Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light
Abstract
1. Introduction
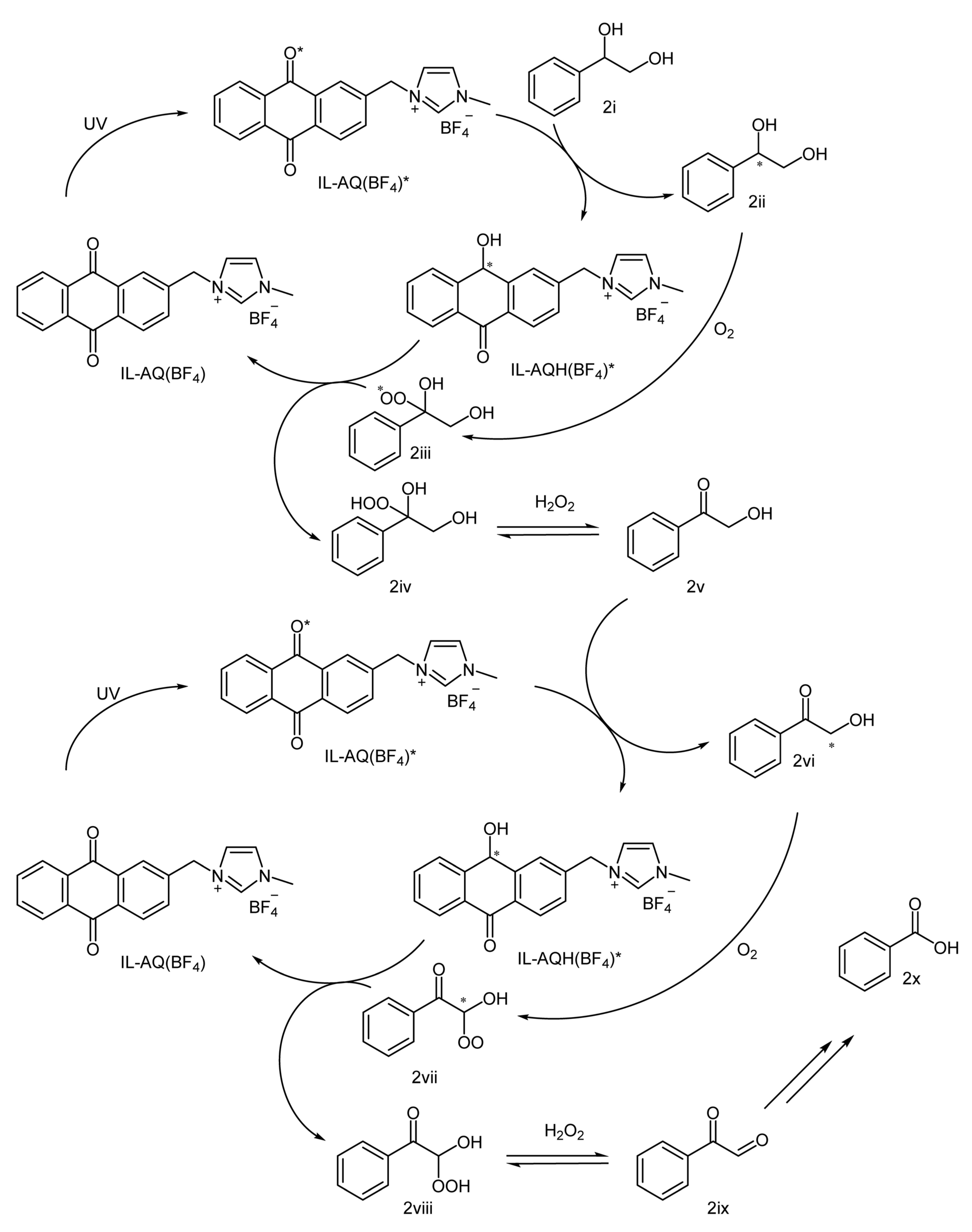
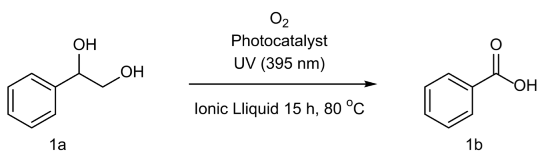
2. Results and Discussion
3. Materials and Methods
3.1. General Information Including Important Notices
3.2. Synthesis of the Substrates
3.2.1. General Procedure
3.2.2. 2-Bromomethyl-9,10-anthraquinone
3.2.3. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Bromide IL-AQ(Br)
3.2.4. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Bis(trifluoromethanesulfonyl) imide IL-AQ (TFSI)
3.2.5. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Tetrafluoroborate IL-AQ (BF4)
3.2.6. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Hexafluorophosphate IL-AQ (PF6)
3.2.7. Benzoic Acid
3.2.8. 4-Chlorobenzoic Acid
3.2.9. 4-Methylbenzoic Acid
3.2.10. 4-Acetoxybenzoic Acid
3.2.11. 4-(Tert-butyl) Benzoic Acid
3.2.12. [1,1′-Biphenyl]-4-carboxylic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chan, T.H. Ionic–liquid–supported synthesis: A novel liquid–phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic Liquids-New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar]
- Koguchi, S. An ionic–liquid–supported 18-crown-6 ether: Recyclable catalyst for acetylation and fluorination in anionic liquid. Trans. Mater. Res. Soc. Jpn. 2013, 38, 35–36. [Google Scholar] [CrossRef]
- Koguchi, S.; Nakamura, K. Ascorbic acid based ionic liquid: Recyclable and efficient catalytic systems for the Huisgen cycloaddition. Synlett 2013, 24, 2305–2309. [Google Scholar] [CrossRef]
- Koguchi, S.; Izawa, K. Ionic liquid-phase synthesis of 1,5-disubstituted 1,2,3-triazoles. ACS Comb. Sci. 2014, 16, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, S.; Mihoya, A.; Mimura, M. Alcohol oxidation via recyclable hydrophobic ionic liquid-supported IBX. Tetrahedron 2016, 72, 7633–7637. [Google Scholar] [CrossRef]
- Koguchi, S.; Shibuya, Y.; Igarashi, Y.; Takemura, H. Ionic liquid–supported 1,3-dimethylimidazolidin-2-one: Application as a reusable halogenation reagent. Synlett 2019, 30, 943–946. [Google Scholar] [CrossRef]
- Mihoya, A.; Koguchi, S.; Shibuya, Y.; Mimura, M.; Oba, M. Oxidation of Thiol Using Ionic Liquid-Supported Organotelluride as a Recyclable Catalyst. Catalysts 2020, 10, 398. [Google Scholar] [CrossRef]
- Mihoya, A.; Shibuya, Y.; Ito, A.; Toyoda, A.; Oba, M.; Koguchi, S. Aerobic Oxidation of Phosphite Esters to Phosphate Esters by Using an Ionic-Liquid-Supported Organotelluride Reusable Catalyst. Synlett 2020, 31, 2043–2045. [Google Scholar]
- Mastsusaki, Y.; Yamaguchi, T.; Tada, N.; Miura, T.; Ito, A. Aerobic Photooxidative Cleavage of Vicinal Diols to Carboxylic Acids Using 2-Chloroanthraquinone. Synlett 2012, 23, 2059–2062. [Google Scholar]

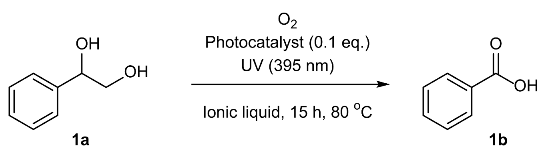 | |||
|---|---|---|---|
| Entry | Photocatalysts | Ionic Liquid | Yield b (%) |
| 1 | IL-AQ(PF6) | (bmim)PF6 | 96 |
| 2 | IL-AQ(TFSI) | (bmim)TFSI | 98 |
| 3 | IL-AQ(BF4) | (bmim)BF4 | quant. |
| 4 | IL-AQ(Br) | (bmim)Br | 99 |
| 5 | IL-AQ(PF6) | (bmim)BF4 | 88 |
| 6 | IL-AQ(TFSI) | (bmim)BF4 | 91 |
| 7 | IL-AQ(Br) | (bmim)BF4 | 60 |
| 8 | anthraquinone | (bmim)BF4 | 98 |
| 9 c | IL-AQ(BF4) | (bmim)BF4 | n. r. |
| 10 | - | (bmim)BF4 | 40 |
| 11 d | IL-AQ(BF4) | (bmim)BF4 | 65 |
 | ||
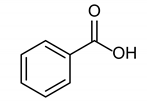 | 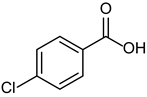 | 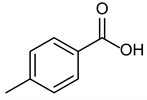 |
| 1b quant. | 2b 98% | 3b quant. |
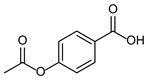 | 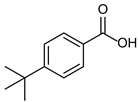 | 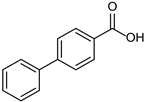 |
| 4b 95% | 5b quant. | 6b 99% |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Condition | 1 Cycle | 2 Cycles | 3 Cycles | 4 Cycles | 5 Cycles |
| 1 | Photocatalyst; IL-AQ(BF4) Ionic Liquid; (bmim)BF4 | quant. | quant. | quant. | 99% | quant. |
| 2 | Photocatalyst; IL-AQ(PF6) Ionic Liquid; (bmim) PF6 | 96% | 96% | 90% | 95% | 97% |
| 3 | Photocatalyst; IL-AQ(TFSI) Ionic Liquid; (bmim) TFSI | 98% | 98% | 72% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koguchi, S.; Fujita, H.; Shibuya, Y. Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. Int. J. Mol. Sci. 2023, 24, 7141. https://doi.org/10.3390/ijms24087141
Koguchi S, Fujita H, Shibuya Y. Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. International Journal of Molecular Sciences. 2023; 24(8):7141. https://doi.org/10.3390/ijms24087141
Chicago/Turabian StyleKoguchi, Shinichi, Haruto Fujita, and Yuga Shibuya. 2023. "Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light" International Journal of Molecular Sciences 24, no. 8: 7141. https://doi.org/10.3390/ijms24087141
APA StyleKoguchi, S., Fujita, H., & Shibuya, Y. (2023). Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. International Journal of Molecular Sciences, 24(8), 7141. https://doi.org/10.3390/ijms24087141






