The Effect of Silencing the Genes Responsible for the Level of Sphingosine-1-phosphate on the Apoptosis of Colon Cancer Cells
Abstract
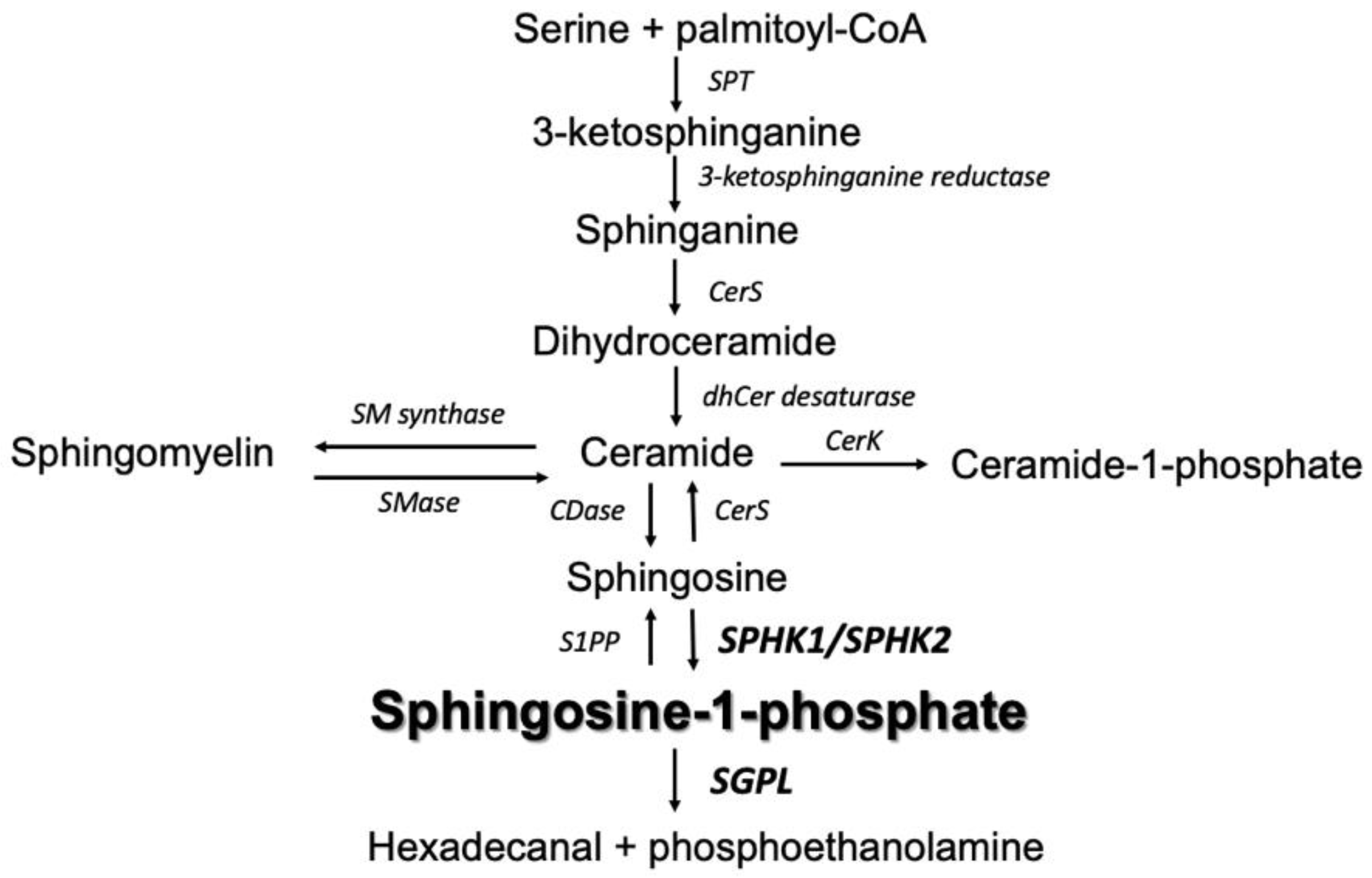
1. Introduction
2. Results
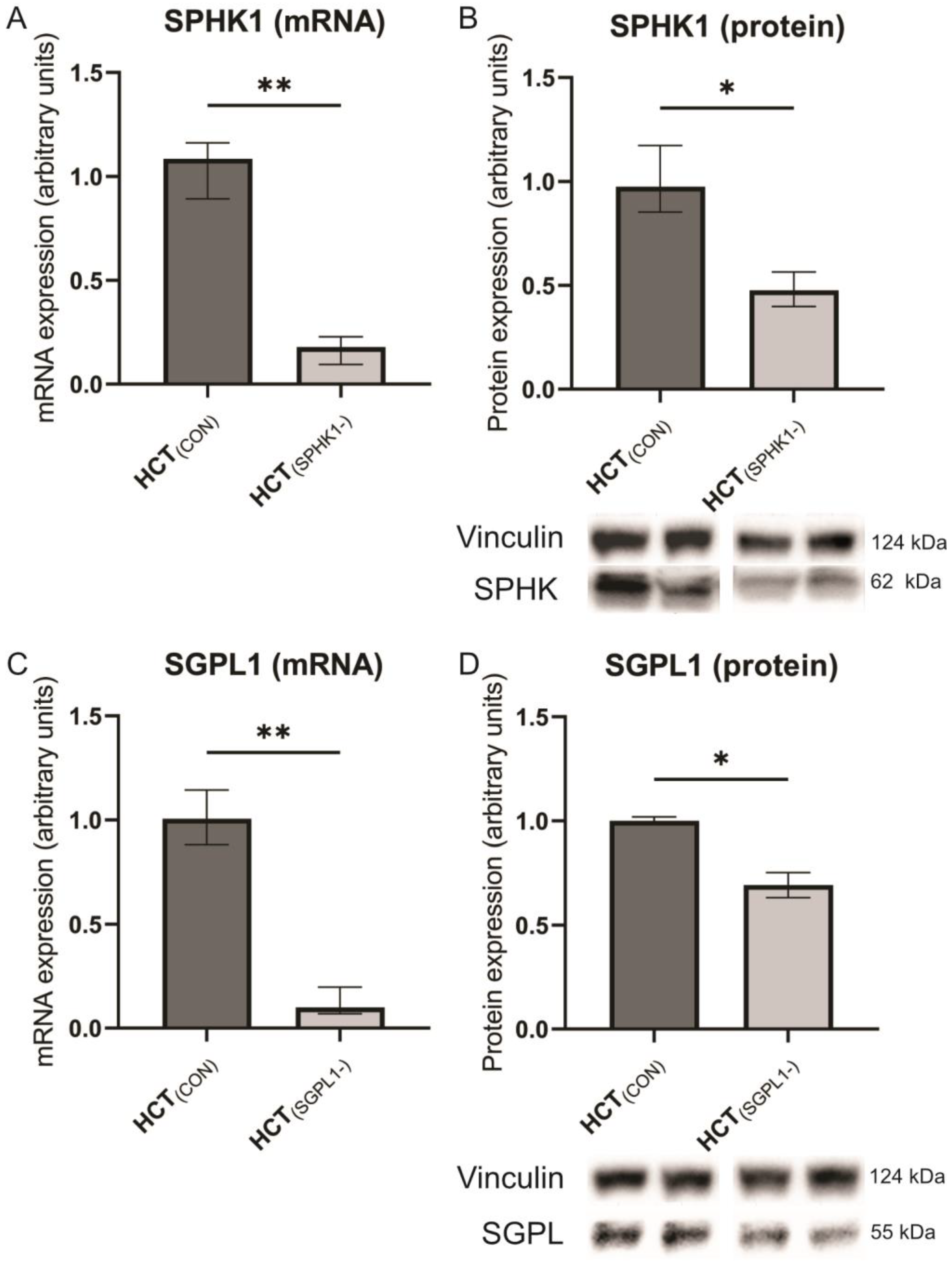
2.1. Gene Silencing
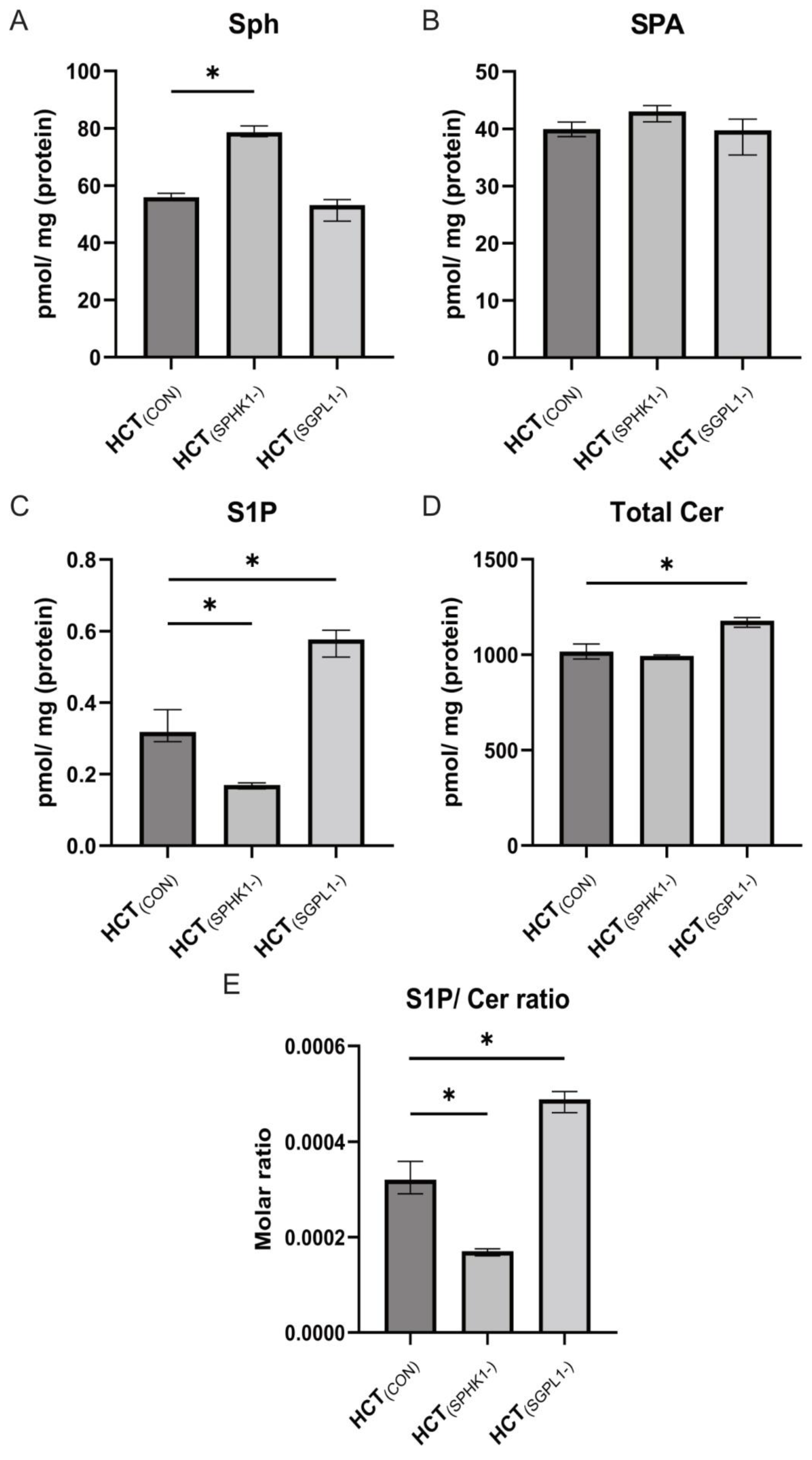
2.2. Sphingolipids Content
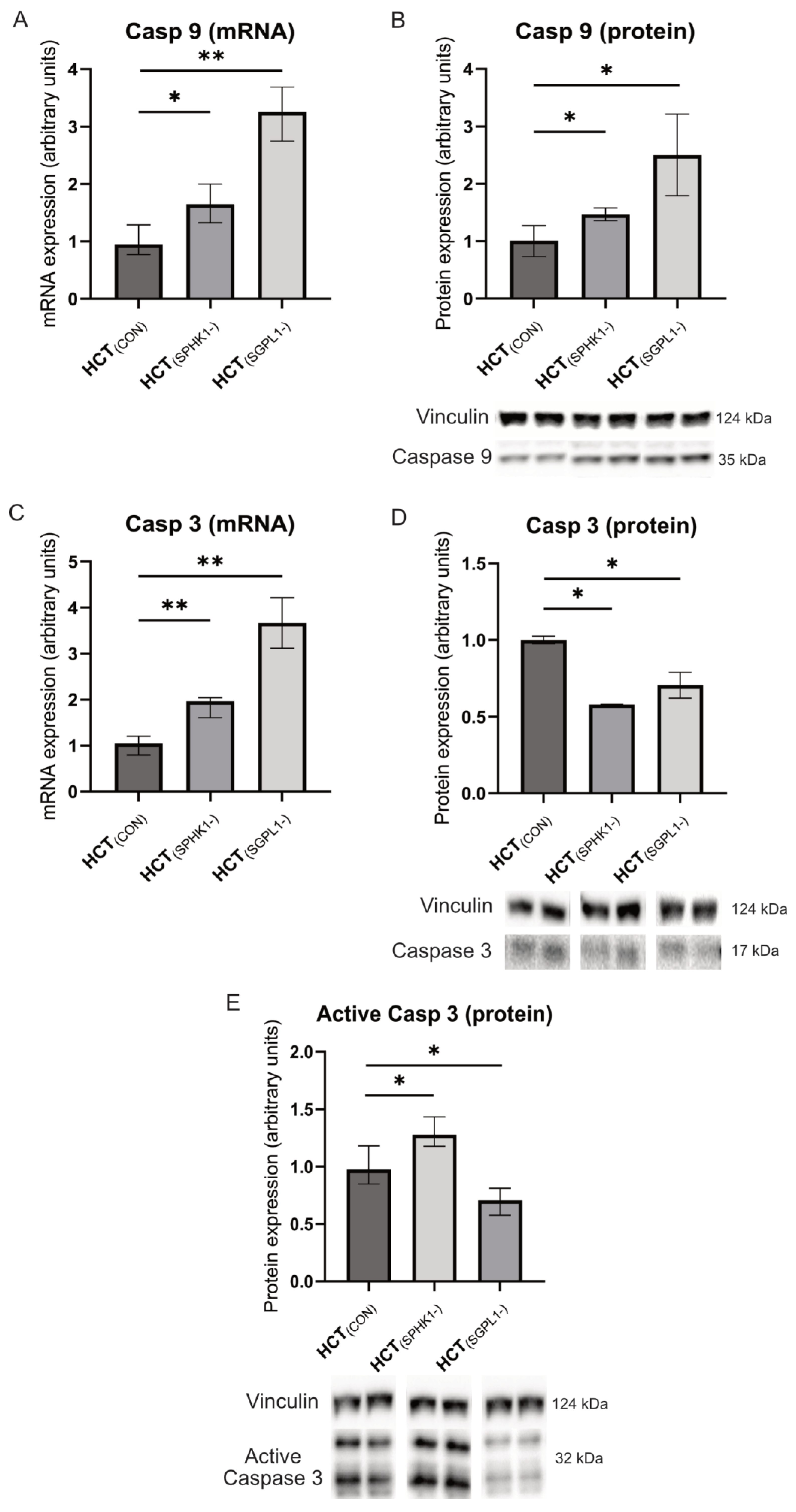
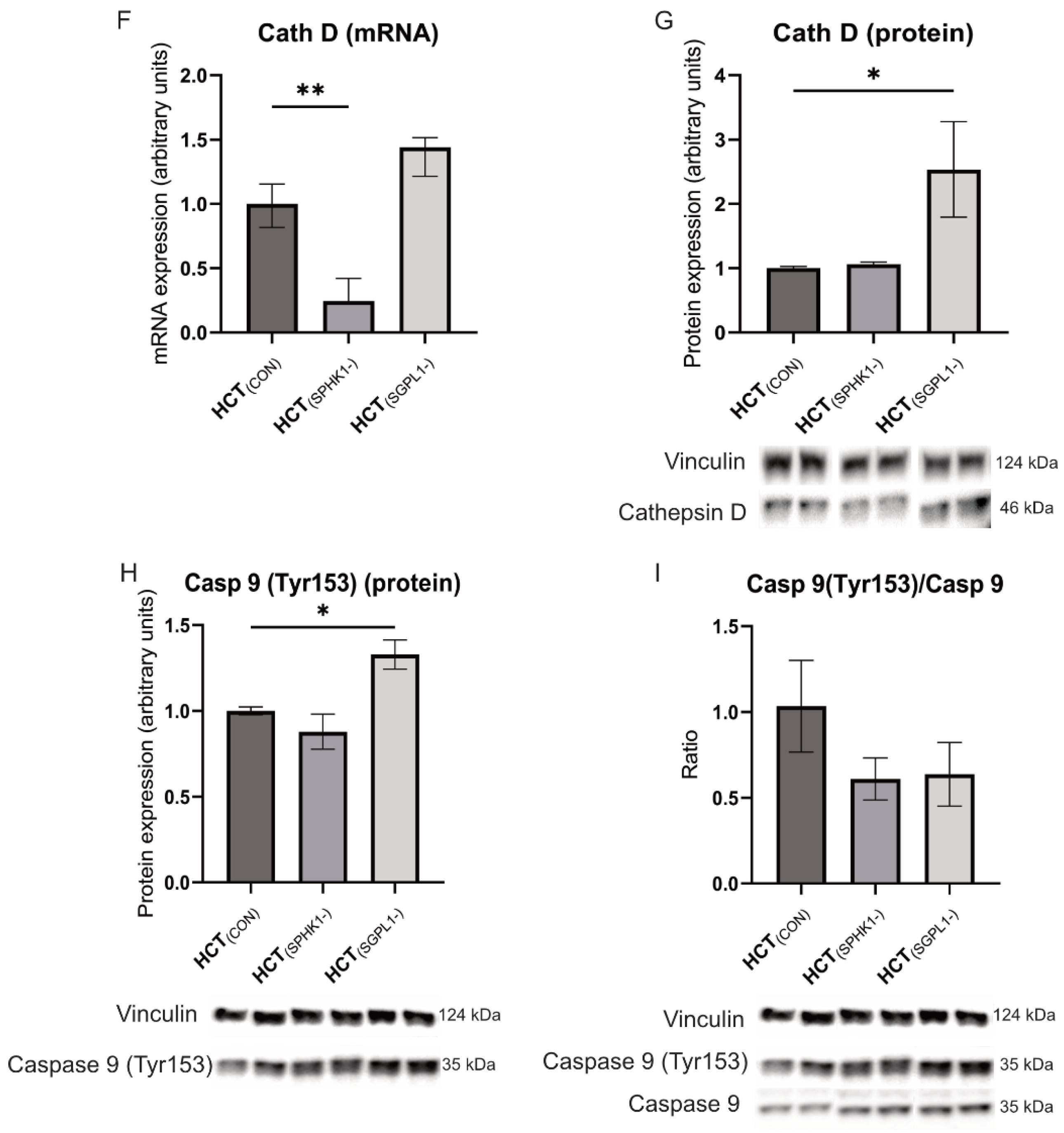
2.3. Caspases and Cathepsin-D
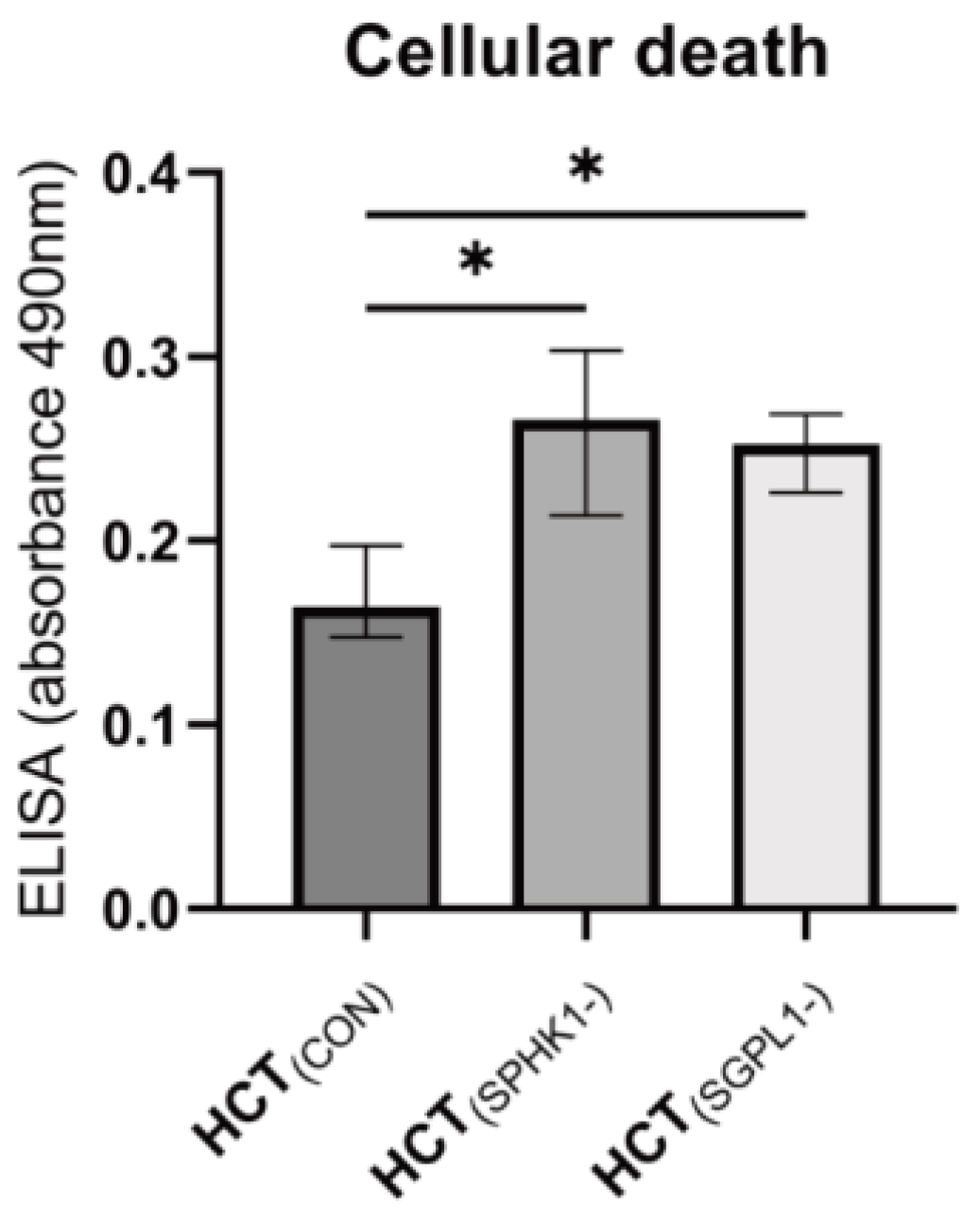
2.4. Cellular Apoptosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. Evaluation of Cellular Apoptosis
4.3. Sphingolipid Measurements
4.4. Western Blotting
4.5. Real-Time PCR
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markowski, A.R.; Blachnio-Zabielska, A.U.; Guzińska-Ustymowicz, K.; Markowska, A.; Pogodzińska, K.; Roszczyc, K.; Zińczuk, J.; Zabielski, P. Ceramides Profile Identifies Patients with More Advanced Stages of Colorectal Cancer. Biomolecules 2020, 10, 632. [Google Scholar] [CrossRef]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.N.; Goel, A.; Blum, H.E.; Boland, C.R. Molecular pathogenesis of colorectal cancer: Implications for molecular diagnosis. Cancer 2005, 104, 2035–2047. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. Functions of ceramide in coordinating cellular responses to stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef]
- Speirs, M.M.P.; Swensen, A.C.; Chan, T.Y.; Jones, P.M.; Holman, J.C.; Harris, M.c.C.B.; Maschek, J.A.; Cox, J.E.; Carson, R.H.; Hill, J.T.; et al. Imbalanced sphingolipid signaling is maintained as a core proponent of a cancerous phenotype in spite of metabolic pressure and epigenetic drift. Oncotarget 2019, 10, 449–479. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.C.; Turner, L.S.; Elojeimy, S.; Beckham, T.H.; Bielawska, A.; Keane, T.E.; Hannun, Y.A.; Norris, J.S. Acid ceramidase upregulation in prostate cancer: Role in tumor development and implications for therapy. Expert Opin. Ther. Targets 2009, 13, 1449–1458. [Google Scholar] [CrossRef]
- Zeng, S.; Liang, Y.; Hu, H.; Wang, F.; Liang, L. Endothelial cell-derived S1P promotes migration and stemness by binding with GPR63 in colorectal cancer. Pathol. Res. Pract. 2022, 240, 154197. [Google Scholar] [CrossRef]
- del Solar, V.; Lizardo, D.Y.; Li, N.; Hurst, J.J.; Brais, C.J.; Atilla-Gokcumen, G.E. Differential Regulation of Specific Sphingolipids in Colon Cancer Cells during Staurosporine-Induced Apoptosis. Chem. Biol. 2015, 22, 1662–1670. [Google Scholar] [CrossRef]
- Kawamori, T.; Osta, W.; Johnson, K.R.; Pettus, B.J.; Bielawski, J.; Tanaka, T.; Wargovich, M.J.; Reddy, B.S.; Hannun, Y.A.; Obeid, L.M.; et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006, 20, 386–388. [Google Scholar] [CrossRef]
- Grbčić, P.; Sedić, M. Sphingosine 1-Phosphate Signaling and Metabolism in Chemoprevention and Chemoresistance in Colon Cancer. Molecules 2020, 25, 2436. [Google Scholar] [CrossRef]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Stunff, H.L.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Ding, G.; Sonoda, H.; Kajimoto, T.; Haga, Y.; Khosrowbeygi, A.; Gao, S.; Miwa, N.; Jahangeer, S.; Nakamura, S.I. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 2005, 280, 36318–36325. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Don, A.S.; Martinez-Lamenca, C.; Webb, W.R.; Proia, R.L.; Roberts, E.; Rosen, H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J. Biol. Chem. 2007, 282, 15833–15842. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zuo, Z.; Hao, C.; Ma, Y. Sphingosine kinase 2 promotes colorectal cancer cell proliferation and invasion by enhancing MYC expression. Tumour Biol. 2016, 37, 8455–8460. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shi, W.N.; Wu, S.H.; Miao, R.R.; Sun, S.Y.; Luo, D.D.; Wan, S.B.; Guo, Z.K.; Wang, W.Y.; Yu, X.F.; et al. SphK2 confers 5-fluorouracil resistance to colorectal cancer via upregulating H3K56ac-mediated DPD expression. Oncogene 2020, 39, 5214–5227. [Google Scholar] [CrossRef]
- Mizutani, N.; Omori, Y.; Tanaka, K.; Ito, H.; Takagi, A.; Kojima, T.; Nakatochi, M.; Ogiso, H.; Kawamoto, Y.; Nakamura, M.; et al. Increased SPHK2 Transcription of Human Colon Cancer Cells in Serum-Depleted Culture: The Involvement of CREB Transcription Factor. J. Cell. Biochem. 2015, 116, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Xun, C.; Chen, M.B.; Qi, L.; Zhang, T.-N.; Peng, X.; Ning, L.; Chen, Z.-X.; Wang, L.-W. Targeting sphingosine kinase 2 (SphK2) by ABC294640 inhibits colorectal cancer cell growth in vitro and in vivo. J. Exp. Clin. Cancer Res. 2015, 34, 94. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Gao, Z.H.; Qu, X.J. Down-regulation of sphingosine kinase 2 (SphK2) increases the effects of all-trans-retinoic acid (ATRA) on colon cancer cells. Biomed. Pharmacother. 2014, 68, 1089–1097. [Google Scholar] [CrossRef]
- Nemoto, S.; Nakamura, M.; Osawa, Y.; Kono, S.; Itoh, Y.; Okano, Y.; Murate, T.; Hara, A.; Ueda, H.; Nozawa, Y.; et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J. Biol. Chem. 2009, 284, 10422–10432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wan, Z.; Liu, S.; Cao, Y.; Zeng, Z. Sphingosine kinase 1 and cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e90362. [Google Scholar] [CrossRef]
- Kawamori, T.; Kaneshiro, T.; Okumura, M.; Maalouf, S.; Uflacker, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.S.; Khin, L.W.; Wong, L.; Yan, B.; Ong, C.W.; Datta, A.; Salto-Tellez, M.; Lam, Y.; Yap, C.T. Sphingosine kinase 1 promotes malignant progression in colon cancer and independently predicts survival of patients with colon cancer by competing risk approach in South asian population. Clin. Transl. Gastroenterol. 2014, 5, e51. [Google Scholar] [CrossRef]
- Oskouian, B.; Saba, J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: Lipid signaling strikes again. Cell Cycle 2007, 6, 522–527. [Google Scholar] [CrossRef]
- Le Stunff, H.; Galve-Roperh, I.; Peterson, C.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J. Cell Biol. 2002, 158, 1039–1049. [Google Scholar] [CrossRef]
- Oskouian, B.; Sooriyakumaran, P.; Borowsky, A.D.; Crans, A.; Dillard-Telm, L.; Tam, Y.Y.; Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17384–17389. [Google Scholar] [CrossRef]
- Saba, J.D. Fifty years of lyase and a moment of truth: Sphingosine phosphate lyase from discovery to disease. J. Lipid Res. 2019, 60, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hertervig, E.; Nilsson, A.; Nyberg, L.; Duan, R.D. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer 1997, 79, 448–453. [Google Scholar] [CrossRef]
- García-Barros, M.; Coant, N.; Kawamori, T.; Wada, M.; Snider, A.J.; Truman, J.P.; Wu, B.X.; Furuya, H.; Clarke, C.J.; Bialkowska, A.B.; et al. Role of neutral ceramidase in colon cancer. FASEB J. 2016, 30, 4159–4171. [Google Scholar] [CrossRef]
- Poreba, M.; Groborz, K.; Navarro, M.; Snipas, S.J.; Drag, M.; Salvesen, G.S. Caspase selective reagents for diagnosing apoptotic mechanisms. Cell Death Differ. 2019, 26, 229–244. [Google Scholar] [CrossRef] [PubMed]
- García-Barros, M.; Coant, N.; Truman, J.P.; Snider, A.J.; Hannun, Y.A. Sphingolipids in colon cancer. Biochim. Biophys. Acta 2014, 1841, 773–782. [Google Scholar] [CrossRef]
- Davaille, J.; Li, L.; Mallat, A.; Lotersztajn, S. Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J. Biol. Chem. 2002, 277, 37323–37330. [Google Scholar] [CrossRef]
- Panneer Selvam, S.; Roth, B.M.; Nganga, R.; Kim, J.; Cooley, M.A.; Helke, K.; Smith, C.D.; Ogretmen, B. Balance between senescence and apoptosis is regulated by telomere damage-induced association between p16 and caspase-3. J. Biol. Chem. 2018, 293, 9784–9800. [Google Scholar] [CrossRef]
- Shin, J.H.; Choi, G.S.; Kang, W.H.; Myung, K.B. Sphingosine 1-phosphate triggers apoptotic signal for B16 melanoma cells via ERK and caspase activation. J. Korean Med. Sci. 2007, 22, 298–304. [Google Scholar] [CrossRef]
- Weigert, A.; Cremer, S.; Schmidt, M.V.; von Knethen, A.; Angioni, C.; Geisslinger, G.; Brüne, B. Cleavage of sphingosine kinase 2 by caspase-1 provokes its release from apoptotic cells. Blood 2010, 115, 3531–3540. [Google Scholar] [CrossRef]
- Trejo-Solis, C.; Escamilla-Ramirez, A.; Jimenez-Farfan, D.; Castillo-Rodriguez, R.A.; Flores-Najera, A.; Cruz-Salgado, A. Crosstalk of the Wnt/β-Catenin Signaling Pathway in the Induction of Apoptosis on Cancer Cells. Pharmaceuticals 2021, 14, 871. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Hounsell, C.; Fan, Y. The Duality of Caspases in Cancer, as Told through the Fly. Int. J. Mol. Sci. 2021, 22, 8927. [Google Scholar] [CrossRef]
- Pérez, E.; Lindblad, J.L.; Bergmann, A. Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila. eLife 2017, 6, e26747. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Woo, S.M.; Im, S.S.; Jang, Y.; Han, E.; Kim, S.H.; Lee, H.; Lee, H.S.; Nam, J.O.; Gabrielson, E.; et al. Cathepsin D as a potential therapeutic target to enhance anticancer drug-induced apoptosis via RNF183-mediated destabilization of Bcl-xL in cancer cells. Cell Death Dis. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.F.; Pereira, H.; Alves, S.; Castro, L.; Baltazar, F.; Chaves, S.R.; Preto, A.; Côrte-Real, M. Cathepsin D protects colorectal cancer cells from acetate-induced apoptosis through autophagy independent degradation of damaged mitochondria. Cell Death Dis. 2015, 6, e1788. [Google Scholar] [CrossRef] [PubMed]
- Zebrakovská, I.; Máša, M.; Srp, J.; Horn, M.; Vávrová, K.; Mareš, M. Complex modulation of peptidolytic activity of cathepsin D by sphingolipids. Biochim. Biophys. Acta 2011, 1811, 1097–1104. [Google Scholar] [CrossRef]
- Cuvillier, O.; Rosenthal, D.S.; Smulson, M.E.; Spiegel, S. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide-mediated apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 1998, 273, 2910–2916. [Google Scholar] [CrossRef]
- Cuvillier, O.; Levade, T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood 2001, 98, 2828–2836. [Google Scholar] [CrossRef]
- Heinrich, M.; Neumeyer, J.; Jakob, M.; Hallas, C.; Tchikov, V.; Winoto-Morbach, S.; Wickel, M.; Schneider-Brachert, W.; Trauzold, A.; Hethke, A.; et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004, 11, 550–563. [Google Scholar] [CrossRef]
- Ullio, C.; Casas, J.; Brunk, U.T.; Sala, G.; Fabriàs, G.; Ghidoni, R.; Bonelli, G.; Baccino, F.M.; Autelli, R. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J. Lipid Res. 2012, 53, 1134–1143. [Google Scholar] [CrossRef]
- Kågedal, K.; Zhao, M.; Svensson, I.; Brunk, U.T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 2001, 359, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Baran, Y.; Salas, A.; Senkal, C.E.; Gunduz, U.; Bielawski, J.; Obeid, L.M.; Ogretmen, B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J. Biol. Chem. 2007, 282, 10922–10934. [Google Scholar] [CrossRef]
- Duan, R.D.; Nilsson, A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 2009, 48, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.; Bai, Y.; Liu, Y.; Zhang, B.; Jin, J. Upregulation of sphingosine kinase 1 is associated with recurrence and poor prognosis in papillary thyroid carcinoma. Oncol. Lett. 2019, 18, 5374–5382. [Google Scholar] [CrossRef]
- Su, Y.J.; Huang, J.A.; Liu, S.Q.; Huang, J.X.; Zhong, Y.Y.; Tang, G.D.; Jiang, H.X. The expression and clinical significance of SphK1 and nuclear factor-KB p65 in human colon carcinoma. Zhonghua Nei Ke Za Zhi 2012, 51, 220–224. [Google Scholar]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/ sphingosine-1-phosphate axis in cancer: Potential target for anti-cancer therapy. Pharmacol. Ther. 2018, 195, 85–99. [Google Scholar] [CrossRef]
- Furuya, H.; Shimizu, Y.; Tamashiro, P.M.; Iino, K.; Bielawski, J.; Chan, O.T.M.; Pagano, I.; Kawamori, T. Sphingosine kinase 1 expression enhances colon tumor growth. J. Transl. Med. 2017, 15, 120. [Google Scholar] [CrossRef]
- Park, S.B.; Choi, B.I.; Lee, B.J.; Kim, N.J.; Jeong, Y.A.; Joo, M.K.; Kim, H.J.; Park, J.J.; Kim, J.S.; Noh, Y.S.; et al. Intestinal epithelial deletion of sphk1 prevents colitis-associated cancer development by inhibition of epithelial stat3 activation. Dig. Dis. Sci. 2020, 65, 2284–2293. [Google Scholar] [CrossRef]
- Chumanevich, A.A.; Poudyal, D.; Cui, X.; Davis, T.; Wood, P.A.; Smith, C.D.; Hofseth, L.J. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 2010, 31, 1787–1793. [Google Scholar] [CrossRef]
- Liu, S.Q.; Xu, C.Y.; Wu, W.H.; Fu, Z.H.; He, S.W.; Qin, M.B.; Huang, J.A. Sphingosine kinase 1 promotes the metastasis of colorectal cancer by inducing the epithelial-mesenchymal transition mediated by the FAK/AKT/MMPs axis. Int. J. Oncol. 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Xu, J.; Xue, S. Increased Sphingosine Kinase 1 Expression Is Associated with Poor Prognosis in Human Solid Tumors: A Meta-Analysis. Dis. Markers 2022, 2022, 8443932. [Google Scholar] [CrossRef] [PubMed]
- Thamilselvan, V.; Li, W.; Sumpio, B.E.; Basson, M.D. Sphingosine-1-phosphate stimulates human Caco-2 intestinal epithelial proliferation via p38 activation and activates ERK by an independent mechanism. In Vitro Cell Dev. Biol. Anim. 2002, 38, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Visentin, B.; Vekich, J.A.; Sibbald, B.J.; Cavalli, A.L.; Moreno, K.M.; Matteo, R.G.; Garland, W.A.; Lu, Y.; Yu, S.; Hall, H.S.; et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 2006, 9, 225–238. [Google Scholar] [CrossRef]
- Greenspon, J.; Li, R.; Xiao, L.; Rao, J.N.; Marasa, B.S.; Strauch, E.D.; Wang, J.Y.; Turner, D.J. Sphingosine-1-Phosphate Protects Intestinal Epithelial Cells from Apoptosis Through the Akt Signaling Pathway. Dig. Dis. Sci. 2009, 54, 499–510. [Google Scholar] [CrossRef]
- Widlak, P.; Garrard, W.T. Discovery, regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J. Cell Biochem. 2005, 94, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- White-Gilbertson, S.; Mullen, T.; Senkal, C.; Lu, P.; Ogretmen, B.; Obeid, L.; Voelkel-Johnson, C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase 3 in colon cancer cells. Oncogene 2009, 28, 1132–1141. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 regulates the migration, invasion, and metastasis of colon cancer cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Shanehbandi, D.; Kermani, T.A.; Sanaat, Z.; Zafari, V.; Hashemzadeh, S. Expression Level of Caspase Genes in Colorectal Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1277–1280. [Google Scholar]
- Ahmed, S.F.M.; Shaheed, C.N.B.; Muhammad, E.M.S. Immunohistochemical Expression of Caspase-3 in Colorectal Carcinoma. SVU-IJMS 2022, 5, 240–251. [Google Scholar] [CrossRef]
- Basu, S.; Cheriyamundath, S.; Gavert, N.; Brabletz, T.; Haase, G.; Ben-Ze’ev, A. Increased expression of cathepsin D is required for L1-mediated colon cancer progression. Oncotarget 2019, 10, 5217–5228. [Google Scholar] [CrossRef] [PubMed]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin D—Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef]
- Beckham, T.H.; Lu, P.; Cheng, J.C.; Zhao, D.; Turner, L.S.; Zhang, X.; Hoffman, S.; Armeson, K.E.; Liu, A.; Marrison, T.; et al. Acid ceramidase-mediated production of sphingosine 1-phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int. J. Cancer 2012, 131, 2034–2043. [Google Scholar] [CrossRef]
- Karaca, I.; Tamboli, I.Y.; Glebov, K.; Richter, J.; Fell, L.H.; Grimm, M.O.; Haupenthal, V.J.; Hartmann, T.; Gräler, M.H.; van Echten-Deckert, G.; et al. Deficiency of sphingosine-1-phosphate lyase impairs lysosomal metabolism of the amyloid precursor protein. J. Biol. Chem. 2014, 289, 16761–16772. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Jeong, H.; Krainc, D. Lysosomal ceramides regulate cathepsin B-mediated processing of saposin C and glucocerebrosidase activity. Hum. Mol. Genet. 2022, 31, 2424–2437. [Google Scholar] [CrossRef]
- Degagné, E.; Pandurangan, A.; Bandhuvula, P.; Kumar, A.; Eltanawy, A.; Zhang, M.; Yoshinaga, Y.; Nefedov, M.; de Jong, P.J.; Fong, L.G.; et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Investig. 2014, 124, 5368–5384. [Google Scholar] [CrossRef] [PubMed]
- Uranbileg, B.; Nishikawa, T.; Ikeda, H.; Kurano, M.; Sato, M.; Saigusa, D.; Aoki, J.; Watanabe, T.; Yatomi, Y. Evidence Suggests Sphingosine 1-Phosphate Might Be Actively Generated, Degraded, and Transported to Extracellular Spaces with Increased S1P2 and S1P3 Expression in Colon Cancer. Clin. Color. Cancer 2018, 17, e171–e182. [Google Scholar] [CrossRef] [PubMed]
- Colié, S.; Van Veldhoven, P.P.; Kedjouar, B.; Bedia, C.; Albinet, V.; Sorli, S.C.; Garcia, V.; Djavaheri-Mergny, M.; Bauvy, C.; Codogno, P.; et al. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 2009, 69, 9346–9353. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Fujiya, M.; Konishi, H.; Murakami, Y.; Iwama, T.; Sasaki, T.; Kunogi, T.; Sakatani, A.; Ando, K.; Ueno, N.; et al. Heterogenous nuclear ribonucleoprotein H1 promotes colorectal cancer progression through the stabilization of mRNA of sphingosine-1-phosphate lyase 1. Int. J. Mol. Sci. 2020, 21, 4515. [Google Scholar] [CrossRef]
- Faqar-Uz-Zaman, W.F.; Schmidt, K.G.; Thomas, D.; Pfeilschifter, J.M.; Radeke, H.H.; Schwiebs, A. S1P lyase siRNA dampens malignancy of DLD-1 colorectal cancer cells. Lipids 2021, 56, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Schwiebs, A.; Herrero San Juan, M.; Schmidt, K.G.; Wiercinska, E.; Anlauf, M.; Ottenlinger, F.; Thomas, D.; Elwakeel, E.; Weigert, A.; Farin, H.F.; et al. Cancer-induced inflammation and inflammation-induced cancer in colon: A role for S1P lyase. Oncogene 2019, 38, 4788–4803. [Google Scholar] [CrossRef]
- Scherr, M.; Morgan, M.A.; Eder, M. Gene silencing mediated by small interfering RNAs in mammalian cells. Curr. Med. Chem. 2003, 10, 245–256. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Persson, X.M.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. 2012, 26, 1134–1140. [Google Scholar] [CrossRef]
| HCT(CON) | HCT(SPHK1-) | HCT(SGPL1-) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ceramide | Q1 | Median | Q3 | Q1 | Median | Q3 | Q1 | Median | Q3 |
| C14:0 | 22.25 | 23.56 | 24.9 | 22.46 | 24.55 | 25.15 | 20.71 | 21.45 | 22.92 |
| C16:0 | 419.2 | 462.6 | 489.7 | 393.3 | 426.6 | 436.5 | 547.8 | 566.8 * | 594.2 |
| C18:1 | 10.51 | 10.78 | 11.08 | 11.9 | 12.75 * | 13.03 | 13.21 | 13.99 * | 14.29 |
| C18:0 | 8.59 | 9.09 | 10.56 | 11.75 | 12.29 * | 14.2 | 14.76 | 16.14 * | 16.55 |
| C20:0 | 3.64 | 3.83 | 4.46 | 3.57 | 3.68 | 4.14 | 4.93 | 5.25 * | 5.43 |
| C22:0 | 19.99 | 23.16 | 24.14 | 24.38 | 26.38 | 27.29 | 30.08 | 31.05 * | 33.51 |
| C24:1 | 402.2 | 409.5 | 412.5 | 391.5 | 407.9 | 439.3 | 409.9 | 421.4 | 450.9 |
| C24:0 | 76.89 | 81.08 | 86.43 | 78.98 | 82.76 | 87.99 | 84.01 | 87.87 | 89.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markowski, A.R.; Żbikowski, A.; Zabielski, P.; Chlabicz, U.; Sadowska, P.; Pogodzińska, K.; Błachnio-Zabielska, A.U. The Effect of Silencing the Genes Responsible for the Level of Sphingosine-1-phosphate on the Apoptosis of Colon Cancer Cells. Int. J. Mol. Sci. 2023, 24, 7197. https://doi.org/10.3390/ijms24087197
Markowski AR, Żbikowski A, Zabielski P, Chlabicz U, Sadowska P, Pogodzińska K, Błachnio-Zabielska AU. The Effect of Silencing the Genes Responsible for the Level of Sphingosine-1-phosphate on the Apoptosis of Colon Cancer Cells. International Journal of Molecular Sciences. 2023; 24(8):7197. https://doi.org/10.3390/ijms24087197
Chicago/Turabian StyleMarkowski, Adam R., Arkadiusz Żbikowski, Piotr Zabielski, Urszula Chlabicz, Patrycja Sadowska, Karolina Pogodzińska, and Agnieszka U. Błachnio-Zabielska. 2023. "The Effect of Silencing the Genes Responsible for the Level of Sphingosine-1-phosphate on the Apoptosis of Colon Cancer Cells" International Journal of Molecular Sciences 24, no. 8: 7197. https://doi.org/10.3390/ijms24087197
APA StyleMarkowski, A. R., Żbikowski, A., Zabielski, P., Chlabicz, U., Sadowska, P., Pogodzińska, K., & Błachnio-Zabielska, A. U. (2023). The Effect of Silencing the Genes Responsible for the Level of Sphingosine-1-phosphate on the Apoptosis of Colon Cancer Cells. International Journal of Molecular Sciences, 24(8), 7197. https://doi.org/10.3390/ijms24087197






