Type I Diabetes in Zebrafish Reduces Sperm Quality and Increases Insulin and Glucose Transporter Transcripts
Abstract
1. Introduction
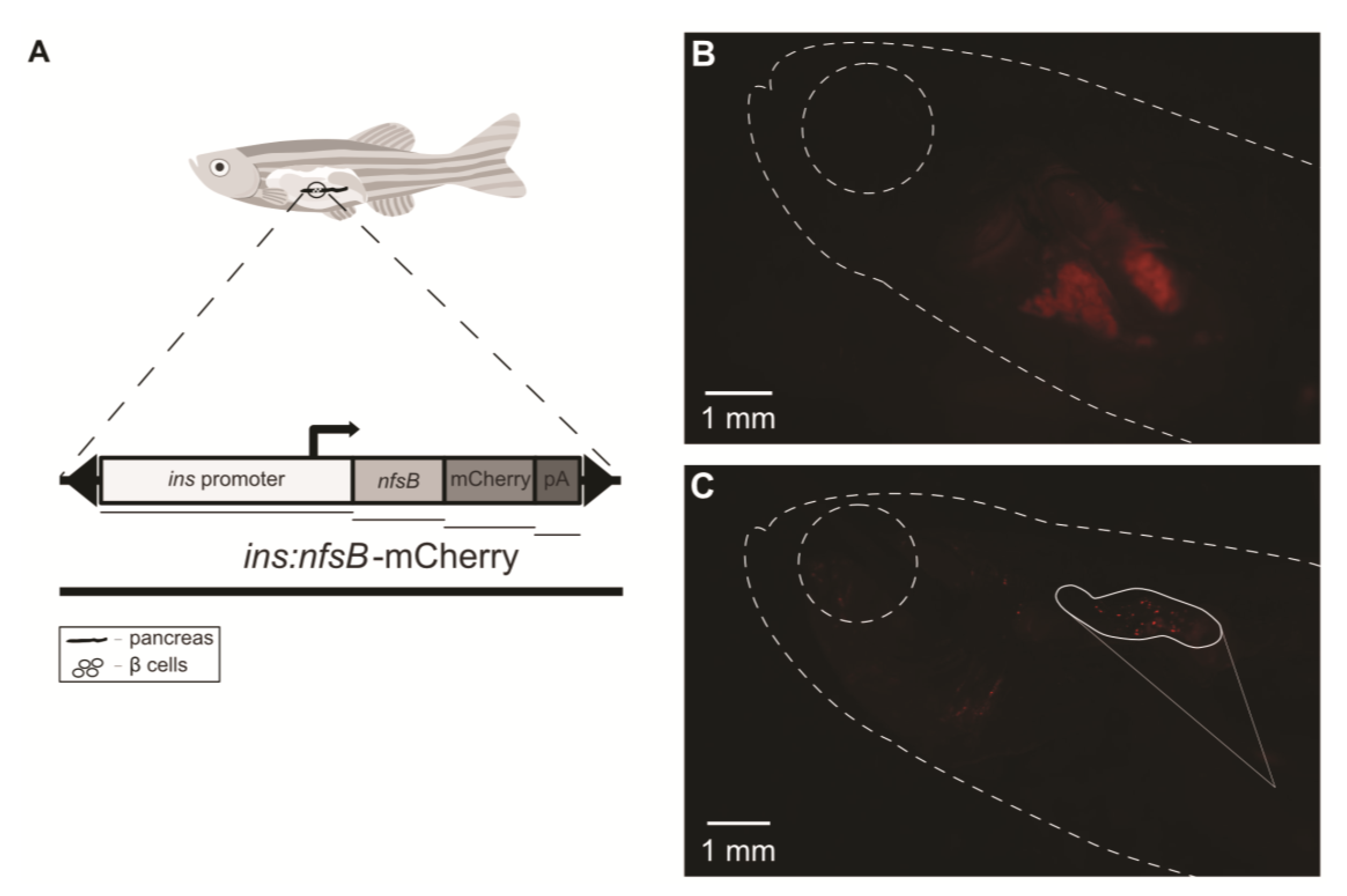
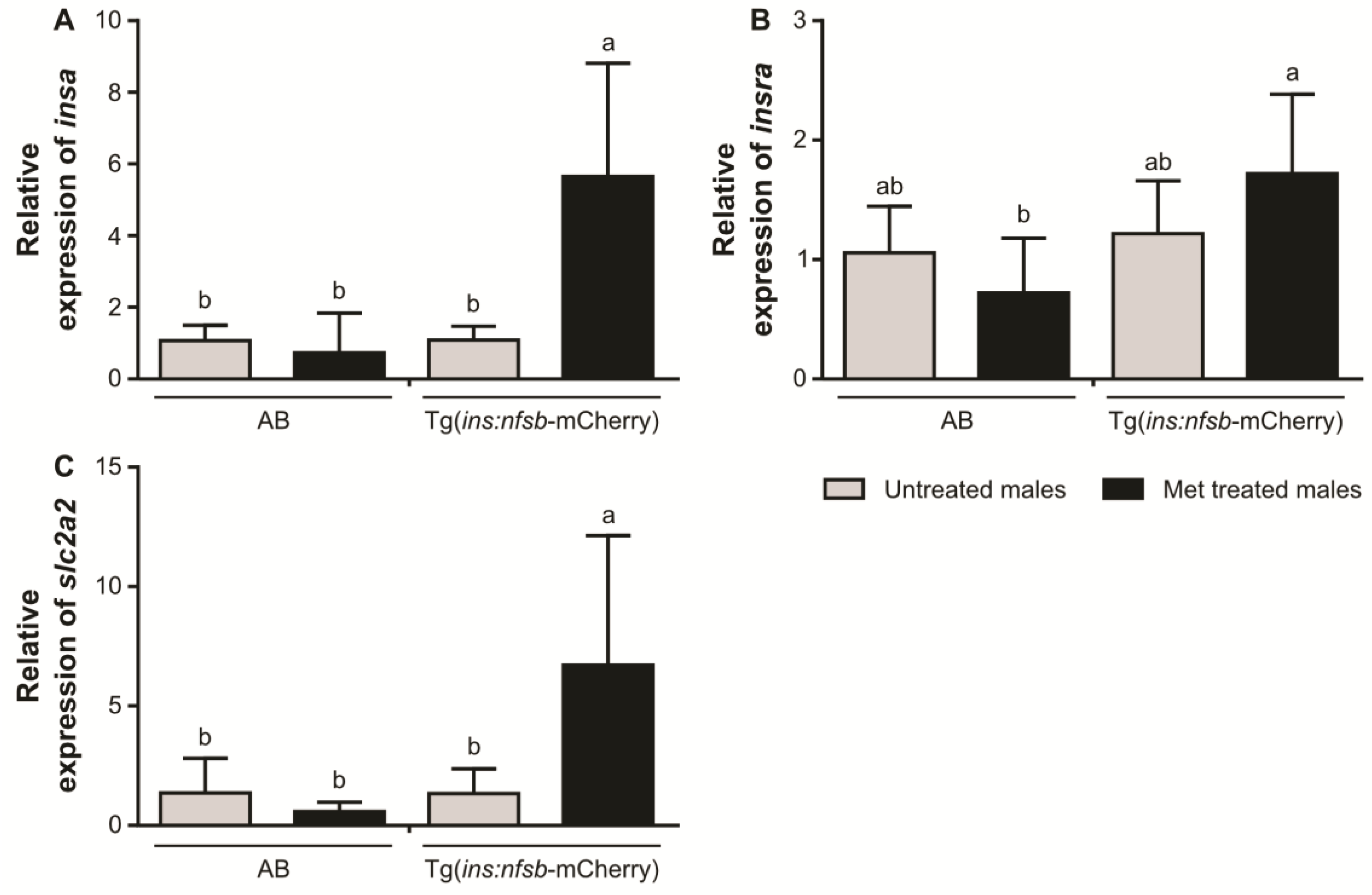
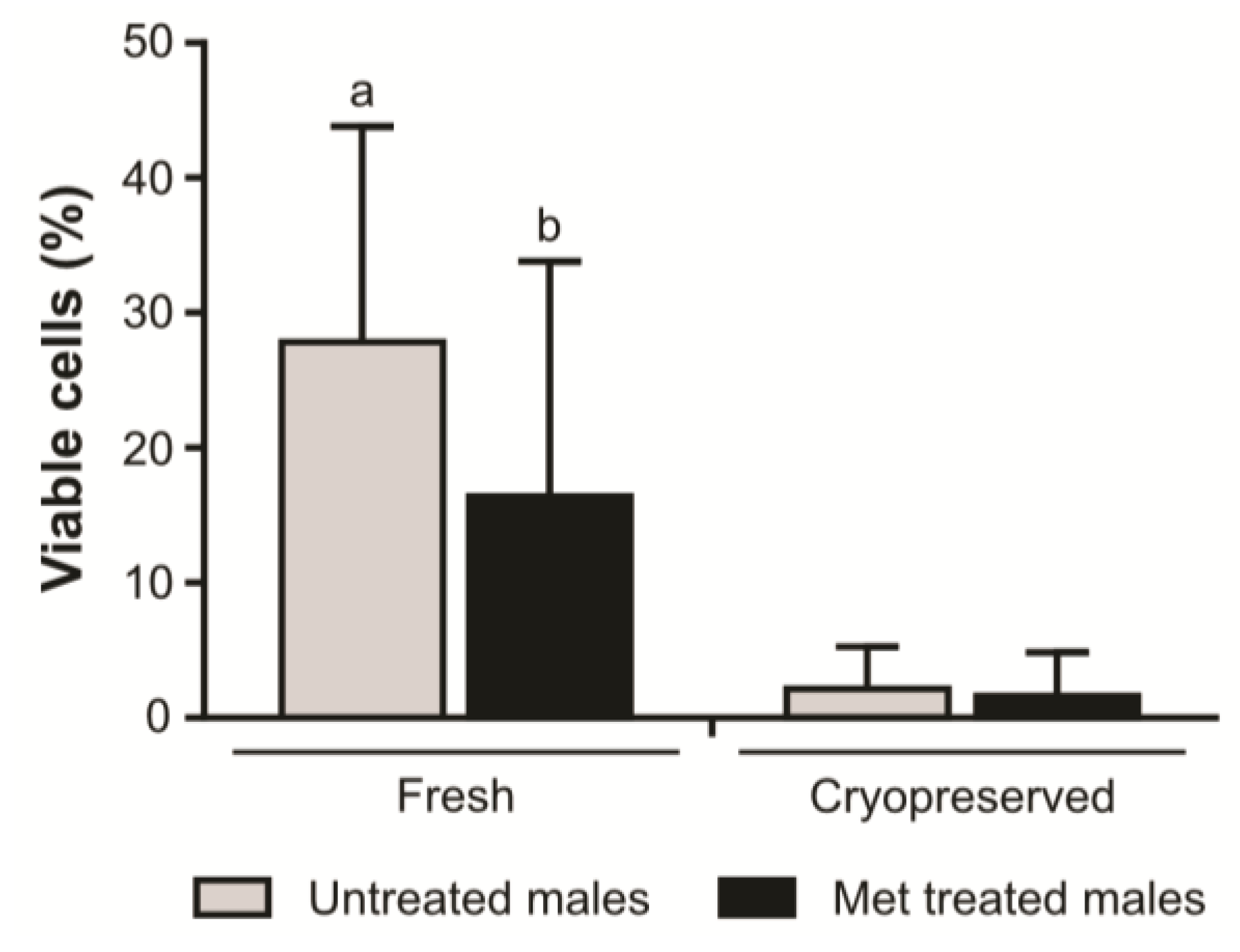
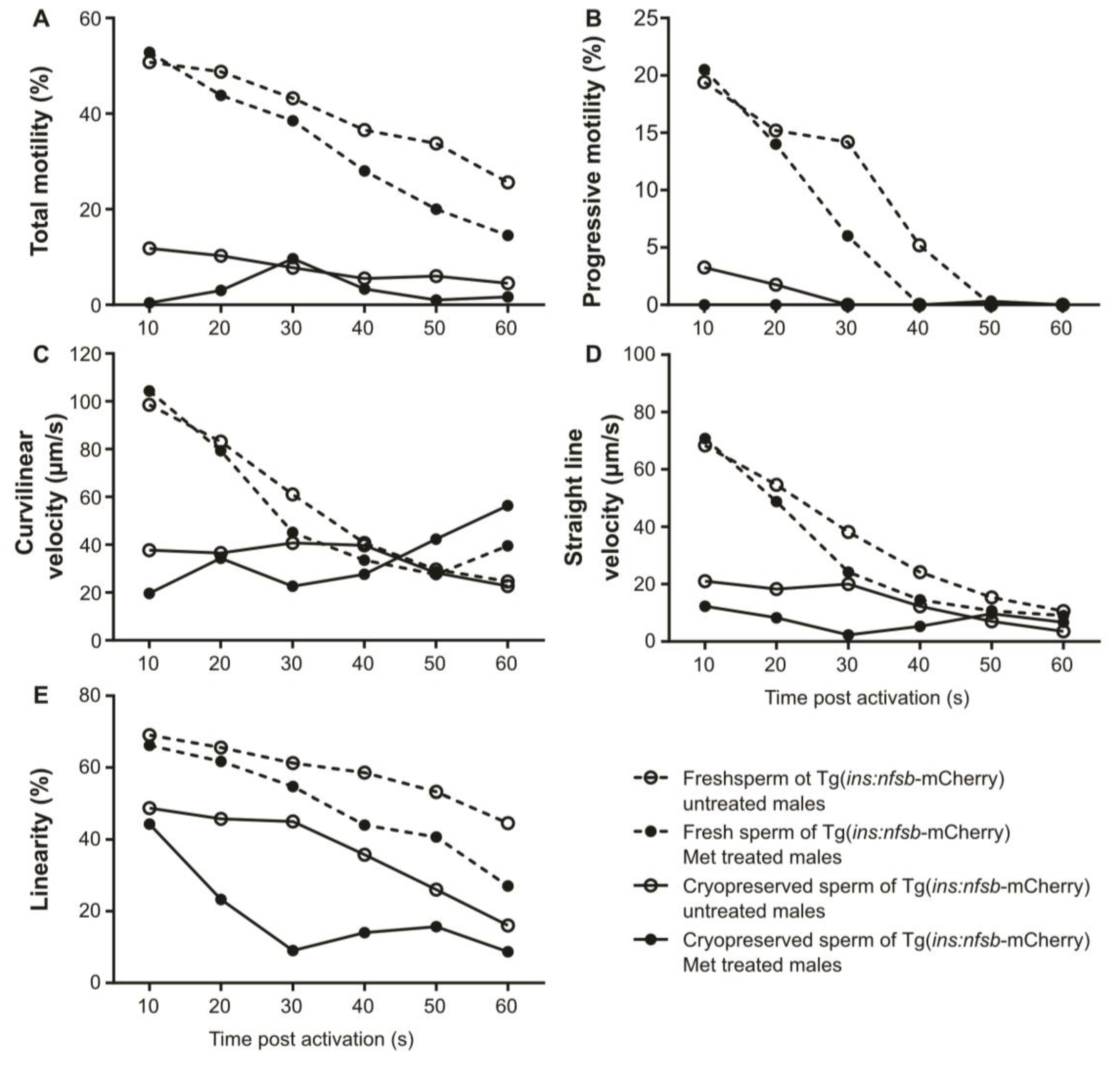
2. Results
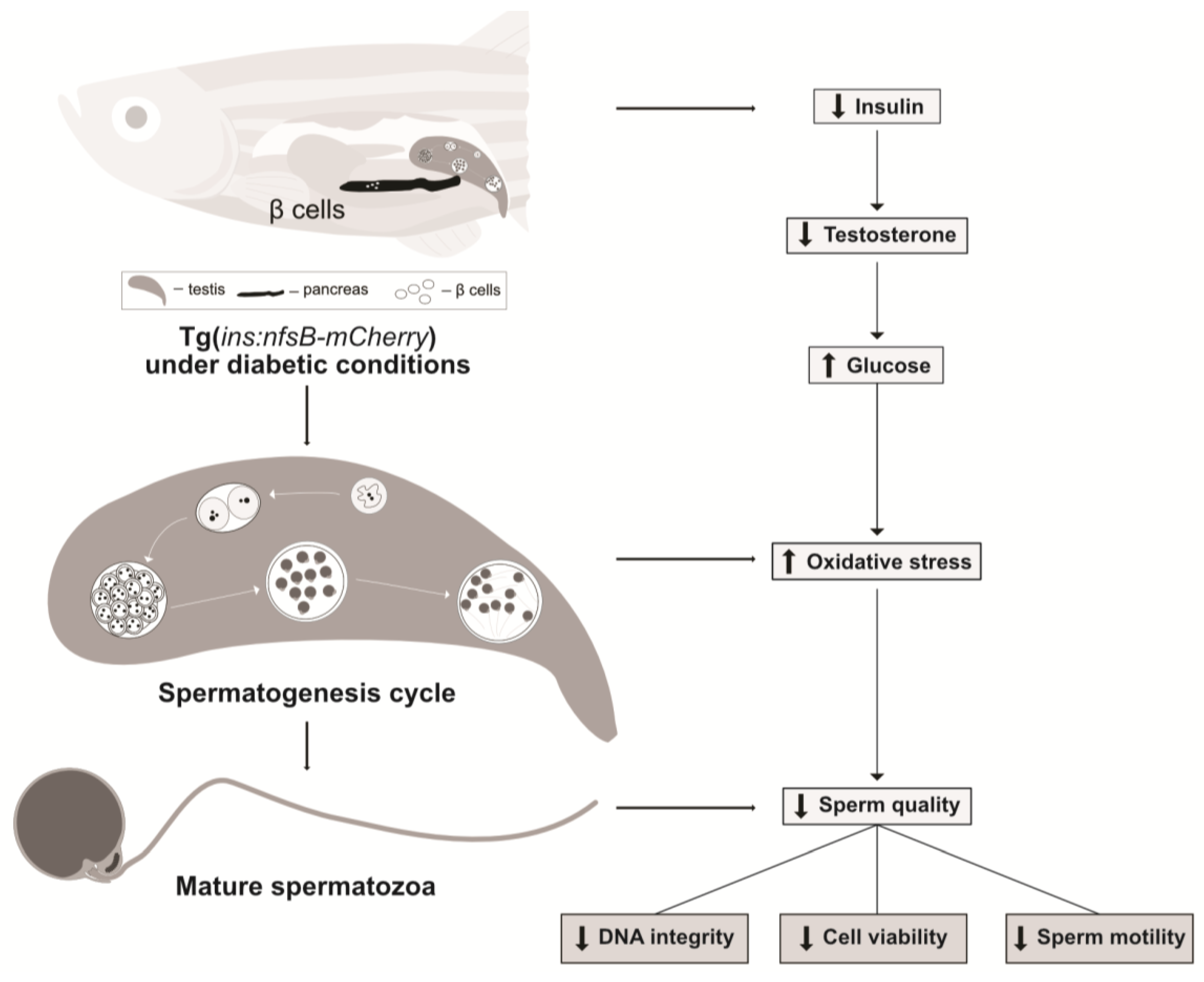
3. Discussion
4. Materials and Methods
4.1. Fish Husbandry
4.2. Experimental Design
4.3. Induction of Diabetes on Tg(ins:nfsb-mCherry) Zebrafish Model
4.4. Sperm Collection
4.5. Pancreatic Fluorescence Observation
4.6. RNA Extraction and Complementary DNA Synthesis
4.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.8. Sperm Plasma Membrane Viability Analysis
4.9. Sperm Cryopreservation and Thawing
4.10. Sperm Motility Analysis
4.11. DNA Integrity Evaluation through Comet Assay
4.12. Data Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell Deficit and Increased β-Cell Apoptosis in Humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary Fats and Prevention of Type 2 Diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, Z.; Huang, G.; Zhou, Z. Incidence and Trend of Type 1 Diabetes and the Underlying Environmental Determinants. Diabetes/Metab. Res. Rev. 2019, 35, e3075. [Google Scholar] [CrossRef]
- Ding, G.-L.; Liu, Y.; Liu, M.-E.; Pan, J.-X.; Guo, M.-X.; Sheng, J.-Z.; Huang, H.-F. The Effects of Diabetes on Male Fertility and Epigenetic Regulation during Spermatogenesis. Asian J. Androl. 2015, 17, 948. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Bergemann, D.; Massoz, L.; Bourdouxhe, J.; Pardo, C.A.C.; Voz, M.L.; Peers, B.; Manfroid, I. Nifurpirinol: A More Potent and Reliable Substrate Compared to Metronidazole for Nitroreductase-Mediated Cell Ablations. Wound Repair Regen. 2018, 26, 238–244. [Google Scholar] [CrossRef]
- Pisharath, H.; Rhee, J.M.; Swanson, M.A.; Leach, S.D.; Parsons, M.J. Targeted Ablation of Beta Cells in the Embryonic Zebrafish Pancreas Using E. Coli Nitroreductase. Mech. Dev. 2007, 124, 218–229. [Google Scholar] [CrossRef]
- Carvalho, F.R.; Fernandes, A.R.; Cancela, M.L.; Gavaia, P.J. Improved Regeneration and de Novo Bone Formation in a Diabetic Zebrafish Model Treated with Paricalcitol and Cinacalcet. Wound Repair Regen. 2017, 25, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Alves, M.G.; Silva, B.M.; Oliveira, P.F. Sperm Glucose Transport and Metabolism in Diabetic Individuals. Mol. Cell. Endocrinol. 2014, 396, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Urner, F.; Sakkas, D. Involvement of the Pentose Phosphate Pathway and Redox Regulation in Fertilization in the Mouse. Mol. Reprod. Dev. 2005, 70, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef]
- López-Escobar, B.; Cano, D.A.; Rojas, A.; de Felipe, B.; Palma, F.; Sánchez-Alcázar, J.A.; Henderson, D.; Ybot-González, P. The Effect of Maternal Diabetes on the Wnt-PCP Pathway during Embryogenesis as Reflected in the Developing Mouse Eye. Dis. Model. Mech. 2015, 8, 157–168. [Google Scholar] [CrossRef]
- Agbaje, I.M.; McVicar, C.M.; Schock, B.C.; McClure, N.; Atkinson, A.B.; Rogers, D.; Lewise, S.E.M. Increased Concentrations of the Oxidative DNA Adduct 7,8-Dihydro-8-Oxo-2-Deoxyguanosine in the Germ-Line of Men with Type 1 Diabetes. Reprod. Biomed. Online 2008, 16, 401–409. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of Obesity on Male Fertility, Sperm Function and Molecular Composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Dias, T.R.; Lopes, G.; Cavaco, J.E.; Socorro, S.; Oliveira, P.F. High-Energy Diets May Induce a Pre-Diabetic State Altering Testicular Glycolytic Metabolic Profile and Male Reproductive Parameters. Andrology 2013, 1, 495–504. [Google Scholar] [CrossRef]
- Cabrita, E.; Robles, V.; Herráez, M. Sperm Quality Assessment. In Methods in Reproductive Aquaculture: Marine and Freshwater Species, 1st ed.; Cabrita, E., Robles, V., Herráez, M., Eds.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Casas, I.; Sancho, S.; Briz, M.; Pinart, E.; Bussalleu, E.; Yeste, M.; Bonet, S. Freezability Prediction of Boar Ejaculates Assessed by Functional Sperm Parameters and Sperm Proteins. Theriogenology 2009, 72, 930–948. [Google Scholar] [CrossRef]
- Ranganathan, P.; Mahran, A.M.; Hallak, J.; Agarwal, A. Sperm Cryopreservation for Men With Nonmalignant, Systemic Diseases: A Descriptive Study. J. Androl. 2002, 23, 71–75. [Google Scholar] [CrossRef]
- Flores, E.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. The Degree of Resistance to Freezing-Thawing Is Related to Specific Changes in the Structures of Motile Sperm Subpopulations and Mitochondrial Activity in Boar Spermatozoa. Theriogenology 2009, 72, 784–797. [Google Scholar] [CrossRef]
- Pérez-Patiño, C.; Li, J.; Barranco, I.; Martínez, E.A.; Rodriguez-Martínez, H.; Roca, J.; Parrilla, I. The Proteome of Frozen-Thawed Pig Spermatozoa Is Dependent on the Ejaculate Fraction Source. Sci. Rep. 2019, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Hernández, M.; Carvajal, G.; Vázquez, J.M.; Martiínez, E.A. Factors Influencing Boar Sperm Cryosurvival1. J. Anim. Sci. 2006, 84, 2692–2699. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Ciereszko, A. Proteomic Characterization of Fresh Spermatozoa and Supernatant after Cryopreservation in Relation to Freezability of Carp (Cyprinus carpio L) Semen. PLoS ONE 2018, 13, e0192972. [Google Scholar] [CrossRef]
- Aquila, S.; Gentile, M.; Middea, E.; Catalano, S.; Andò, S. Autocrine Regulation of Insulin Secretion in Human Ejaculated Spermatozoa. Endocrinology 2005, 146, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Carpino, A.; Rago, V.; Guido, C.; Casaburi, I.; Aquila, S. Insulin and IR-β in Pig Spermatozoa: A Role of the Hormone in the Acquisition of Fertilizing Ability. Int. J. Androl. 2010, 33, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Andò, S.; Aquila, S. Arguments Raised by the Recent Discovery That Insulin and Leptin Are Expressed in and Secreted by Human Ejaculated Spermatozoa. Mol. Cell. Endocrinol. 2005, 245, 1–6. [Google Scholar] [CrossRef]
- Bucci, D.; Rodriguez-Gil, J.E.; Vallorani, C.; Spinaci, M.; Galeati, G.; Tamanini, C. GLUTs and Mammalian Sperm Metabolism. J. Androl. 2011, 32, 348–355. [Google Scholar] [CrossRef]
- Scheepers, A.; Joost, H.G.; Schurmann, A. The Glucose Transporter Families SGLT and GLUT: Molecular Basis of Normal and Aberrant Function. J. Parenter. Enter. Nutr. 2004, 28, 364–371. [Google Scholar] [CrossRef]
- Lawrence, C. Chapter 24—New Frontiers for Zebrafish Management. Methods Cell Biol. 2016, 135, 483–508. [Google Scholar] [CrossRef]
- Hoo, J.Y.; Kumari, Y.; Shaikh, M.F.; Hue, S.M.; Goh, B.H. Zebrafish: A Versatile Animal Model for Fertility Research. BioMed Res. Int. 2016, 2016, 9732780. [Google Scholar] [CrossRef]
- Patton, E.E.; Tobin, D.M. Spotlight on Zebrafish: The next Wave of Translational Research. Dis. Model. Mech. 2019, 12, dmm039370. [Google Scholar] [CrossRef]
- Vignera, S.; Calogero, A.; Condorelli, R.; Lanzafame, F.; Giammusso, B.; Vicari, E. Andrological Characterization of the Patient with Diabetes Mellitus. Minerva Endocrinol. 2009, 34, 1–9. [Google Scholar]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Martinvalet, D.; Zhu, P.; Lieberman, J. Granzyme A Induces Caspase-Independent Mitochondrial Damage, a Required First Step for Apoptosis. Immunity 2005, 22, 355–370. [Google Scholar] [CrossRef]
- Zheng, X.; Narayanan, S.; Xu, C.; Angelstig, S.E.; Grünler, J.; Zhao, A.; Di Toro, A.; Bernardi, L.; Mazzone, M.; Carmeliet, P.; et al. Repression of Hypoxia-Inducible Factor-1 Contributes to Increased Mitochondrial Reactive Oxygen Species Production in Diabetes. Elife 2022, 11, e70714. [Google Scholar] [CrossRef]
- Mallidis, C.; Agbaje, I.; O’Neill, J.; McClure, N. The Influence of Type 1 Diabetes Mellitus on Spermatogenic Gene Expression. Fertil. Steril. 2009, 92, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Ingermann, R.L.; Schultz, C.L.F.; Kanuga, M.K.; Wilson-Leedy, J.G. Metabolism of Motile Zebrafish Sperm. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Gallego, V.; Asturiano, J.F. Sperm Motility in Fish: Technical Applications and Perspectives through CASA-Mot Systems. Reprod. Fertil. Dev. 2018, 30, 820–832. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Alves, M.G.; Rato, L.; Laurentino, S.; Silva, J.; Sá, R.; Barros, A.; Sousa, M.; Carvalho, R.A.; Cavaco, J.E.; et al. Effect of Insulin Deprivation on Metabolism and Metabolism-Associated Gene Transcript Levels of in Vitro Cultured Human Sertoli Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Page-McCaw, P.S.; Chen, W.; Cone, R.D. Leptin Signaling Regulates Glucose Homeostasis, but Not Adipostasis, in the Zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, 3084–3089. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhai, G.; Su, J.; Yang, B.; Jin, J.; Liu, H.; Yin, Z.; Xie, S.; Han, D. Different Roles of Insulin Receptor a and b in Maintaining Blood Glucose Homeostasis in Zebrafish. Gen. Comp. Endocrinol. 2018, 269, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Martins, G.; Quinzico, I.; Nogueira, R.; Gavaia, P.J.; Cabrita, E. Electric Ultrafreezer (−150 °C) as an Alternative for Zebrafish Sperm Cryopreservation and Storage. Fish Physiol. Biochem. 2018, 44, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Vogel, S.S.; Liu, N.; Melton, D.A.; Lin, S. Analysis of Pancreatic Development in Living Transgenic Zebrafish Embryos. Mol. Cell. Endocrinol. 2001, 177, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the Pancreas in Adult Zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef]
- Leal, M.C.; Cardoso, E.R.; Nóbrega, R.H.; Batlouni, S.R.; Bogerd, J.; França, L.R.; Schulz, R.W. Histological and Stereological Evaluation of Zebrafish (Danio Rerio) Spermatogenesis with an Emphasis on Spermatogonial Generations1. Biol. Reprod. 2009, 81, 177–187. [Google Scholar] [CrossRef]
- Eames, S.C.; Philipson, L.H.; Prince, V.E.; Kinkel, M.D. Blood Sugar Measurement in Zebrafish Reveals Dynamics of Glucose Homeostasis. Zebrafish 2010, 7, 205–213. [Google Scholar] [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef]
- Wilson, J.M.; Bunte, R.M.; Carty, A.J. Evaluation of Rapid Cooling and Tricaine Methanesulfonate (MS222) as Methods of Euthanasia in Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 785–789. [Google Scholar]
- Jing, R.; Huang, C.; Bai, C.; Tanguay, R.; Dong, Q. Optimization of Activation, Collection, Dilution, and Storage Methods for Zebrafish Sperm. Aquaculture 2009, 290, 165–171. [Google Scholar] [CrossRef]
- Hagedorn, M.; Carter, V.L. Zebrafish Reproduction: Revisiting in Vitro Fertilization to Increase Sperm Cryopreservation Success. PLoS ONE 2011, 6, e21059. [Google Scholar] [CrossRef] [PubMed]
- McCurley, A.T.; Callard, G.V. Characterization of Housekeeping Genes in Zebrafish: Male-Female Differences and Effects of Tissue Type, Developmental Stage and Chemical Treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.; Tiersch, T.R. Sources of Variation in Flow Cytometric Analysis of Aquatic Species Sperm: The Effect of Cryoprotectants on Flow Cytometry Scatter Plots and Subsequent Population Gating. Aquaculture 2012, 370–371, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, E.; Robles, V.; Cuñado, S.; Wallace, J.C.; Sarasquete, C.; Herráez, M.P. Evaluation of Gilthead Sea Bream, Sparus Aurata, Sperm Quality after Cryopreservation in 5 mL Macrotubes. Cryobiology 2005, 50, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Martins, G.; Eufrásio, A.; Silva, T.; Cabrita, E.; Gavaia, P. Selection Criteria of Zebrafish Male Donors for Sperm Cryopreservation. Zebrafish 2019, 16, 189–196. [Google Scholar] [CrossRef]
- Reinardy, H.C.; Skippins, E.; Henry, T.B.; Jha, A.N. Assessment of DNA Damage in Sperm after Repeated Non-Invasive Sampling in Zebrafish Danio rerio. J. Fish Biol. 2013, 82, 1074–1081. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
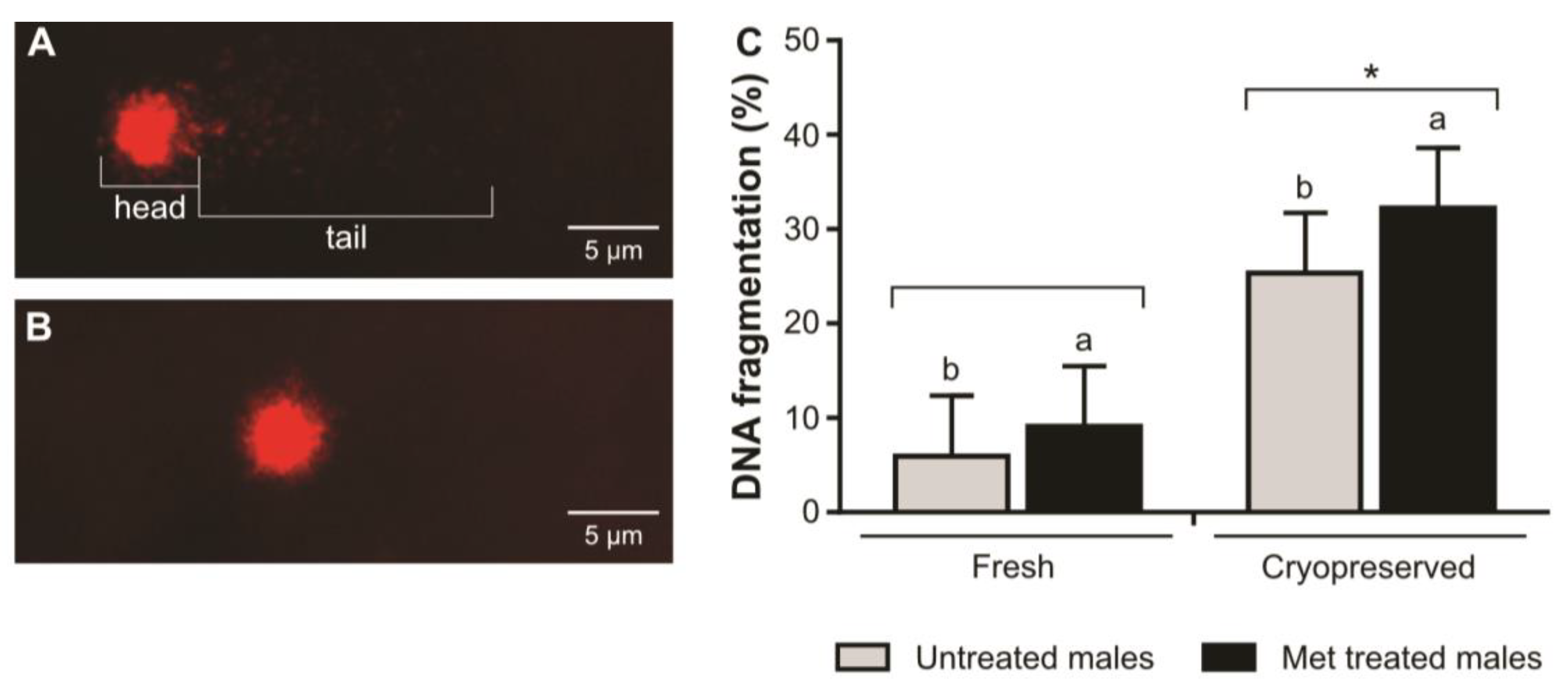
| Repeated Measures ANOVA | TM (%) | PM (%) | VCL (µm/s) | VSL (µm/s) | PM (%) |
|---|---|---|---|---|---|
| Treatment (control/diabetic) | 0.017 * | 0.050 * | 0.023 * | 0.018 * | 0.006 * |
| Sperm (fresh/cryopreserved) | <0.001 * | <0.001 * | 0.045 * | 0.005 * | 0.005 * |
| Treatment × sperm | 0.530 | 0.165 | 0.717 | 0.943 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diogo, P.; Martins, G.; Simão, M.; Marreiros, A.; Eufrásio, A.C.; Cabrita, E.; Gavaia, P.J. Type I Diabetes in Zebrafish Reduces Sperm Quality and Increases Insulin and Glucose Transporter Transcripts. Int. J. Mol. Sci. 2023, 24, 7035. https://doi.org/10.3390/ijms24087035
Diogo P, Martins G, Simão M, Marreiros A, Eufrásio AC, Cabrita E, Gavaia PJ. Type I Diabetes in Zebrafish Reduces Sperm Quality and Increases Insulin and Glucose Transporter Transcripts. International Journal of Molecular Sciences. 2023; 24(8):7035. https://doi.org/10.3390/ijms24087035
Chicago/Turabian StyleDiogo, Patrícia, Gil Martins, Márcio Simão, Ana Marreiros, Ana Catarina Eufrásio, Elsa Cabrita, and Paulo Jorge Gavaia. 2023. "Type I Diabetes in Zebrafish Reduces Sperm Quality and Increases Insulin and Glucose Transporter Transcripts" International Journal of Molecular Sciences 24, no. 8: 7035. https://doi.org/10.3390/ijms24087035
APA StyleDiogo, P., Martins, G., Simão, M., Marreiros, A., Eufrásio, A. C., Cabrita, E., & Gavaia, P. J. (2023). Type I Diabetes in Zebrafish Reduces Sperm Quality and Increases Insulin and Glucose Transporter Transcripts. International Journal of Molecular Sciences, 24(8), 7035. https://doi.org/10.3390/ijms24087035








