The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence
Abstract
1. Introduction
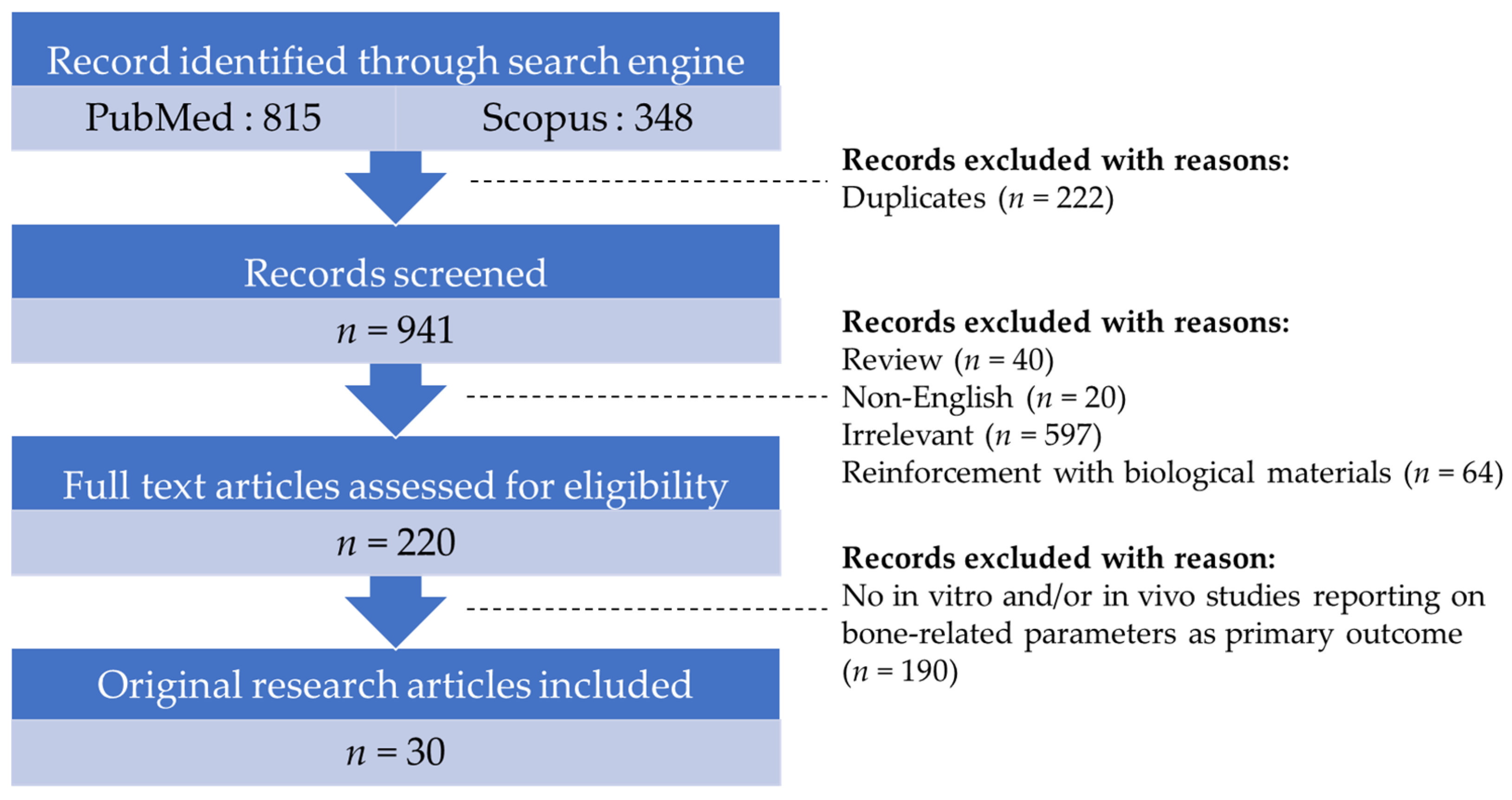
2. Literature Search
3. The Enhancement of CPC Using Synthetic Materials
3.1. Synthetic Polymers
| Enhancer | Type of Study | Cell Culture/Animal Model | Findings | References |
|---|---|---|---|---|
| Dense & irregularly shaped PLGA | In vitro | Mouse bone mesenchymal stem cell | Cell proliferation: ↑, setting time: ↔, degradation rate: ↑, compressive strength: ↑, good biocompatibility | [12] |
| Porous PLGA | Cell proliferation: ↑, final setting time: ↑, compressive strength: ↓, good biocompatibility | |||
| PLGA | In vitro | hUCMSCs | Flexural strength: ↑, work-of-fracture: ↑, cell proliferation: ↑, ALP: ↑, OCN: ↑, COL1: ↑, mineralisation: ↑ | [13] |
| PLGA | In vitro | rMSCs | Bone regeneration: ↑ | [14] |
| In vivo | Defect in the femora of New Zealand white rabbits | Cell proliferation: ↑, ALP activity: ↑ | ||
| PLGA | In vivo | Bone defect at L4 lumbar vertebral body in aged osteopenicfemale sheep | BV/TV: ↑, BMD: ↑, biomechanical compression strength: ↑, bone erosion: ↓, osteoid volume: ↑, osteoid surface: ↑ | [15] |
| PEGS | In vitro | rMSCs | Cell viability: ↑, cell proliferation: ↑, cell attachment: ↑, COL1: ↑, Runx-2: ↑, OCN: ↑ | [16] |
| In vivo | Calvarial defect model of male Sprague- Dawley rats | BV/TV: ↑, Tb.Th: ↑, mineralisation: ↑ | ||
| PAAS | In vitro | Mouse bone marrow stromal cells | Setting time: ↑, compressive strength: ↑, cell proliferation: ↑ | [18] |
3.2. Biomimetic Materials
| Enhancer | Type of Study | Cell Culture/Animal Model | Findings | References |
|---|---|---|---|---|
| Synthetic collagen I mimetic P-15 | In vitro | Human mesenchymal stem cell | ALP: ↑, OPN: ↑, Runx-2: ↑, COL1: ↑, osteonectin: ↑, OCN: ↑, calcium deposit: ↑ | [21] |
| In vivo | Sheep vertebra of non- osteoporotic and osteoporotic model | Pull-out strength: ↑ | ||
| Trimethyl chitosan | In vitro | MG-63 cells | Setting time: ↑, specific surface area: ↓, wettability: ↑, compressive strength: ↔, elastic modulus: ↑, bending strength: ↑, cell viability: ↑, biocompatibility: ↑, load-bearing capacity: ↑ | [23] |
| Chondroitin sulphate | In vitro | Bone mesenchymal stem cells | Setting time: ↑, injectability: ↑, fibronectin adsorption: ↑, cell proliferation: ↑, Runx-2: ↑, COL1: ↑, OCN: ↑, ALP: ↑, OPN: ↑ | [25] |
3.3. Chemical Elements and Compounds
| Enhancer | Type of Study | Cell Culture/Animal Model | Findings | References |
|---|---|---|---|---|
| Strontium | In vitro | MG-63 cells | Setting time: ↓, compressive strength: ↔, cell proliferation: ↑, ALP activity: ↑ | [28] |
| Strontium | In vitro | Primary human mesenchymal stromal cells | Setting time: ↔, compressive strength: ↑, cell proliferation: ↑, ALP activity: ↑, | [27] |
| In vivo | Vertebral body reconstruction in an 80-year-old male cadaver | Viscosity: ↑, tendency to leak out: ↓ | ||
| Strontium | In vivo | Calvarial bone defect in male Sprague-Dawley rats | Bone formation: ↑, BMP-2: ↑, OCN: ↑, OPG: ↑ | [30] |
| Strontium | In vivo | Two bone defects, one at distal femoral condyle and one at proximal tibial metaphysis of adult female merino sheep | B.Ar/T.Ar: ↑, material resorption: ↔, osteoclast number: ↔ | [29] |
| Strontium ranelate | In vitro | Mouse bone marrow mesenchymal stem cells | Cell spreading area: ↑, cell proliferation: ↑, COL1: ↑, ALP: ↑, OCN: ↑, Runx-2: ↑ | [31] |
| In vitro | RAW264.7 cells | TRAP: ↓, CTSK: ↓, MMP-9: ↓, Car2: ↓ | ||
| Selenium | In vivo | Bone defect at femoral epiphysis ofovariectomised rats | BMD: ↑, BV/TV: ↑, Tb.N: ↑, Conn.D: ↑, Tb.Th: ↑, Tb.Sp: ↓, bone regeneration: ↑, biomaterial degradation: ↑, MAR: ↑, SOD: ↑, CAT: ↓, GPX: ↑, OPG: ↑, RANKL: ↓ | [33] |
| Iron | In vitro | Mouse bone marrow stromal cells | Setting time: ↓, injectability: ↑, compressive strength: ↑, cell proliferation: ↑, ALP: ↑, COL1: ↑, OPN: ↑, Runx-2: ↑ | [36] |
| Human umbilical vein endothelial cells | VEGF: ↑, eNOS: ↑ | |||
| Iron | In vivo | Four bone defects created at the proximal and distal extremities of the humerus and femur in female Romanian alpine sheep | New bone and blood vessel formation: ↑, cells at resorption zone: ↑, osteoid formation: ↑, no inflammation & necrosis | [37] |
| Zinc | In vitro | Mouse bone mesenchymal stem cells | Cell proliferation: ↑, ALP activity: ↑, COL1: ↑, Runx-2: ↑ | [40] |
| Zinc | In vitro | MC3T3-E1 cells | Setting time: ↓, tensile strength: ↑, cell viability: ↑, cell proliferation: ↑, ALP activity: ↑ | [41] |
| Magnesium | In vitro | Rat bone marrow stromal cells | Fibronectin adsorption: ↑, cell attachment: ↑, integrin α5β1 expression: ↑, ALP: ↑, COL1: ↑, OCN: ↑ | [44] |
| In vivo | Calvarial defect of 4-month-old Sprague Dawley rats | New bone formation: ↑, material residue: ↓ | ||
| Magnesium | In vitro | MG-63 cells | Cell proliferation: ↑ | [45] |
| In vivo | Calvarial defect in New Zealand rabbits | New bone formation: ↑ | ||
| Copper | In vitro | Mouse bone marrow mesenchymal stem cells | Setting time: ↑, compressive strength: ↑, porosity: ↔, injectability: ↑, adhesion activity: ↑, cell proliferation: ↑, COL1: ↑, OCN: ↑, ALP: ↑ | [47] |
| Human umbilical vein endothelial cells | eNOS: ↑, VEGF: ↑, bFGF: ↑, nitric oxide: ↑ | |||
| Lithium chloride | In vitro | MC3T3-E1 cells | Cell proliferation: ↑, ALP activity: ↑, mineralisation: ↑, COL1: ↑, OCN: ↑, OPG: ↑, Runx-2: ↑, p-β-catenin: ↓, p-GSK3β: ↑ | [50] |
| In vivo | Bone defect at the medial tibial shaft of female ovariectomised Sprague-Dawley rats | BMD: ↑, new bone formation: ↑, the gap was occupied by new bone. | ||
| Silicon carbide whiskers | In vitro | MC3T3-E1 cells | Flexural strength: ↑, work-of-fracture: ↑, elastic modulus: ↑, hardness: ↑, cell adhesion: ↔, cell viability: ↔, cell proliferation: ↔ | [52] |
| Calcium silicate | In vitro | MC3T3-E1 cells | Cell proliferation: ↑, ALP activity: ↑ | [55] |
| Human umbilical vein endothelial cell |
3.4. Combination between Two or More Synthetic Materials
| Enhancer | Type of Study | Cell Culture/Animal Model | Findings | References |
|---|---|---|---|---|
| PLGA + wollastonite | In vitro | Mouse bone mesenchymal stem cells | Flexibility: ↑, cell attachment: ↑, cell proliferation: ↑, Runx-2: ↑, COL1: ↑, BSP: ↑ | [57] |
| In vivo | Bone defect at femoral condyle of New Zealand rabbits | New bone formation: ↑, material residual: ↓, bone matrix: ↑, new blood vessel: ↑ | ||
| PLGA + PFCE + gold nanoparticles | In vivo | Bone defect at femoral condyle of male Wistar rats | New bone formation: ↑ | [60] |
| PLGA + silicon/zinc | In vitro | rMSCs | Setting time: ↑, injectability: ↑, compressive strength: ↑, BMP-2: ↑ | [61] |
| RAW 264.7 cells | cell adhesion & spreading: ↑, TNF-α: ↓, IL-6: ↓, IL-10: ↑, TGF-1β: ↑, VEGF, PDGF-BB: ↑ | |||
| In vivo | Bone defect at femur of male Sprague- Dawley rats | New bone formation: ↑, BV/TV: ↑, residual material: ↓ | ||
| PLGA microspheres + simvastatin + nanostrontium | In vivo | Parietal bone defect in male New Zealand white rabbits | New bone area: ↑, good biocompatibility | [30] |
| PLGA + alendronate | In vivo | Bone defect at both femoral condyles of ovariectomised female Wistar rats | Setting time: ↑, compressive strength: ↓, bone formation: ↑, BMD: ↑ | [3] |
| Dexamethasone + Laponite® nanoplates | In vitro | MG63 cells | Compressive strength: ↑, setting time: ↓, cell proliferation: ↑ | [68] |
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- van Houdt, C.I.; Gabbai-Armelin, P.R.; Lopez-Perez, P.M.; Ulrich, D.J.; Jansen, J.A.; Renno, A.C.M.; van den Beucken, J.J. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef] [PubMed]
- Canillas, M.; Pena, P.; Antonio, H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín De La Soc. Española De Cerámica Y Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Kloss, F.R.; Offermanns, V.; Kloss-Brandstätter, A. Comparison of allogeneic and autogenous bone grafts for augmentation of alveolar ridge defects-A 12-month retrospective radiographic evaluation. Clin. Oral Implant. Res. 2018, 29, 1163–1175. [Google Scholar] [CrossRef]
- Schroeder, J.E.; Mosheiff, R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef]
- Parchi, P.D.; Simonetti, M.; Bonicoli, E.; Piolanti, N.; Scaglione, M. Synthetic Bone Grafting in Aseptic Loosening of Acetabular Cup: Good Clinical and Radiological Outcomes in Contained Bone Defects at Medium-Term Follow Up. Int. J. Environ. Res. Public Health 2020, 17, 5624. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Wong, S.K.; Wong, Y.H.; Chin, K.Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Lim, J.V.; Bee, S.T.; Tin Sin, L.; Ratnam, C.T.; Abdul Hamid, Z.A. A Review on the Synthesis, Properties, and Utilities of Functionalized Carbon Nanoparticles for Polymer Nanocomposites. Polymers 2021, 13, 3547. [Google Scholar] [CrossRef]
- Namazi, H. Polymers in our daily life. BioImpacts BI 2017, 7, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; He, F.; Ye, J. Physicochemical Properties, In Vitro Degradation, and Biocompatibility of Calcium Phosphate Cement Incorporating Poly(lactic-co-glycolic acid) Particles with Different Morphologies: A Comparative Study. ACS Omega 2021, 6, 8322–8331. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Chen, W.; Weir, M.D.; Thein-Han, W.; Xu, H.H. Effects of electrospun submicron fibers in calcium phosphate cement scaffold on mechanical properties and osteogenic differentiation of umbilical cord stem cells. Acta Biomater. 2011, 7, 4037–4044. [Google Scholar] [CrossRef]
- He, F.; Ye, J. In vitro degradation, biocompatibility, and in vivo osteogenesis of poly(lactic-co-glycolic acid)/calcium phosphate cement scaffold with unidirectional lamellar pore structure. J. Biomed. Mater. Res. Part A 2012, 100, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Maenz, S.; Brinkmann, O.; Kunisch, E.; Horbert, V.; Gunnella, F.; Bischoff, S.; Schubert, H.; Sachse, A.; Xin, L.; Günster, J.; et al. Enhanced bone formation in sheep vertebral bodies after minimally invasive treatment with a novel, PLGA fiber-reinforced brushite cement. Spine J. Off. J. N. Am. Spine Soc. 2017, 17, 709–719. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. PEGylated poly(glycerol sebacate)-modified calcium phosphate scaffolds with desirable mechanical behavior and enhanced osteogenic capacity. Acta Biomater. 2016, 44, 110–124. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Wang, Z.; Zhang, J.; Zhu, J.; Ma, Y.; Yang, Z.; Yuan, Y. Optimized Synthesis of Biodegradable Elastomer PEGylated Poly(glycerol sebacate) and Their Biomedical Application. Polymers 2019, 11, 965. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Ye, J. Preparation, characterization and in vitro cell performance of anti-washout calcium phosphate cement modified by sodium polyacrylate. RSC Adv. 2017, 7, 32842–32849. [Google Scholar] [CrossRef]
- Glaser, D.E.; Viney, C. Biomimetic materials. In Biomaterials science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 349–360. [Google Scholar]
- Gomar, F.; Orozco, R.; Villar, J.L.; Arrizabalaga, F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial. Int. Orthop. 2007, 31, 93–99. [Google Scholar] [CrossRef]
- Krenzlin, H.; Foelger, A.; Mailänder, V.; Blase, C.; Brockmann, M.; Düber, C.; Ringel, F.; Keric, N. Novel Biodegradable Composite of Calcium Phosphate Cement and the Collagen I Mimetic P-15 for Pedicle Screw Augmentation in Osteoporotic Bone. Biomedicines 2021, 9, 1392. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Gallinetti, S.; Mestres, G.; Canal, C.; Persson, C.; Ginebra, M.P. A novel strategy to enhance interfacial adhesion in fiber-reinforced calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2017, 75, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Awofiranye, A.E.; Hudson, J.; Tithi, A.D.; Linhardt, R.J.; Vongsangnak, W.; Koffas, M.A. Chondroitin sulfate and its derivatives: A review of microbial and other production methods. Fermentation 2022, 8, 323. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Zhang, J.; Ye, J. Enhanced Osteogenesis of Injectable Calcium Phosphate Bone Cement Mediated by Loading Chondroitin Sulfate. ACS Biomater. Sci. Eng. 2019, 5, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef] [PubMed]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-modified premixed calcium phosphate cements for the therapy of osteoporotic bone defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef]
- Kuang, G.M.; Yau, W.P.; Lam, W.M.; Wu, J.; Chiu, K.Y.; Lu, W.W.; Pan, H. An effective approach by a chelate reaction in optimizing the setting process of strontium-incorporated calcium phosphate bone cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 778–787. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium(II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 106–117. [Google Scholar] [CrossRef]
- Masaeli, R.; Kashi, T.S.J.; Dinarvand, R.; Tahriri, M.; Rakhshan, V.; Esfandyari-Manesh, M. Preparation, characterization and evaluation of drug release properties of simvastatin-loaded PLGA microspheres. Iran. J. Pharm. Res. IJPR 2016, 15, 205. [Google Scholar]
- Wu, T.; Yang, S.; Lu, T.; He, F.; Zhang, J.; Shi, H.; Lin, Z.; Ye, J. Strontium ranelate simultaneously improves the radiopacity and osteogenesis of calcium phosphate cement. Biomed. Mater. 2019, 14, 035005. [Google Scholar] [CrossRef]
- Yang, T.; Lee, S.Y.; Park, K.C.; Park, S.H.; Chung, J.; Lee, S. The Effects of Selenium on Bone Health: From Element to Therapeutics. Molecules 2022, 27, 392. [Google Scholar] [CrossRef] [PubMed]
- Li, T.L.; Tao, Z.S.; Wu, X.J.; Yang, M.; Xu, H.G. Selenium-modified calcium phosphate cement can accelerate bone regeneration of osteoporotic bone defect. J. Bone Miner. Metab. 2021, 39, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Parelman, M.; Stoecker, B.; Baker, A.; Medeiros, D. Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp. Biol. Med. 2006, 231, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M.; Stoecker, B.; Plattner, A.; Jennings, D.; Haub, M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J. Nutr. 2004, 134, 3061–3067. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Liu, J.; Yu, T.; Shen, Z.; Ye, J. Good hydration and cell-biological performances of superparamagnetic calcium phosphate cement with concentration-dependent osteogenesis and angiogenesis induced by ferric iron. J. Mater. Chem. B 2015, 3, 8782–8795. [Google Scholar] [CrossRef]
- Vlad, M.D.; Şindilar, E.V.; Mariñoso, M.L.; Poeată, I.; Torres, R.; López, J.; Barracó, M.; Fernández, E. Osteogenic biphasic calcium sulphate dihydrate/iron-modified α-tricalcium phosphate bone cement for spinal applications: In vivo study. Acta Biomater. 2010, 6, 607–616. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of nutritional zinc in the prevention of osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Ceylan, M.N.; Akdas, S.; Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol. Trace Elem. Res. 2021, 199, 535–549. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, J.; Zhu, Y.; Chen, L.; Ye, J. Zinc doping induced differences in the surface composition, surface morphology and osteogenesis performance of the calcium phosphate cement hydration products. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110065. [Google Scholar] [CrossRef]
- Horiuchi, S.; Hiasa, M.; Yasue, A.; Sekine, K.; Hamada, K.; Asaoka, K.; Tanaka, E. Fabrications of zinc-releasing biocement combining zinc calcium phosphate to calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2014, 29, 151–160. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Tartara, A.; Gasparri, C.; Perna, S.; Infantino, V.; Riva, A.; Petrangolini, G.; Peroni, G. An update on magnesium and bone health. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2021, 34, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, X.; Lin, D.; Shi, H.; Yuan, Y.; Tang, W.; Zhou, H.; Guo, H.; Qian, J.; Liu, C. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials 2015, 53, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements for enhanced bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 403–410. [Google Scholar] [CrossRef]
- Qu, X.; He, Z.; Qiao, H.; Zhai, Z.; Mao, Z.; Yu, Z.; Dai, K. Serum copper levels are associated with bone mineral density and total fracture. J. Orthop. Transl. 2018, 14, 34–44. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved osteogenesis and angiogenesis of a novel copper ions doped calcium phosphate cement. Mater. Sci. Eng. C 2020, 114, 111032. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The Skeletal-Protecting Action and Mechanisms of Action for Mood-Stabilizing Drug Lithium Chloride: Current Evidence and Future Potential Research Areas. Front. Pharmacol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and Therapeutic Targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 7, 45204. [Google Scholar] [CrossRef]
- Qian, M.; Xu, X.; Qin, Z.; Yan, S. Silicon carbide whiskers enhance mechanical and anti-wear properties of PA6 towards potential applications in aerospace and automobile fields. Compos. Part B Eng. 2019, 175, 107096. [Google Scholar] [CrossRef]
- Xu, H.H.; Smith, D.T.; Simon, C.G. Strong and bioactive composites containing nano-silica-fused whiskers for bone repair. Biomaterials 2004, 25, 4615–4626. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Tag El-deen, D.M.; Taha, M.A. A Review on Calcium Silicate Ceramics: Properties, Limitations, and Solutions for Their Use in Biomedical Applications. Silicon 2022, 1–13. [Google Scholar] [CrossRef]
- Srinath, P.; Abdul Azeem, P.; Venugopal Reddy, K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, J.; Zhou, H.; Yuan, Y.; Mao, Y.; Liu, C. In vitro osteoblast-like and endothelial cells’ response to calcium silicate/calcium phosphate cement. Biomed. Mater. 2010, 5, 35004. [Google Scholar] [CrossRef]
- Zenebe, C.G. A Review on the Role of Wollastonite Biomaterial in Bone Tissue Engineering. BioMed Res. Int. 2022, 2022, 4996530. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Fan, P.; He, F.; Ye, J. Novel Strategy to Accelerate Bone Regeneration of Calcium Phosphate Cement by Incorporating 3D Plotted Poly(lactic-co-glycolic acid) Network and Bioactive Wollastonite. Adv. Healthc. Mater. 2019, 8, e1801325. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Nicholson, M.L. The role of perfluorocarbon in organ preservation. Transplantation 2010, 89, 1169–1175. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, J.; Guo, A.; Ren, R.; He, W.; Liu, S.; Liu, Y. Nanoscale perfluorocarbon expediates bone fracture healing through selectively activating osteoblastic differentiation and functions. J. Nanobiotechnol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Mastrogiacomo, S.; Dou, W.; Koshkina, O.; Boerman, O.C.; Jansen, J.A.; Heerschap, A.; Srinivas, M.; Walboomers, X.F. Perfluorocarbon/Gold Loading for Noninvasive in Vivo Assessment of Bone Fillers Using (19)F Magnetic Resonance Imaging and Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 22149–22159. [Google Scholar] [CrossRef]
- Liang, W.; Gao, M.; Lou, J.; Bai, Y.; Zhang, J.; Lu, T.; Sun, X.; Ye, J.; Li, B.; Sun, L. Integrating silicon/zinc dual elements with PLGA microspheres in calcium phosphate cement scaffolds synergistically enhances bone regeneration. J. Mater. Chem. B 2020, 8, 3038–3049. [Google Scholar] [CrossRef]
- Hasan, W.N.W.; Chin, K.Y.; Jolly, J.J.; Ghafar, N.A.; Soelaiman, I.N. Identifying Potential Therapeutics for Osteoporosis by Exploiting the Relationship between Mevalonate Pathway and Bone Metabolism. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, G.D.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Girardelli, M.; Kleiner, G.; Knowles, A.; Valencic, E.; Crovella, S.; Marcuzzi, A. Alendronate, a double-edged sword acting in the mevalonate pathway. Mol. Med. Rep. 2015, 12, 4238–4242. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A fresh look at the Laponite phase diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Choi, D.; Heo, J.; Aviles Milan, J.; Oreffo, R.O.C.; Dawson, J.I.; Hong, J.; Kim, Y.-H. Structured nanofilms comprising Laponite® and bone extracellular matrix for osteogenic differentiation of skeletal progenitor cells. Mater. Sci. Eng. C 2021, 118, 111440. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, M.; Yamada, T.; Taniyama, T.; Masaoka, T.; Xuetao, W.; Yoshii, T.; Horie, M.; Yasuda, H.; Uemura, T.; Okawa, A.; et al. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS ONE 2015, 10, e0116462. [Google Scholar] [CrossRef]
- Roozbahani, M.; Kharaziha, M. Dexamethasone loaded Laponite(®)/porous calcium phosphate cement for treatment of bone defects. Biomed. Mater. 2019, 14, 055008. [Google Scholar] [CrossRef]
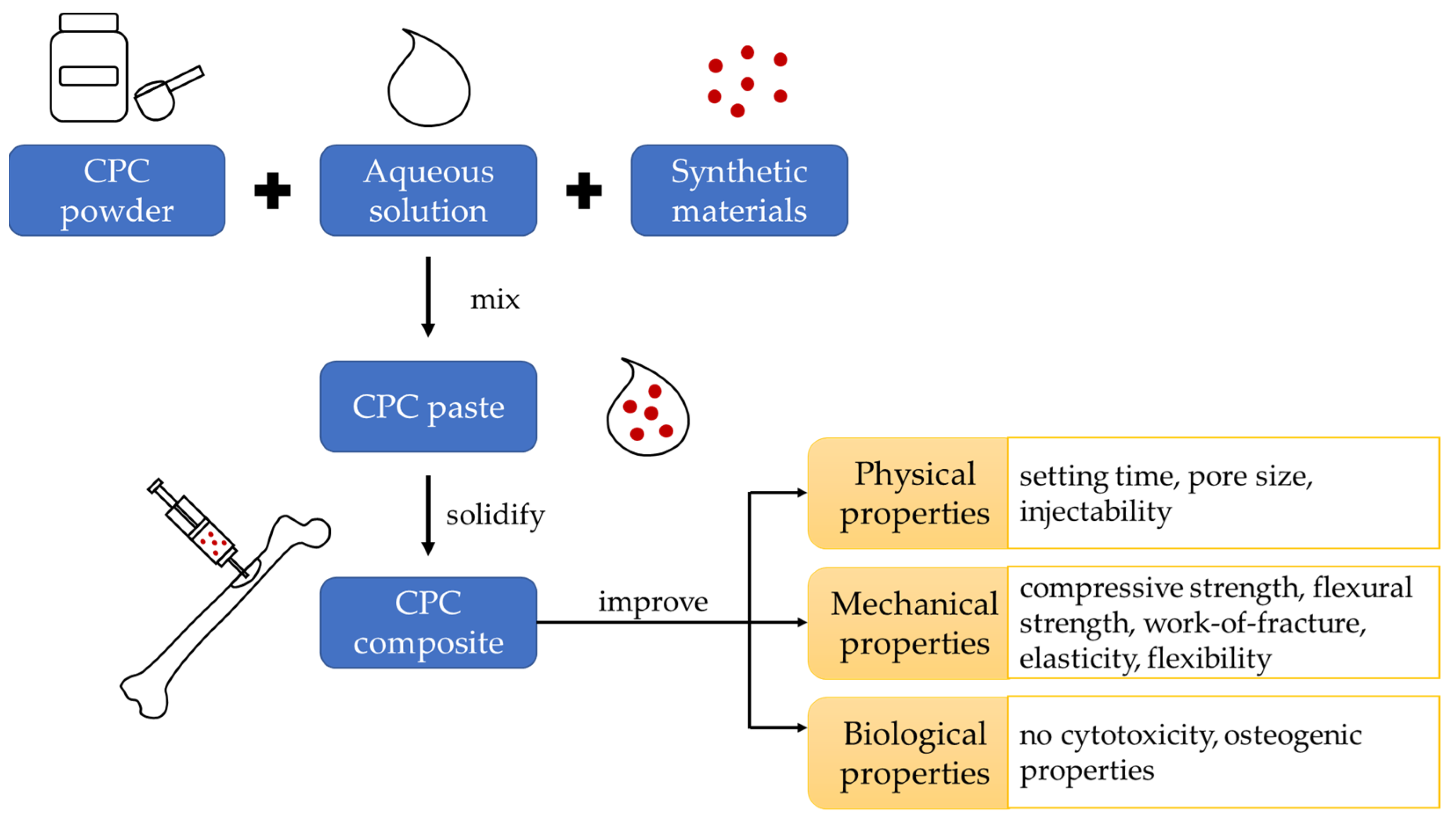
| Polymers | Biomimetic Materials | Chemical Elements/ Compound | Combination of Synthetic Materials | |
|---|---|---|---|---|
| Physical properties | ↑ setting time ↓ pore size | ↑ injectability ↑ setting time | ↑ injectability (iron and copper) ↓ setting time (except copper increased in setting time) | ↑ injectability ↑ setting time (except dexamethasone + Laponite® decreased in setting time) |
| Mechanical properties | ↑ compressive strength ↑ flexural strength ↑ work-of-fracture | ↑ compressive strength (except trimethyl chitosan, no improvement in compressive strength) ↑ elasticity | ↑ compressive strength (except strontium decreased in compressive strength with an increasing percentage of strontium) ↑ flexural strength | ↑ compressive strength (except PLGA + alendronate decreased in compressive strength) ↑ flexibility |
| Biological properties | No cytotoxicity ↑ osteogenesis ↑ bone density ↓ bone erosion ↑ mineralisation ↑ osteogenic differentiation | No cytotoxicity ↑ osteogenesis ↑ bone density (except synthetic collagen I mimetic P-15, no change in bone density) ↑ osteogenic differentiation | No cytotoxicity ↑ osteogenesis ↑ bone density ↑ osteogenic differentiation | No cytotoxicity ↑ osteogenesis ↑ bone density ↑ osteogenic differentiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Dali, S.S.; Wong, S.K.; Chin, K.-Y.; Ahmad, F. The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence. Int. J. Mol. Sci. 2023, 24, 7161. https://doi.org/10.3390/ijms24087161
Md Dali SS, Wong SK, Chin K-Y, Ahmad F. The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence. International Journal of Molecular Sciences. 2023; 24(8):7161. https://doi.org/10.3390/ijms24087161
Chicago/Turabian StyleMd Dali, Siti Sarah, Sok Kuan Wong, Kok-Yong Chin, and Fairus Ahmad. 2023. "The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence" International Journal of Molecular Sciences 24, no. 8: 7161. https://doi.org/10.3390/ijms24087161
APA StyleMd Dali, S. S., Wong, S. K., Chin, K.-Y., & Ahmad, F. (2023). The Osteogenic Properties of Calcium Phosphate Cement Doped with Synthetic Materials: A Structured Narrative Review of Preclinical Evidence. International Journal of Molecular Sciences, 24(8), 7161. https://doi.org/10.3390/ijms24087161








