Alveolar–Capillary Barrier Protection In Vitro: Lung Cell Type-Specific Effects and Molecular Mechanisms Induced by 1α, 25-Dihydroxyvitamin D3
Abstract
1. Introduction
2. Results
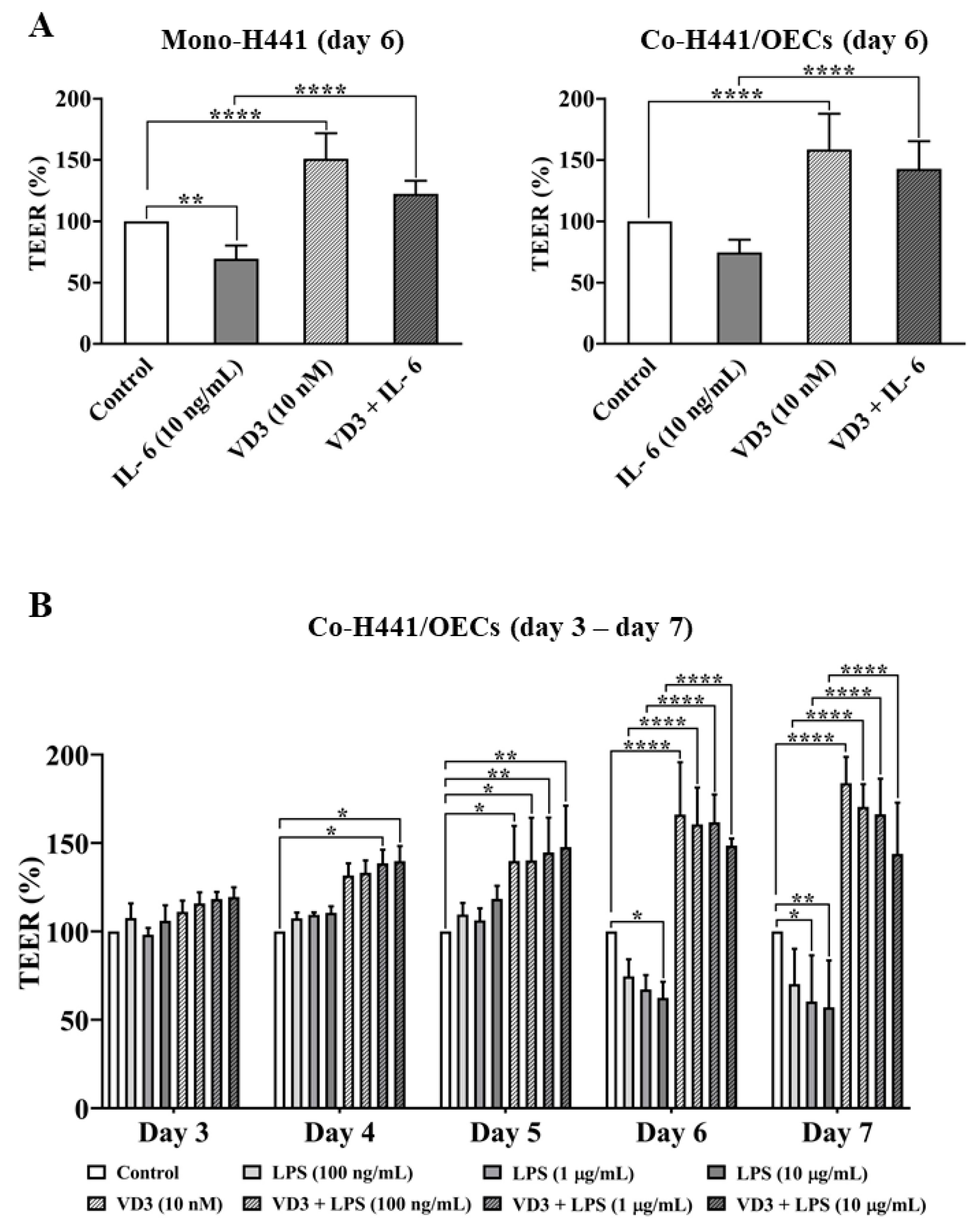
2.1. Effect of VD3 on the Barrier Integrity of the Alveolar–Capillary Interface In Vitro
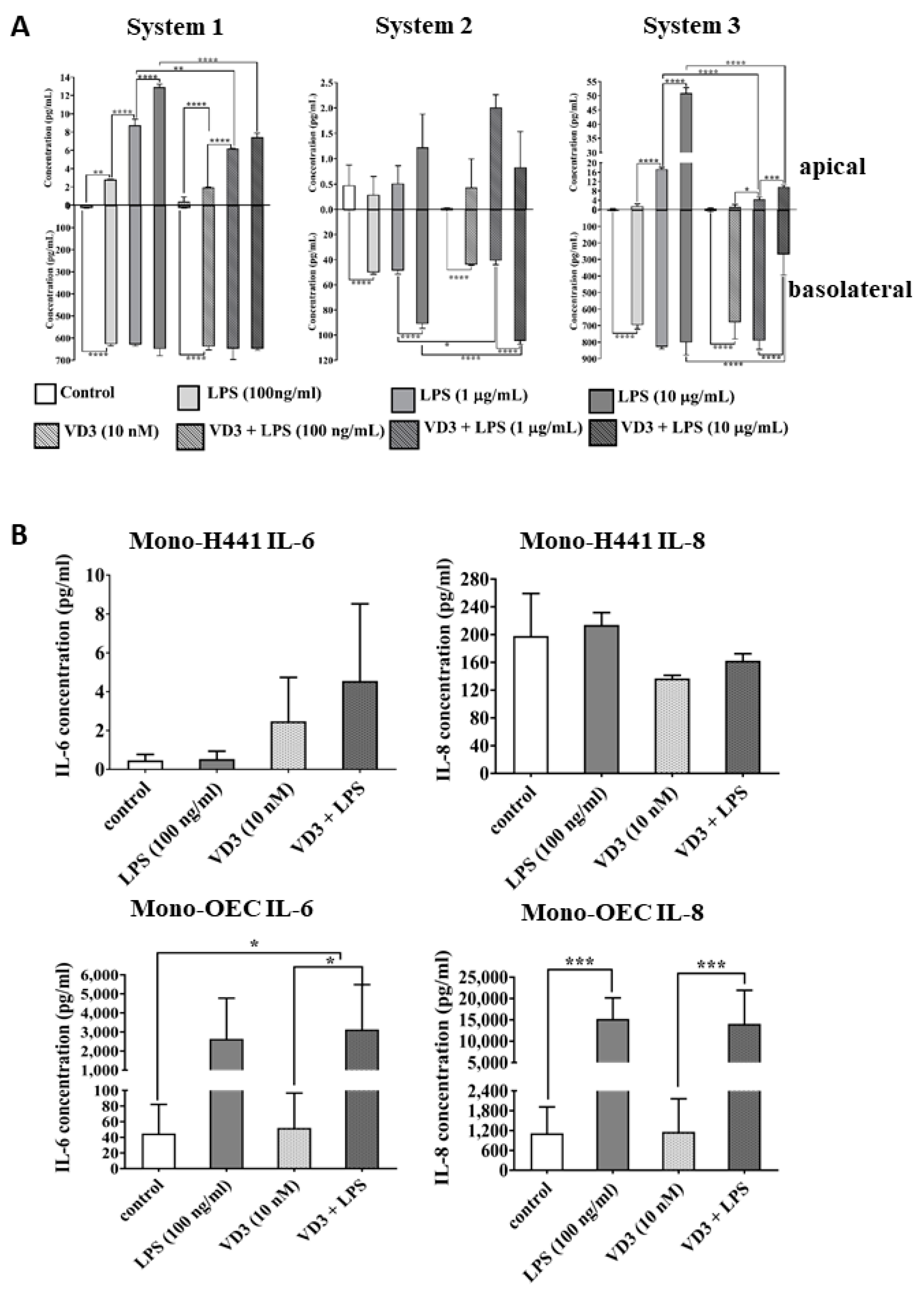
2.2. The Impact of VD3 on the Inflammatory Response in NCI-H441 Cells and OECs in Response to LPS Exposure
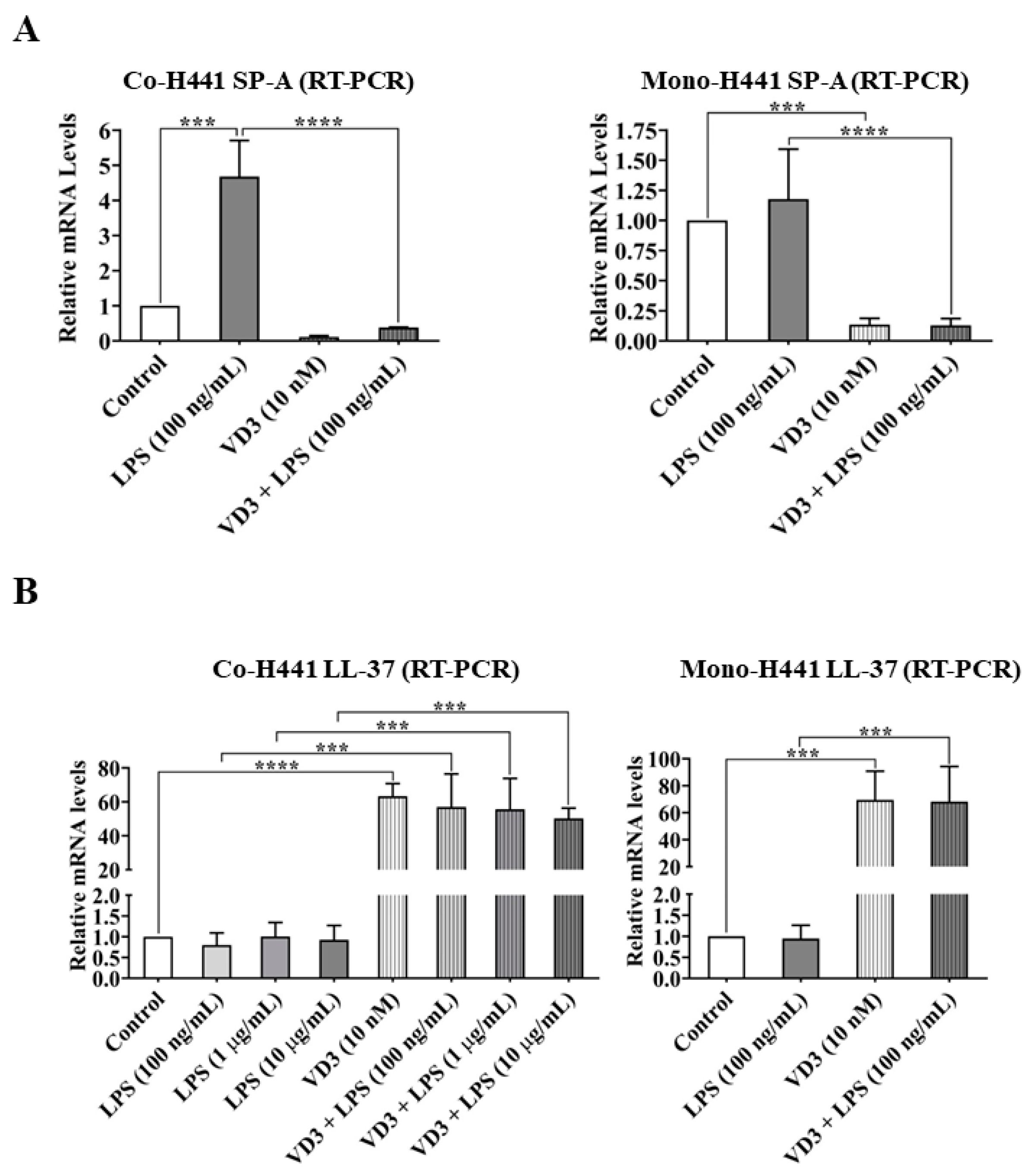
2.3. Effects of VD3 on Gene Expression of Epithelial Defense and Immune Regulators
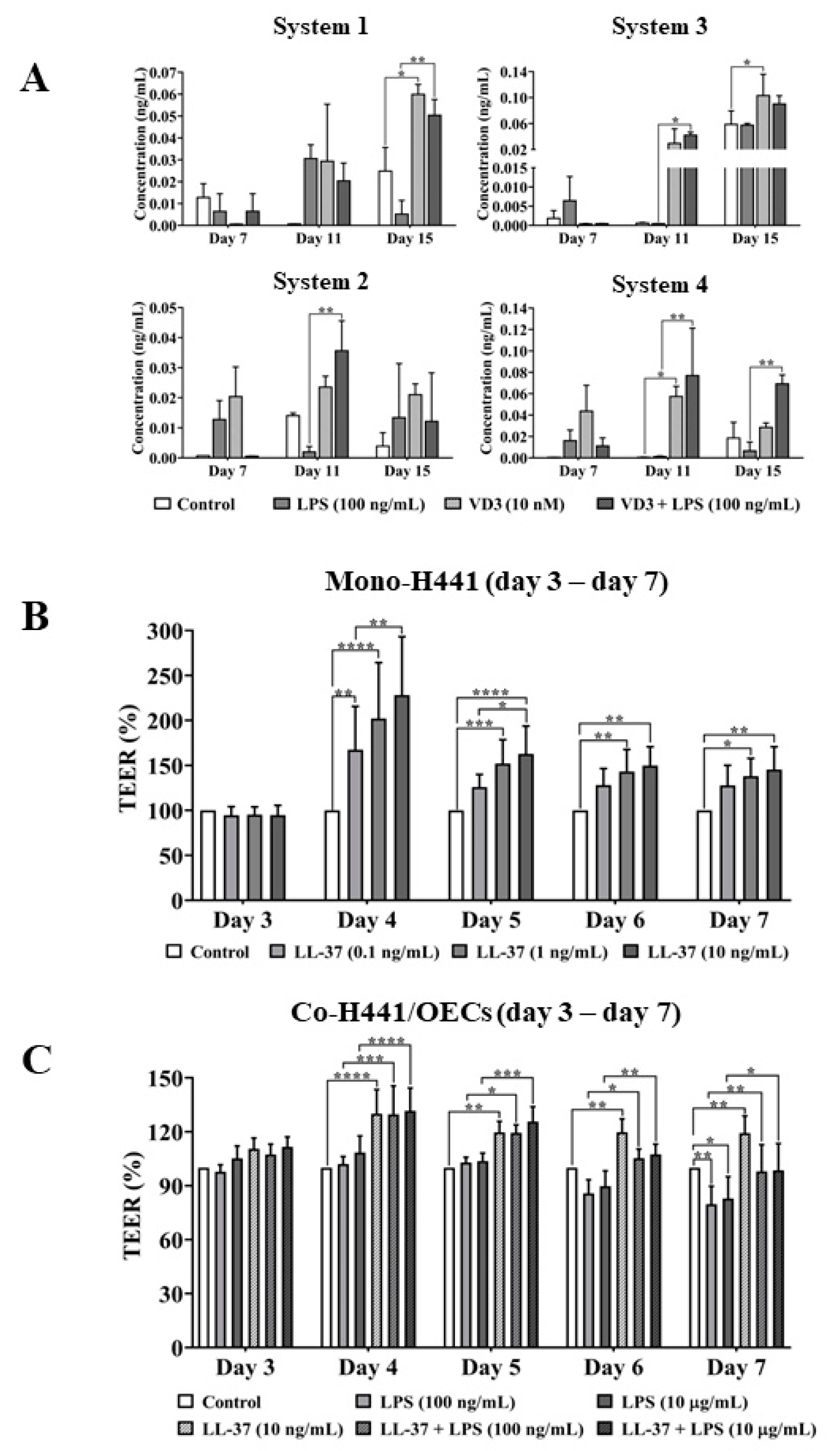
2.4. VD3-Mediated Effect of Cathelicidin on Epithelial Barrier Protection
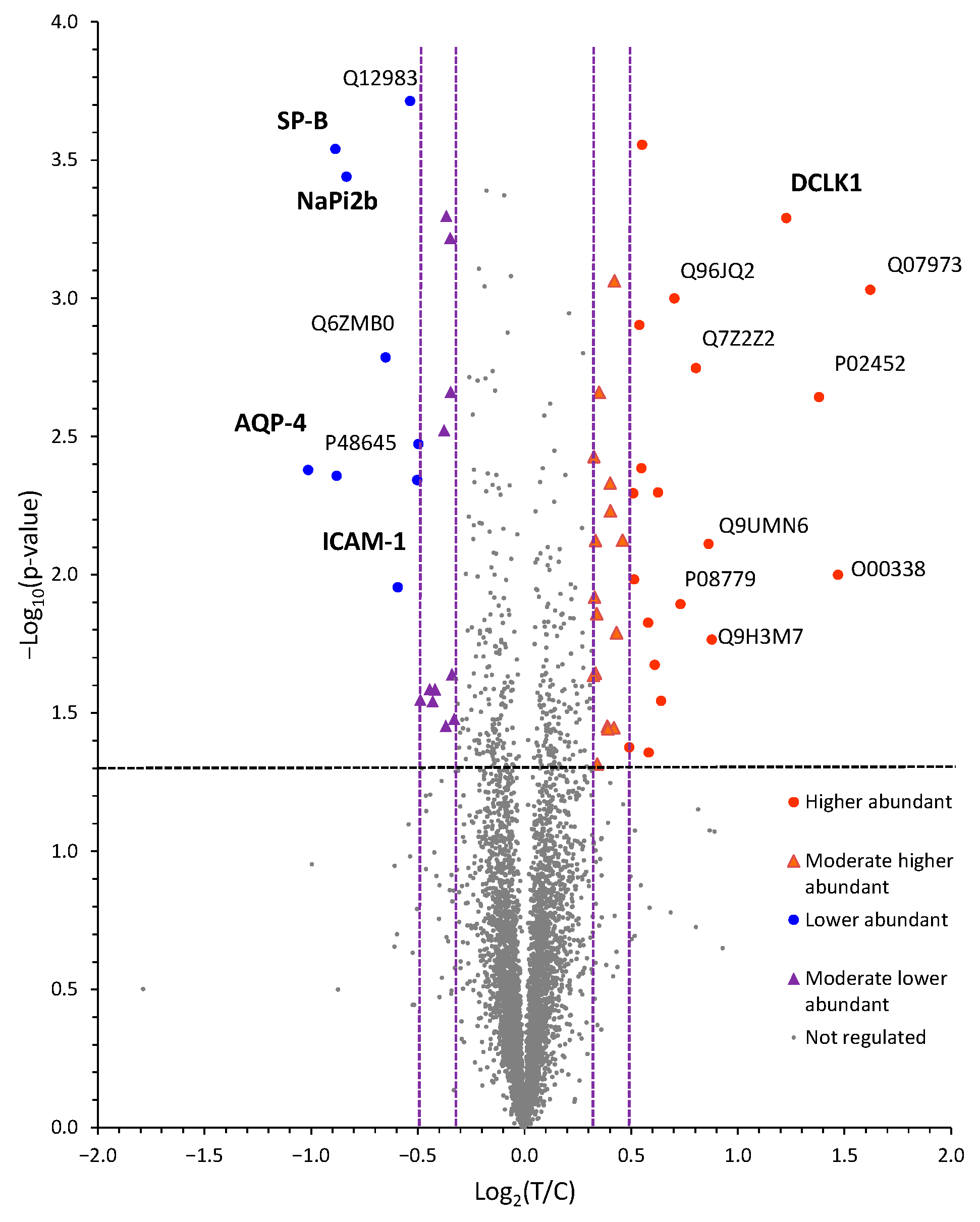
2.5. Quantitative Proteomics Identifies VD3-Induced Changes in Protein Abundances in NCI-H441 Epithelial Cells
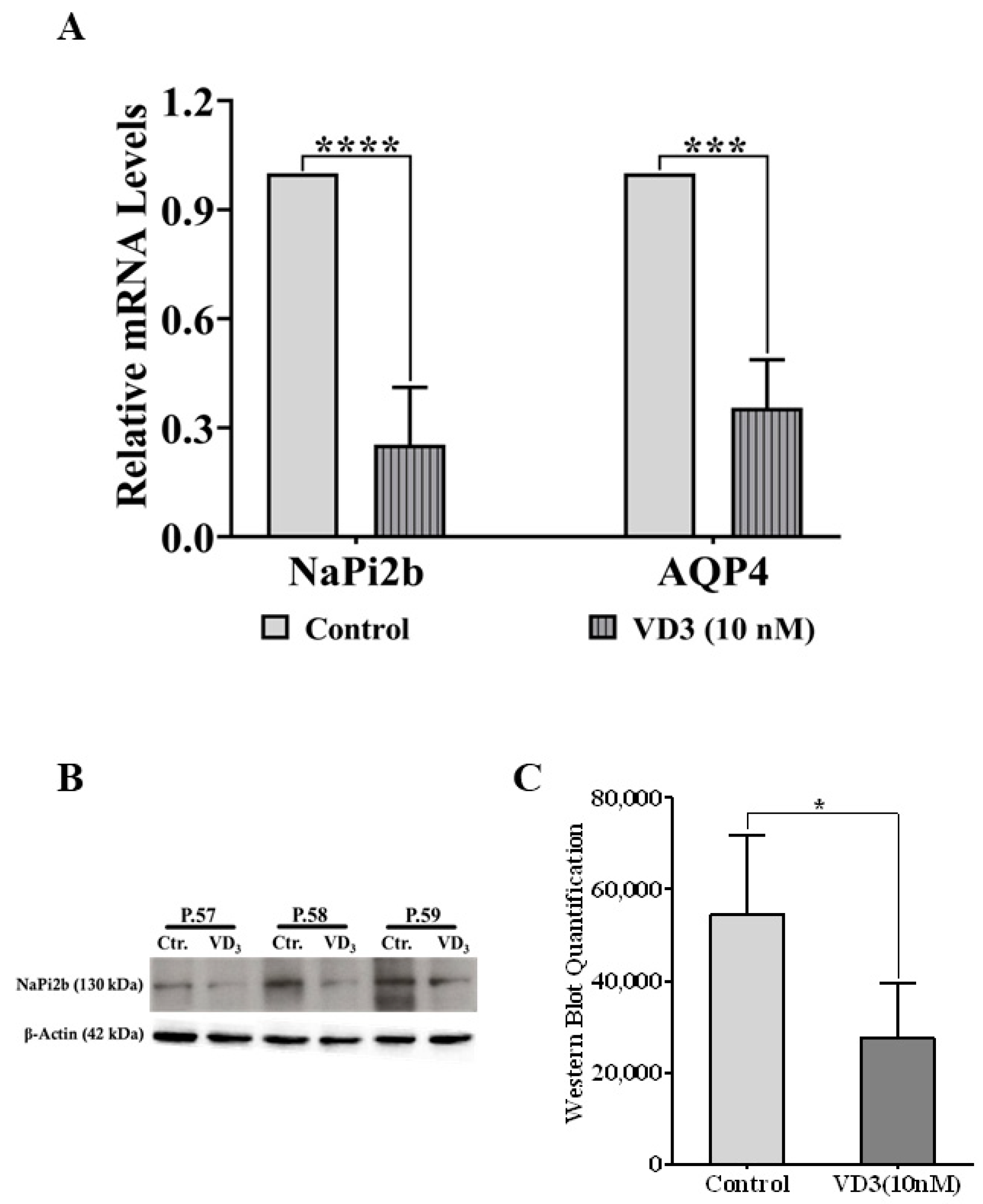
2.6. Verification of VD3-Regulated Target Molecules in NCI-H441 Identified by Proteomics
3. Discussion
4. Materials and Methods
4.1. Cells and General Cell Culture
Reagents
4.2. Mono- and Co-Culture of NCI-H441 Cells with OECs for Barrier Property Assessment
4.3. Trans-Epithelial Electrical Resistance (TEER) Measurement
4.4. Endothelial Cell Barrier Monitoring
4.5. Immunofluorescence Staining and Imaging
4.6. Semi-Quantitative Real-Time Polymerase Chain Reaction (Real-Time PCR)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Sample Preparation for Quantitative Proteome Analysis
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehr, P.; Bachofen, M.; Weibel, E.R. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Respir. Physiol. 1978, 32, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Foong, R.E.; Zosky, G.R. Vitamin D deficiency and the lung: Disease initiator or disease modifier? Nutrients 2013, 5, 2880–2900. [Google Scholar] [CrossRef]
- Takahashi, S.; Maeda, T.; Sano, Y.; Nishihara, H.; Takeshita, Y.; Shimizu, F.; Kanda, T. Active form of vitamin D directly protects the blood-brain barrier in multiple sclerosis. Clin. Exp. Neuroimmunol. 2017, 8, 244–254. [Google Scholar] [CrossRef]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef]
- Lykkedegn, S.; Sorensen, G.L.; Beck-Nielsen, S.S.; Christesen, H.T. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L587–L602. [Google Scholar] [CrossRef]
- Kho, A.T.; Sharma, S.; Qiu, W.; Gaedigk, R.; Klanderman, B.; Niu, S.; Anderson, C.; Leeder, J.S.; Weiss, S.T.; Tantisira, K.G. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med. Genomics. 2013, 6, 47. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66 (Suppl. 2), S116–S124. [Google Scholar] [CrossRef]
- Marin, L.; Dufour, M.E.; Nguyen, T.M.; Tordet, C.; Garabedian, M. Maturational changes induced by 1 alpha,25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am. J. Physiol. 1993, 265, L45–L52. [Google Scholar] [CrossRef]
- Edelson, J.D.; Chan, S.; Jassal, D.; Post, M.; Tanswell, A.K. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim. Biophys. Acta 1994, 1221, 159–166. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Javadian, Y.; Monadi, M.; Dankob, Y.; Firouzjahi, A. Vitamin D status and distribution in patients with chronic obstructive pulmonary disease versus healthy controls. Casp. J. Intern. Med. 2015, 6, 93–97. [Google Scholar]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Moosavi, S.A.J.; Shoushtari, M.H. The Effects of Vitamin D Supplementation on Pulmonary Function of Chronic Obstructive Pulmonary Disease Patients, before and after Clinical Trial. Diseases 2015, 3, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.; Dancer, R.C.; Lax, S.; Cooper, M.S.; Martineau, A.R.; Fraser, W.D.; Tucker, O.; Alderson, D.; Perkins, G.D.; Gao-Smith, F.; et al. Vitamin D to prevent acute lung injury following oesophagectomy (VINDALOO): Study protocol for a randomised placebo controlled trial. Trials 2013, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Yumrutepe, T.; Aytemur, Z.A.; Baysal, O.; Taskapan, H.; Taskapan, C.M.; Hacievliyagil, S.S. Relationship between vitamin D and lung function, physical performance and balance on patients with stage I-III chronic obstructive pulmonary disease. Rev. Assoc. Med. Bras. 2015, 61, 132–138. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Srinivasan, M.; Parwani, A.V.; Hershberger, P.A.; Lenzner, D.E.; Weissfeld, J.L. Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer. J. Steroid Biochem. Mol. Biol. 2011, 123, 30–36. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef]
- MacDonald, P.N.; Dowd, D.R.; Nakajima, S.; Galligan, M.A.; Reeder, M.C.; Haussler, C.A.; Ozato, K.; Haussler, M.R. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol. Cell Biol. 1993, 13, 5907–5917. [Google Scholar] [PubMed]
- Carlberg, C.; Bendik, I.; Wyss, A.; Meier, E.; Sturzenbecker, L.J.; Grippo, J.F.; Hunziker, W. Two nuclear signalling pathways for vitamin D. Nature 1993, 361, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect. Immun. 2008, 76, 3837–3843. [Google Scholar] [CrossRef]
- Carlberg, C.; Molnar, F. Current status of vitamin D signaling and its therapeutic applications. Curr. Top. Med. Chem. 2012, 12, 528–547. [Google Scholar] [CrossRef] [PubMed]
- Janga, H.; Cassidy, L.; Wang, F.; Spengler, D.; Oestern-Fitschen, S.; Krause, M.F.; Seekamp, A.; Tholey, A.; Fuchs, S. Site-specific and endothelial-mediated dysfunction of the alveolar-capillary barrier in response to lipopolysaccharides. J. Cell Mol. Med. 2018, 22, 982–998. [Google Scholar] [CrossRef] [PubMed]
- Spengler, D.; Winoto-Morbach, S.; Kupsch, S.; Vock, C.; Blochle, K.; Frank, S.; Rintz, N.; Diekotter, M.; Janga, H.; Weckmann, M.; et al. Novel therapeutic roles for surfactant-inositols and -phosphatidylglycerols in a neonatal piglet ARDS model: A translational study. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L32–L53. [Google Scholar] [CrossRef]
- Thompson, A.; Schafer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Johnstone, R.; Mohammed, A.K.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef]
- Lin, P.T.; Gleeson, J.G.; Corbo, J.C.; Flanagan, L.; Walsh, C.A. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J. Neurosci. 2000, 20, 9152–9161. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [PubMed]
- Tzilas, V.; Bouros, E.; Barbayianni, I.; Karampitsakos, T.; Kourtidou, S.; Ntassiou, M.; Ninou, I.; Aidinis, V.; Bouros, D.; Tzouvelekis, A. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2019, 55, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Sabzeghabaei, A.; Valaei Barhagh, H.; Soltani, S. The Correlation between Serum Level of Vitamin D and Outcome of Sepsis Patients; a Cross-Sectional Study. Arch. Acad. Emerg. Med. 2019, 7, e1. [Google Scholar] [PubMed]
- De Haan, K.; Groeneveld, A.B.J.; de Geus, H.R.H.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Fedecostante, M.; Oost, M.J.; Steenhuis, S.K.P.; Lentjes, E.; Maitimu-Smeele, I.; Janssen, M.J.; Hilbrands, L.B.; Masereeuw, R. Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. Int. J. Mol. Sci. 2017, 18, 2531. [Google Scholar] [CrossRef]
- Mieremet, A.; van Dijk, R.; Gooris, G.; Bouwstra, J.A.; El Ghalbzouri, A. Shedding light on the effects of 1,25-dihydroxyvitamin D3 on epidermal lipid barrier formation in three-dimensional human skin equivalents. J. Steroid Biochem. Mol. Biol. 2019, 189, 19–27. [Google Scholar] [CrossRef]
- Stamme, C.; Wright, J.R. Surfactant protein A enhances the binding and deacylation of E. coli LPS by alveolar macrophages. Am. J. Physiol. 1999, 276 Pt 1, L540–L547. [Google Scholar]
- Borron, P.; McIntosh, J.C.; Korfhagen, T.R.; Whitsett, J.A.; Taylor, J.; Wright, J.R. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L840–L847. [Google Scholar] [CrossRef]
- Crouch, E.; Wright, J.R. Surfactant proteins a and d and pulmonary host defense. Annu. Rev. Physiol. 2001, 63, 521–554. [Google Scholar] [CrossRef]
- Crouch, E.C. Collectins and pulmonary host defense. Am. J. Respir. Cell Mol. Biol. 1998, 19, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Phokela, S.S.; Peleg, S.; Moya, F.R.; Alcorn, J.L. Regulation of human pulmonary surfactant protein gene expression by 1alpha,25-dihydroxyvitamin D3. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L617–L626. [Google Scholar] [CrossRef] [PubMed]
- Amiry-Moghaddam, M.; Ottersen, O.P. The molecular basis of water transport in the brain. Nat. Rev. Neurosci. 2003, 4, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef]
- Verkman, A.S. Knock-out models reveal new aquaporin functions. Handb. Exp. Pharmacol. 2009, 190, 359–381. [Google Scholar]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Srivastava, A.K. DCAMKL1, a brain-specific transmembrane protein on 13q12.3 that is similar to doublecortin (DCX). Genomics 1999, 56, 121–126. [Google Scholar] [CrossRef]
- Yi, H.; Hu, W.; Chen, S.; Lu, Z.; Wang, Y. Cathelicidin-WA Improves Intestinal Epithelial Barrier Function and Enhances Host Defense against Enterohemorrhagic Escherichia coli O157:H7 Infection. J. Immunol. 2017, 198, 1696–1705. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins--new players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.; Muley, T.; Meister, M.; Herpel, E.; Pathil, A.; Hoffmann, H.; Schnabel, P.A.; Bender, C.; Buness, A.; Schirmacher, P.; et al. Loss of aquaporin-4 expression and putative function in non-small cell lung cancer. BMC Cancer 2011, 11, 161. [Google Scholar] [CrossRef]
- Rangel, L.B.; Sherman-Baust, C.A.; Wernyj, R.P.; Schwartz, D.R.; Cho, K.R.; Morin, P.J. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene 2003, 22, 7225–7232. [Google Scholar] [CrossRef]
- Jarzab, B.; Wiench, M.; Fujarewicz, K.; Simek, K.; Jarzab, M.; Oczko-Wojciechowska, M.; Wloch, J.; Czarniecka, A.; Chmielik, E.; Lange, D.; et al. Gene expression profile of papillary thyroid cancer: Sources of variability and diagnostic implications. Cancer Res. 2005, 65, 1587–1597. [Google Scholar] [CrossRef]
- Xu, H.; Bai, L.; Collins, J.F.; Ghishan, F.K. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics 1999, 62, 281–284. [Google Scholar] [CrossRef]
- Frigeri, A.; Gropper, M.A.; Turck, C.W.; Verkman, A.S. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 1995, 92, 4328–4331. [Google Scholar] [CrossRef]
- Bai, C.; Fukuda, N.; Song, Y.; Ma, T.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Investig. 1999, 103, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Weygant, N.; May, R.; Chandrakesan, P.; Madhoun, M.; Ali, N.; Sureban, S.M.; An, G.; Schlosser, M.J.; Houchen, C.W. Ablation of Doublecortin-Like Kinase 1 in the Colonic Epithelium Exacerbates Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2015, 10, e0134212. [Google Scholar] [CrossRef]
- Middelhoff, M.; Westphalen, C.B.; Hayakawa, Y.; Yan, K.S.; Gershon, M.D.; Wang, T.C.; Quante, M. Dclk1-expressing tuft cells: Critical modulators of the intestinal niche? Am. J. Physiology. Gastrointest. Liver Physiol. 2017, 313, G285–G299. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, B.; Reid, L. The alveolar brush cell in rat lung—A third pneumonocyte. J. Ultrastruct. Res. 1968, 23, 71–80. [Google Scholar] [CrossRef]
- Sbarbati, A.; Osculati, F. A new fate for old cells: Brush cells and related elements. J. Anat. 2005, 206, 349–358. [Google Scholar] [CrossRef]
- Kasper, M.; Hofer, D.; Woodcock-Mitchell, J.; Migheli, A.; Attanasio, A.; Rudolf, T.; Muller, M.; Drenckhahn, D. Colocalization of cytokeratin 18 and villin in type III alveolar cells (brush cells) of the rat lung. Histochemistry 1994, 101, 57–62. [Google Scholar] [CrossRef]
- Krasteva, G.; Canning, B.J.; Hartmann, P.; Veres, T.Z.; Papadakis, T.; Mühlfeld, C.; Schliecker, K.; Tallini, Y.N.; Braun, A.; Hackstein, H.; et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc. Natl. Acad. Sci. USA 2011, 108, 9478–9483. [Google Scholar] [CrossRef]
- Hijiya, K. Electron microscope study of the alveolar brush cell. J. Electron Microsc. 1978, 27, 223–227. [Google Scholar]
- Hijiya, K.; Okada, Y.; Tankawa, H. Ultrastructural study of the alveolar brush cell. J. Electron Microsc. 1977, 26, 321–329. [Google Scholar]
- Gerbe, F.; van Es, J.H.; Makrini, L.; Brulin, B.; Mellitzer, G.; Robine, S.; Romagnolo, B.; Shroyer, N.F.; Bourgaux, J.F.; Pignodel, C.; et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 2011, 192, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Peregrina, K.; Houston, M.; Daroqui, C.; Dhima, E.; Sellers, R.S.; Augenlicht, L.H. Vitamin D is a determinant of mouse intestinal Lgr5 stem cell functions. Carcinogenesis 2015, 36, 25–31. [Google Scholar] [CrossRef]
- DiMaio, M.F.; Kattan, M.; Ciurea, D.; Gil, J.; Dische, R. Brush cells in the human fetal trachea. Pediatr. Pulmonol. 1990, 8, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Fuchs, S.; Hermanns, M.I.; Kirkpatrick, C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006, 326, 79–92. [Google Scholar] [CrossRef]
- Fuchs, S.; Hofmann, A.; Kirkpatrick, C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007, 13, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells cells for endothelialization as a potential source of autologous of silk fibroin biomaterials. Biomaterials 2006, 27, 5399–5408. [Google Scholar] [CrossRef] [PubMed]
- Wessel, D.; Flugge, U.I. A Method for the Quantitative Recovery of Protein in Dilute-Solution in the Presence of Detergents and Lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Delmotte, N.; Lasaosa, M.; Tholey, A.; Heinzle, E.; Huber, C.G. Two-dimensional reversed-phase x ion-pair reversed-phase HPLC: An alternative approach to high-resolution peptide separation for shotgun proteome analysis. J. Proteome. Res. 2007, 6, 4363–4373. [Google Scholar] [CrossRef]
- Treitz, C.; Cassidy, L.; Hockendorf, A.; Leippe, M.; Tholey, A. Quantitative proteome analysis of Caenorhabditis elegans upon exposure to nematicidal Bacillus thuringiensis. J. Proteomics. 2015, 113, 337–350. [Google Scholar] [CrossRef]
- Vizcaino, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Rios, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Savitski, M.M.; Mathieson, T.; Zinn, N.; Sweetman, G.; Doce, C.; Becher, I.; Pachl, F.; Kuster, B.; Bantscheff, M. Measuring and Managing Ratio Compression for Accurate iTRAQ/TMT Quantification. J. Proteome. Res. 2013, 12, 3586–3598. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids. Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, J.; Kaleja, P.; Ückert, L.; Nezaratizadeh, N.; Krantz, S.; Krause, M.F.; Fitschen-Oestern, S.; Seekamp, A.; Cassidy, L.; Tholey, A.; et al. Alveolar–Capillary Barrier Protection In Vitro: Lung Cell Type-Specific Effects and Molecular Mechanisms Induced by 1α, 25-Dihydroxyvitamin D3. Int. J. Mol. Sci. 2023, 24, 7298. https://doi.org/10.3390/ijms24087298
Xiong J, Kaleja P, Ückert L, Nezaratizadeh N, Krantz S, Krause MF, Fitschen-Oestern S, Seekamp A, Cassidy L, Tholey A, et al. Alveolar–Capillary Barrier Protection In Vitro: Lung Cell Type-Specific Effects and Molecular Mechanisms Induced by 1α, 25-Dihydroxyvitamin D3. International Journal of Molecular Sciences. 2023; 24(8):7298. https://doi.org/10.3390/ijms24087298
Chicago/Turabian StyleXiong, Junyu, Patrick Kaleja, Larissa Ückert, Niloufar Nezaratizadeh, Stefanie Krantz, Martin Friedrich Krause, Stefanie Fitschen-Oestern, Andreas Seekamp, Liam Cassidy, Andreas Tholey, and et al. 2023. "Alveolar–Capillary Barrier Protection In Vitro: Lung Cell Type-Specific Effects and Molecular Mechanisms Induced by 1α, 25-Dihydroxyvitamin D3" International Journal of Molecular Sciences 24, no. 8: 7298. https://doi.org/10.3390/ijms24087298
APA StyleXiong, J., Kaleja, P., Ückert, L., Nezaratizadeh, N., Krantz, S., Krause, M. F., Fitschen-Oestern, S., Seekamp, A., Cassidy, L., Tholey, A., & Fuchs, S. (2023). Alveolar–Capillary Barrier Protection In Vitro: Lung Cell Type-Specific Effects and Molecular Mechanisms Induced by 1α, 25-Dihydroxyvitamin D3. International Journal of Molecular Sciences, 24(8), 7298. https://doi.org/10.3390/ijms24087298






