Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2
Abstract
1. Introduction
2. Results
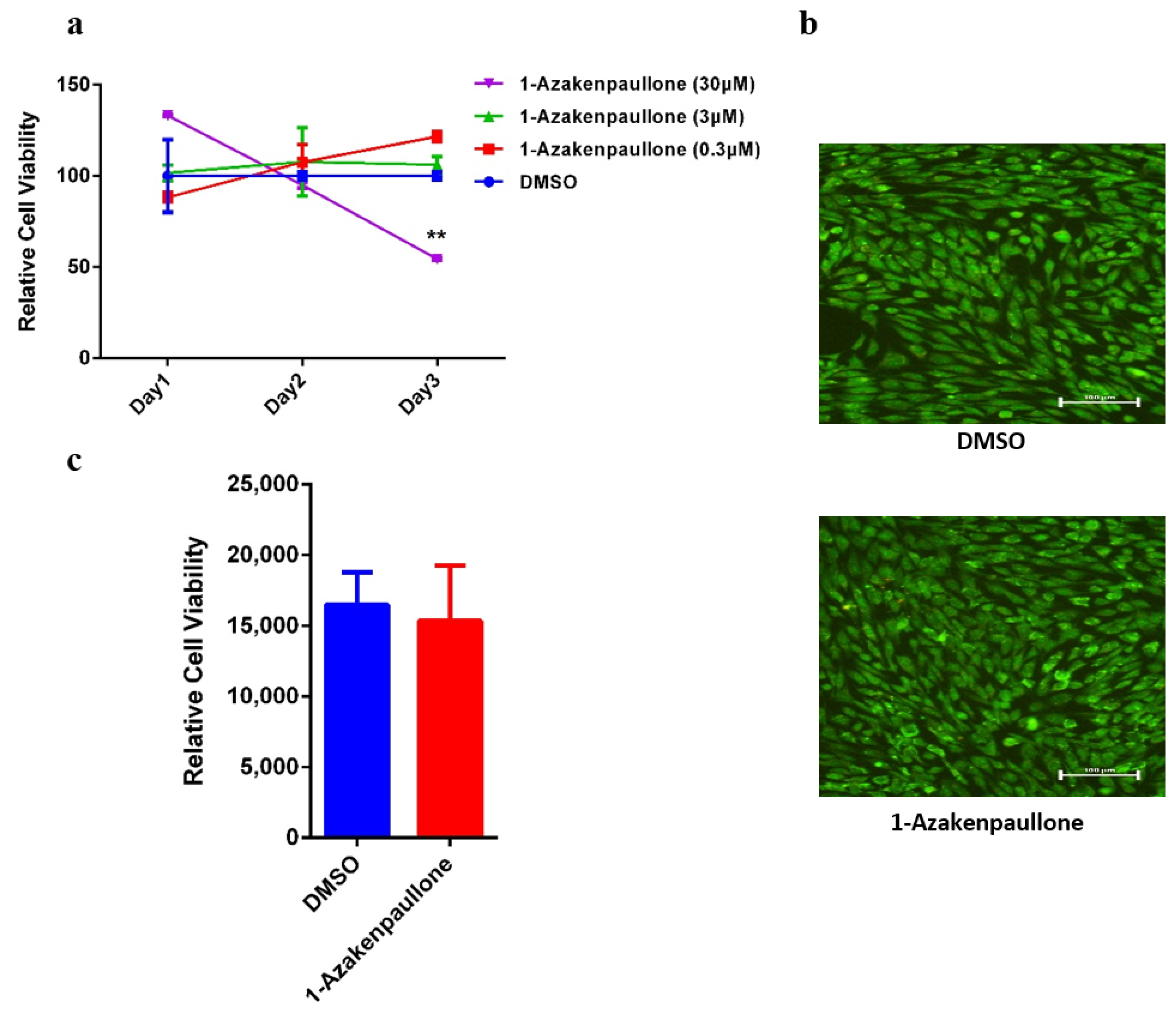
2.1. 1-Azakenpaullone Supports the Proliferation of Human MSCs
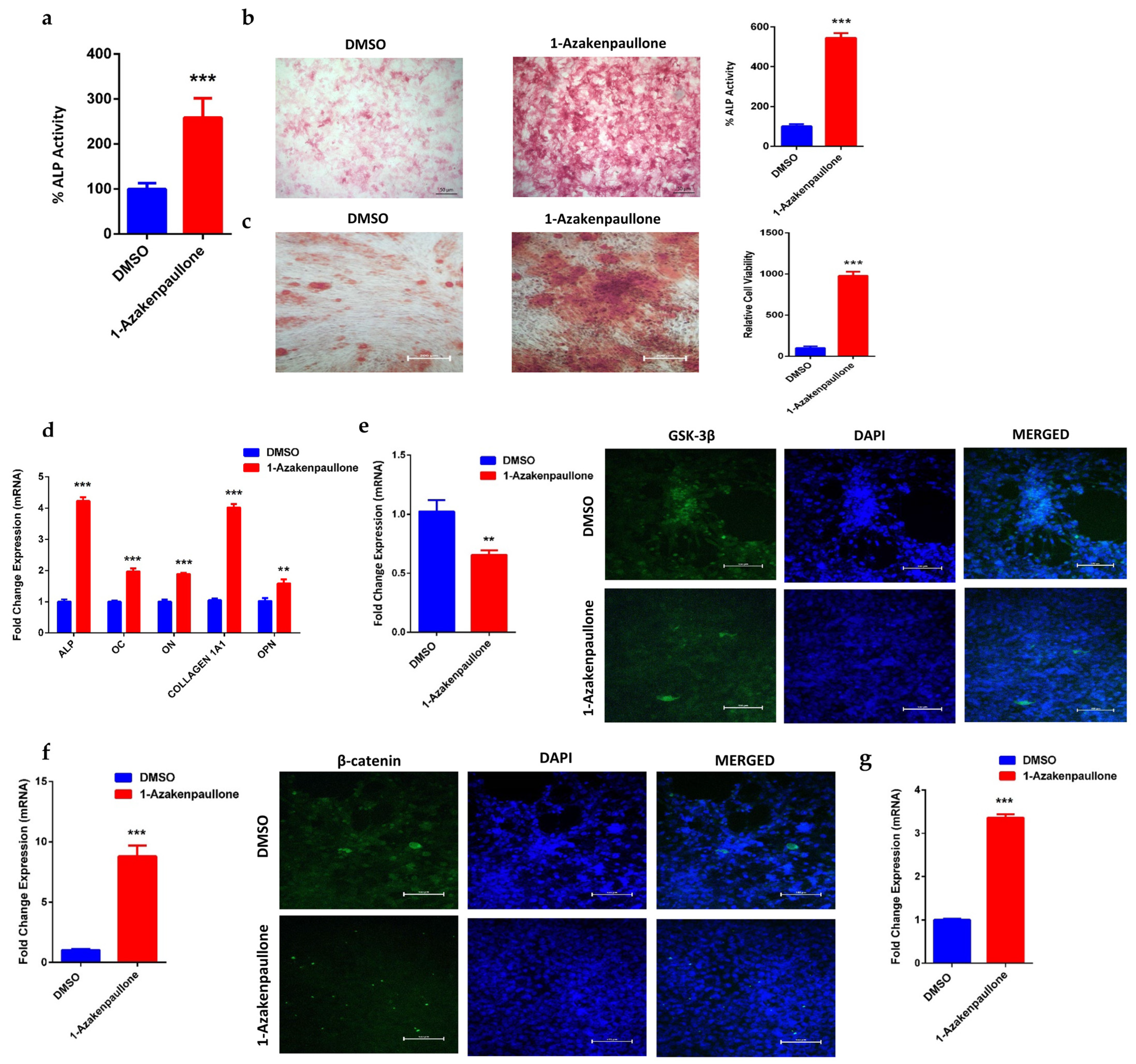
2.2. 1-Azakenpaullone Enhances Osteoblast Differentiation of Human MSCs
2.3. 1-Azakenpaullone Promotes Osteoblastic Differentiation of Human MSCs via Accumulation of β-Catenin, Which Upregulates the Expression of Runx2
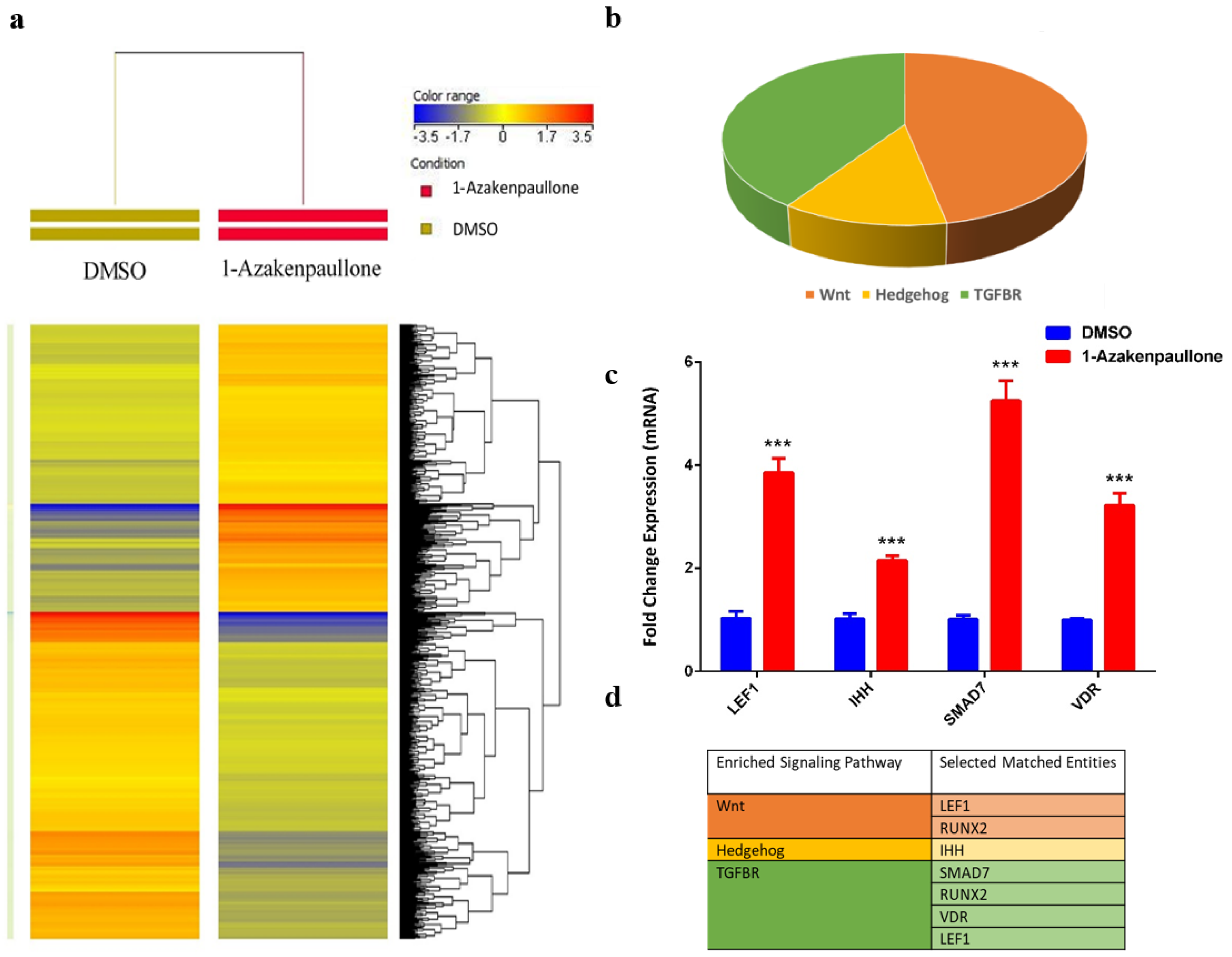
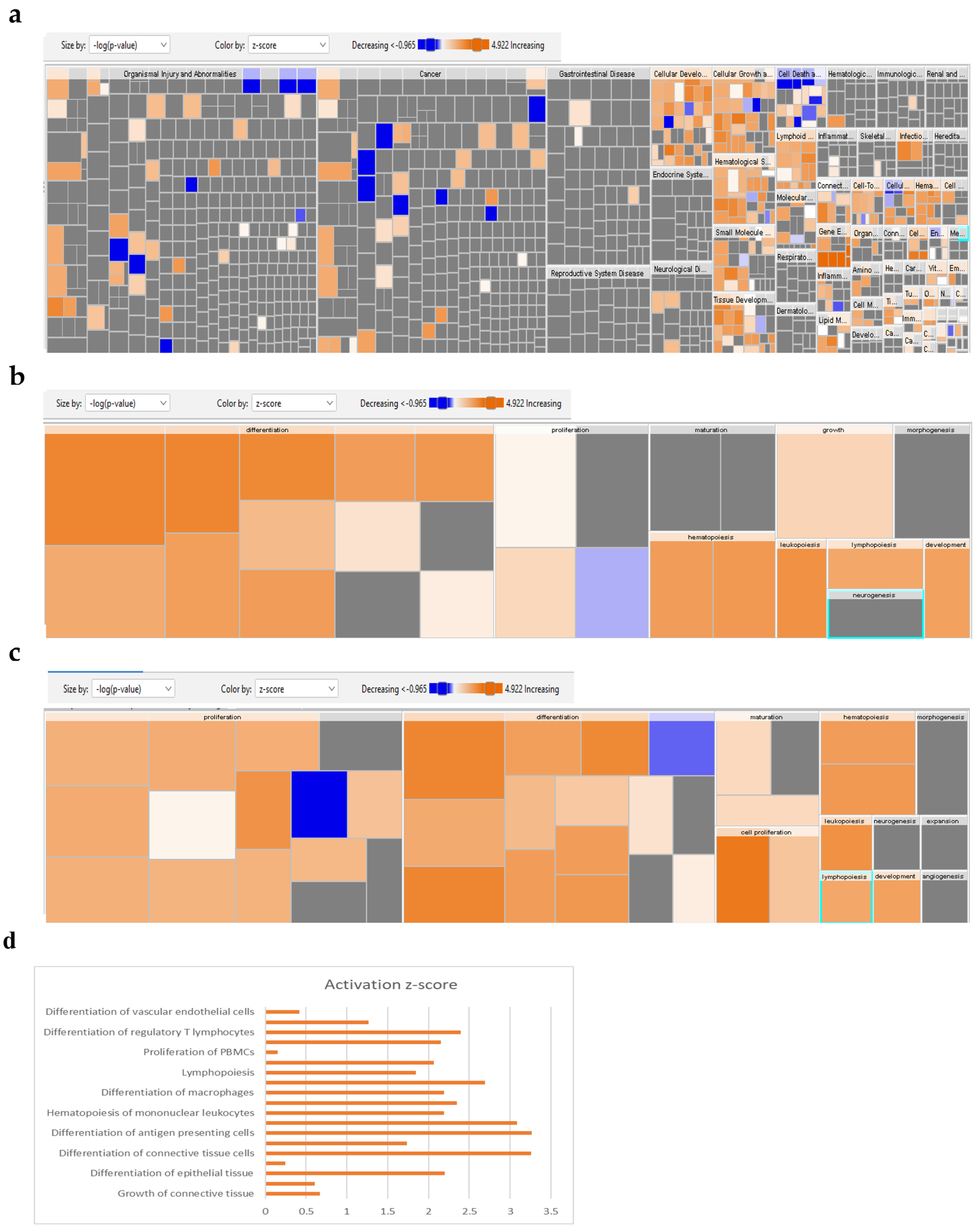
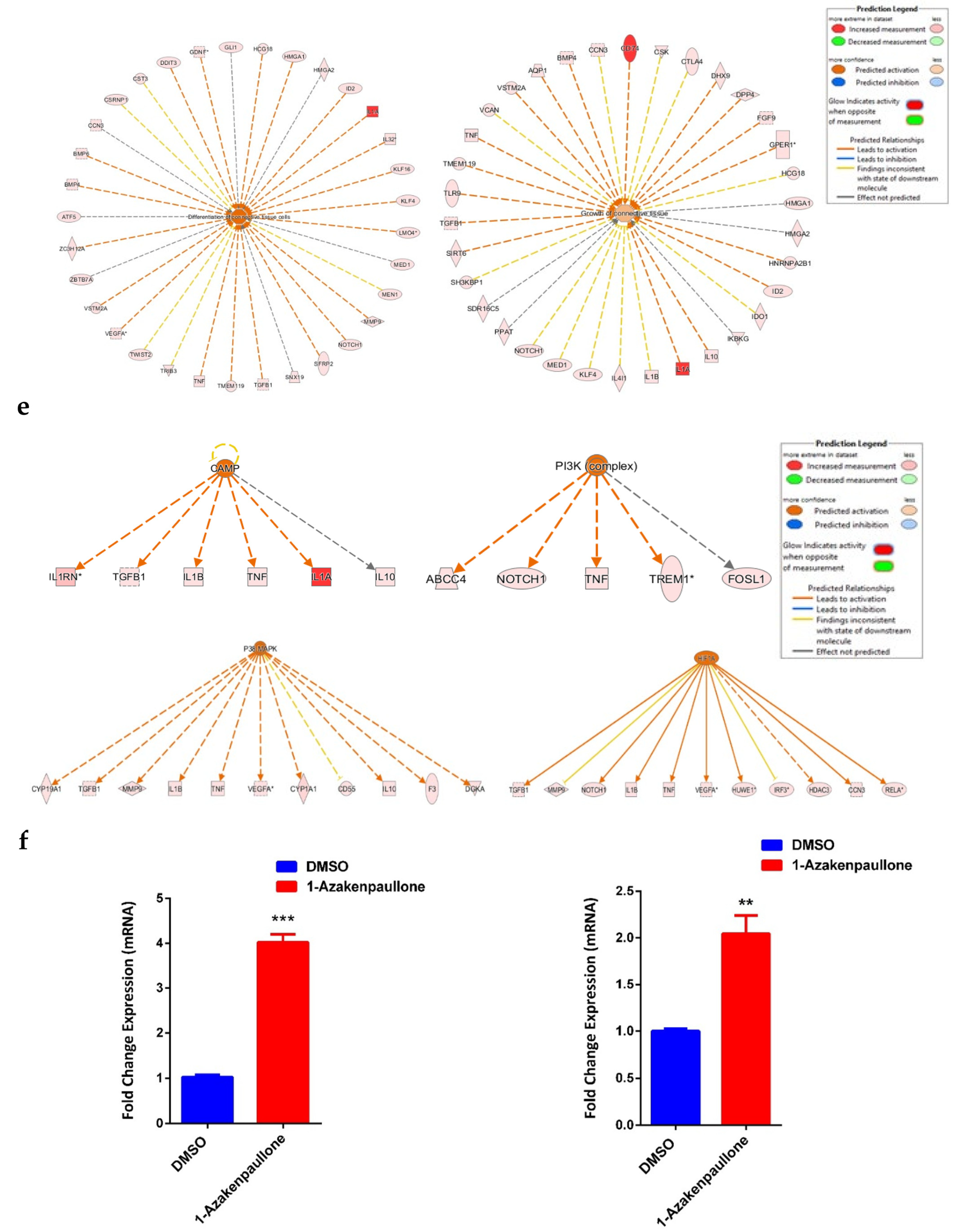
2.4. Global Gene Expression Suggests Several Differentially Expressed Signaling Pathways in 1-Azakenpaullone-Treated Human MSCs
3. Discussion
4. Methods
4.1. Cell Culture
4.2. Osteoblastic Differentiation
4.3. Measurement of Apoptosis
4.4. Quantification of Alkaline Phosphatase Activity
4.5. Alkaline Phosphatase Staining
4.6. Alizarin Red S Staining for Mineralization
4.7. RNA Extraction and cDNA Synthesis
4.8. Quantitative Real-Time Polymerase Chain Reaction
4.9. Immunocytochemistry
4.10. Gene Expression Profiling by Microarray
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kugimiya, F.; Kawaguchi, H.; Ohba, S.; Kawamura, N.; Hirata, M.; Chikuda, H.; Azuma, Y.; Woodgett, J.R.; Nakamura, K.; Chung, U.I. GSK-3beta controls osteogenesis through regulating Runx2 activity. PLoS ONE 2007, 2, e837. [Google Scholar] [CrossRef]
- Wei, J.; Wang, J.; Zhang, J.; Yang, J.; Wang, G.; Wang, Y. Development of inhibitors targeting glycogen synthase kinase-3beta for human diseases: Strategies to improve selectivity. Eur. J. Med. Chem. 2022, 236, 114301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, P.; Liu, Y.; Zhou, Y. GSK3 inhibitor AR-A014418 promotes osteogenic differentiation of human adipose-derived stem cells via ERK and mTORC2/Akt signaling pathway. Biochem. Biophys. Res. Commun. 2017, 490, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.; Eturi, A.; De Souza, A.; Pamarthy, S.; Tavora, F.; Giles, F.J.; Carneiro, B.A. Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol. Ther. 2019, 20, 1047–1056. [Google Scholar] [CrossRef]
- Chen, E.Y.; DeRan, M.T.; Ignatius, M.S.; Grandinetti, K.B.; Clagg, R.; McCarthy, K.M.; Lobbardi, R.M.; Brockmann, J.; Keller, C.; Wu, X.; et al. Glycogen synthase kinase 3 inhibitors induce the canonical WNT/beta-catenin pathway to suppress growth and self-renewal in embryonal rhabdomyosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Ghanghas, P.; Rana, C.; Sanyal, S.N. Role of GSK-3beta in Regulation of Canonical Wnt/beta-catenin Signaling and PI3-K/Akt Oncogenic Pathway in Colon Cancer. Cancer Investig. 2017, 35, 473–483. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Shimozaki, S.; Yamamoto, N.; Domoto, T.; Nishida, H.; Hayashi, K.; Kimura, H.; Takeuchi, A.; Miwa, S.; Igarashi, K.; Kato, T.; et al. Efficacy of glycogen synthase kinase-3beta targeting against osteosarcoma via activation of beta-catenin. Oncotarget 2016, 7, 77038–77051. [Google Scholar] [CrossRef] [PubMed]
- Holmes, T.; O’Brien, T.A.; Knight, R.; Lindeman, R.; Symonds, G.; Dolnikov, A. The role of glycogen synthase kinase-3beta in normal haematopoiesis, angiogenesis and leukaemia. Curr. Med. Chem. 2008, 15, 1493–1499. [Google Scholar] [CrossRef]
- Miyashita, K.; Nakada, M.; Shakoori, A.; Ishigaki, Y.; Shimasaki, T.; Motoo, Y.; Kawakami, K.; Minamoto, T. An emerging strategy for cancer treatment targeting aberrant glycogen synthase kinase 3 beta. Anticancer. Agents Med. Chem. 2009, 9, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Srivani, G.; Sharvirala, R.; Veerareddy, P.R.; Pal, D.; Kiran, G. GSK-3 Inhibitors as New Leads to Treat Type-II Diabetes. Curr. Drug. Targets 2021, 22, 1555–1567. [Google Scholar] [CrossRef]
- MacAulay, K.; Woodgett, J.R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert. Opin. Ther. Targets 2008, 12, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Cescon, E.; Grieco, I.; Spalluto, G.; Federico, S. Glycogen Synthase Kinase 3beta Involvement in Neuroinflammation and Neurodegenerative Diseases. Curr. Med. Chem. 2022, 29, 4631–4697. [Google Scholar] [CrossRef]
- Kandar, C.C.; Sen, D.; Maity, A. Anti-inflammatory Potential of GSK-3 Inhibitors. Curr. Drug. Targets 2021, 22, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell. Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yoshida, K.; Nishizawa, T.; Otani, K.; Yamashita, Y.; Okabe, H.; Hadano, Y.; Kayama, T.; Kurosaka, D.; Saito, M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022, 23, 2871. [Google Scholar] [CrossRef] [PubMed]
- Almasoud, N.; Binhamdan, S.; Younis, G.; Alaskar, H.; Alotaibi, A.; Manikandan, M.; Alfayez, M.; Kassem, M.; AlMuraikhi, N. Author Correction: Tankyrase inhibitor XAV-939 enhances osteoblastogenesis and mineralization of human skeletal (mesenchymal) stem cells. Sci. Rep. 2021, 11, 4559. [Google Scholar] [CrossRef]
- AlMuraikhi, N.; Ali, D.; Alshanwani, A.; Vishnubalaji, R.; Manikandan, M.; Atteya, M.; Siyal, A.; Alfayez, M.; Aldahmash, A.; Kassem, M.; et al. Stem cell library screen identified ruxolitinib as regulator of osteoblastic differentiation of human skeletal stem cells. Stem Cell. Res. Ther. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- AlMuraikhi, N.; Alaskar, H.; Binhamdan, S.; Alotaibi, A.; Kassem, M.; Alfayez, M. JAK2 Inhibition by Fedratinib Reduces Osteoblast Differentiation and Mineralisation of Human Mesenchymal Stem Cells. Molecules 2021, 26, 606. [Google Scholar] [CrossRef] [PubMed]
- AlMuraikhi, N.; Almasoud, N.; Binhamdan, S.; Younis, G.; Ali, D.; Manikandan, M.; Vishnubalaji, R.; Atteya, M.; Siyal, A.; Alfayez, M.; et al. Hedgehog Signaling Inhibition by Smoothened Antagonist BMS-833923 Reduces Osteoblast Differentiation and Ectopic Bone Formation of Human Skeletal (Mesenchymal) Stem Cells. Stem Cells Int. 2019, 2019, 3435901. [Google Scholar] [CrossRef] [PubMed]
- Elsafadi, M.; Manikandan, M.; Almalki, S.; Mobarak, M.; Atteya, M.; Iqbal, Z.; Hashmi, J.A.; Shaheen, S.; Alajez, N.; Alfayez, M.; et al. TGFbeta1-Induced Differentiation of Human Bone Marrow-Derived MSCs Is Mediated by Changes to the Actin Cytoskeleton. Stem Cells Int. 2018, 2018, 6913594. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Irelli, A.; Sirufo, M.M.; Scipioni, T.; De Pietro, F.; Pancotti, A.; Ginaldi, L.; De Martinis, M. mTOR Links Tumor Immunity and Bone Metabolism: What are the Clinical Implications? Int. J. Mol. Sci. 2019, 20, 5841. [Google Scholar] [CrossRef] [PubMed]
- AlMuraikhi, N.; Ali, D.; Vishnubalaji, R.; Manikandan, M.; Atteya, M.; Siyal, A.; Alfayez, M.; Aldahmash, A.; Kassem, M.; Alajez, N.M. Notch Signaling Inhibition by LY411575 Attenuates Osteoblast Differentiation and Decreased Ectopic Bone Formation Capacity of Human Skeletal (Mesenchymal) Stem Cells. Stem Cells Int. 2019, 2019, 3041262. [Google Scholar] [CrossRef]
- Kunick, C.; Lauenroth, K.; Leost, M.; Meijer, L.; Lemcke, T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 beta. Bioorg Med. Chem. Lett. 2004, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730. [Google Scholar]
- Miyashita, K.; Kawakami, K.; Nakada, M.; Mai, W.; Shakoori, A.; Fujisawa, H.; Hayashi, Y.; Hamada, J.; Minamoto, T. Potential therapeutic effect of glycogen synthase kinase 3beta inhibition against human glioblastoma. Clin. Cancer Res. 2009, 15, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Z.; Duan, C.; Liu, W.; Sun, J.; Han, B. Role of TCF/LEF Transcription Factors in Bone Development and Osteogenesis. Int. J. Med. Sci. 2018, 15, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Chung, U.I.; Ohba, S.; McMahon, J.; Kronenberg, H.M.; McMahon, A.P. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 2004, 131, 1309–1318. [Google Scholar] [CrossRef]
- Li, N.; Lee, W.Y.; Lin, S.E.; Ni, M.; Zhang, T.; Huang, X.R.; Lan, H.Y.; Li, G. Partial loss of Smad7 function impairs bone remodeling, osteogenesis and enhances osteoclastogenesis in mice. Bone 2014, 67, 46–55. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Udagawa, N.; Horibe, K.; Mizoguchi, T.; Yamamoto, Y.; Nakamura, T.; Hosoya, A.; Kato, S.; Suda, T.; Takahashi, N. VDR in Osteoblast-Lineage Cells Primarily Mediates Vitamin D Treatment-Induced Increase in Bone Mass by Suppressing Bone Resorption. J. Bone Miner. Res. 2017, 32, 1297–1308. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Kang, C.H.; Lee, M.H.; Choi, Y.H.; Lee, C.M.; Lee, S.; Kim, G.Y. Fisetin promotes osteoblast differentiation and osteogenesis through GSK-3beta phosphorylation at Ser9 and consequent beta-catenin activation, inhibiting osteoporosis. Biochem. Pharmacol. 2021, 192, 114676. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Shen, Y.S.; He, M.C.; Yang, F.; Yang, P.; Pang, F.X.; He, W.; Cao, Y.M.; Wei, Q.S. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2019, 112, 108746. [Google Scholar] [CrossRef] [PubMed]
- Etich, J.; Rehberg, M.; Eckes, B.; Sengle, G.; Semler, O.; Zaucke, F. Signaling pathways affected by mutations causing osteogenesis imperfecta. Cell. Signal. 2020, 76, 109789. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, L.H.; Secreto, F.J.; Westendorf, J.J. Wnt signaling as a therapeutic target for bone diseases. Expert. Opin. Ther. Targets 2009, 13, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guan, G.F.; Chen, J.; Hu, B.; Sun, C.; Ma, Q.; Wen, Y.H.; Qiu, X.C.; Zhou, Y. Aberrant CXCR4 and beta-catenin expression in osteosarcoma correlates with patient survival. Oncol. Lett. 2015, 10, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Zhao, Z.Q.; Wang, Y.Q.; Li, W.G.; Su, Q.; Jia, Q.; Zhang, J.J.; Zhang, X.L.; Yin, J.Q.; Shen, J.N. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/beta-catenin signaling pathway. Cell. Death Dis. 2018, 9, 343. [Google Scholar] [CrossRef]
- Sun, N.Y.; Liu, X.L.; Gao, J.; Wu, X.H.; Dou, B. Astragaloside-IV modulates NGF-induced osteoblast differentiation via the GSK3 beta/beta-catenin signalling pathway. Mol. Med. Rep. 2021, 23, 19. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.L.; Hu, X.T.; Wang, X.T.; Chen, A.M. Metformin promotes differentiation of human bone marrow derived mesenchymal stem cells into osteoblast via GSK3 beta inhibition. Eur. Rev. Med. Pharmaco 2018, 22, 7962–7968. [Google Scholar]
- Zhao, R.B.; Li, Y.S.; Lin, Z.Y.; Wan, J.; Xu, C.; Zeng, Y.; Zhu, Y. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3 beta/beta-catenin signaling pathway. Biochem. Bioph Res. Co. 2016, 477, 749–754. [Google Scholar] [CrossRef]
- Baron, R.; Rawadi, G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.H.; Onyia, J.E.; Zeng, Q.; Tian, X.; Liu, M.; Halladay, D.L.; Frolik, C.A.; Engler, T.; Wei, T.; Kriauciunas, A.; et al. Orally bioavailable GSK-3alpha/beta dual inhibitor increases markers of cellular differentiation in vitro and bone mass in vivo. J. Bone Miner. Res. 2006, 21, 910–920. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Haack-Sorensen, M.; Burns, J.S.; Elsnab, B.; Jakob, F.; Hokland, P.; Kassem, M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem. Biophys. Res. Commun. 2005, 326, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Elango, R.; Al-Toub, M.; Manikandan, M.; Al-Rikabi, A.; Harkness, L.; Ditzel, N.; Atteya, M.; Hamam, R.; Alfayez, M.; et al. Neoplastic Transformation of Human Mesenchymal Stromal Cells Mediated via LIN28B. Sci. Rep. 2019, 9, 8101. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Manikandan, M.; Fahad, M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Alajez, N.M. Molecular profiling of ALDH1(+) colorectal cancer stem cells reveals preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget 2018, 9, 13551–13564. [Google Scholar] [CrossRef] [PubMed]
- Calvano, S.E.; Xiao, W.; Richards, D.R.; Felciano, R.M.; Baker, H.V.; Cho, R.J.; Chen, R.O.; Brownstein, B.H.; Cobb, J.P.; Tschoeke, S.K.; et al. A network-based analysis of systemic inflammation in humans. Nature 2005, 437, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Official Symbol | Accession Number | Product Length | Forward Primer | Reverse Primer |
|---|---|---|---|---|---|
| GAPDH | GAPDH | NM_001256799.3 | 81 | CTGGTAAAGTGGATATTGTTGCCAT | TGGAATCATATTGGAACATGTAAACC |
| ALP | ALPL | NM_001127501.4 | 86 | GGAACTCCTGACCCTTGACC | 5′TCCTGTTCAGCTCGTACTGC3′ |
| OC | BGLAP | NM_001199662.1 | 102 | GGCAGCGAGGTAGTGAAGAG | CTCACACACCTCCCTCCTG |
| ON | SPARC | NM_001309444.2 | 95 | GAGGAAACCGAAGAGGAGG | 5′GGGGTGTTGTTCTCATCCAG3′ |
| RUNX2 | RUNX2 | NM_001015051.4 | 78 | GTAGATGGACCTCGGGAACC | 5′GAGGCGGTCAGAGAACAAAC3′ |
| OPN | SPP1 | NM_000582.3 | 348 | GGTGATGTCCTCGTCTGTA | CCAAGTAAGTCCAACGAAAG |
| COL1A1 | COL1A1 | NM_000088.4 | 52 | 5′GAGTGCTGTCCCGTCTGC3′ | 5′TTTCTTGGTCGGTGGGTG3′ |
| GSK-3β | GSK3B | NM_001146156.2 | 106 | 5′GGAACTCCAACAAGGGAGCA3′ | 5′TTCGGGGTCGGAAGACCTTA3′ |
| β-catenin | CTNNB1 | NM_001330729.2 | 108 | 5′ATGGAGCCGGACAGAAAAGC3′ | 5′CTTGCCACTCAGGGAAGGA3′ |
| LEF1 | LEF1 | NM_001130713.3 | 134 | 5′CTTTATCCAGGCTGGTCTGC | 5′TCGTTTTCCACCATGTTTCA |
| IHH | IHH | NM_001346281.2 | 109 | CAGCGATGTGCTCATTTTCCT | AAGGCTCTCAGCCTGTGAGG |
| SMAD7 | SMAD7 | NM_005904.4 | 95 | CCCATCACCTTAGCCGACTC | TGGACAGTCTGCAGTTGGTTT |
| VDR | VDR | NM_001017535.2 | 87 | CTCTGATAGCCTCATGCCAGG | ACCCAAAGGCTTCCCAAAGAG |
| TGFB1 | TGFB1 | NM_000660.7 | 300 | 5′GAAACCCACAACGAAATC3′ | 5′AATTTCCCCTCCACGGCT3′ |
| TNF | TNF | NM_000594.4 | 139 | 5′ACTTTGGAGTGATCGGCC3′ | 5′GCTTGAGGGTTTGCTACAAC3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMuraikhi, N.; Binhamdan, S.; Alaskar, H.; Alotaibi, A.; Tareen, S.; Muthurangan, M.; Alfayez, M. Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2. Int. J. Mol. Sci. 2023, 24, 7164. https://doi.org/10.3390/ijms24087164
AlMuraikhi N, Binhamdan S, Alaskar H, Alotaibi A, Tareen S, Muthurangan M, Alfayez M. Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2. International Journal of Molecular Sciences. 2023; 24(8):7164. https://doi.org/10.3390/ijms24087164
Chicago/Turabian StyleAlMuraikhi, Nihal, Sarah Binhamdan, Hanouf Alaskar, Amal Alotaibi, Sumaiya Tareen, Manikandan Muthurangan, and Musaad Alfayez. 2023. "Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2" International Journal of Molecular Sciences 24, no. 8: 7164. https://doi.org/10.3390/ijms24087164
APA StyleAlMuraikhi, N., Binhamdan, S., Alaskar, H., Alotaibi, A., Tareen, S., Muthurangan, M., & Alfayez, M. (2023). Inhibition of GSK-3β Enhances Osteoblast Differentiation of Human Mesenchymal Stem Cells through Wnt Signalling Overexpressing Runx2. International Journal of Molecular Sciences, 24(8), 7164. https://doi.org/10.3390/ijms24087164





