Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections
Abstract
1. Introduction
2. Androgen Deprivation Therapy and Cardiovascular Complications
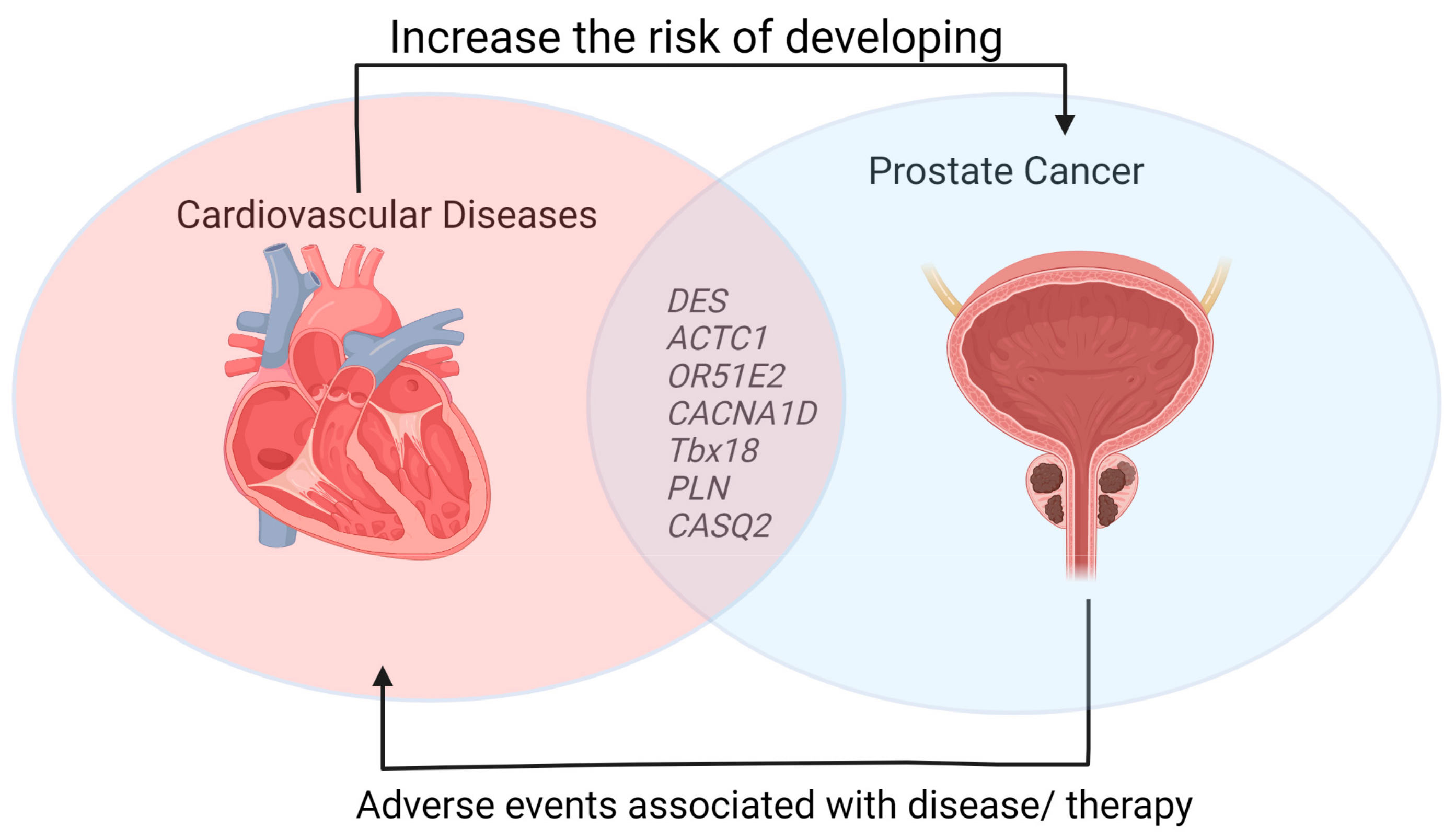
3. Cardiovascular Diseases and Prostate Cancer
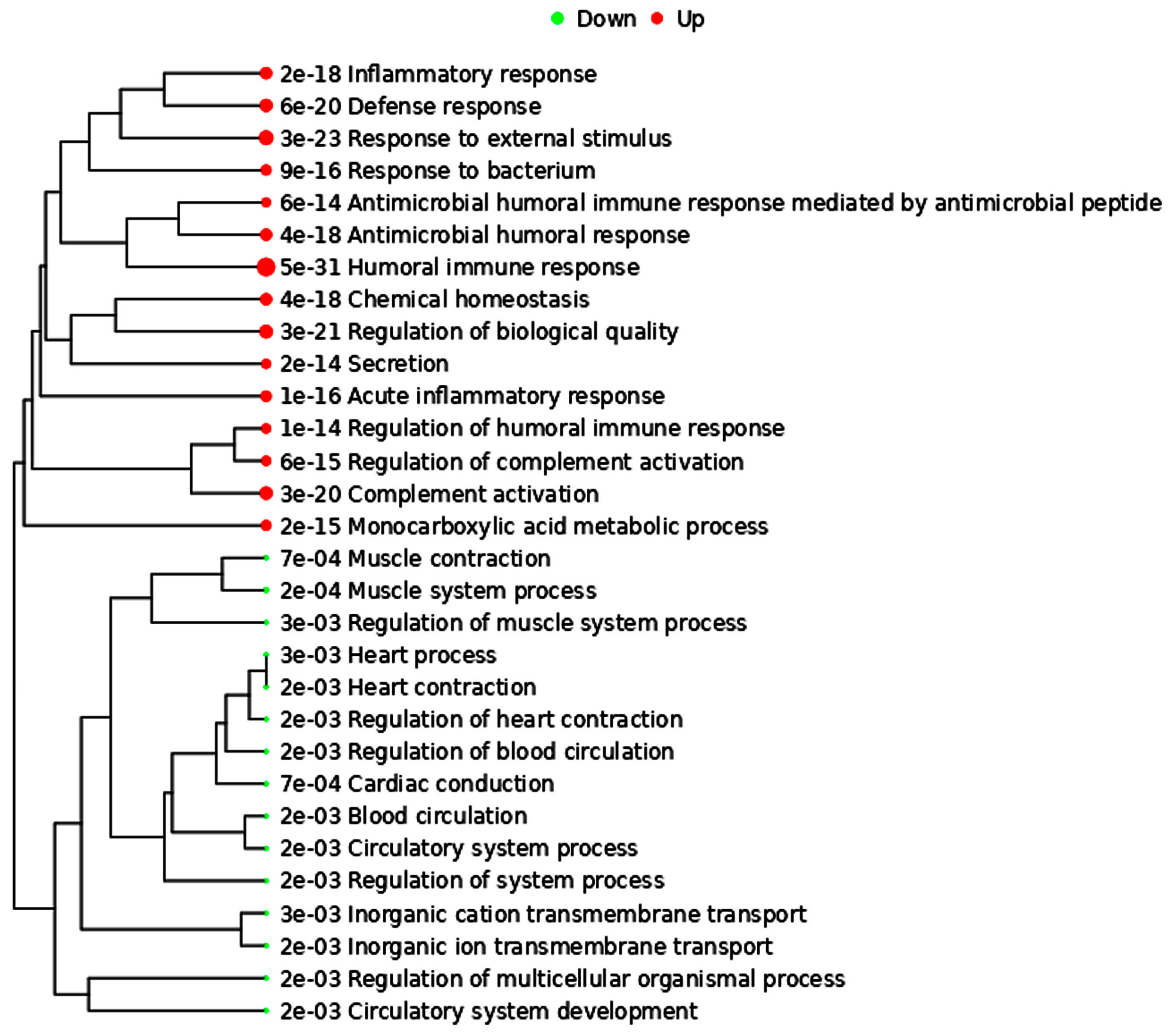
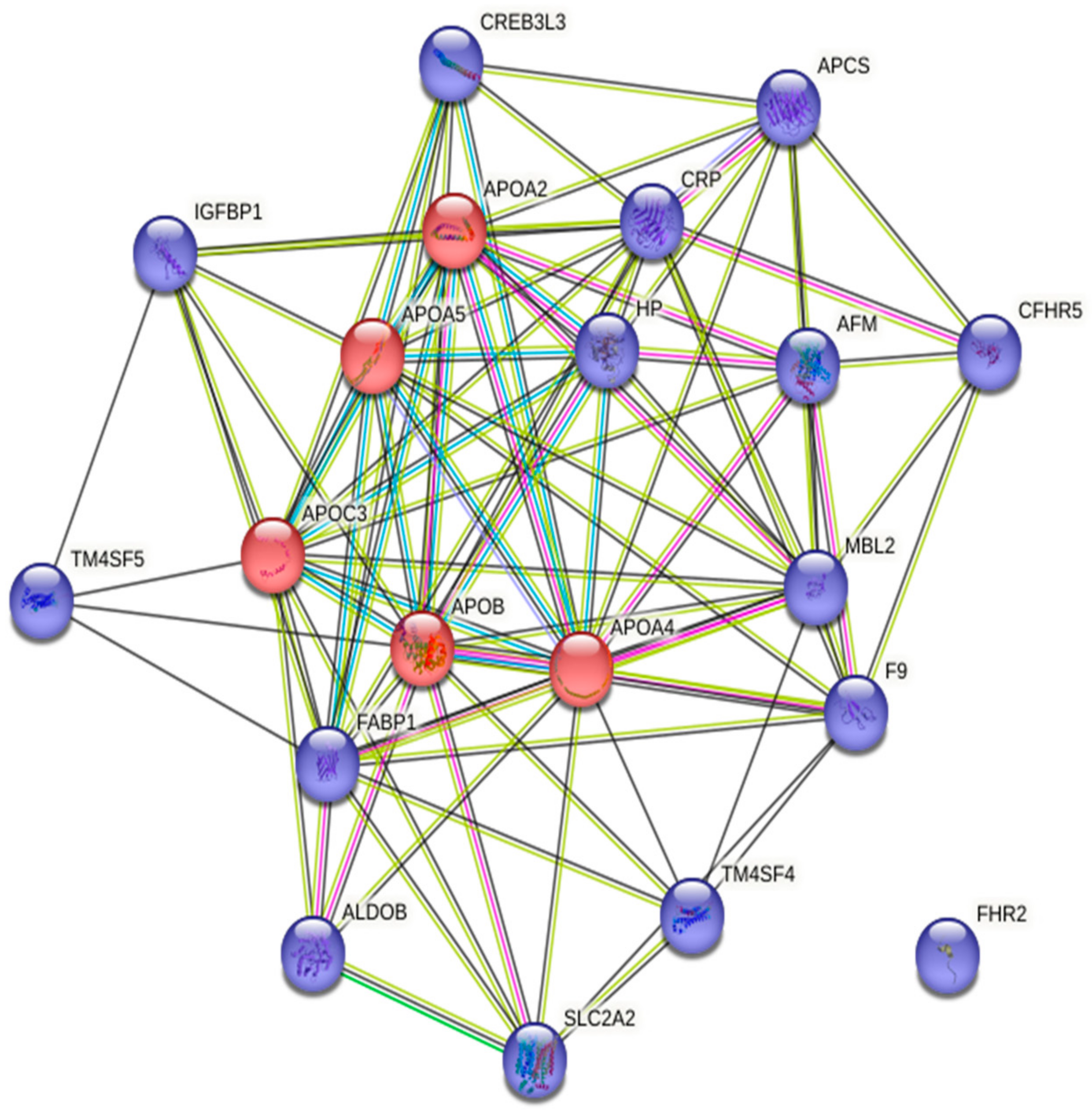
4. Molecular Link between Cardiovascular Diseases and Prostate Cancer
5. Cardiovascular Events Associated with Second-Generation ADTs: Results from Clinical Trials with CRPC Patients
| Drug | Trial Number and Description | Status | Phase | Intervention | Enrollment | Cardiovascular Events | References |
|---|---|---|---|---|---|---|---|
| Enzalutamide | NCT00974311 (AFFIRM study; multinational randomized, double-blind, placebo-controlled) | Start date: 30 September 2009 End date: 2 November 2017 | III | Arm 1: Enzalutamide Arm 2: Placebo | 1199 participants |
| [98] |
| NCT01212991(PREVAIL study; multinational randomized, double-blind, placebo-controlled) | Start date: 16 September 2010 End date: 14 February 2019 | III | Arm 1: Enzalutamide Arm 2: Placebo | 1717 participants |
| [99] | |
| NCT02116582(multi-center, single arm) | Start date: 23 May 2014 End date: 29 September 2017 | IV | Arm 1: Enzalutamide | 215 participants |
| [100] | |
| NCT01995513 (PLATO trial randomized, double-blind, placebo-controlled) | Start date: 22 October 2013 End date: 31 August 2022 | IV | Arm 1: Enzalutamide + Abiraterone + Prednisone Arm 2: Placebo + Abiraterone + Prednisone | 509 participants |
| [101] | |
| Abiraterone acetate | NCT00887198(randomized, double-blind, placebo-controlled) | Start date: 28 April 2009 End date: 25 May 2017 | III | Arm 1: Placebo + prednisone Arm 2: Abiraterone acetate + prednisone | 1088 participants |
| [102] |
| NCT01715285(LATITUDE study; randomized, double-blind, placebo-controlled) | Start date: 12 February 2013 End date: 13 February 2022 | III | Arm 1: Abiraterone acetate + Prednisone + ADT Arm 2: Placebo + ADT | 1209 participants |
| [103] | |
| NCT00638690 (randomized, double-blind, placebo-controlled) | Start date: May 2008 End date: October 2012 | III | Arm 1: Abiraterone acetate plus prednisone/ prednisolone Arm 2: Placebo plus prednisone/prednisolone | 1195 participants |
| [104] | |
| Apalutamide | NCT01946204(SPARTAN study; multicenter randomized, double-blind, placebo-controlled) | Start date: 14 October 2013 Estimated End date: 8 November 2023 | III | Arm 1: Apalutamide Arm 2: Placebo | 1207 participants |
| [105,106] |
| NCT02257736 (randomized, double-blind, placebo-controlled) | Start date: 26 November 2014 Estimated End date: 5 April 2023 | III | Arm 1: Apalutamide + Abiraterone acetate + Prednisone Arm 2: Placebo + Abiraterone acetate + Prednisone | 982 participants |
| [107] | |
| Darolutamide | NCT02200614 (ARAMIS trial; multinational, randomized, double-blind, placebo-controlled) | Start date: 12 September 2014 End date: 14 June 2021 | III | Arm 1: Darolutamide Arm 2: Placebo | 1509 participants |
| [108] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Merino, M.; Pinto, A.; González, R.; Espinosa, E. Antiangiogenic Agents and Endothelin Antagonists in Advanced Castration Resistant Prostate Cancer. Eur. J. Cancer 2011, 47, 1846–1851. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.-P.; Zietman, A.L.; et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N. Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Cannata, D.H.; Kirschenbaum, A.; Levine, A.C. Androgen Deprivation Therapy as Primary Treatment for Prostate Cancer. J. Clin. Endocrinol. Metab. 2012, 97, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.J.; Tindall, D.J. Androgen Receptor: Past, Present and Future. Curr. Drug Targets 2013, 14, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Smith, A.D.; Ferraldeschi, R.; Al-Lazikani, B.; Workman, P.; de Bono, J.S. Drug Discovery in Advanced Prostate Cancer: Translating Biology into Therapy. Nat. Rev. Drug Discov. 2016, 15, 699–718. [Google Scholar] [CrossRef]
- Katsogiannou, M.; Ziouziou, H.; Karaki, S.; Andrieu, C.; Henry de Villeneuve, M.; Rocchi, P. The Hallmarks of Castration-Resistant Prostate Cancers. Cancer Treat. Rev. 2015, 41, 588–597. [Google Scholar] [CrossRef]
- Haque, R.; UlcickasYood, M.; Xu, X.; Cassidy-Bushrow, A.E.; Tsai, H.-T.; Keating, N.L.; Van Den Eeden, S.K.; Potosky, A.L. Cardiovascular Disease Risk and Androgen Deprivation Therapy in Patients with Localised Prostate Cancer: A Prospective Cohort Study. Br. J. Cancer 2017, 117, 1233–1240. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Mahar, A.L.; Satkunasivam, R.; Herschorn, S.; Kodama, R.T.; Lee, Y.; Kulkarni, G.S.; Narod, S.A.; Nam, R.K. Cardiovascular and Skeletal-Related Events Following Localized Prostate Cancer Treatment: Role of Surgery, Radiotherapy, and Androgen Deprivation. Urology 2016, 97, 145–152. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy: Observational Study of Veterans With Prostate Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 39–46. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Bria, E.; Romano, M.; Fantinel, E.; Bimbatti, D.; Muraglia, A.; Porcaro, A.B.; Siracusano, S.; Brunelli, M.; et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin. Genitourin. Cancer 2018, 16, e645–e653. [Google Scholar] [CrossRef]
- Kao, H.-H.; Kao, L.-T.; Li, I.-H.; Pan, K.-T.; Shih, J.-H.; Chou, Y.-C.; Wu, S.-T. Androgen Deprivation Therapy Use Increases the Risk of Heart Failure in Patients With Prostate Cancer: A Population-Based Cohort Study. J. Clin. Pharmacol. 2019, 59, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Makarov, D.V.; Partin, A.W. Weighing the Risks: Prostate Cancer versus Cardiovascular Disease. Rev. Urol. 2008, 10, 171–173. [Google Scholar]
- Zhu, X.; Wu, S. Increased Risk of Hypertension with Enzalutamide in Prostate Cancer: A Meta-Analysis. Cancer Investig. 2019, 37, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Thomsen, H.; Brandt, A.; Sundquist, J.; Hemminki, K. What Do Prostate Cancer Patients Die Of? Oncology 2011, 16, 175–181. [Google Scholar] [CrossRef]
- Conteduca, V.; Di Lorenzo, G.; Tartarone, A.; Aieta, M. The Cardiovascular Risk of Gonadotropin Releasing Hormone Agonists in Men with Prostate Cancer: An Unresolved Controversy. Crit. Rev. Oncol./Hematol. 2013, 86, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Donkena, K.V.; Yuan, H.; Young, C.Y. Recent Advances in Understanding Hormonal Therapy Resistant Prostate Cancer. CCDT 2010, 10, 402–410. [Google Scholar] [CrossRef]
- Ohwaki, K.; Endo, F.; Hattori, K. Abdominal Obesity, Hypertension, Antihypertensive Medication Use and Biochemical Recurrence of Prostate Cancer after Radical Prostatectomy. Eur. J. Cancer 2015, 51, 604–609. [Google Scholar] [CrossRef]
- Santala, E.E.; Rannikko, A.; Murtola, T.J. Antihypertensive Drugs and Prostate Cancer Survival after Radical Prostatectomy in Finland-A Nationwide Cohort Study. Int. J. Cancer 2019, 144, 440–447. [Google Scholar] [CrossRef]
- Perron, L.; Bairati, I.; Harel, F.; Meyer, F. Antihypertensive Drug Use and the Risk of Prostate Cancer (Canada). Cancer Causes Control 2004, 15, 535–541. [Google Scholar] [CrossRef]
- Ronquist, G.; Rodríguez, L.A.G.; Ruigómez, A.; Johansson, S.; Wallander, M.-A.; Frithz, G.; Svärdsudd, K. Association between Captopril, Other Antihypertensive Drugs and Risk of Prostate Cancer. Prostate 2004, 58, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Wilson, K.M.; Steingrimsdottir, L.; Aspelund, T.; Batista, J.L.; Fall, K.; Giovannucci, E.; Sigurdardottir, L.G.; et al. Midlife Metabolic Factors and Prostate Cancer Risk in Later Life. Int. J. Cancer 2018, 142, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Navin, S.; Ioffe, V. The Association between Hypertension and Prostate Cancer. Rev. Urol. 2017, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bolton, E.C.; Jones, J.O. Androgens and Androgen Receptor Signaling in Prostate Tumorigenesis. J. Mol. Endocrinol. 2015, 54, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 1941, 43, 209. [Google Scholar] [CrossRef]
- Choi, E.; Buie, J.; Camacho, J.; Sharma, P.; de Riese, W.T.W. Evolution of Androgen Deprivation Therapy (ADT) and Its New Emerging Modalities in Prostate Cancer: An Update for Practicing Urologists, Clinicians and Medical Providers. Res. Rep. Urol. 2022, 14, 87–108. [Google Scholar] [CrossRef]
- Goodale, T.; Sadhu, A.; Petak, S.; Robbins, R. Testosterone and the Heart. Methodist. Debakey Cardiovasc. J. 2017, 13, 68–72. [Google Scholar] [CrossRef]
- Smith, J.C.; Bennett, S.; Evans, L.M.; Kynaston, H.G.; Parmar, M.; Mason, M.D.; Cockcroft, J.R.; Scanlon, M.F.; Davies, J.S. The Effects of Induced Hypogonadism on Arterial Stiffness, Body Composition, and Metabolic Parameters in Males with Prostate Cancer. J. Clin. Endocrinol. Metab. 2001, 86, 4261–4267. [Google Scholar] [CrossRef]
- Maggio, M.; Basaria, S. Welcoming Low Testosterone as a Cardiovascular Risk Factor. Int. J. Impot. Res. 2009, 21, 261–264. [Google Scholar] [CrossRef]
- Hu, J.-R.; Duncan, M.S.; Morgans, A.K.; Brown, J.D.; Meijers, W.C.; Freiberg, M.S.; Salem, J.-E.; Beckman, J.A.; Moslehi, J.J. Cardiovascular Effects of Androgen Deprivation Therapy in Prostate Cancer: Contemporary Meta-Analyses. ATVB 2020, 40, e55–e64. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Sasse, A.D.; Wagner, A.A.; Peixoto, G.; Kataguiri, A.; Neto, A.S.; Bianco, B.A.V.; Chang, P.; Pompeo, A.C.L.; Tobias-Machado, M. Cardiovascular Events Associated with Androgen Deprivation Therapy in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. World J. Urol. 2015, 33, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhu, S.; Zhao, J.; Vados, L.; Wang, L.; Zhao, Y.; Zhao, D.; Niu, Y. Stroke Related to Androgen Deprivation Therapy for Prostate Cancer: A Meta-Analysis and Systematic Review. BMC Cancer 2016, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, S.; Sun, L.; Meng, F.; Zhao, L.; Zhao, Y.; Tian, H.; Li, P.; Niu, Y. Androgen Deprivation Therapy for Prostate Cancer Is Associated with Cardiovascular Morbidity and Mortality: A Meta-Analysis of Population-Based Observational Studies. PLoS ONE 2014, 9, e107516. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and Cardiovascular Disease during Androgen Deprivation Therapy for Prostate Cancer. J. Clin. Oncol. 2006, 24, 4448–4456. [Google Scholar] [CrossRef]
- Chen, D.-Y.; See, L.-C.; Liu, J.-R.; Chuang, C.-K.; Pang, S.-T.; Hsieh, I.-C.; Wen, M.-S.; Chen, T.-H.; Lin, Y.-C.; Liaw, C.-C.; et al. Risk of Cardiovascular Ischemic Events After Surgical Castration and Gonadotropin-Releasing Hormone Agonist Therapy for Prostate Cancer: A Nationwide Cohort Study. J. Clin. Oncol. 2017, 35, 3697–3705. [Google Scholar] [CrossRef]
- Lopes, R.D.; Higano, C.S.; Slovin, S.F.; Nelson, A.J.; Bigelow, R.; Sørensen, P.S.; Melloni, C.; Goodman, S.G.; Evans, C.P.; Nilsson, J.; et al. Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Prostate Cancer: The Primary Results of the PRONOUNCE Randomized Trial. Circulation 2021, 144, 1295–1307. [Google Scholar] [CrossRef]
- Zengerling, F.; Jakob, J.J.; Schmidt, S.; Meerpohl, J.J.; Blümle, A.; Schmucker, C.; Mayer, B.; Kunath, F. Degarelix for Treating Advanced Hormone-Sensitive Prostate Cancer. Cochrane Database Syst. Rev. 2021, 2021, CD012548. [Google Scholar] [CrossRef]
- Melloni, C.; Slovin, S.F.; Blemings, A.; Goodman, S.G.; Evans, C.P.; Nilsson, J.; Bhatt, D.L.; Zubovskiy, K.; Olesen, T.K.; Dugi, K.; et al. Cardiovascular Safety of Degarelix Versus Leuprolide for Advanced Prostate Cancer. JACC CardioOncol. 2020, 2, 70–81. [Google Scholar] [CrossRef]
- Shore, N.D.; Sutton, J. Plain Language Summary of the HERO Study Comparing Relugolix with Leuprolide for Men with Advanced Prostate Cancer. Future Oncol. 2022, 18, 2575–2584. [Google Scholar] [CrossRef]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; Akaza, H.; Bossi, A.; van Veenhuyzen, D.F.; Selby, B.; et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Saigal, C.S.; Gore, J.L.; Krupski, T.L.; Hanley, J.; Schonlau, M.; Litwin, M.S.; The Urologic Diseases in America Project. Androgen Deprivation Therapy Increases Cardiovascular Morbidity in Men with Prostate Cancer. Cancer 2007, 110, 1493–1500. [Google Scholar] [CrossRef]
- Stocks, T.; Hergens, M.-P.; Englund, A.; Ye, W.; Stattin, P. Blood Pressure, Body Size and Prostate Cancer Risk in the Swedish Construction Workers Cohort. Int. J. Cancer 2010, 127, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, S. Risk of Hypertension in Cancer Patients Treated with Abiraterone: A Meta-Analysis. Clin. Hypertens. 2019, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.E.; Olmos, D.; de Bono, J.S. CYP17 Blockade by Abiraterone: Further Evidence for Frequent Continued Hormone-Dependence in Castration-Resistant Prostate Cancer. Br. J. Cancer 2009, 100, 671–675. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lakely, B.; Nguyen, H.M.; Yang, Y.C.; da Costa, R.M.G.; Kaipainen, A.; et al. Molecular Profiling Stratifies Diverse Phenotypes of Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer. J. Clin. Investig. 2019, 129, 4492–4505. [Google Scholar] [CrossRef]
- Muniyan, S.; Xi, L.; Datta, K.; Das, A.; Teply, B.A.; Batra, S.K.; Kukreja, R.C. Cardiovascular Risks and Toxicity—The Achilles Heel of Androgen Deprivation Therapy in Prostate Cancer Patients. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188383. [Google Scholar] [CrossRef]
- Thomas, J.-A.; Gerber, L.; Bañez, L.L.; Moreira, D.M.; Rittmaster, R.S.; Andriole, G.L.; Freedland, S.J. Prostate Cancer Risk in Men with Baseline History of Coronary Artery Disease: Results from the REDUCE Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 576–581. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berrington de González, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total Cholesterol and Cancer Risk in a Large Prospective Study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef]
- Iso, H.; Ikeda, A.; Inoue, M.; Sato, S.; Tsugane, S.; JPHC Study Group. Serum Cholesterol Levels in Relation to the Incidence of Cancer: The JPHC Study Cohorts. Int. J. Cancer 2009, 125, 2679–2686. [Google Scholar] [CrossRef]
- Platz, E.A.; Till, C.; Goodman, P.J.; Parnes, H.L.; Figg, W.D.; Albanes, D.; Neuhouser, M.L.; Klein, E.A.; Thompson, I.M.; Kristal, A.R. Men with Low Serum Cholesterol Have a Lower Risk of High-Grade Prostate Cancer in the Placebo Arm of the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2807–2813. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.; Shufelt, C.; Iribarren, C.; Merz, C.N.B. Sex Hormones and the QT Interval: A Review. J. Womens Health 2012, 21, 933–941. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Yang, T.; Moslehi, J.J.; Waintraub, X.; Gandjbakhch, E.; Bachelot, A.; Hidden-Lucet, F.; Hulot, J.-S.; Knollmann, B.C.; Lebrun-Vignes, B.; et al. Androgenic Effects on Ventricular Repolarization: A Translational Study From the International Pharmacovigilance Database to IPSC-Cardiomyocytes. Circulation 2019, 140, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Bretagne, M.; Lebrun-Vignes, B.; Pariente, A.; Shaffer, C.M.; Malouf, G.G.; Dureau, P.; Potey, C.; Funck-Brentano, C.; Roden, D.M.; Moslehi, J.J.; et al. Heart Failure and Atrial Tachyarrhythmia on Abiraterone: A Pharmacovigilance Study. Arch. Cardiovasc. Dis. 2020, 113, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, L.; Yin, H.; Benayoun, S.; Renoux, C.; Boivin, J.-F.; Suissa, S. Androgen-Deprivation Therapy and the Risk of Stroke in Patients with Prostate Cancer. Eur. Urol. 2011, 60, 1244–1250. [Google Scholar] [CrossRef]
- Hu, J.C.; Williams, S.B.; O’Malley, A.J.; Smith, M.R.; Nguyen, P.L.; Keating, N.L. Androgen-Deprivation Therapy for Nonmetastatic Prostate Cancer Is Associated with an Increased Risk of Peripheral Arterial Disease and Venous Thromboembolism. Eur. Urol. 2012, 61, 1119–1128. [Google Scholar] [CrossRef]
- Sun, M.; Choueiri, T.K.; Hamnvik, O.-P.R.; Preston, M.A.; De Velasco, G.; Jiang, W.; Loeb, S.; Nguyen, P.L.; Trinh, Q.-D. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: Effects of Androgen-Deprivation Therapy. JAMA Oncol. 2016, 2, 500–507. [Google Scholar] [CrossRef]
- Yun, S.J.; Kim, S.-K.; Kim, J.; Cha, E.-J.; Kim, J.-S.; Kim, S.-J.; Ha, Y.-S.; Kim, Y.-H.; Jeong, P.; Kang, H.W.; et al. Transcriptomic Features of Primary Prostate Cancer and Their Prognostic Relevance to Castration-Resistant Prostate Cancer. Oncotarget 2017, 8, 114845–114855. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R Package for Identifying Differentially Expressed Genes from RNA-Seq Data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Storey, J.D. The Positive False Discovery Rate: A Bayesian Interpretation and the q-Value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: Pathology and Mechanisms. Acta Neuropathol. 2013, 125, 47–75. [Google Scholar] [CrossRef]
- Elsnicova, B.; Hornikova, D.; Tibenska, V.; Kolar, D.; Tlapakova, T.; Schmid, B.; Mallek, M.; Eggers, B.; Schlötzer-Schrehardt, U.; Peeva, V.; et al. Desmin Knock-Out Cardiomyopathy: A Heart on the Verge of Metabolic Crisis. Int. J. Mol. Sci. 2022, 23, 12020. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.Y.; van Hessem, L.; Jongbloed, J.D.H.; de Walle, H.E.K.; Capetanaki, Y.; van der Kooi, A.J.; van Langen, I.M.; van den Berg, M.P.; van Tintelen, J.P. Desmin-Related Myopathy. Clin. Genet. 2011, 80, 354–366. [Google Scholar] [CrossRef]
- Kramer, P.L.; Luty, J.A.; Litt, M. Regional Localization of the Gene for Cardiac Muscle Actin (ACTC) on Chromosome 15q. Genomics 1992, 13, 904–905. [Google Scholar] [CrossRef]
- Frank, D.; Yusuf Rangrez, A.; Friedrich, C.; Dittmann, S.; Stallmeyer, B.; Yadav, P.; Bernt, A.; Schulze-Bahr, E.; Borlepawar, A.; Zimmermann, W.-H.; et al. Cardiac α-Actin (ACTC1) Gene Mutation Causes Atrial-Septal Defects Associated with Late-Onset Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2019, 12, e002491. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hirono, K.; Nakamura, K.; Suzuki, T.; Hata, Y.; Nishida, N. A Novel ACTC1 Mutation in a Young Boy with Left Ventricular Noncompaction and Arrhythmias. Hear. Case Rep. 2016, 2, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Jovancevic, N.; Dendorfer, A.; Matzkies, M.; Kovarova, M.; Heckmann, J.C.; Osterloh, M.; Boehm, M.; Weber, L.; Nguemo, F.; Semmler, J.; et al. Medium-Chain Fatty Acids Modulate Myocardial Function via a Cardiac Odorant Receptor. Basic Res. Cardiol. 2017, 112, 13. [Google Scholar] [CrossRef] [PubMed]
- Scholl, U.I.; Goh, G.; Stölting, G.; de Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.W.; et al. Somatic and Germline CACNA1D Calcium Channel Mutations in Aldosterone-Producing Adenomas and Primary Aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef]
- Yang, Y.; Song, J.-Y.; Wang, S.; Wang, Y.; Song, Q.-Y.; Dong, Y.-H.; Li, C.-X.; Wang, H.-J.; Ma, J. Combined Effects of the Rs9810888 Polymorphism in Calcium Voltage-Gated Channel Subunit Alpha1 D (CACNA1D) and Lifestyle Behaviors on Blood Pressure Level among Chinese Children. PLoS ONE 2019, 14, e0216950. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.-K.; Cheng, L.; Mao, G.; Chen, H.; Wu, X.; Hong, H.; Wang, C.; Lin, P.; Chen, J.; et al. Dominant Role of CACNA1D Exon Mutations for Blood Pressure Regulation. J. Hypertens. 2022, 40, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.; Haenig, B.; Kispert, A. Cloning and Expression Analysis of the Mouse T-Box Gene Tbx18. Mech. Dev. 2001, 100, 83–86. [Google Scholar] [CrossRef]
- Wu, S.-P.; Dong, X.-R.; Regan, J.N.; Su, C.; Majesky, M.W. Tbx18 Regulates Development of the Epicardium and Coronary Vessels. Dev. Biol. 2013, 383, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Hajighasemi, S.; Khori, V.; Soleimani, M.; Rajaei, M.; Rabbani, S.; Atashi, A.; Ghiaseddin, A.; Saeid, A.K.; Ahmadi Tafti, H.; et al. Functional Biological Pacemaker Generation by T-Box18 Protein Expression via Stem Cell and Viral Delivery Approaches in a Murine Model of Complete Heart Block. Pharmacol. Res. 2019, 141, 443–450. [Google Scholar] [CrossRef]
- Rodriguez, P.; Kranias, E.G. Phospholamban: A Key Determinant of Cardiac Function and Dysfunction. Arch. Mal. Coeur Vaiss. 2005, 98, 1239–1243. [Google Scholar]
- Haghighi, K.; Bidwell, P.; Kranias, E.G. Phospholamban Interactome in Cardiac Contractility and Survival: A New Vision of an Old Friend. J. Mol. Cell. Cardiol. 2014, 77, 160–167. [Google Scholar] [CrossRef]
- Haghighi, K.; Kolokathis, F.; Gramolini, A.O.; Waggoner, J.R.; Pater, L.; Lynch, R.A.; Fan, G.-C.; Tsiapras, D.; Parekh, R.R.; Dorn, G.W.; et al. A Mutation in the Human Phospholamban Gene, Deleting Arginine 14, Results in Lethal, Hereditary Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2006, 103, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, M.; Kryshtal, D.O.; Knollmann, B.C. Calsequestrin Mutations and Catecholaminergic Polymorphic Ventricular Tachycardia. Pediatr. Cardiol. 2012, 33, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, A.; Lacombe, V.A.; Belevych, A.E.; Brunello, L.; Carnes, C.A.; Janssen, P.M.L.; Knollmann, B.C.; Periasamy, M.; Gyørke, S. Up-Regulation of Sarcoplasmic Reticulum Ca2+ Uptake Leads to Cardiac Hypertrophy, Contractile Dysfunction and Early Mortality in Mice Deficient in CASQ2. Cardiovasc. Res. 2013, 98, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; de la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and Lipoproteins in Cardiovascular Diseases: A Classification. Trends Endocrinol. Metab. 2022, 33, 409–423. [Google Scholar] [CrossRef]
- Gofman, J.W.; Lindgren, F.; Elliott, H.; Mantz, W.; Hewitt, J.; Strisower, B.; Herring, V.; Lyon, T.P. The Role of Lipids and Lipoproteins in Atherosclerosis. Science 1950, 111, 166–186. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Dominguez-Viqueira, W.; Liu, M.; Awasthi, S.; Abraham-Miranda, J.; Keske, A.; Steiner, K.K.; Noel, L.; Serna, A.N.; Dhillon, J.; et al. Macrophage-Derived Cholesterol Contributes to Therapeutic Resistance in Prostate Cancer. Cancer Res. 2021, 81, 5477–5490. [Google Scholar] [CrossRef]
- Carter, A.M. Complement Activation: An Emerging Player in the Pathogenesis of Cardiovascular Disease. Scientifica 2012, 2012, 402783. [Google Scholar] [CrossRef]
- Malik, G.; Ward, M.D.; Gupta, S.K.; Trosset, M.W.; Grizzle, W.E.; Adam, B.-L.; Diaz, J.I.; Semmes, O.J. Serum Levels of an Isoform of Apolipoprotein A-II as a Potential Marker for Prostate Cancer. Clin. Cancer Res. 2005, 11, 1073–1085. [Google Scholar] [CrossRef]
- Bisgaier, C.L.; Glickman, R.M. Intestinal Synthesis, Secretion, and Transport of Lipoproteins. Annu. Rev. Physiol. 1983, 45, 625–636. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.A.; Ouatas, T.; Krauwinkel, W.; Ohtsu, Y.; van der Walt, J.-S.; Beddo, V.; de Vries, M.; Mordenti, J. Clinical Pharmacokinetic Studies of Enzalutamide. Clin. Pharm. 2015, 54, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.-E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour Activity of MDV3100 in Castration-Resistant Prostate Cancer: A Phase 1–2 Study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, E.; Titus, M.; Wen, S.; Hoang, A.; Karlou, M.; Ashe, R.; Tu, S.M.; Aparicio, A.; Troncoso, P.; Mohler, J.; et al. Molecular Characterization of Enzalutamide-Treated Bone Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2015, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005, Comment on Parker, C.; Sartor, O. N. Engl. J. Med. 2011, 365, 767; Comment on Sonpavde, G. N. Engl. J. Med. 2011, 365, 766–767; Reply on Sonpavde, G. N. Engl. J. Med. 2011, 365, 767–768. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Fizazi, K.; Blue, I.; Nowak, J.T. Darolutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer: A Patient Perspective of the ARAMIS Trial. Future Oncol. 2021, 17, 1699–1707. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.-E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef]
- Loriot, Y.; Miller, K.; Sternberg, C.N.; Fizazi, K.; De Bono, J.S.; Chowdhury, S.; Higano, C.S.; Noonberg, S.; Holmstrom, S.; Mansbach, H.; et al. Effect of Enzalutamide on Health-Related Quality of Life, Pain, and Skeletal-Related Events in Asymptomatic and Minimally Symptomatic, Chemotherapy-Naive Patients with Metastatic Castration-Resistant Prostate Cancer (PREVAIL): Results from a Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 509–521. [Google Scholar] [CrossRef]
- de Bono, J.S.; Chowdhury, S.; Feyerabend, S.; Elliott, T.; Grande, E.; Melhem-Bertrandt, A.; Baron, B.; Hirmand, M.; Werbrouck, P.; Fizazi, K. Antitumour Activity and Safety of Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer Previously Treated with Abiraterone Acetate Plus Prednisone for ≥24 Weeks in Europe. Eur. Urol. 2018, 74, 37–45. [Google Scholar] [CrossRef]
- Attard, G.; Borre, M.; Gurney, H.; Loriot, Y.; Andresen-Daniil, C.; Kalleda, R.; Pham, T.; Taplin, M.-E.; PLATO collaborators. Abiraterone Alone or in Combination with Enzalutamide in Metastatic Castration-Resistant Prostate Cancer with Rising Prostate-Specific Antigen during Enzalutamide Treatment. J. Clin. Oncol. 2018, 36, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Carles, J.; Gschwend, J.E.; Van Poppel, H.; Diels, J.; Brookman-May, S.D. The Phase 3 COU-AA-302 Study of Abiraterone Acetate Plus Prednisone in Men with Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer: Stratified Analysis Based on Pain, Prostate-Specific Antigen, and Gleason Score. Eur. Urol. 2018, 74, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Baciarello, G.; Özgüroğlu, M.; Mundle, S.; Leitz, G.; Richarz, U.; Hu, P.; Feyerabend, S.; Matsubara, N.; Chi, K.N.; Fizazi, K. Impact of Abiraterone Acetate plus Prednisone in Patients with Castration-Sensitive Prostate Cancer and Visceral Metastases over Four Years of Follow-up: A Post-Hoc Exploratory Analysis of the LATITUDE Study. Eur. J. Cancer 2022, 162, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Harland, S.; Staffurth, J.; Molina, A.; Hao, Y.; Gagnon, D.D.; Sternberg, C.N.; Cella, D.; Fizazi, K.; Logothetis, C.J.; Kheoh, T.; et al. Effect of Abiraterone Acetate Treatment on the Quality of Life of Patients with Metastatic Castration-Resistant Prostate Cancer after Failure of Docetaxel Chemotherapy. Eur. J. Cancer 2013, 49, 3648–3657. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-Free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef]
- Saad, F.; Cella, D.; Basch, E.; Hadaschik, B.A.; Mainwaring, P.N.; Oudard, S.; Graff, J.N.; McQuarrie, K.; Li, S.; Hudgens, S.; et al. Effect of Apalutamide on Health-Related Quality of Life in Patients with Non-Metastatic Castration-Resistant Prostate Cancer: An Analysis of the SPARTAN Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2018, 19, 1404–1416. [Google Scholar] [CrossRef]
- Saad, F.; Efstathiou, E.; Attard, G.; Flaig, T.W.; Franke, F.; Goodman, O.B.; Oudard, S.; Steuber, T.; Suzuki, H.; Wu, D.; et al. Apalutamide plus Abiraterone Acetate and Prednisone versus Placebo plus Abiraterone and Prednisone in Metastatic, Castration-Resistant Prostate Cancer (ACIS): A Randomised, Placebo-Controlled, Double-Blind, Multinational, Phase 3 Study. Lancet Oncol. 2021, 22, 1541–1559. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Trial Number and Description | Status | Phase | Intervention | Enrollment | Cardiovascular Events | References |
|---|---|---|---|---|---|---|---|
| 1. Degarelix (GnRH Receptor Antagonist) 2. Leuprolide (GnRH Receptor Agonist) | NCT02663908 (PRONOUNCE study; multi-center, randomized, assessor-blind, controlled) | Start date: 19 April 2016 Estimated End date: 29 March 2021 | III | Arm 1: Degarelix Arm 2: Leuprolide | 545 participants |
| [37,38,39] |
| 1. Relugolix (GnRH Receptor Antagonist) 2. Leuprolide Acetate(GnRH Receptor Agonist) | NCT03085095 (HERO study; multinational, randomized, open-label, parallel-group) | Start date: 18 April 2017 Estimated End date: 26 November 2021 | III | Arm1: Relugolix Arm 2: Leuprolide Acetate | 1134 participants |
| [40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakkat, S.; Pramanik, P.; Singh, S.; Singh, A.P.; Sarkar, C.; Chakroborty, D. Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections. Int. J. Mol. Sci. 2023, 24, 6984. https://doi.org/10.3390/ijms24086984
Kakkat S, Pramanik P, Singh S, Singh AP, Sarkar C, Chakroborty D. Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections. International Journal of Molecular Sciences. 2023; 24(8):6984. https://doi.org/10.3390/ijms24086984
Chicago/Turabian StyleKakkat, Sooraj, Paramahansa Pramanik, Seema Singh, Ajay Pratap Singh, Chandrani Sarkar, and Debanjan Chakroborty. 2023. "Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections" International Journal of Molecular Sciences 24, no. 8: 6984. https://doi.org/10.3390/ijms24086984
APA StyleKakkat, S., Pramanik, P., Singh, S., Singh, A. P., Sarkar, C., & Chakroborty, D. (2023). Cardiovascular Complications in Patients with Prostate Cancer: Potential Molecular Connections. International Journal of Molecular Sciences, 24(8), 6984. https://doi.org/10.3390/ijms24086984








