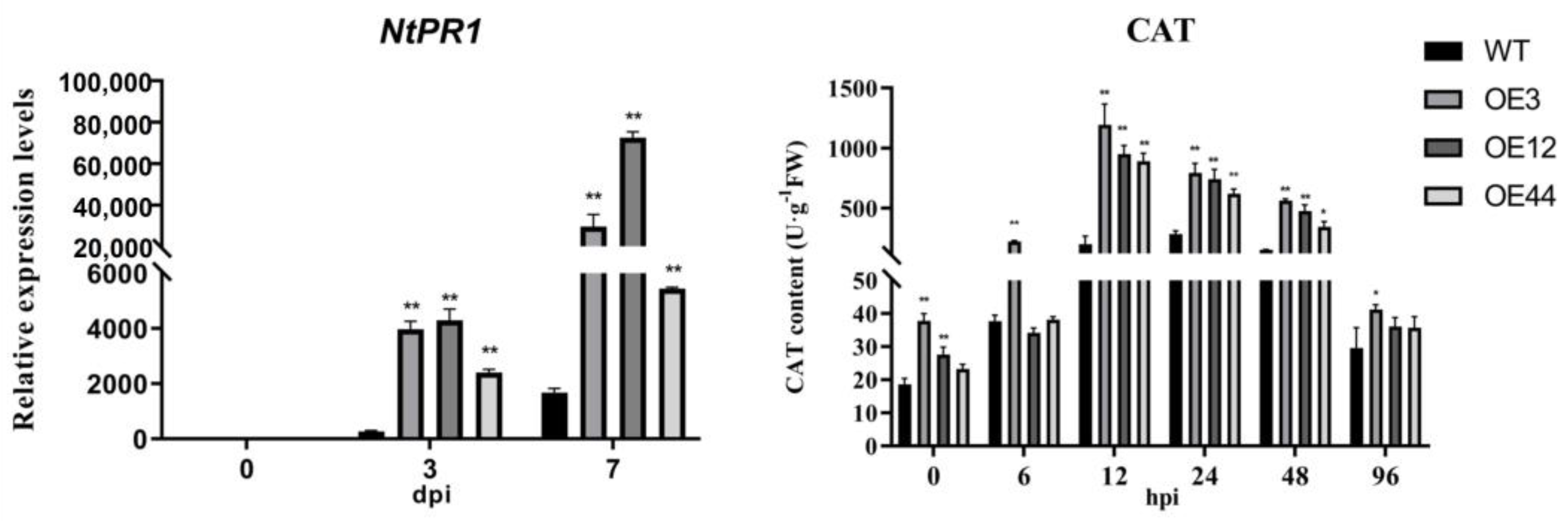
2.6. NpPP2-B10 Enhanced the Expression of Disease Resistance-Related Genes and the Activity of Disease Resistance-Related Enzymes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Pathogen Infection and Hormone Treatment
4.3. RNA Extraction, Reverse Transcription, and qPCR
4.4. Overexpression of NpPP2-B10 in Cultivated Tobacco
4.5. E. coli Expression and Purification of the Recombinant Protein
4.6. Detection of Lectin Activity and Content
4.7. Yeast Two-Hybrid Assay and BiFC
4.8. Detection of Disease Resistance-Related Enzyme Activities
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bass, W.T.; Cornelius, P.L.; Nesmith, W.C.; Li, B.C. Resistance to tobacco black shank (Phytophthora parasitica var. nicotianae) in Nicotiana species. In Vitro Cellular & Developmental Biology-Animal; Springer: New York, NY, USA, 2005; Volume 41, p. 53A. [Google Scholar]
- Xiao, B.; Drake, K.; Vontimitta, V.; Tong, Z.; Zhang, X.; Li, M.; Leng, X.; Li, Y.; Lewis, R.S. Location of genomic regions contributing to Phytophthora nicotianae resistance in tobacco cultivar Florida 301. Crop Sci. 2013, 53, 473–481. [Google Scholar] [CrossRef]
- Vontimitta, V.; Lewis, R.S. Mapping of quantitative trait loci affecting resistance to Phytophthora nicotianae in tobacco (Nicotiana tabacum L.) line Beinhart-1000. Mol. Breed. 2012, 29, 89–98. [Google Scholar] [CrossRef]
- Litton, C.C.; Collins, G.B.; Legg, P.D. Reaction of Nicotiana tabacum and other Nicotiana species to race 0 and 1 of Phytophthora parasitica var. nicotianae. Tob. Sci. 1970, 14, 144–146. [Google Scholar]
- Li, B.C.; Bass, W.T.; Cornelius, P.L. Resistance to tobacco black shank in Nicotiana species. Crop Sci. 2006, 46, 554–560. [Google Scholar] [CrossRef]
- Drake, K.E.; Moore, J.M.; Bertrand, P.; Fortnum, B.; Peterson, P.; Lewis, R.S. Black shank resistance and agronomic performance of flue-cured tobacco lines and hybrids carrying the introgressed Nicotiana rustica region, Wz. Crop Sci. 2015, 55, 79–86. [Google Scholar] [CrossRef]
- Goins, B.; Apple, J.L. Inheritance and phenotypic expression of a dominant factor for black shank resistance from Nicotiana plumbaginifolia in a N. tabacum milieu. Tob. Sci. 1970, 170, 7–21. [Google Scholar]
- Johnson, E.S.; Wolff, M.F.; Wernsman, E.A.; Atchley, W.R.; Shew, H.D. Origin of the black shank resistance gene, Ph, in tobacco cultivar Coker 371-gold. Plant Dis. 2002, 86, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Wang, J.; Yang, Y.; Shang, W.; Guo, Q.; Liang, G. Resistance of Nicotiana tabacum to Phytophthora parasitica var. nicotianae race 0 is enhanced by the addition of N. plumbaginifolia chromosome 9 with a slight effect on host genomic expression. Crop Sci. 2019, 59, 2667–2678. [Google Scholar] [CrossRef]
- Hernández, I.; Chacón, O.; Rodriguez, R.; Portieles, R.; López, Y.; Pujol, M.; Borrás-Hidalgo, O. Black shank resistant tobacco by silencing of glutathione S-transferase. Biochem. Biophys. Res. Commun. 2009, 387, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Portieles, R.; Canales, E.; Chacon, O.; Silva, Y.; Hernández, I.; López, Y.; Rodríguez, M.; Terauchi, R.; Matsumura, H.; Borroto, C.; et al. Expression of a Nicotiana tabacum pathogen-induced gene is involved in the susceptibility to black shank. Funct. Plant Biol. 2016, 43, 534–541. [Google Scholar] [CrossRef]
- Shang, Z. Recent research advance on pathogen and disease occurrence of tobacco black shank disease and its integrated control. J. Agric. Sci. Technol. 2007, 9, 73–76. [Google Scholar]
- Chen, R. Tobacco Pest Control; Shandong Science and Technology Press: Jinan, China, 1989; pp. 1–10. [Google Scholar]
- Wang, Z. Tobacco Black Shank Disease (Chinese Agricultural Encyclopedia, Volume of Phytopathology); Agricultural Press: Beijing, China, 1996; pp. 534–535. [Google Scholar]
- Lannoo, N.; Van Damme, E.J.M. Nucleocytoplasmic plant lectins. Biochim. Biophys. Acta 2010, 1800, 190–201. [Google Scholar] [CrossRef]
- Lannoo, N.; Van Damme, E.J.M. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 397, 1–16. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma counteracts the challenge of Phytophthora nicotianae infections on tomato by modulating plant defense mechanisms and the expression of Crinkler, necrosis-inducing Phytophthora protein 1, and cellulose-binding elicitor lectin pathogenic effectors. Front. Plant Sci. 2020, 11, 583539. [Google Scholar]
- Van Damme, E.J.M. 35 years in plant lectin research: A journey from basic science to applications in agriculture and medicine. Glycoconj. J. 2022, 39, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tang, Z. Plant Physiology and Molecular Biology; Science Press: Beijing, China, 1998. [Google Scholar]
- Chen, Y.; Peumans, W.J.; Hause, B.; Bras, J.; Kumar, M.; Proost, P.; Barre, A.; Rougé, P.; Van Damme, E.J.M. Jasmonate methyl ester induces the synthesis of a cytoplasmic/nuclear chitooligosaccharide-binding lectin in tobacco leaves. FASEB J. 2002, 16, 905–907. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Lannoo, N.; Peumans, W.J. Plant lectins. Adv. Bot. Res. 2008, 48, 107–209. [Google Scholar]
- Eggermont, L.; Stefanowicz, K.; Van Damme, E.J.M. Nictaba homologs from Arabidopsis thaliana are involved in plant stress responses. Front. Plant Sci. 2018, 8, 2218. [Google Scholar] [CrossRef]
- Read, S.M.; Northcote, D.H. Subunit structure and interactions ofthe phloem proteins of Cucurbita maxima (pumpkin). Eur. J. Biochem. 1983, 134, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Boltz, K.A.; Lee, S.Y. Molecular chaperone function of Arabidopsis thaliana phloem protein 2-A1, encodes a protein similar to phloem lectin. Biochem. Biophys. Res. Commun. 2014, 443, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Bobbili, K.B.; Pohlentz, G.; Narahari, A.; Sharma, K.; Surolia, A.; Mormann, M.; Swamy, M.J. Coccinia indica agglutinin, a 17kDa PP2 like phloem lectin: Affinity purification, primary structure and formation of self-assembled filaments. Int. J. Biol. Macromol. 2018, 108, 1227–1236. [Google Scholar] [CrossRef]
- Delporte, A.; Van Holle, S.; Lannoo, N.; Van Damme, E.J.M. The tobacco lectin, prototype of the family of Nictaba-Related Proteins. Curr. Protein Pept. Sci. 2015, 16, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, N.; Peumans, W.J.; Van Damme, E.J.M. Do F-box proteins with a C-terminal domain homologous with the tobacco lectin play a role in protein degradation in plants? Biochem. Soc. Trans. 2008, 36, 843–847. [Google Scholar] [CrossRef]
- Stefanowicz, K.; Lannoo, N.; Zhao, Y.; Eggermont, L.; Van Hove, J.; Atalah, B.A.; Van Damme, E.J.M. Glycan-binding F-box protein from Arabidopsis thaliana protects plants from Pseudomonas syringae infection. BMC Plant Biol. 2016, 16, 213. [Google Scholar] [CrossRef] [PubMed]
- Plener, L.; Manfredi, P.; Valls, M.; Genin, S. PrhG, a transcriptional regulator responding to growth conditions, is involved in the control of the type III secretion system regulon in Ralstonia solanacearum. J. Bacteriol. 2010, 192, 1011–1019. [Google Scholar] [CrossRef]
- Schouppe, D.; Rougé, P.; Lasanajak, Y.; Barre, A.; Smith, D.F.; Proost, P.; Van Damme, E.J.M. Mutational analysis of the carbohydrate binding activity of the tobacco lectin. Glycoconj. J. 2010, 27, 613–623. [Google Scholar] [CrossRef]
- Sano, K.; Ogawa, H. Hemagglutination (inhibition) assay. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1200, pp. 47–52. [Google Scholar]
- Li, X.; Sun, Y.; Liu, N.; Wang, P.; Pei, Y.; Liu, D.; Ma, X.; Ge, X.; Li, F.; Hou, Y. Enhanced resistance to Verticillium dahliae mediated by an F-box protein GhACIF1 from Gossypium hirsutum. Plant Sci. 2019, 284, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Tang, Y.; Ding, W. NtPR1a regulates resistance to Ralstonia solanacearum in Nicotiana tabacum via activating the defense-related genes. Biochem. Biophys. Res. Commun. 2019, 508, 940–945. [Google Scholar] [CrossRef]
- Xu, Z. General Plant Pathology; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Chen, J.; Wang, G.; Cheng, S. Progress about catalase function in plant stress reactions. Acta Bot. Boreali-Occident. Sin. 2008, 28, 188–193. [Google Scholar]
- Chen, R.; Guo, W.; Yin, Y.; Gong, Z. A novel F-box protein CaF-box is involved in responses to plant hormones and abiotic stress in pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2014, 15, 2413–2430. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, C.; Fan, R.; Yu, X.; Wang, X.; Zhao, W.; Wei, X.; Kang, Z.; Liu, D. TaPP2-A13 gene shows induced expression pattern in wheat responses to stresses and interacts with adaptor protein SKP1 from SCF complex. Acta Agron. Sin. 2021, 47, 224–236. [Google Scholar] [CrossRef]
- Delporte, A.; De Vos, W.H.; Van Damme, E.J.M. In vivo interaction between the tobacco lectin and the core histone proteins. J. Plant Physiol. 2014, 171, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wu, B.; Li, H.; Huang, J.; Zheng, C. Genome-wide identification and characterisation of F-box family in maize. Mol. Genet. Genom. 2013, 288, 559–577. [Google Scholar] [CrossRef]
- Li, H.; Wei, C.; Meng, Y.; Fan, R.; Zhao, W.; Wang, X.; Yu, X.; Laroche, A.; Kang, Z.; Liu, D. Identification and expression analysis of some wheat F-box subfamilies during plant development and infection by Puccinia triticina. Plant Physiol. Biochem. 2020, 155, 535–548. [Google Scholar] [CrossRef]
- Achor, D.; Welker, S.; Ben-Mahmoud, S.; Wang, C.; Folimonova, S.Y.; Dutt, M.; Gowda, S.; Levy, A. Dynamics of Candidatus liberibacter asiaticus movement and sieve-pore plugging in citrus sink cells. Plant Physiol. 2020, 182, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of plant lectin research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.; Ameye, M.; Audenaert, K.; Van Damme, E.J.M. Overexpression of F-Box Nictaba promotes defense and anthocyanin accumulation in Arabidopsis thaliana after Pseudomonas syringae infection. Front. Plant Sci. 2021, 12, 692606. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; El-Shetehy, M.; Gamir, J.; Austerlitz, T.; Dahlin, P.; Wieczorek, K.; Künzler, M.; Mauch, F. Expression of a fungal lectin in Arabidopsis enhances plant growth and resistance towards microbial pathogens and a plant-parasitic nematode. Front. Plant Sci. 2021, 12, 657451. [Google Scholar] [CrossRef] [PubMed]
- Grappin, P.; Bouinot, D.; Sotta, B.; Miginiac, E.; Jullien, M. Control of seed dormancy in Nicotiana plumbaginifolia: Post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 2000, 210, 279–285. [Google Scholar] [CrossRef]
- Xie, Z.; Wen, G.; Yang, Y.; Wang, H.; Wang, J.; Lei, C.; Guo, Q.; Dang, J.; Liang, G. The FBA motif-containing protein NpFBA1 causes leaf curling and reduces resistance to black shank disease in tobacco. Agronomy 2021, 11, 2478. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Dang, J.; Xie, Z.; Wang, J.; Jiang, P.; Guo, Q.; Liang, G. Molecular karyotypes of loquat (Eriobotrya japonica) aneuploids can be detected by using SSR markers combined with quantitative PCR irrespective of heterozygosity. Plant Methods 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, Z.; Zhang, Y.; Chen, L.; Wang, J.; Xu, L.; Zhang, Y.; He, M. Identification of odorant-binding and chemosensory protein genes and the ligand affinity of two of the encoded proteins suggest a complex olfactory perception system in Periplaneta americana. Insect Mol. Biol. 2017, 26, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muñoz, J.L.; García-Molina, F.; García-Ruiz, P.A.; Arribas, E.; Tudela, J.; García-Cánovas, F.; Rodríguez-López, J.N. Enzymatic and chemical oxidation of trihydroxylated phenols. Food Chem. 2009, 113, 435–444. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 121–126. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, G.; Xie, Z.; Yang, Y.; Yang, Y.; Guo, Q.; Liang, G.; Dang, J. NpPP2-B10, an F-Box-Nictaba Gene, Promotes Plant Growth and Resistance to Black Shank Disease Incited by Phytophthora nicotianae in Nicotiana tabacum. Int. J. Mol. Sci. 2023, 24, 7353. https://doi.org/10.3390/ijms24087353
Wen G, Xie Z, Yang Y, Yang Y, Guo Q, Liang G, Dang J. NpPP2-B10, an F-Box-Nictaba Gene, Promotes Plant Growth and Resistance to Black Shank Disease Incited by Phytophthora nicotianae in Nicotiana tabacum. International Journal of Molecular Sciences. 2023; 24(8):7353. https://doi.org/10.3390/ijms24087353
Chicago/Turabian StyleWen, Guo, Zhongyi Xie, Yao Yang, Yuxue Yang, Qigao Guo, Guolu Liang, and Jiangbo Dang. 2023. "NpPP2-B10, an F-Box-Nictaba Gene, Promotes Plant Growth and Resistance to Black Shank Disease Incited by Phytophthora nicotianae in Nicotiana tabacum" International Journal of Molecular Sciences 24, no. 8: 7353. https://doi.org/10.3390/ijms24087353
APA StyleWen, G., Xie, Z., Yang, Y., Yang, Y., Guo, Q., Liang, G., & Dang, J. (2023). NpPP2-B10, an F-Box-Nictaba Gene, Promotes Plant Growth and Resistance to Black Shank Disease Incited by Phytophthora nicotianae in Nicotiana tabacum. International Journal of Molecular Sciences, 24(8), 7353. https://doi.org/10.3390/ijms24087353





