Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection
Abstract
:1. Introduction
2. How Do Microorganisms Become Uropathogens?
2.1. Uropathogenic Escherichia coli (UPEC)
2.2. The Virulence Factors of UPEC
3. Acute Uncomplicated Cystitis
3.1. The Adaptive Evolution of UPEC in UTI to Thrive within Urothelial Cells
3.2. Host Defenses by Immune Response
3.3. Urinary Microbiome in Acute Cystitis
| Urobiome | Predominantly Found in | Clinical Significance | Reference |
|---|---|---|---|
| Shigella | Women |
| [75] |
| Lactobacillus | Women |
| [76,77,78,79] |
| Gardnerella | Women |
| [70,80] |
| Prevotella | Women |
| [58] |
| Streptococcus | Women |
| [81] |
| Corynebacterium | Men |
| [82,83] |
4. MDR Uropathogens
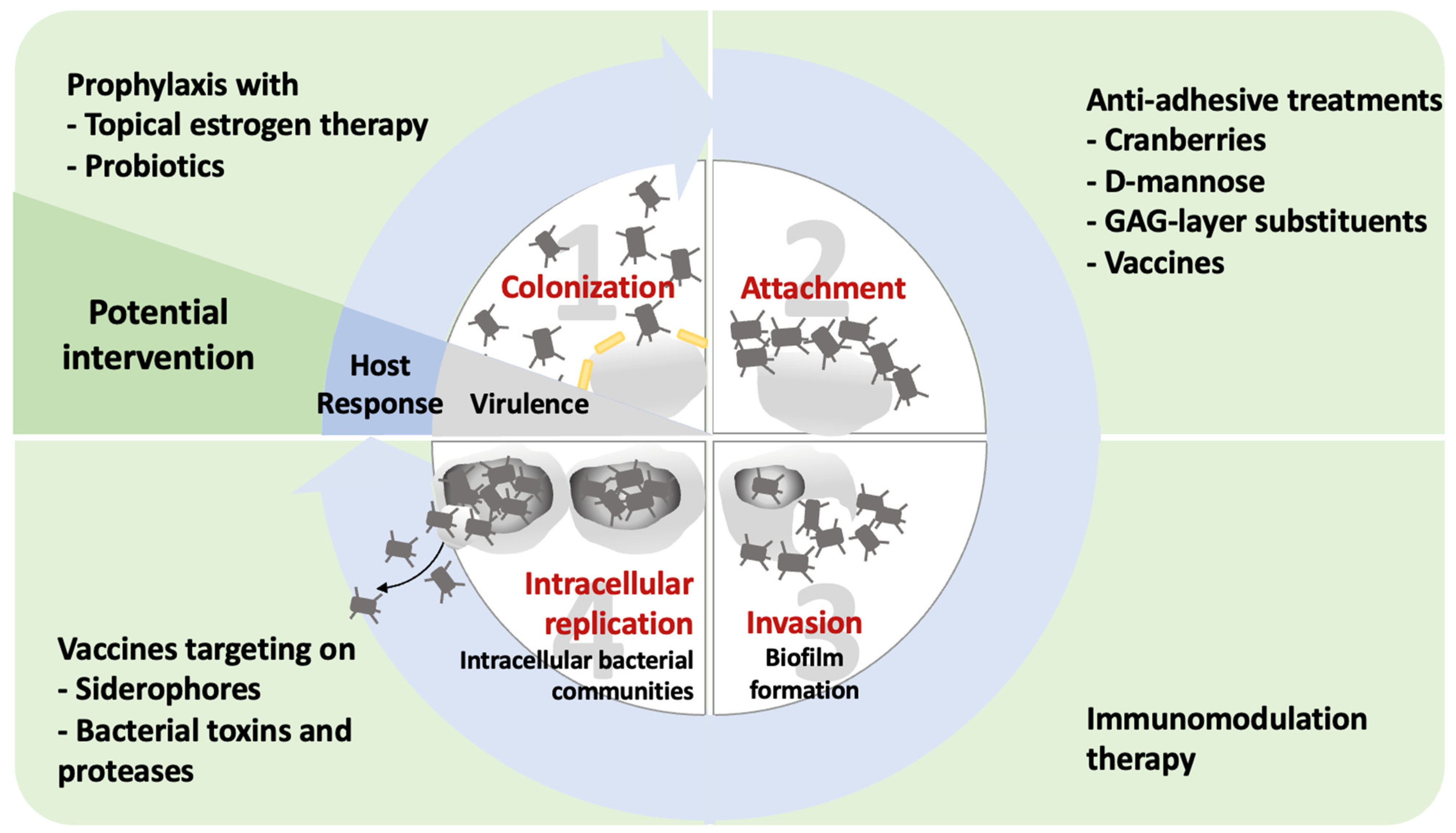
5. Emerging Prevention and Treatment Options Targeting UPEC
5.1. Decrease in the Periurethral Colonization of Uropathogens
5.1.1. Topical Estrogen Therapy and Vaginal Laser Therapy
5.1.2. Prophylaxis with Probiotics
5.2. Antiadhesive Treatments
5.2.1. Cranberries (Vaccinium macrocarpon)
5.2.2. D-Mannose
5.2.3. GAG Layer Substituents
5.3. Alternative Antibacterial Management
5.3.1. Methenamine Hippurate
5.3.2. Immunomodulation Therapy
5.3.3. Vaccine Prophylaxis
Vaccines Targeting Bacterial Adhesion
Vaccines Containing Bacterial Extracts
Vaccines Targeting Bacterial Toxins and Proteases
Vaccines Targeting Siderophores
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Brown, J.; Wyman, J.F.; Berry, A.; Newman, D.K.; Stapleton, A.E. Treatment and Prevention of Recurrent Lower Urinary Tract Infections in Women: A Rapid Review with Practice Recommendations. J. Urol. 2018, 200, 1174–1191. [Google Scholar] [CrossRef]
- Goebel, M.C.; Trautner, B.W.; Grigoryan, L. The Five Ds of Outpatient Antibiotic Stewardship for Urinary Tract Infections. Clin. Microbiol. Rev. 2021, 34, e0000320. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Edlund, C.; Nauclér, P.; Svensson, M.; Ternhag, A. Cost-Effectiveness Analysis of Temocillin Treatment in Patients with Febrile UTI Accounting for the Emergence of Antibiotic Resistance. Appl. Health Econ. Health Policy 2022, 20, 835–843. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antibiotic Resistance; WHO: Geneva, Switzerland, 2018.
- Kang, C.; Kim, J.; Park, D.W.; Kim, B.N.; Ha, U.S.; Lee, S.J.; Yeo, J.K.; Min, S.K.; Lee, H.; Wie, S.H. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infect. Chemother. 2018, 50, 67–100. [Google Scholar] [CrossRef]
- Zare, M.; Vehreschild, M.J.G.T.; Wagenlehner, F. Management of uncomplicated recurrent urinary tract infections. BJU Int. 2022, 129, 668–678. [Google Scholar] [CrossRef]
- Cai, T. Recurrent uncomplicated urinary tract infections: Definitions and risk factors. GMS Infect Dis. 2021, 27, 9. [Google Scholar]
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249. [Google Scholar] [CrossRef]
- Javadi, M.; Bouzari, S.; Oloomi, M. Horizontal Gene Transfer and the Diversity of Escherichia coli. In Escherichia coli—Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Samie, A., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Ooka, T.; Iguchi, A.; Toh, H.; Asadulghani, M.; Oshima, K.; Kodama, T.; Abe, H.; Nakayama, K.; Kurokawa, K.; et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106, 17939–17944. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar]
- Aubron, C.; Glodt, J.; Matar, C.; Huet, O.; Borderie, D.; Dobrindt, U.; Duranteau, J.; Denamur, E.; Conti, M.; Bouvet, O. Variation in endogenous oxidative stress in Escherichia coli natural isolates during growth in urine. BMC Microbiol. 2012, 12, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, N.; Agarwal, A.; Grover, M.; Singh, S. Uropathogenic Escherichia coli. In Enterobacteria; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Hochhut, B.; Wilde, C.; Balling, G.; Middendorf, B.; Dobrindt, U.; Brzuszkiewicz, E.; Gottschalk, G.; Carniel, E.; Hacker, J. Role of pathogenicity island-associated integrases in the genome plasticity of uropathogenic Escherichia coli strain 536. Mol. Microbiol. 2006, 61, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Brzuszkiewicz, E.; Brüggemann, H.; Liesegang, H.; Emmerth, M.; Olschläger, T.; Nagy, G.; Albermann, K.; Wagner, C.; Buchrieser, C.; Emody, L.; et al. How to become a uropathogen: Comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc. Natl. Acad. Sci. USA 2006, 103, 12879–12884. [Google Scholar] [CrossRef] [Green Version]
- Luo, C.; Hu, G.Q.; Zhu, H. Genome reannotation of Escherichia coli CFT073 with new insights into virulence. BMC Genom. 2009, 10, 552. [Google Scholar] [CrossRef] [Green Version]
- Ulett, G.C.; Mabbett, A.N.; Fung, K.C.; Webb, R.I.; Schembri, M.A. The role of F9 fimbriae of uropathogenic Escherichia coli in biofilm formation. Microbiology 2007, 153, 2321–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjifrangiskou, M.; Gu, A.P.; Pinkner, J.S.; Kostakioti, M.; Zhang, E.W.; Greene, S.E.; Hultgren, S.J. Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J. Bacterial. 2012, 194, 6195–6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, D.; Chan, A.C.; Murphy, M.E.; Lilie, H.; Grass, G.; Nies, D.H. Characterization of a dipartite iron uptake system from uropathogenic Escherichia coli strain F11. J. Biol. Chem. 2011, 286, 25317–25330. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic, E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143–154. [Google Scholar] [CrossRef]
- Klein, R.D.; Hultgren, S.J. Urinary tract infections: Microbial pathogenesis, host-pathogen interactions and new treatment strategies. Nat. Rev. Microbiol. 2020, 18, 211–226. [Google Scholar] [CrossRef]
- Chen, M.; Tofighi, R.; Bao, W.; Aspevall, O.; Jahnukainen, T.; Gustafsson, L.E.; Ceccatelli, S.; Celsi, G. Carbon monoxide prevents apoptosis induced by uropathogenic Escherichia coli toxins. Pediatr. Nephrol. 2006, 21, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.M.; Dozois, C.M.; Avelar-González, F.J.; Hernández-Cuellar, E.; Pokharel, P.; de Santiago, A.S.; Guerrero-Barrera, A.L. The Vacuolating Autotransporter Toxin (Vat) of Escherichia coli Causes Cell Cytoskeleton Changes and Produces Non-lysosomal Vacuole Formation in Bladder Epithelial Cells. Front. Cell. Infect. Microbiol. 2020, 10, 299. [Google Scholar] [CrossRef]
- Lie’vin-Le Moal, V.; Comenge, Y.; Ruby, V.; Amsellem, R.; Nicolas, V.; Servin, A.L. Secreted autotransporter toxin (Sat) triggers autophagy in epithelial cells that relies on cell detachment. Cell. Microbiol. 2011, 13, 992–1013. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.R.; Cappello, R.; Navarro-García, F.; Nataro, J.P. Functional comparison of serine protease autotransporters of enterobacteriaceae. Infect. Immun. 2002, 70, 7105–7113. [Google Scholar] [CrossRef] [Green Version]
- Marrs, C.F.; Zhang, L.; Foxman, B. Escherichia coli mediated urinary tract infections: Are there distinct uropathogenic E. coli (UPEC) pathotypes? FEMS Microbiol. Lett. 2005, 252, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Tassell, A.H.-V.; Urvil, P.T.; Lea, S.; Pettigrew, D.; Anderson, K.L.; Samet, A.; Kur, J.; Matthews, S.; Nowicki, S.; et al. Hydrophilic domain II of Escherichia coli Dr fimbriae facilitates cell invasion. Infect. Immun. 2005, 73, 6119–6126. [Google Scholar] [CrossRef] [Green Version]
- Pizarro-Cerdá, J.; Cossart, P. Bacterial adhesion and entry into host cells. Cell 2006, 124, 715–727. [Google Scholar] [CrossRef] [Green Version]
- Ambite, I.; Butler, D.S.C.; Stork, C.; Grönberg-Hernández, J.; Köves, B.; Zdziarski, J.; Pinkner, J.; Hultgren, S.J.; Dobrindt, U.; Wullt, B.; et al. Fimbriae reprogram host gene expression—Divergent effects of P and type 1 fimbriae. PLoS Pathog. 2019, 15, e1007671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, M.; Hoschutzky, H.; Jann, K.; Van Die, I.; Hacker, J. Gene clusters for S fimbrial adhesin (sfa) and F1C (foc) of Escherichia coli: Comparative aspects of structure and function. J. Bacteriol. 1998, 170, 3983–3990. [Google Scholar] [CrossRef] [Green Version]
- Siitonen, A.; Martikainen, R.; Ikaheimo, R.; Palmgren, J.; Mkela, P.H. Virulence-associated characteristics of Escherichia coli in urinary tract infection: A statistical analysis with special attention to type 1C fimbriation. Microb. Pathog. 1993, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ristow, L.C.; Welch, R.A. Hemolysin of uropathogenic Escherichia coli: A cloak or a dagger? Biochim. Biophys. Acta 2016, 1858, 538–545. [Google Scholar] [CrossRef]
- Carlini, F.; Maroccia, Z.; Fiorentini, C.; Travaglione, S.; Fabbri, A. Effects of the Escherichia coli Bacterial Toxin Cytotoxic Necrotizing Factor 1 on Different Human and Animal Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12610. [Google Scholar] [CrossRef] [PubMed]
- Piteau, M.; Papatheodorou, P.; Schwan, C.; Schlosser, A.; Aktories, K.; Schmidt, G. Lu/BCAM adhesion glycoprotein is a receptor for Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1). PLoS Pathog. 2014, 10, e1003884. [Google Scholar] [CrossRef]
- Reppin, F.; Cochet, S.; El Nemer, W.; Fritz, G.; Schmidt, G. High Affinity Binding of Escherichia coli Cytotoxic Necrotizing Factor 1 (CNF1) to Lu/BCAM Adhesion Glycoprotein. Toxins 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, R.A. Uropathogenic Escherichia coli-Associated Exotoxins. Microbiol. Spectr. 2016, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurbeck, P.R.; Dinh, P.C., Jr.; Walk, S.T.; Stapleton, A.E.; Hooton, T.M.; Nolan, L.K.; Kim, K.S.; Johnson, J.R.; Mobley, H.L.T. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 2012, 80, 4115–4122. [Google Scholar] [CrossRef] [Green Version]
- Nichols, K.B.; Totsika, M.; Moriel, D.G.; Lo, A.W.; Yang, J.; Wurpel, D.J.; Rossiter, A.E.; Strugnell, R.A.; Henderson, I.R.; Ulett, G.C.; et al. Molecular characterization of the vacuolating autotransporter toxin in uropathogenic Escherichia coli. J. Bacteriol. 2016, 198, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Nava-Acosta, R.; Navarro-Garcia, F. Cytokeratin 8 is an epithelial cell receptor for pet, a cytotoxic serine protease autotransporter of Enterobacteriaceae. mBio 2013, 4, e00838-13. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [Green Version]
- Beebout, C.J.; Robertson, G.L.; Reinfeld, B.I.; Blee, A.M.; Morales, G.H.; Brannon, J.R.; Chazin, W.J.; Rathmell, W.K.; Rathmell, J.C.; Gama, V.; et al. Uropathogenic Escherichia coli subverts mitochondrial metabolism to enable intracellular bacterial pathogenesis in urinary tract infection. Nat. Microbiol. 2022, 7, 1348–1360. [Google Scholar] [CrossRef]
- Henderson, J.P.; Crowley, J.R.; Pinkner, J.S.; Walker, J.N.; Tsukayama, P.; Stamm, W.E.; Hooton, T.M.; Hultgren, S.J. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog. 2009, 5, e1000305. [Google Scholar] [CrossRef]
- Sabri, M.; Houle, S.; Dozois, C.M. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect. Immun. 2009, 77, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Juárez-Hernández, R.E.; Miller, M.J. Exploiting bacterial iron acquisition: Siderophore conjugates. Future Med. Chem. 2012, 4, 297–313. [Google Scholar] [CrossRef]
- Ambite, I.; Puthia, M.; Nagy, K.; Cafaro, C.; Nadeem, A.; Butler, D.S.C.; Rydström, G.; Filenko, N.A.; Wullt, B.; Miethke, T.; et al. Molecular basis of acute cystitis reveals susceptibility genes and immunotherapeutic targets. PLoS Pathog. 2016, 12, e1005848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Duncan, M.J.; Li, G.; Chan, C.; Grady, R.; Stapleton, A.; Abraham, S.N. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathog. 2007, 3, e60. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Bishop, B.L.; Li, G.; Grady, R.; Stapleton, A.; Abraham, S.N. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14966–14971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klumpp, D.J.; Rycyk, M.T.; Chen, M.C.; Thumbikat, P.; Sengupta, S.; Schaeffer, A.J. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect. Immun. 2006, 74, 5106–5113. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Bauckman, K.A.; Ross, A.S.B.; Symington, J.W.; Ligon, M.M.; Scholtes, G.; Kumar, A.; Chang, H.-W.; Twentyman, J.; Fashemi, B.E.; et al. A non-canonical autophagy-dependent role of the ATG16L1(T300A) variant in urothelial vesicular trafficking and uropathogenic Escherichia coli persistence. Autophagy 2019, 15, 527–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zasloff, M. The antibacterial shield of the human urinary tract. Kidney Int. 2013, 83, 548–550. [Google Scholar] [CrossRef] [Green Version]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Haley, K.P.; Skaar, E.P. A battle for iron: Host sequestration and Staphylococcus aureus acquisition. Microbes Infect. 2012, 14, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Sorić Hosman, I.; Cvitković Roić, A.; Lamot, L. A Systematic Review of the (Un)known Host Immune Response Biomarkers for Predicting Recurrence of Urinary Tract Infection. Front. Med. 2022, 9, 931717. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Kenneally, C.; Murphy, C.P.; Sleator, R.D.; Culligan, E.P. The urinary microbiome and biological therapeutics: Novel therapies for urinary tract infections. Microbiol. Res. 2022, 259, 127010. [Google Scholar] [CrossRef]
- Fouts, D.E.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.J.; Huang, S.T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceprnja, M.; Oros, D.; Melvan, E.; Svetlicic, E.; Skrlin, J.; Barisic, K.; Starcevic, L.; Zucko, J.; Starcevic, A. Modeling of urinary microbiota associated with cystitis. Front. Cell. Infect. Microbiol. 2021, 11, 643638. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, T.K.; Hilt, E.E.; Thomas-White, K.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. The urobiome of continent adult women: A cross-sectional study. BJOG 2020, 127, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol. 2015, 213, e1–e11. [Google Scholar] [CrossRef] [Green Version]
- Marrazzo, J.M. Interpreting the epidemiology and natural history of bacterial vaginosis: Are we still confused? Anaerobe 2011, 17, 186–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadieux, P.A.; Burton, J.P.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60 (Suppl. 6), 13–18. [Google Scholar] [PubMed]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum—Production genetic organization. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Komesu, Y.M.; Richter, H.E.; Carper, B.; Dinwiddie, D.L.; Lukacz, E.S.; Siddiqui, N.Y.; Sung, V.W.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; et al. The urinary microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int. Urogynecol. J. 2018, 29, 1785–1795. [Google Scholar] [CrossRef]
- Stapleton, A.E. The vaginal microbiota and urinary tract infection. Microbiol. Spectr. 2016, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, N.M.; O’Brien, V.P.; Lewis, A.L. Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog. 2017, 13, e1006238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, V.P.; Joens, M.S.; Lewis, A.L.; Gilbert, N.M. Recurrent Escherichia coli urinary tract infection triggered by gardnerella vaginalis bladder exposure in mice. J. Vis. Exp. 2020, 4, e61967. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4680–4687. [Google Scholar] [CrossRef] [Green Version]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a cause of bacterial vaginosis: Appraisal of the evidence from in vivo models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. mBio 2014, 5, e01283-14. [Google Scholar] [CrossRef] [Green Version]
- Baka, S.; Spathi, A.; Tsouma, I.; Kouskouni, E. Symptomatic Shigella sonnei urinary tract infection in pregnancy. Clin. Exp. Obstet. Gynecol. 2013, 40, 116–117. [Google Scholar] [PubMed]
- Amabebe, E.; Anumba, D. The vaginal microenvironment: The physiologic role of lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, A.E.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.S.; Silverstroni, A.; Stapleton, A.E. Cytoprotective effect of Lactobacillus crispatus CTV-05 against uropathogenic E. coli. Pathogens 2016, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Lim, I.S.; Lee, H.S.; Kim, W.Y. The effect of lactic acid bacteria isolates on the urinary tract pathogens to infants In vitro. J. Korean Med. Sci. 2009, 24, S57–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [Green Version]
- Price, T.K.; Wolff, B.; Halverson, T.; Limeira, R.; Brubaker, L.; Dong, Q.; Mueller, E.R.; Wolfe, A.J. Temporal dynamics of the adult female lower urinary tract microbiota. mBio 2020, 11, e00475-20. [Google Scholar] [CrossRef]
- Sokol-Leszczynska, B.; Leszczynski, P.; Lachowicz, D.; Rostkowska, O.; Niemczyk, M.; Piecha, T.; van Belkum, A.; Sawicka-Grzelak, A.; Mlynarczyk, G. Corynebac-terium coyleae as potential urinary tract pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1339–1342. [Google Scholar] [CrossRef] [Green Version]
- Brubaker, L.; Wolfe, A.J. The urinary microbiota: A paradigm shift for bladder disorders? Curr. Opin. Obstet. Gynecol. 2016, 28, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Beerepoot, M.A.J.; Geerlings, S.V.; Van Haarst, E.P.; Van Charante, N.M.; Ter Riet, G. Nonantibiotic prophylaxis for recurrent urinary tract infections: A systematic review and meta-analysis of randomized controlled trials. J. Urol. 2013, 190, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anger, J.T.; Bixler, B.R.; Holmes, R.S.; Lee, U.J.; Santiago-Lastra, Y.; Selph, S.S. Updates to Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. J. Urol. 2022, 208, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, J.; Marijam, A.; Joshi, A.V.; Mitrani-Gold, F.S.; Everson, K.; Tuly, R.; Rosenquist, P.; Gillam, M.; Ruiz, M.E. Impact of suboptimal or inappropriate treatment on healthcare resource use and cost among patients with uncomplicated urinary tract infection: An analysis of integrated delivery network electronic health records. Antimicrob. Resist. Infect. Control. 2022, 11, 133. [Google Scholar] [CrossRef]
- Moon, R.C.; Marijam, A.; Mitrani-Gold, F.S.; Gibbons, D.C.; Kartashov, A.; Rosenthal, N.A.; Joshi, A.V. Treatment patterns, healthcare resource use, and costs associated with uncomplicated urinary tract infection among female patients in the United States. PLoS ONE 2022, 17, e0277713. [Google Scholar] [CrossRef]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A.A.-O. Incidence and diversity of antimicrobial multidrug resistance profiles of uropathogenic bacteria. Biomed. Res. Int. 2015, 2015, 354084. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef]
- Dantas, G.; Sommer, M.O.A.; Oluwasegun, R.D.; Church, G.M. Bacteria Subsisting on Antibiotics. Science 2008, 320, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Gniadkowski, M. Evolution of Extended-Spectrum b-Lactamases by Mutation. Clin. Microbiol. Infect. 2008, 14, 11–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, C.; Levy, S.B. Many Chromosomal Genes Modulate Mara-Mediated Multidrug Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 2125–2134. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Vavra, M.; Schweigger, T.M.; Rossen, J.W.A.; Matsumura, Y.; Kern, W.V. Contribution of AcrAB-TolC to Multidrug Resistance in an Escherichia coli Sequence Type 131 Isolate. Int. J. Antimicrob. Agents 2017, 50, 477–481. [Google Scholar] [CrossRef]
- Atac, N.; Kurt-Azap, O.; Dolapci, I.; Yesilkaya, A.; Ergonul, O.; Gonen, M.; Can, F. The Role of AcrAB–TolC Efflux Pumps on Quinolone Resistance of E. coli ST131. Curr. Microbiol. 2018, 75, 1661–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, N.; Suhani, S.; Purkaystha, A.; Begum, M.K.; Raihan, T.; Alam, M.J.; Islam, K.; Azad, A.K. Identification of AcrAB-TolC Efflux Pump Genes and Detection of Mutation in Efflux Repressor AcrR From Omeprazole Responsive Multidrug-Resistant Escherichia coli Isolates Causing Urinary Tract Infections. Microbiol. Insights 2019, 12, 1178636119889629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, S.V.; Dixon, L.; Benoit, M.R.; Brodie, E.L.; Keyhan, M.; Hu, P.; Ackerley, D.F.; Andersen, G.L.; Matin, A. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob. Agents Chemother. 2007, 51, 3650–3658. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.H.; Wang, K.C.; Lee, L.W.; Huang, Y.N.; Yeh, K.S. A constitutively mannose-sensitive agglutinating Salmonella enterica subsp. enterica serovar typhimurium strain, carrying a transposon in the fimbrial usher gene stbC, exhibits multidrug resistance and flagellated phenotypes. Sci. World J. 2012, 2012, 280264. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Choe, H.S.; Na, Y.G.; Kim, K.H.; Kim, J.H.; Chung, H.; Chung, J.M.; Jung, J.H.; Choi, H.; Lee, S.-J.; et al. 2017 Guidelines of the Korean association of urogenital tract infection and inflammation: Recurrent urinary tract infection. Urogenit. Tract Infect. 2017, 12, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Raz, R.; Stamm, W.E. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N. Engl. J. Med. 1993, 329, 753–756. [Google Scholar] [CrossRef] [Green Version]
- Lüthje, P.; Brauner, H.; Ramos, N.L.; Övregaard, A.; Gläser, R.; Hirschberg, A.L.; Aspenström, P.; Brauner, A. Estrogen supports urothelial defense mechanisms. Sci. Transl. Med. 2013, 5, 190ra80. [Google Scholar] [CrossRef]
- Lüthje, P.; Hirschberg, A.L.; Brauner, A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas 2014, 77, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Su, T.H.; Lau, H.H. Estrogen for the prevention of recurrent urinary tract infections in postmenopausal women: A meta-analysis of randomized controlled trials. Int. Urogynecol. J. 2021, 32, 17–25. [Google Scholar] [CrossRef]
- Leckie, K.J. What is the evidence for the role of oestrogen in the prevention of recurrent urinary tract infections in postmenopausal women? An evidence-based review. J. Clin. Gerontol. Geriatr. 2010, 1, 31. [Google Scholar] [CrossRef] [Green Version]
- Dueñas-Garcia, O.F.; Sullivan, G.; Hall, C.D.; Flynn, M.K.; O’Dell, K. Pharmacological agents to decrease new episodes of recurrent lower urinary tract infections in postmenopausal women. A systematic review. Female Pelvic Med. Reconstruct. Surg. 2016, 22, 63–69. [Google Scholar] [CrossRef]
- Antoniou, V.; Somani, B.K. Topical and Oral Oestrogen for Recurrent Urinary Tract Infection-Evidence-based Review of Literature, Treatment Recommendations, and Correlation with the European Association of Urology Guidelines on Urological Infections. Eur. Urol. Focus 2022, 8, 1768–1774. [Google Scholar] [CrossRef]
- Perrotta, C.; Aznar, M.; Mejia, R.; Albert, X.; Ng, C.W. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst. Rev. 2008, 2, CD005131. [Google Scholar] [CrossRef] [PubMed]
- Fractional CO2 Vaginal LASER Therapy for Recurrent Urinary Tract Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT04301934 (accessed on 1 June 2020).
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.P.; Cadieux, P.A.; Reid, G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ. Microbiol. 2003, 69, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Grin, P.; Kowalewska, P.M.; Alhazzan, W.; Fox-Robichaud, A.E. Lactobacillus for preventing recurrent urinary tract infections in women: Meta-analysis. Can. J. Urol. 2013, 20, 6607. [Google Scholar]
- Ng, Q.X.; Peters, C.; Venkatanarayanan, N.; Goh, Y.Y.; Ho, C.Y.X.; Yeo, W.-S. Use of Lactobacillus spp. to prevent recurrent urinary tract infections in females. Med. Hypotheses 2018, 114, 49–54. [Google Scholar] [CrossRef]
- Emami, E.; Mt Sherwin, C.; Heidari-Soureshjani, S. Effect of probiotics on urinary tract infections in children: A systematic review and meta-analysis. Curr. Rev. Clin. Exp. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Abdullatif, V.A.; Sur, R.L.; Eshaghian, E.; Gaura, K.A.; Goldman, B.; Panchatsharam, P.K.; Williams, N.J.; Abbott, J.E. Efficacy of Probiotics as Prophylaxis for Urinary Tract Infections in Premenopausal Women: A Systematic Review and Meta-Analysis. Cureus 2021, 13, e18843. [Google Scholar] [CrossRef]
- Canales, J.; Rada, G. Are probiotics effective in preventing urinary tract infection? Medwave 2018, 18, e7186. [Google Scholar] [CrossRef]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. European Urological Association: Guidelines on Urological Infections. 2022. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urological-Infections-2022.pdf (accessed on 1 March 2022).
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.; Chan, M.; Tufenkji, N. Inhibition of Escherichia coli CFT073 fliC expression and motility by cranberry materials. Appl. Environ. Microbiol. 2011, 77, 6852–6857. [Google Scholar] [CrossRef] [Green Version]
- O’May, C.; Tufenkji, N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011, 77, 3061–3067. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, S.; Thamil Selvan, G.; Bhaskaran, S.; Hari, N.; Solomon, A.P. Reciprocal Cooperation of Type A Procyanidin and Nitrofurantoin Against Multi-Drug Resistant (MDR) UPEC: A pH-Dependent Study. Front. Cell Infect. Microbiol. 2020, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.; Sendker, J.; Dobrindt, U.; Hensel, A. Influence of cranberry extract on tamm-horsfall protein in human urine and its antiadhesive activity against uropathogenic Escherichia coli. Planta Med. 2019, 85, 126–138. [Google Scholar] [CrossRef]
- Scharf, B.; Schmidt, T.J.; Rabbani, S.; Stork, C.; Dobrindt, U.; Sendker, J.; Ernst, B.; Hensel, A. Antiadhesive natural products against uropathogenic E. coli: What can we learn from cranberry extract? J. Ethnopharmacol. 2020, 257, 112889. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Okshevsky, M.; Déziel, E.; Tufenkji, N. Proanthocyanidin interferes with intrinsic antibiotic resistance mechanisms of gram-negative bacteria. Adv. Sci. 2019, 6, 1802333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, S.; Swamy, S.S.; Kaur, G.; Princy, S.A.; Balamurugan, P. Synergism between quorum sensing inhibitors and antibiotics: Combating the antibiotic resistance crisis. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Singapore, 2018; pp. 205–225. [Google Scholar] [CrossRef]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD001321. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry Reduces the Risk of Urinary Tract Infection Recurrence in Otherwise Healthy Women: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [Green Version]
- Luís, Â.; Domingues, F.; Pereira, L. Can Cranberries Contribute to Reduce the Incidence of Urinary Tract Infections? A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Clinical Trials. J. Urol. 2017, 198, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Fang, C.C.; Chen, N.C.; Liu, S.S.H.; Yu, P.H.; Wu, T.Y.; Chen, W.-T.; Lee, C.C.; Chen, S.C. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: A systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar] [CrossRef] [Green Version]
- Tambunan, M.P.; Rahardjo, H.E. Cranberries for women with recurrent urinary tract infection: A meta-analysis. Med. J. Indones. 2019, 28, 268–275. [Google Scholar] [CrossRef]
- Liska, D.J.; Kern, H.J.; Maki, K.C. Cranberries and Urinary Tract Infections: How Can the Same Evidence Lead to Conflicting Advice? Adv. Nutr. 2016, 7, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Pinkner, J.S.; Ford, B.; Chorell, E.; Crowley, J.M.; Cusumano, C.K.; Campbell, S.; Henderson, J.P.; Hultgren, S.J.; Janetka, J.W. Lead optimization studies on FimH antagonists: Discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J. Med. Chem. 2012, 55, 3945–3959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Pinkner, J.S.; Ford, B.; Obermann, R.; Nolan, W.; Wildman, S.A.; Hobbs, D.; Ellenberger, T.; Cusumano, C.K.; Hultgren, S.J.; et al. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 2010, 53, 4779–4792. [Google Scholar] [CrossRef] [Green Version]
- Cusumano, C.K.; Pinkner, J.S.; Han, Z.; Greene, S.A.; Ford, B.A.; Crowley, J.R.; Henderson, J.P.; Janetka, J.W.; Hultgren, S.J. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl. Med. 2011, 3, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Luthi, C.; Scharenberg, M.; Bezenuon, J.; et al. FimH antagonists for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef]
- Guiton, P.S.; Cusumano, C.K.; Kline, K.A.; Dodson, K.W.; Han, Z.; Janetka, J.W.; Henderson, J.P.; Caparon, M.G.; Hultgren, S.J. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob. Agents Chemother. 2012, 56, 4738–4745. [Google Scholar] [CrossRef] [Green Version]
- Totsika, M.; Kostakioti, M.; Hannan, T.J.; Upton, M.; Beatson, S.A.; Janetka, J.W.; Hultgren, S.J.; Schembri, M.A. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J. Infect. Dis. 2013, 208, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Kranz, J.; Schmidt, S.; Schneidewind, L. Current Evidence on Nonantibiotic Prevention of Recurrent Urinary Tract Infections. Eur. Urol. Focus 2019, 5, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Lenger, S.; Bradley, M.S.; Thomas, D.A.; Bertolet, M.H.; Lowder, J.L.; Sutcliffe, S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020, 223, 265. [Google Scholar] [CrossRef] [PubMed]
- Ala-Jaakkola, R.; Laitila, A.; Ouwehand, A.C.; Lehtoranta, L. Role of D-mannose in urinary tract infections—A narrative review. Nutr. J. 2022, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, R.; Jones, P.; Somani, B.K. Role of D-Mannose in the Prevention of Recurrent Urinary Tract Infections: Evidence from a Systematic Review of the Literature. Eur. Urol. Focus 2021, 7, 1166–1169. [Google Scholar] [CrossRef]
- Damiano, R.; Quarto, G.; Bava, I.; Ucciero, G.; De Domenico, R.; Palumbo, M.I.; Autorino, R. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: A placebo-controlled randomised trial. Eur. Urol. 2011, 59, 645, Corrigendum in Eur. Urol. 2011, 60, 193. [Google Scholar] [CrossRef]
- De Vita, D.; Giordano, S. Effectiveness of intravesical hyaluronic acid/chondroitin sulfate in recurrent bacterial cystitis: A randomized study. Int. Urogynecol. J. 2012, 23, 1707–1713. [Google Scholar] [CrossRef]
- Goddard, J.C.; Janssen, D.A.W. Intravesical hyaluronic acid and chondroitin sulfate for recurrent urinary tract infections: Systematic review and meta-analysis. Int. Urogynecol. J. 2018, 29, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.B.; Bhuta, T.; Simpson, J.M.; Craig, J. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD003265. [Google Scholar] [CrossRef]
- Bakhit, M.; Krzyzaniak, N.; Hilder, J.; Clark, J.; Scott, A.M.; Del Mar, C. Use of methenamine hippurate to prevent urinary tract infections in community adult women: A systematic review and meta-analysis. Br. J. Gen. Pract. 2021, 71, e528. [Google Scholar] [CrossRef]
- Harding, C.; Mossop, H.; Homer, T.; Chadwick, T.; King, W.; Carnell, S.; Lecouturier, J.; Abouhajar, A.; Vale, L.; Watson, G.; et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: Multicentre, open label, randomised, non-inferiority trial. BMJ 2022, 376, e068229. [Google Scholar] [CrossRef]
- Botros, C.; Lozo, S.; Iyer, S.; Warren, A.; Goldberg, R.; Tomezsko, J.; Sasso, K.; Sand, P.; Gafni-Kane, A.; Biener, A.; et al. Methenamine hippurate compared with trimethoprim for the prevention of recurrent urinary tract infections: A randomized clinical trial. Int. Urogynecol. J. 2022, 33, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chancellor, M.B.; Lamb, L.E.; Ward, E.P.; Bartolone, S.N.; Carabulea, A.; Sharma, P.; Janicki, J.; Smith, C.; Laudano, M.; Abraham, N.; et al. Comparing concentration of urinary inflammatory cytokines in interstitial cystitis, overactive bladder, urinary tract infection, and bladder cancer. Urol. Sci. 2022, 33, 199–204. [Google Scholar] [CrossRef]
- Mayer-Barber, K.D.; Andrade, B.B.; Barber, D.L.; Hieny, S.; Feng, C.; Caspar, P.; Oland, S.; Gordon, S.; Sher, A. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity 2011, 35, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.; Sharma, A.; Jen, R.; Hirschfeld, A.F.; Chilvers, M.; Lavoie, P.M.; Turvey, S.E. Inflammasome- mediated IL-1β production in humans with cystic fibrosis. PLoS ONE 2012, 7, e37689. [Google Scholar] [CrossRef] [Green Version]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.-M.; Bézie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19:a cohort study. Lancet Rheumatol. 2020, 2, e393–e400. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome- dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef] [Green Version]
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G.; et al. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient- level meta- analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Schembri, M.A. Fimbriae-assisted bacterial surface display of heterologous peptides. Int. J. Med. Microbiol. 2000, 290, 215–221. [Google Scholar] [CrossRef]
- Goluszko, P.; Goluszko, E.; Nowicki, B.; Nowicki, S.; Popo, V.; Wang, H.Q. Vaccination with purified Dr fimbraie reduces mortality associated with chronic urinary tract infection due to Escherichia coli bearing Dr adhesin. Infect. Immun. 2005, 73, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Asadi Karam, M.R.; Oloomi, M.; Mahdavi, M.; Habibi, M.; Bouzari, S. Vaccination with recombinant FimH fused with flagellin enhances cellular and humoral immunity against urinary tract infection in mice. Vaccine 2013, 31, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Langermann, S.; Palaszynski, S.; Barnhart, M.; Auguste, G.; Pinkner, J.S.; Burlein, J.; Barren, P.; Koenig, S.; Leath, S.; Jones, C.H.; et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997, 276, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Savar, N.S.; Jahanian-Najafabadi, A.; Mahdavi, M.; Shokrgozar, M.A.; Jafari, A.; Bouzari, S. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J. Biotechnol. 2014, 175, 31–37. [Google Scholar] [CrossRef]
- Eldridge, G.R.; Hughey, H.; Rosenberger, L.; Martin, S.M.; Shapiro, A.M.; D’Antonio, E.; Krejci, K.G.; Shore, N.; Peterson, J.; Lukes, A.S.; et al. Safety and immunogenicity of an adjuvanted Escherichia coli adhesin vaccine in healthy women with and without histories of recurrent urinary tract infections: Results from a first-in-human phase 1 study. Hum. Vaccin. Immunother. 2021, 17, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Cho, Y.H.; Matsumoto, T.; Schaeffer, A.J. Immunoactive prophylaxis of recurrent urinary tract infections: A meta-analysis. Int. J. Antimicrob. Agents 2009, 33, 111–119. [Google Scholar] [CrossRef]
- Aziminia, N.; Hadjipavlou, M.; Philippou, Y.; Pandian, S.S.; Malde, S.; Hammadeh, M.Y. Vaccines for the prevention of recurrent urinary tract infections: A systematic review. BJU Int. 2019, 123, 753–768. [Google Scholar] [CrossRef] [Green Version]
- Prattley, S.; Geraghty, R.; Moore, M.; Somani, B.K. Role of Vaccines for Recurrent Urinary Tract Infections: A Systematic Review. Eur. Urol. Focus 2020, 6, 593–604. [Google Scholar] [CrossRef] [PubMed]
- O’Hanley, P.; Lalonde, G.; Ji, G. Alpha-hemolysin contributes to the pathogenicity of piliated digalactoside-binding Escherichia coli in the kidney: Efficacy of an α-hemolysin vaccine in preventing renal injury in the BALB/c mouse model of pyelonephritis. Infect. Immun. 1991, 59, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alamuri, P.; Eaton, K.A.; Himpsl, S.D.; Smith, S.N.; Mobley, H.L. Vaccination with proteus toxic agglutinin, a hemolysin-independent cytotoxin in vivo, protects against Proteus mirabilis urinary tract infection. Infect. Immun. 2009, 77, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumbaugh, A.R.; Smith, S.N.; Mobley, H.L. Immunization with the yersiniabactin receptor, FyuA, protects against pyelonephritis in a murine model of urinary tract infection. Infect. Immun. 2013, 81, 3309–3316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alteri, C.J.; Hagan, E.C.; Sivick, K.E.; Smith, S.N.; Mobley, H.L. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009, 5, e1000586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
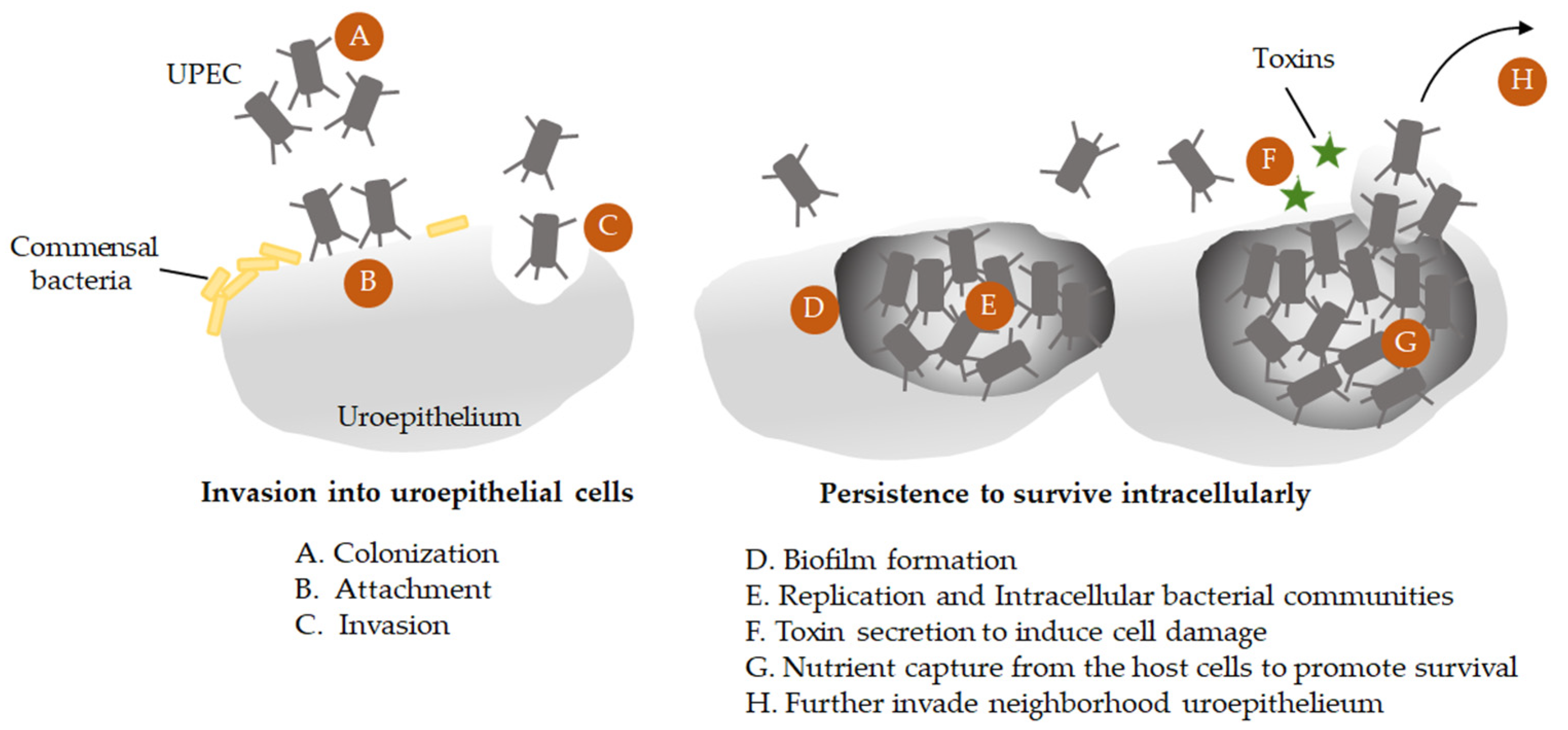
| Strain | Serotype | Iron Uptake System | Source | Characteristics | Reference |
|---|---|---|---|---|---|
| 536 | O6:K15:H31 | Yersiniabactin, enterobactin, salmochelin, and hemin uptake system | From a patient with acute pyelonephritis | Increased hemolytic activity (stronger than CFT073) | [15,16] |
| CFT073 | O6:K2:H1 | Enterobactin, salmochelin, aerobactin, hemin uptake system, and iron/manganese transport | Blood and urine of hospitalized women with acute pyelonephritis | Increased hemolytic activity. Encoding a gene cluster (c1931-c1936), that can produce F9 fimbria | [17,18] |
| UTI89 | O18:K1:H17 | Yersiniabactin, enterobactin, salmochelin, hemin uptake system, and iron/manganese transport | Urine of a patient with acute cystitis | Decreased endogenous production of ROS 1 Multiple genes related to biofilm formation were identified | [13,19] |
| F11 | O139:H38 | FetMP system | From a patient with acute uncomplicated cystitis and bacteriuria | Able to use both oxidation states of iron efficiently under conditions of ever-changing Fe(II)/Fe(III) ratios, aiding in colonization of the urinary tract | [20] |
| ABU83972 | Ont:K5 | Yersiniabactin, enterobactin, salmochelin, aerobactin, hemin uptake system, and iron/manganese transport | Urine of a female patient with asymptomatic bacteriuria | Rapid growth in urine | [20] |
| Virulence Factors | Type of Virulence Factors | Receptor | Gene | Characteristics | Reference |
|---|---|---|---|---|---|
| Adhesins | Type I fimbriae | Monomannose moiety of the tetraspanin molecule uroplakin 1a (UP1a) | fim B, fim E, fim H, and Pil | . Mannose-sensitive . Responsible for colonization, invasion, and persistence of uroepithelial cells . The most frequently expressed virulence factor, accounting for 80 to 100% of UPEC . Less prevalent in pyelonephritogenic UPEC but prevalent in urinary catheter-related bacteria | [27,28] |
| P fimbriae | Globoseries glycosphingolipid receptors (Gal-Gal) | PapG and papGAP | . Mannose-insensitive . Able to bind to glycosphingolipids of the kidney epithelium . A super-virulence factor that transits the response from asymptomatic bacteriuria to acute pyelonephritis in the host . Associated with the severity of UTI | [29,30] | |
| Dr adhesins | Type 4 collagen and Dr blood group antigen | AfaE1–5, AfaF, and Drb operon | . Invasion of epithelial cells of the bladder . Consists of both fimbrial and afimbrial adhesins | [28] | |
| S and F1C fimbriae | Sialyl-α-2-3 galactoside | Sfa/fac | . Associated with pyelonephritis and cystitis . Expressed by approximately 14% of UPEC | [31,32] | |
| Toxins | α-hemolysin (HlyA) | CD11a/CD18 (LFA-1) and glycophorin | hly | . Isolated from more than 70% of patients with pyelonephritis . Isolated from 31–48% of patients with acute cystitis . Associated with renal complications, including permanent renal scarring, in up to 50% of pyelonephritis cases | [33] |
| Cytotoxic necrotizing factor 1 | Lu/BCAM adhesion glycoprotein | cnf1 | . Able to modulate the activity of Rho GTPases | [34,35,36] | |
| Autotransporters | Vacuolating autotransporter toxin (VAT) | Cytokeratin 8 | vat | . Identified in 20–36% of UPEC . Isolated from 59–68% of patients with pyelonephritis and urosepsis | [24,37,38,39,40] |
| Secreted autotransporter toxin (SAT) | Cytokeratin 8 | Sat-encoding gene (sat) | . Isolated from 68% of patients with pyelonephritis | [40] |
| Aim of UPEC | Strategies of UPEC | Mechanism | Reference |
|---|---|---|---|
| Antagonism of uroepithelial cell apoptosis | Aerobic respiration | Cytochrome bd (reducing the efficiency of mitochondrial respiration) → stabilize HIF-1 → promote aerobic glycolysis | [43] |
| Maintenance of uropathogens’ cellular function | Nutrient acquisition | Iron: extracellular insoluble/biounavailable Fe3+ + siderophore → soluble/bioavailable siderophore- Fe3+ complex → transport into cell → intracellular iron assimilation from the siderophore–Fe3+ complex → intracellular iron usage and storage | [46] |
| Enhancement of uroepithelial cell exfoliation | Toxin secretion | Toxins → cell damage and apoptosis→ release of iron and nutrients from host cells | [14] |
| Steps of UPEC Pathogenesis | Normal Defenses of Hosts |
|---|---|
| Colonization of uropathogens in the urethra, periurethra, and vagina |
|
| Fim-H-mediated adherence to epithelial cells of the bladder |
|
| Biofilm elaboration and intracellular replication |
|
| Formation of bladder intracellular bacterial communities for invasion and replication |
|
| Siderophores for iron acquisition to maintain survival |
|
| Zinc acquisition to maintaining survival |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-C.; Lee, W.-C.; Chuang, Y.-C. Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection. Int. J. Mol. Sci. 2023, 24, 7055. https://doi.org/10.3390/ijms24087055
Chen Y-C, Lee W-C, Chuang Y-C. Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection. International Journal of Molecular Sciences. 2023; 24(8):7055. https://doi.org/10.3390/ijms24087055
Chicago/Turabian StyleChen, Yu-Chen, Wei-Chia Lee, and Yao-Chi Chuang. 2023. "Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection" International Journal of Molecular Sciences 24, no. 8: 7055. https://doi.org/10.3390/ijms24087055
APA StyleChen, Y.-C., Lee, W.-C., & Chuang, Y.-C. (2023). Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection. International Journal of Molecular Sciences, 24(8), 7055. https://doi.org/10.3390/ijms24087055






