Inhibitory Synaptic Influences on Developmental Motor Disorders
Abstract
1. Introduction
2. Inhibitory Neurotransmission
2.1. GABA- and Glycinergic Neurotransmission
2.2. Glycinergic Neurotransmission
2.3. Developmental Aspects of GABA and Glycine as Signalling Molecules and Neurotransmitters
2.4. Developmental Aspects of Chloride Channel Expression
3. Inhibitory Influences on Rett Syndrome
3.1. Rett Syndrome
3.2. Limb and Respiratory Neuromotor Deficits in Rett Syndrome and Their Relationships to Inhibitory Neurotransmission
3.3. Chloride Homeostasis in Rett Syndrome
4. Inhibitory Influences on Spastic Cerebral Palsy
4.1. Spastic Cerebral Palsy
4.2. Animal Models of Cerebral Palsy and Criteria for Validation
4.3. Evidence for Inhibitory Deficits in Spastic Cerebral Palsy
4.4. Enhancing Inhibition as Therapies in Spastic Cerebral Palsy
4.5. Motor Neurons and the Spinal Cord as a Locus of Pathophysiology in Spastic Cerebral Palsy
4.6. Prospects for Progress in Understanding the Pathogenesis and Improving Patient Outcomes in Spastic Cerebral Palsy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fogarty, M.J.; Sieck, G.C. Diaphragm muscle adaptations in health and disease. Drug Discov. Today Dis. Model. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Pitts, T.; Iceman, K.E. Deglutition and the Regulation of the Swallow Motor Pattern. Physiology 2023, 38, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Hacker, P.M. The motor system in neuroscience: A history and analysis of conceptual developments. Prog. Neurobiol. 2002, 67, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Nielsen, J.B. Spinal control of locomotion—from cat to man. Acta Physiol. 2007, 189, 111–121. [Google Scholar] [CrossRef]
- Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef]
- Grillner, S.; Wallen, P.; Saitoh, K.; Kozlov, A.; Robertson, B. Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res. Rev. 2008, 57, 2–12. [Google Scholar] [CrossRef]
- Maas, E. Speech and nonspeech: What are we talking about? Int. J. Speech Lang. Pathol. 2017, 19, 345–359. [Google Scholar] [CrossRef]
- Eccles, J.C.; Sherrington, C.S. Numbers and contraction values of individual motor units examined in some muscles of the limb. Am. J. Physiol. 1930, 253, 210–218. [Google Scholar]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Sieck, G.C. Evolution and Functional Differentiation of the Diaphragm Muscle of Mammals. Compr. Physiol. 2019, 9, 715–766. [Google Scholar]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Elbasiouny, S.M.; Rymer, W.Z.; Heckman, C.J. Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: A simulation study. J. Neurophysiol. 2012, 107, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.K.; Heckman, C.J. Synaptic control of the shape of the motoneuron pool input-output function. J. Neurophysiol. 2017, 117, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Kubota, K.; Kobayakawa, K.; Saito, T.; Hara, M.; Kijima, K.; Maeda, T.; Katoh, H.; Ohkawa, Y.; Nakashima, Y.; et al. Pathological changes of distal motor neurons after complete spinal cord injury. Mol. Brain 2019, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Mantilla, C.B.; Sieck, G.C. Glutamatergic Input Varies with Phrenic Motor Neuron Size. J. Neurophysiol. 2019, 122, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Hammond, L.A.; Kanjhan, R.; Bellingham, M.C.; Noakes, P.G. A method for the three-dimensional reconstruction of Neurobiotin-filled neurons and the location of their synaptic inputs. Front. Neural. Circuits 2013, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Kanjhan, R.; Bellingham, M.C.; Noakes, P.G. Glycinergic Neurotransmission: A Potent Regulator of Embryonic Motor Neuron Dendritic Morphology and Synaptic Plasticity. J. Neurosci. 2016, 36, 80–87. [Google Scholar] [CrossRef]
- Rozani, I.; Tsapara, G.; Witts, E.C.; Deaville, S.J.; Miles, G.B.; Zagoraiou, L. Pitx2 cholinergic interneurons are the source of C bouton synapses on brainstem motor neurons. Sci. Rep. 2019, 9, 4936. [Google Scholar] [CrossRef]
- Buls Wollman, L.; Clarke, J.; DeLucia, C.M.; Levine, R.B.; Fregosi, R.F. Developmental Nicotine Exposure Alters Synaptic Input to Hypoglossal Motoneurons and Is Associated with Altered Function of Upper Airway Muscles. eNeuro 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Thakre, P.P.; Bellingham, M.C. Capsaicin Enhances Glutamatergic Synaptic Transmission to Neonatal Rat Hypoglossal Motor Neurons via a TRPV1-Independent Mechanism. Front. Cell. Neurosci. 2017, 11, 383. [Google Scholar] [CrossRef]
- Lau, C.; Thakre, P.P.; Bellingham, M.C. Alfaxalone Causes Reduction of Glycinergic IPSCs, but Not Glutamatergic EPSCs, and Activates a Depolarizing Current in Rat Hypoglossal Motor Neurons. Front. Cell. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef]
- Thakre, P.P.; Bellingham, M.C. Capsaicin causes robust reduction in glycinergic transmission to rat hypoglossal motor neurons via a TRPV1-independent mechanism. J. Neurophysiol. 2019, 121, 1535–1542. [Google Scholar] [CrossRef]
- Bellingham, M.C. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J. Neurophysiol. 1999, 82, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.F.; Funk, G.D.; Bellingham, M.C. Muscarinic acetylcholine receptors enhance neonatal mouse hypoglossal motoneuron excitability in vitro. J. Appl. Physiol. 2012, 113, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C.; Berger, A.J. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J. Neurophysiol. 1996, 76, 3758–3770. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.H.; Bellingham, M.C.; Berger, A.J. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J. Neurophysiol. 1996, 76, 799–807. [Google Scholar] [CrossRef]
- Lorier, A.R.; Funk, G.D.; Greer, J.J. Opiate-induced suppression of rat hypoglossal motoneuron activity and its reversal by ampakine therapy. PLoS ONE 2010, 5, e8766. [Google Scholar] [CrossRef]
- Parkis, M.A.; Dong, X.; Feldman, J.L.; Funk, G.D. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: Phase-specific control of excitability. J. Neurosci. 1999, 19, 2368–2380. [Google Scholar] [CrossRef]
- Rekling, J.C.; Funk, G.D.; Bayliss, D.A.; Dong, X.W.; Feldman, J.L. Synaptic control of motoneuronal excitability. Physiol. Rev. 2000, 80, 767–852. [Google Scholar] [CrossRef]
- Flynn, J.R.; Conn, V.L.; Boyle, K.A.; Hughes, D.I.; Watanabe, M.; Velasquez, T.; Goulding, M.D.; Callister, R.J.; Graham, B.A. Anatomical and Molecular Properties of Long Descending Propriospinal Neurons in Mice. Front Neuroanat 2017, 11, 5. [Google Scholar] [CrossRef]
- Smith, J.C.; Ellenberger, H.H.; Ballanyi, K.; Richter, D.W.; Feldman, J.L. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science 1991, 254, 726–729. [Google Scholar] [CrossRef]
- Leiras, R.; Cregg, J.M.; Kiehn, O. Brainstem Circuits for Locomotion. Ann. Rev. Neurosci. 2022, 45, 63–85. [Google Scholar] [CrossRef]
- Grillner, S.; Kozlov, A. The CPGs for Limbed Locomotion-Facts and Fiction. Int. J. Mol. Sci. 2021, 22, 5882. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S. The execution of movement: A spinal affair. J. Neurophysiol. 2021, 125, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; El Manira, A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed]
- Bolneo, E.; Chau, P.Y.S.; Noakes, P.G.; Bellingham, M.C. Investigating the Role of GABA in Neural Development and Disease Using Mice Lacking GAD67 or VGAT Genes. Int. J. Mol. Sci. 2022, 23, 7965. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Karlen-Amarante, M.; Wang, J.J.; Bush, N.E.; Carroll, M.S.; Weese-Mayer, D.E.; Huff, A. The Pathophysiology of Rett Syndrome With a Focus on Breathing Dysfunctions. Physiology 2020, 35, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.E.; Fogarty, M.J.; Sieck, G.C. A Critical Evaluation of Current Concepts in Cerebral Palsy. Physiology 2019, 34, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Shaye, H.; Stauch, B.; Gati, C.; Cherezov, V. Molecular mechanisms of metabotropic GABA(B) receptor function. Sci. Adv. 2021, 7, eabg3362. [Google Scholar] [CrossRef]
- Bowery, N.G.; Smart, T.G. GABA and glycine as neurotransmitters: A brief history. Br. J. Pharmacol. 2006, 147, S109–S119. [Google Scholar] [CrossRef] [PubMed]
- Misgeld, U.; Bijak, M.; Jarolimek, W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog. Neurobiol. 1995, 46, 423–462. [Google Scholar] [CrossRef] [PubMed]
- Dutar, P.; Nicoll, R.A. A physiological role for GABAB receptors in the central nervous system. Nature 1988, 332, 156–158. [Google Scholar] [CrossRef]
- Mueller, A.L.; Taube, J.S.; Schwartzkroin, P.A. Development of Hyperpolarizing Inhibitory Postsynaptic Potentials and Hyperpolarizing Response to Gamma-Aminobutyric Acid in Rabbit Hippocampus Studied Invitro. J. Neurosci. 1984, 4, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Obata, K. The inhibitory action of -aminobutyric acid, a probable synaptic transmitter. Int. Rev. Neurobiol. 1972, 15, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Ito, M.; Ochi, R.; Sato, N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp. Brain Res. 1967, 4, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.; Weidner, J.M.; Jin, Z.; Korpi, E.R.; Birnir, B. GABAergic signalling in the immune system. Acta Physiol. 2015, 213, 819–827. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. A gut feeling about GABA: Focus on GABA(B) receptors. Front. Pharmacol. 2010, 1, 124. [Google Scholar] [CrossRef]
- Ben-Othman, N.; Vieira, A.; Courtney, M.; Record, F.; Gjernes, E.; Avolio, F.; Hadzic, B.; Druelle, N.; Napolitano, T.; Navarro-Sanz, S.; et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-like Cell Neogenesis. Cell 2017, 168, 73–85.e11. [Google Scholar] [CrossRef]
- Hagan, D.W.; Ferreira, S.M.; Santos, G.J.; Phelps, E.A. The role of GABA in islet function. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, R.L.; Garry, D.G.; Brelje, T.C. Structural and Functional Considerations of Gaba in Islets of Langerhans—Beta-Cells and Nerves. Diabetes 1991, 40, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhao, J.; Zheng, Y.; Guo, S.; Schrodi, S.J.; He, D. Understanding the function of the GABAergic system and its potential role in rheumatoid arthritis. Front. Immunol. 2023, 14, 1114350. [Google Scholar] [CrossRef] [PubMed]
- Pin, J.P.; Bettler, B. Organization and functions of mGlu and GABA(B) receptor complexes. Nature 2016, 540, 60–68. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Benes, F.M.; Berretta, S. GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001, 25, 1–27. [Google Scholar] [CrossRef]
- Kelsom, C.; Lu, W. Development and specification of GABAergic cortical interneurons. Cell. Biosci. 2013, 3, 19. [Google Scholar] [CrossRef]
- Anwyl, R. Modulation of vertebrate neuronal calcium channels by transmitters. Brain Res. Brain Res. Rev. 1991, 16, 265–281. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Houser, C.R.; Tobin, A.J. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J. Neurochem. 1991, 56, 720–723. [Google Scholar] [CrossRef]
- Eaton, M.J.; Plunkett, J.A.; Karmally, S.; Martinez, M.A.; Montanez, K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J. Chem. Neuroanat. 1998, 16, 57–72. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef] [PubMed]
- McIntire, S.L.; Reimer, R.J.; Schuske, K.; Edwards, R.H.; Jorgensen, E.M. Identification and characterization of the vesicular GABA transporter. Nature 1997, 389, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, A.; Rostaing, P.; Bedet, C.; Levi, S.; Isambert, M.F.; Henry, J.P.; Triller, A.; Gasnier, B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J. Cell Sci. 1999, 112 Pt 6, 811–823. [Google Scholar] [CrossRef]
- Jin, X.T.; Galvan, A.; Wichmann, T.; Smith, Y. Localization and Function of GABA Transporters GAT-1 and GAT-3 in the Basal Ganglia. Front. Syst. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Motiwala, Z.; Aduri, N.G.; Shaye, H.; Han, G.W.; Lam, J.H.; Katritch, V.; Cherezov, V.; Gati, C. Structural basis of GABA reuptake inhibition. Nature 2022, 606, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Danbolt, N.C. GABA and Glutamate Transporters in Brain. Front. Endocrinol. 2013, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Daemen, M.A.; Hoogland, G.; Cijntje, J.M.; Spincemaille, G.H. Upregulation of the GABA-transporter GAT-1 in the spinal cord contributes to pain behaviour in experimental neuropathy. Neurosci. Lett. 2008, 444, 112–115. [Google Scholar] [CrossRef]
- Guo, H.; Yuan, X.S.; Zhou, J.C.; Chen, H.; Li, S.Q.; Qu, W.M.; Huang, Z.L. Whole-Brain Monosynaptic Inputs to Hypoglossal Motor Neurons in Mice. Neurosci. Bull. 2020, 36, 585–597. [Google Scholar] [CrossRef]
- Curtis, D.R.; Felix, D. GABA and prolonged spinal inhibition. Nat. New Biol. 1971, 231, 187–188. [Google Scholar] [CrossRef]
- Goulding, M.; Bourane, S.; Garcia-Campmany, L.; Dalet, A.; Koch, S. Inhibition downunder: An update from the spinal cord. Curr. Opin. Neurobiol. 2014, 26, 161–166. [Google Scholar] [CrossRef]
- Gamlin, C.R.; Yu, W.Q.; Wong, R.O.L.; Hoon, M. Assembly and maintenance of GABAergic and Glycinergic circuits in the mammalian nervous system. Neural. Dev. 2018, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Baertsch, N.A.; Baertsch, H.C.; Ramirez, J.M. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat. Commun. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Alstermark, B. Not GABA but glycine mediates segmental, propriospinal, and bulbospinal postsynaptic inhibition in adult mouse spinal forelimb motor neurons. J. Neurosci. 2015, 35, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Betley, J.N.; Wright, C.V.; Kawaguchi, Y.; Erdelyi, F.; Szabo, G.; Jessell, T.M.; Kaltschmidt, J.A. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 2009, 139, 161–174. [Google Scholar] [CrossRef]
- Fink, A.J.; Croce, K.R.; Huang, Z.J.; Abbott, L.F.; Jessell, T.M.; Azim, E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 2014, 509, 43–48. [Google Scholar] [CrossRef]
- Rudomin, P.; Schmidt, R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999, 129, 1–37. [Google Scholar] [CrossRef]
- Caron, G.; Bilchak, J.N.; Cote, M.P. Direct evidence for decreased presynaptic inhibition evoked by PBSt group I muscle afferents after chronic SCI and recovery with step-training in rats. J. Physiol. 2020, 598, 4621–4642. [Google Scholar] [CrossRef]
- Glykys, J.; Mody, I. Activation of GABA(A) receptors: Views from outside the synaptic cleft. Neuron 2007, 56, 763–770. [Google Scholar] [CrossRef]
- Bryson, A.; Reid, C.; Petrou, S. Fundamental Neurochemistry Review: GABA(A) receptor neurotransmission and epilepsy: Principles, disease mechanisms and pharmacotherapy. J. Neurochem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef]
- Sigel, E.; Buhr, A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997, 18, 425–429. [Google Scholar] [CrossRef]
- Sigel, E.; Luscher, B.P. A closer look at the high affinity benzodiazepine binding site on GABAA receptors. Curr. Top Med. Chem. 2011, 11, 241–246. [Google Scholar] [CrossRef]
- Benke, D.; Fakitsas, P.; Roggenmoser, C.; Michel, C.; Rudolph, U.; Mohler, H. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J. Biol. Chem. 2004, 279, 43654–43660. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M.; Brunig, I. Formation and plasticity of GABAergic synapses: Physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 2003, 98, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Carunchio, I.; Mollinari, C.; Pieri, M.; Merlo, D.; Zona, C. GAB(A) receptors present higher affinity and modified subunit composition in spinal motor neurons from a genetic model of amyotrophic lateral sclerosis. Eur. J. Neurosci. 2008, 28, 1275–1285. [Google Scholar] [CrossRef]
- Davenport, C.M.; Rajappa, R.; Katchan, L.; Taylor, C.R.; Tsai, M.C.; Smith, C.M.; de Jong, J.W.; Arnold, D.B.; Lammel, S.; Kramer, R.H. Relocation of an Extrasynaptic GABA(A) Receptor to Inhibitory Synapses Freezes Excitatory Synaptic Strength and Preserves Memory. Neuron 2021, 109, 123–134.e4. [Google Scholar] [CrossRef] [PubMed]
- Varoqueaux, F.; Jamain, S.; Brose, N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur. J. Cell. Biol. 2004, 83, 449–456. [Google Scholar] [CrossRef]
- Ali, H.; Marth, L.; Krueger-Burg, D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci. Signal 2020, 13, eabd8379. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, T.; Choudhury, P.; Tong, C.K.; Conway, C.M.; Scherrer, G.; Flood, P.D.; Mukai, J.; MacDermott, A.B. Inhibition Mediated by Glycinergic and GABAergic Receptors on Excitatory Neurons in Mouse Superficial Dorsal Horn Is Location-Specific but Modified by Inflammation. J. Neurosci. 2017, 37, 2336–2348. [Google Scholar] [CrossRef]
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793. [Google Scholar] [CrossRef]
- Gradwell, M.A.; Boyle, K.A.; Callister, R.J.; Hughes, D.I.; Graham, B.A. Heteromeric alpha/beta glycine receptors regulate excitability in parvalbumin-expressing dorsal horn neurons through phasic and tonic glycinergic inhibition. J. Physiol. 2017, 595, 7185–7202. [Google Scholar] [CrossRef]
- Anderson, W.B.; Graham, B.A.; Beveridge, N.J.; Tooney, P.A.; Brichta, A.M.; Callister, R.J. Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons. Mol. Pain 2009, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Momiyama, A. Single-Channel Currents Underlying Glycinergic Inhibitory Postsynaptic Responses in Spinal Neurons. Neuron 1991, 7, 965–969. [Google Scholar] [CrossRef]
- Schneider, S.P.; Fyffe, R.E. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurons. J. Neurophysiol. 1992, 68, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Oleskevich, S.; Alvarez, F.J.; Walmsley, B. Glycinergic miniature synaptic currents and receptor cluster sizes differ between spinal cord interneurons. J. Neurophysiol. 1999, 82, 312–319. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Berger, A.J. Cotransmission of GABA and glycine to brain stem motoneurons. J. Neurophysiol. 1999, 82, 1638–1641. [Google Scholar] [CrossRef]
- Moore, M.J.; Caspary, D.M. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J. Neurosci. 1983, 3, 237–242. [Google Scholar] [CrossRef]
- Tadros, M.A.; Farrell, K.E.; Schofield, P.R.; Brichta, A.M.; Graham, B.A.; Fuglevand, A.J.; Callister, R.J. Intrinsic and synaptic homeostatic plasticity in motoneurons from mice with glycine receptor mutations. J. Neurophysiol. 2014, 111, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Hulsmann, S.; Hagos, L.; Eulenburg, V.; Hirrlinger, J. Inspiratory Off-Switch Mediated by Optogenetic Activation of Inhibitory Neurons in the preBotzinger Complex In Vivo. Int. J. Mol. Sci. 2021, 22, 2019. [Google Scholar] [CrossRef]
- Fortuna, M.G.; Kugler, S.; Hulsmann, S. Probing the function of glycinergic neurons in the mouse respiratory network using optogenetics. Respir. Physiol. Neurobiol. 2019, 265, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, N.A.; Busselberg, D.; Molkov, Y.I.; Bischoff, A.M.; Smith, J.C.; Richter, D.W.; Rybak, I.A. Effects of glycinergic inhibition failure on respiratory rhythm and pattern generation. Prog. Brain Res. 2014, 209, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004, 84, 1051–1095. [Google Scholar] [CrossRef] [PubMed]
- Mahendrasingam, S.; Wallam, C.A.; Hackney, C.M. Two approaches to double post-embedding immunogold labeling of freeze-substituted tissue embedded in low temperature Lowicryl HM20 resin. Brain Res. Brain Res. Protoc. 2003, 11, 134–141. [Google Scholar] [CrossRef]
- Poyatos, I.; Ponce, J.; Aragon, C.; Gimenez, C.; Zafra, F. The glycine transporter GLYT2 is a reliable marker for glycine-immunoreactive neurons. Mol. Brain Res. 1997, 49, 63–70. [Google Scholar] [CrossRef]
- Eulenburg, V.; Huelsmann, S. Synergistic Control of Transmitter Turnover at Glycinergic Synapses by GlyT1, GlyT2, and ASC-1. Int. J. Mol. Sci. 2022, 23, 2561. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, J.T.; Liu, Y.; Wang, Y.; Paladugu, N.; Johnson, A.E.; Rothstein, J.D.; du Lac, S.; Mattson, M.P.; Hoke, A. The astrocytic transporter SLC7A10 (Asc-1) mediates glycinergic inhibition of spinal cord motor neurons. Sci. Rep. 2016, 6, 35592. [Google Scholar] [CrossRef] [PubMed]
- Mesuret, G.; Khabbazzadeh, S.; Bischoff, A.M.; Safory, H.; Wolosker, H.; Hulsmann, S. A neuronal role of the Alanine-Serine-Cysteine-1 transporter (SLC7A10, Asc-1) for glycine inhibitory transmission and respiratory pattern. Sci. Rep. 2018, 8, 8536. [Google Scholar] [CrossRef] [PubMed]
- Jonas, P.; Bischofberger, J.; Sandkuhler, J. Corelease of two fast neurotransmitters at a central synapse. Science 1998, 281, 419–424. [Google Scholar] [CrossRef]
- Russier, M.; Kopysova, I.L.; Ankri, N.; Ferrand, N.; Debanne, D. GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J. Physiol. 2002, 541, 123–137. [Google Scholar] [CrossRef]
- Sagne, C.; El Mestikawy, S.; Isambert, M.F.; Hamon, M.; Henry, J.P.; Giros, B.; Gasnier, B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997, 417, 177–183. [Google Scholar] [CrossRef]
- Kuhse, J.; Betz, H.; Kirsch, J. The inhibitory glycine receptor: Architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr. Opin. Neurobiol. 1995, 5, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.; Nguyen, L.; Rigo, J.M. Glycine receptors and brain development. Front. Cell. Neurosci. 2013, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Betz, H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J. Neurosci. 1995, 15, 4148–4156. [Google Scholar] [CrossRef] [PubMed]
- Kneussel, M.; Betz, H. Receptors, gephyrin and gephyrin-associated proteins: Novel insights into the assembly of inhibitory postsynaptic membrane specializations. J. Physiol. 2000, 525 Pt 1, 1–9. [Google Scholar] [CrossRef]
- Pless, S.A.; Dibas, M.I.; Lester, H.A.; Lynch, J.W. Conformational variability of the glycine receptor M2 domain in response to activation by different agonists. J. Biol. Chem. 2007, 282, 36057–36067. [Google Scholar] [CrossRef]
- Lewis, T.M.; Schofield, P.R.; McClellan, A.M.L. Kinetic determinants of agonist action at the recombinant human glycine receptor. J. Physiol. 2003, 549, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.J.; Westmoreland, J.J.; Summers, R.; Condie, B.G. Cleft Palate Is Caused by CNS Dysfunction in Gad1 and Viaat Knockout Mice. PLoS ONE 2010, 5, e9758. [Google Scholar] [CrossRef]
- Tsunekawa, N.; Arata, A.; Obata, K. Development of spontaneous mouth/tongue movement and related neural activity, and their repression in fetal mice lacking glutamate decarboxylase 67. Eur. J. Neurosci. 2005, 21, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.G.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; Obata, K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. USA 1997, 94, 6496–6499. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.B.; Eyjolfsson, E.M.; Smeland, O.B.; Nilsen, L.H.; Schousboe, I.; Schousboe, A.; Sonnewald, U.; Waagepetersen, H.S. Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J. Cerebr. Blood F Met. 2011, 31, 494–503. [Google Scholar] [CrossRef]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.; Ji, F.Y.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 1996, 229, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kakizaki, T.; Fujihara, K.; Miyata, S.; Zhang, Y.; Suto, T.; Kato, D.; Saito, S.; Shibasaki, K.; Ishizaki, Y.; et al. Impact of GAD65 and/or GAD67 deficiency on perinatal development in rats. FASEB J. 2022, 36, e22123. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, T.; Oriuchi, N.; Yanagawa, Y. Gad65/Gad67 Double Knockout Mice Exhibit Intermediate Severity in Both Cleft Palate and Omphalocele Compared with Gad67 Knockout and Vgat Knockout Mice. Neuroscience 2015, 288, 86–93. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Kanjhan, R.; Yanagawa, Y.; Noakes, P.G.; Bellingham, M.C. Alterations in hypoglossal motor neurons due to GAD67 and VGAT deficiency in mice. Exp. Neurol. 2017, 289, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Arata, A.; Kanbara-Kume, N.; Saito, K.; Yanagawa, Y.; Obata, K. Respiratory activity in brainstem of fetal mice lacking glutamate decarboxylase 65/67 and vesicular GABA transporter. Neuroscience 2007, 146, 1044–1052. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Smallcombe, K.L.; Yanagawa, Y.; Obata, K.; Bellingham, M.C.; Noakes, P.G. Genetic deficiency of GABA differentially regulates respiratory and non-respiratory motor neuron development. PLoS ONE 2013, 8, e56257. [Google Scholar] [CrossRef]
- Muller, I.; Caliskan, G.; Stork, O. The GAD65 knock out mouse—A model for GABAergic processes in fear- and stress-induced psychopathology. Genes Brain Behav. 2015, 14, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Stork, O.; Ji, F.Y.; Kaneko, K.; Stork, S.; Yoshinobu, Y.; Moriya, T.; Shibata, S.; Obata, K. Postnatal development of a GABA deficit and disturbance of neural functions in mice lacking GAD65. Brain Res. 2000, 865, 45–58. [Google Scholar] [CrossRef]
- MacFarlane, A.J.; Liu, X.W.; Perry, C.A.; Flodby, P.; Allen, R.H.; Stabler, S.P.; Stover, P.J. Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J. Biol. Chem. 2008, 283, 25846–25853. [Google Scholar] [CrossRef]
- Saito, K.; Kakizaki, T.; Hayashi, R.; Nishimaru, H.; Furukawa, T.; Nakazato, Y.; Takamori, S.; Ebihara, S.; Uematsu, M.; Mishina, M.; et al. The physiological roles of vesicular GABA transporter during embryonic development: A study using knockout mice. Mol. Brain 2010, 3, 40. [Google Scholar] [CrossRef]
- Wojcik, S.M.; Katsurabayashi, S.; Guillemin, I.; Friauf, E.; Rosenmund, C.; Brose, N.; Rhee, J.S. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 2006, 50, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Inanlou, M.R.; Kablar, B. Contractile activity of skeletal musculature involved in breathing is essential for normal lung cell differentiation, as revealed in Myf5-/-: MyoD-/- embryos. Dev. Dyn. 2005, 233, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Banks, G.B.; Kanjhan, R.; Wiese, S.; Kneussel, M.; Wong, L.M.; O’Sullivan, G.; Sendtner, M.; Bellingham, M.C.; Betz, H.; Noakes, P.G. Glycinergic and GABAergic synaptic activity differentially regulate motoneuron survival and skeletal muscle innervation. J. Neurosci. 2005, 25, 1249–1259, Erratum in J. Neurosci. 2005, 25, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Homanics, G.E.; DeLorey, T.M.; Firestone, L.L.; Quinlan, J.J.; Handforth, A.; Harrison, N.L.; Krasowski, M.D.; Rick, C.E.M.; Korpi, E.R.; Makela, R.; et al. Mice devoid of gamma-aminobutyrate type A receptor beta 3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl. Acad. Sci. USA 1997, 94, 4143–4148. [Google Scholar] [CrossRef]
- Culiat, C.T.; Stubbs, L.J.; Woychik, R.P.; Russell, L.B.; Johnson, D.K.; Rinchik, E.M. Deficiency of the Beta-3 Subunit of the Type-a Gamma-Aminobutyric-Acid Receptor Causes Cleft-Palate in Mice. Nat. Genet. 1995, 11, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Gunther, U.; Benson, J.; Benke, D.; Fritschy, J.M.; Reyes, G.; Knoflach, F.; Crestani, F.; Aguzzi, A.; Arigoni, M.; Lang, Y.; et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 1995, 92, 7749–7753. [Google Scholar] [CrossRef]
- Sur, C.; Wafford, K.A.; Reynolds, D.S.; Hadingham, K.L.; Bromidge, F.; Macaulay, A.; Collinson, N.; O’Meara, G.; Howell, O.; Newman, R.; et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J. Neurosci. 2001, 21, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.; Vogel, N.; Villmann, C. Glycine receptor mutants of the mouse: What are possible routes of inhibitory compensation? Front. Mol. Neurosci. 2012, 5, 98. [Google Scholar] [CrossRef]
- Shiang, R.; Ryan, S.G.; Zhu, Y.Z.; Hahn, A.F.; Oconnell, P.; Wasmuth, J.J. Mutations in the Alpha-1 Subunit of the Inhibitory Glycine Receptor Cause the Dominant Neurologic Disorder, Hyperekplexia. Nat. Genet. 1993, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Young-Pearse, T.L.; Ivic, L.; Kriegstein, A.R.; Cepko, C.L. Characterization of mice with targeted deletion of glycine receptor alpha 2. Mol. Cell. Biol. 2006, 26, 5728–5734. [Google Scholar] [CrossRef]
- Harvey, R.J.; Depner, U.B.; Wassle, H.; Ahmadi, S.; Heindl, C.; Reinold, H.; Smart, T.G.; Harvey, K.; Schutz, B.; Abo-Salem, O.M.; et al. GlyR alpha3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004, 304, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.R.; Huber, K.M.; Sudhof, T.C. Neuroligin-2 deletion selectively decreases inhibitory synaptic transmission originating from fast-spiking but not from somatostatin-positive interneurons. J. Neurosci. 2009, 29, 13883–13897. [Google Scholar] [CrossRef] [PubMed]
- Pohl, T.T.; Hornberg, H. Neuroligins in neurodevelopmental conditions: How mouse models of de novo mutations can help us link synaptic function to social behavior. Neuronal Signal 2022, 6, NS20210030. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Tabuchi, K.; Bolliger, M.F.; Blaiss, C.A.; Brose, N.; Liu, X.; Sudhof, T.C.; Powell, C.M. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009, 8, 114–126. [Google Scholar] [CrossRef]
- Grosskreutz, Y.; Betz, H.; Kneussel, M. Rescue of molybdenum cofactor biosynthesis in gephyrin-deficient mice by a Cnx1 transgene. Biochem. Biophys. Res. Commun. 2003, 301, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Tintrup, H.; Kirsch, J.; Nichol, M.C.; Kuhse, J.; Betz, H.; Sanes, J.R. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 1998, 282, 1321–1324. [Google Scholar] [CrossRef]
- Levi, S.; Logan, S.M.; Tovar, K.R.; Craig, A.M. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J. Neurosci. 2004, 24, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, F.; Eggersmann, F.R.; Merkler, Y.; Kloppenburg, P.; Schwarz, G. Automated Image Analysis Reveals Different Localization of Synaptic Gephyrin C4 Splice Variants. eNeuro 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuljis, D.A.; Micheva, K.D.; Ray, A.; Wegner, W.; Bowman, R.; Madison, D.V.; Willig, K.I.; Barth, A.L. Gephyrin-Lacking PV Synapses on Neocortical Pyramidal Neurons. Int. J. Mol. Sci. 2021, 22, 10032. [Google Scholar] [CrossRef]
- Garcia, J.D.; Gookin, S.E.; Crosby, K.C.; Schwartz, S.L.; Tiemeier, E.; Kennedy, M.J.; Dell’Acqua, M.L.; Herson, P.S.; Quillinan, N.; Smith, K.R. Stepwise disassembly of GABAergic synapses during pathogenic excitotoxicity. Cell Rep. 2021, 37, 110142. [Google Scholar] [CrossRef]
- Tyagarajan, S.K.; Fritschy, J.M. Gephyrin: A master regulator of neuronal function? Nat. Rev. Neurosci. 2014, 15, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Safory, H.; Neame, S.; Shulman, Y.; Zubedat, S.; Radzishevsky, I.; Rosenberg, D.; Sason, H.; Engelender, S.; Avital, A.; Hulsmann, S.; et al. The alanine-serine-cysteine-1 (Asc-1) transporter controls glycine levels in the brain and is required for glycinergic inhibitory transmission. EMBO Rep. 2015, 16, 590–598. [Google Scholar] [CrossRef]
- Yang, P.; Cai, G.; Cai, Y.; Fei, J.; Liu, G. Gamma aminobutyric acid transporter subtype 1 gene knockout mice: A new model for attention deficit/hyperactivity disorder. Acta Biochim. Biophys. Sin. 2013, 45, 578–585. [Google Scholar] [CrossRef]
- Fischer, F.P.; Kasture, A.S.; Hummel, T.; Sucic, S. Molecular and Clinical Repercussions of GABA Transporter 1 Variants Gone Amiss: Links to Epilepsy and Developmental Spectrum Disorders. Front. Mol. Biosci. 2022, 9, 834498. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Zhou, X.; Wang, C.; Gong, X.; Chen, B.; Chen, Y. Hyperactivity and impaired attention in Gamma aminobutyric acid transporter subtype 1 gene knockout mice. Acta Neuropsychiatr. 2015, 27, 368–374. [Google Scholar] [CrossRef]
- Gomeza, J.; Ohno, K.; Hulsmann, S.; Armsen, W.; Eulenburg, V.; Richter, D.W.; Laube, B.; Betz, H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 2003, 40, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Gomeza, J.; Hulsmann, S.; Ohno, K.; Eulenburg, V.; Szoke, K.; Richter, D.; Betz, H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 2003, 40, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Eulenburg, V.; Retiounskaia, M.; Papadopoulos, T.; Gomeza, J.; Betz, H. Glial Glycine Transporter 1 Function Is Essential for Early Postnatal Survival but Dispensable in Adult Mice. Glia 2010, 58, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.X.; Stricker, C.; Ziskind-Conhaim, L. Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks. J. Neurophysiol. 2001, 86, 492–502. [Google Scholar] [CrossRef]
- Ehrlich, I.; Lohrke, S.; Friauf, E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl- regulation. J. Physiol. 1999, 520, 121–137. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Delpy, A.; Allain, A.E.; Meyrand, P.; Branchereau, P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J. Physiol. 2008, 586, 1059–1075. [Google Scholar] [CrossRef]
- Baccei, M.L.; Fitzgerald, M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J. Neurosci. 2004, 24, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Wu, W.L.; Ziskind-Conhaim, L.; Sweet, M.A. Early development of glycine- and GABA-mediated synapses in rat spinal cord. J. Neurosci. 1992, 12, 3935–3945. [Google Scholar] [CrossRef]
- Singer, J.H.; Talley, E.M.; Bayliss, D.A.; Berger, A.J. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J. Neurophysiol. 1998, 80, 2608–2620. [Google Scholar] [CrossRef]
- Brickley, S.G.; Cull-Candy, S.G.; Farrant, M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. 1996, 497 Pt 3, 753–759. [Google Scholar] [CrossRef]
- Tyzio, R.; Holmes, G.L.; Ben-Ari, Y.; Khazipov, R. Timing of the developmental switch in GABA(A) mediated signaling from excitation to inhibition in CA3 rat hippocampus using gramicidin perforated patch and extracellular recordings. Epilepsia 2007, 48 (Suppl. S5), 96–105. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Owens, D.F.; Boyce, L.H.; Davis, M.B.; Kriegstein, A.R. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996, 16, 6414–6423. [Google Scholar] [CrossRef] [PubMed]
- Backus, K.H.; Deitmer, J.W.; Friauf, E. Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurones. J. Physiol. 1998, 507 Pt 3, 783–794. [Google Scholar] [CrossRef]
- Ito, S.; Cherubini, E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J. Physiol. 1991, 440, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chattipakorn, S.C.; McMahon, L.L. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J. Neurophysiol. 2006, 95, 2366–2379. [Google Scholar] [CrossRef]
- Lam, P.; Newland, J.; Faull, R.L.M.; Kwakowsky, A. Cation-Chloride Cotransporters KCC2 and NKCC1 as Therapeutic Targets in Neurological and Neuropsychiatric Disorders. Molecules 2023, 28, 1344. [Google Scholar] [CrossRef]
- Lam, P.; Vinnakota, C.; Guzman, B.C.; Newland, J.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Beta-Amyloid (Abeta(1-42)) Increases the Expression of NKCC1 in the Mouse Hippocampus. Molecules 2022, 27, 2440. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.H.; Tesic, V.; Tat, Q.L.; Chastain, S.; Quillinan, N.; Jevtovic-Todorovic, V. Sevoflurane-Induced Dysregulation of Cation-Chloride Cotransporters NKCC1 and KCC2 in Neonatal Mouse Brain. Mol. Neurobiol. 2020, 57, 1–10. [Google Scholar] [CrossRef]
- Duarte, S.T.; Armstrong, J.; Roche, A.; Ortez, C.; Perez, A.; O’Callaghan Mdel, M.; Pereira, A.; Sanmarti, F.; Ormazabal, A.; Artuch, R.; et al. Abnormal expression of cerebrospinal fluid cation chloride cotransporters in patients with Rett syndrome. PLoS ONE 2013, 8, e68851. [Google Scholar] [CrossRef]
- Tyzio, R.; Nardou, R.; Ferrari, D.C.; Tsintsadze, T.; Shahrokhi, A.; Eftekhari, S.; Khalilov, I.; Tsintsadze, V.; Brouchoud, C.; Chazal, G.; et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 2014, 343, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Loscher, W.; Puskarjov, M.; Kaila, K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 2013, 69, 62–74. [Google Scholar] [CrossRef]
- Kahle, K.T.; Staley, K.J.; Nahed, B.V.; Gamba, G.; Hebert, S.C.; Lifton, R.P.; Mount, D.B. Roles of the cation-chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 2008, 4, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Amici, M.; Sobrero, F.; Spinelli, G.; Di Angelantonio, S.; Ragozzino, D.; Mascia, A.; Scoppetta, C.; Esposito, V.; Miledi, R.; et al. Anomalous levels of Cl− transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. USA 2006, 103, 8465–8468. [Google Scholar] [CrossRef]
- Sen, A.; Martinian, L.; Nikolic, M.; Walker, M.C.; Thom, M.; Sisodiya, S.M. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res 2007, 74, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Serranilla, M.; Woodin, M.A. Striatal Chloride Dysregulation and Impaired GABAergic Signaling Due to Cation-Chloride Cotransporter Dysfunction in Huntington’s Disease. Front. Cell. Neurosci. 2022, 15, 817013. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.; Josiah, S.S.; Zhang, J. The Therapeutic Potential of Neuronal K-Cl Co-Transporter KCC2 in Huntington’s Disease and Its Comorbidities. Int. J. Mol. Sci. 2020, 21, 9142. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Chang, Y.G.; Liu, Y.C.; Wang, K.Y.; Chen, H.M.; Lee, D.J.; Yang, S.S.; Tsai, C.H.; Lien, C.C.; Chern, Y. Enhanced Na(+)-K(+)-2Cl(−) cotransporter 1 underlies motor dysfunction in huntington’s disease. Mov. Disord. 2019, 34, 845–857. [Google Scholar] [CrossRef]
- Dargaei, Z.; Bang, J.Y.; Mahadevan, V.; Khademullah, C.S.; Bedard, S.; Parfitt, G.M.; Kim, J.C.; Woodin, M.A. Restoring GABAergic inhibition rescues memory deficits in a Huntington’s disease mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E1618–E1626. [Google Scholar] [CrossRef]
- Hinz, L.; Torrella Barrufet, J.; Heine, V.M. KCC2 expression levels are reduced in post mortem brain tissue of Rett syndrome patients. Acta Neuropathol. Commun. 2019, 7, 196. [Google Scholar] [CrossRef]
- Arion, D.; Lewis, D.A. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen. Psychiatry 2011, 68, 21–31. [Google Scholar] [CrossRef]
- Tao, R.; Li, C.; Newburn, E.N.; Ye, T.; Lipska, B.K.; Herman, M.M.; Weinberger, D.R.; Kleinman, J.E.; Hyde, T.M. Transcript-specific associations of SLC12A5 (KCC2) in human prefrontal cortex with development, schizophrenia, and affective disorders. J. Neurosci. 2012, 32, 5216–5222. [Google Scholar] [CrossRef]
- Talifu, Z.; Pan, Y.; Gong, H.; Xu, X.; Zhang, C.; Yang, D.; Gao, F.; Yu, Y.; Du, L.; Li, J. The role of KCC2 and NKCC1 in spinal cord injury: From physiology to pathology. Front. Physiol. 2022, 13, 1045520. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.L.; Seven, Y.B.; Baker, T.L.; Mitchell, G.S. Cervical spinal contusion alters Na(+)-K(+)-2Cl- and K(+)-Cl- cation-chloride cotransporter expression in phrenic motor neurons. Respir. Physiol. Neurobiol. 2019, 261, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Beverungen, H.; Klaszky, S.C.; Klaszky, M.; Cote, M.P. Rehabilitation Decreases Spasticity by Restoring Chloride Homeostasis through the Brain-Derived Neurotrophic Factor-KCC2 Pathway after Spinal Cord Injury. J. Neurotraum. 2020, 37, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.P.; Gandhi, S.; Zambrotta, M.; Houle, J.D. Exercise modulates chloride homeostasis after spinal cord injury. J. Neurosci. 2014, 34, 8976–8987. [Google Scholar] [CrossRef] [PubMed]
- Bilchak, J.N.; Yeakle, K.; Caron, G.; Malloy, D.; Cote, M.P. Enhancing KCC2 activity decreases hyperreflexia and spasticity after chronic spinal cord injury. Exp Neurol 2021, 338, 113605. [Google Scholar] [CrossRef] [PubMed]
- Chesler, M. Regulation and modulation of pH in the brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef] [PubMed]
- Voipio, J.; Kaila, K. GABAergic excitation and K(+)-mediated volume transmission in the hippocampus. Prog. Brain Res. 2000, 125, 329–338. [Google Scholar] [CrossRef]
- Staley, K.J.; Proctor, W.R. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl- and HCO3- transport. J. Physiol. 1999, 519 Pt 3, 693–712. [Google Scholar] [CrossRef]
- Staley, K.J.; Soldo, B.L.; Proctor, W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 1995, 269, 977–981. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fenoglio, K.A.; Kerrigan, J.F.; Rho, J.M. Bicarbonate contributes to GABAA receptor-mediated neuronal excitation in surgically resected human hypothalamic hamartomas. Epilepsy Res. 2009, 83, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, E.; Gaiarsa, J.L.; Ben-Ari, Y. GABA: An excitatory transmitter in early postnatal life. Trends Neurosci. 1991, 14, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rett, A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr 1966, 116, 723–726. [Google Scholar] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Pascual-Alonso, A.; Martinez-Monseny, A.F.; Xiol, C.; Armstrong, J. MECP2-Related Disorders in Males. Int. J. Mol. Sci. 2021, 22, 9610. [Google Scholar] [CrossRef]
- Luikenhuis, S.; Giacometti, E.; Beard, C.F.; Jaenisch, R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6033–6038. [Google Scholar] [CrossRef]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Mullaney, B.C.; Johnston, M.V.; Blue, M.E. Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience 2004, 123, 939–949. [Google Scholar] [CrossRef]
- Shahbazian, M.D.; Antalffy, B.; Armstrong, D.L.; Zoghbi, H.Y. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum. Mol. Genet. 2002, 11, 115–124. [Google Scholar] [CrossRef]
- Percy, A.K.; Lane, J.B.; Childers, J.; Skinner, S.; Annese, F.; Barrish, J.; Caeg, E.; Glaze, D.G.; MacLeod, P. Rett syndrome: North American database. J. Child. Neurol. 2007, 22, 1338–1341. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.; Schanen, N.C.; Zappella, M.; et al. Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Schule, B.; Armstrong, D.D.; Vogel, H.; Oviedo, A.; Francke, U. Severe congenital encephalopathy caused by MECP2 null mutations in males: Central hypoxia and reduced neuronal dendritic structure. Clin. Genet. 2008, 74, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.E.; Merritt, J.K.; Erickson, K.R.; Neul, J.L. Safety and efficacy of genetic MECP2 supplementation in the R294X mouse model of Rett syndrome. Genes. Brain Behav. 2022, 21, e12739. [Google Scholar] [CrossRef] [PubMed]
- Vermudez, S.A.D.; Gogliotti, R.G.; Arthur, B.; Buch, A.; Morales, C.; Moxley, Y.; Rajpal, H.; Conn, P.J.; Niswender, C.M. Profiling beneficial and potential adverse effects of MeCP2 overexpression in a hypomorphic Rett syndrome mouse model. Genes. Brain Behav. 2022, 21, e12752. [Google Scholar] [CrossRef]
- Tropea, D.; Giacometti, E.; Wilson, N.R.; Beard, C.; McCurry, C.; Fu, D.D.; Flannery, R.; Jaenisch, R.; Sur, M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Panayotis, N.; Ehinger, Y.; Felix, M.S.; Roux, J.C. State-of-the-art therapies for Rett syndrome. Dev. Med. Child. Neurol. 2023, 65, 162–170. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef]
- Glaze, D.G.; Neul, J.L.; Percy, A.; Feyma, T.; Beisang, A.; Yaroshinsky, A.; Stoms, G.; Zuchero, D.; Horrigan, J.; Glass, L.; et al. A Double-Blind, Randomized, Placebo-Controlled Clinical Study of Trofinetide in the Treatment of Rett Syndrome. Pediatr. Neurol. 2017, 76, 37–46. [Google Scholar] [CrossRef]
- Li, W. Excitation and Inhibition Imbalance in Rett Syndrome. Front. Neurosci. 2022, 16, 825063. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Sieck, G.C. Tongue muscle contractile, fatigue, and fiber type properties in rats. J. Appl. Physiol. 2021, 131, 1043–1055. [Google Scholar] [CrossRef]
- Holstege, G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog. Brain Res. 2014, 209, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Samaco, R.C.; Hogart, A.; LaSalle, J.M. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum. Mol. Genet. 2005, 14, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Minh, V.C.N.; Du, F.; Felice, C.A.; Shan, X.W.; Nigam, A.; Mandel, G.; Robinson, J.K.; Ballas, N. MeCP2 Is Critical for Maintaining Mature Neuronal Networks and Global Brain Anatomy during Late Stages of Postnatal Brain Development and in the Mature Adult Brain. J. Neurosci. 2012, 32, 10021–10034. [Google Scholar] [CrossRef]
- Chao, H.T.; Chen, H.M.; Samaco, R.C.; Xue, M.S.; Chahrour, M.; Yoo, J.; Neul, J.L.; Gong, S.C.; Lu, H.C.; Heintz, N.; et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010, 468, 263–269. [Google Scholar] [CrossRef]
- Ure, K.; Lui, H.; Wang, W.; Ito-Ishida, A.; Wu, Z.Y.; He, L.J.; Sztainberg, Y.; Chen, W.; Tang, J.R.; Zoghbi, H.Y. Restoration of Mecp2 expression in GABAergic neurons is sufficient to rescue multiple disease features in a mouse model of Rett syndrome. Elife 2016, 5, e14198. [Google Scholar] [CrossRef]
- Wood, L.; Gray, N.W.; Zhou, Z.; Greenberg, M.E.; Shepherd, G.M. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J. Neurosci. 2009, 29, 12440–12448. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Kells, P.A.; Osgood, A.C.; Gautam, S.H.; Shew, W.L. Collapse of complexity of brain and body activity due to excessive inhibition and MeCP2 disruption. Proc. Natl. Acad. Sci. USA 2021, 118, e2106378118. [Google Scholar] [CrossRef]
- Bernardo, P.; Cobb, S.; Coppola, A.; Tomasevic, L.; Di Lazzaro, V.; Bravaccio, C.; Manganelli, F.; Dubbioso, R. Neurophysiological Signatures of Motor Impairment in Patients with Rett Syndrome. Ann. Neurol. 2020, 87, 763–773. [Google Scholar] [CrossRef]
- Eyre, J.A.; Kerr, A.M.; Miller, S.; O’Sullivan, M.C.; Ramesh, V. Neurophysiological observations on corticospinal projections to the upper limb in subjects with Rett syndrome. J. Neurol. Neurosurg. Psychiatry 1990, 53, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Singer, H.S.; Horska, A.; Kline, T.; Ryan, M.; Edden, R.A.; Mahone, E.M. GABA and Glutamate in Children with Primary Complex Motor Stereotypies: An 1H-MRS Study at 7T. AJNR Am. J. Neuroradiol. 2016, 37, 552–557. [Google Scholar] [CrossRef]
- Hines, R.M.; Wu, L.; Hines, D.J.; Steenland, H.; Mansour, S.; Dahlhaus, R.; Singaraja, R.R.; Cao, X.; Sammler, E.; Hormuzdi, S.G.; et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J. Neurosci. 2008, 28, 6055–6067. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.D.; Dashevskiy, T.; Mendoza, J.; Garcia, A.J., 3rd; Ramirez, J.M.; Shea-Brown, E. Different roles for inhibition in the rhythm-generating respiratory network. J. Neurophysiol. 2017, 118, 2070–2088. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, V.; Koizumi, H.; Mosher, B.; Koshiya, N.; Tariq, M.F.; Bezdudnaya, T.G.; Zhang, R.; Molkov, Y.I.; Rybak, I.A.; Smith, J.C. Perturbations of Respiratory Rhythm and Pattern by Disrupting Synaptic Inhibition within Pre-Botzinger and Botzinger Complexes. eNeuro 2016, 3, 1–24. [Google Scholar] [CrossRef]
- Baertsch, N.A.; Severs, L.J.; Anderson, T.M.; Ramirez, J.M. A spatially dynamic network underlies the generation of inspiratory behaviors. Proc. Natl. Acad. Sci. USA 2019, 116, 7493–7502. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Mantilla, C.B.; Sieck, G.C. Breathing: Motor Control of Diaphragm Muscle. Physiology 2018, 33, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Huff, A.; Karlen-Amarante, M.; Pitts, T.; Ramirez, J.M. Optogenetic stimulation of pre-Botzinger complex reveals novel circuit interactions in swallowing-breathing coordination. Proc. Natl. Acad. Sci. USA 2022, 119, e2121095119. [Google Scholar] [CrossRef]
- d’Orsi, G.; Trivisano, M.; Luisi, C.; Demaio, V.; Di Claudio, M.T.; Pascarella, M.G.; Sciruicchio, V.; Galeone, D.; La Neve, A.; Scarpelli, F.; et al. Epileptic seizures, movement disorders, and breathing disturbances in Rett syndrome: Diagnostic relevance of video-polygraphy. Epilepsy Behav. 2012, 25, 401–407. [Google Scholar] [CrossRef]
- Mesuret, G.; Dannenberg, J.; Arnoldt, M.; Grutzner, A.A.; Niebert, M.; Hulsmann, S. Breathing disturbances in a model of Rett syndrome: A potential involvement of the glycine receptor alpha3 subunit? Respir. Physiol. Neurobiol. 2018, 248, 43–47. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Karlen-Amarante, M.; Wang, J.J.; Huff, A.; Burgraff, N. Breathing disturbances in Rett syndrome. Handb. Clin. Neurol. 2022, 189, 139–151. [Google Scholar] [CrossRef]
- Stettner, G.M.; Huppke, P.; Gartner, J.; Richter, D.W.; Dutschmann, M. Disturbances of breathing in Rett syndrome: Results from patients and animal models. Adv. Exp. Med. Biol. 2008, 605, 503–507. [Google Scholar] [CrossRef]
- Tarquinio, D.C.; Hou, W.; Neul, J.L.; Berkmen, G.K.; Drummond, J.; Aronoff, E.; Harris, J.; Lane, J.B.; Kaufmann, W.E.; Motil, K.J.; et al. The course of awake breathing disturbances across the lifespan in Rett syndrome. Brain Dev. 2018, 40, 515–529. [Google Scholar] [CrossRef]
- Voituron, N.; Zanella, S.; Menuet, C.; Dutschmann, M.; Hilaire, G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir. Physiol. Neurobiol. 2009, 168, 109–118. [Google Scholar] [CrossRef]
- Voituron, N.; Zanella, S.; Menuet, C.; Lajard, A.M.; Dutschmann, M.; Hilaire, G. Early abnormalities of post-sigh breathing in a mouse model of Rett syndrome. Respir. Physiol. Neurobiol. 2010, 170, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.M.; Dutschmann, M.; Ramirez, J.M.; Hilaire, G. Breathing disorders in Rett syndrome: Progressive neurochemical dysfunction in the respiratory network after birth. Respir. Physiol. Neurobiol. 2009, 168, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Ward, C.S.; Neul, J.L. Breathing challenges in Rett syndrome: Lessons learned from humans and animal models. Respir. Physiol. Neurobiol. 2013, 189, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.S.; Ramirez, J.M.; Weese-Mayer, D.E. Diurnal variation in autonomic regulation among patients with genotyped Rett syndrome. J. Med. Genet. 2020, 57, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Weese-Mayer, D.E.; Lieske, S.P.; Boothby, C.M.; Kenny, A.S.; Bennett, H.L.; Silvestri, J.M.; Ramirez, J.M. Autonomic nervous system dysregulation: Breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 2006, 60, 443–449. [Google Scholar] [CrossRef]
- Bassett, E.; Heinle, R.; Johnston, D. Sleep Apnea in Patients With Rett Syndrome: Roles for Polysomnography and Adenotonsillectomy. J. Child. Neurol. 2016, 31, 1633–1634. [Google Scholar] [CrossRef] [PubMed]
- Sarber, K.M.; Howard, J.J.M.; Dye, T.J.; Pascoe, J.E.; Simakajornboon, N. Sleep-Disordered Breathing in Pediatric Patients With Rett Syndrome. J. Clin. Sleep Med. 2019, 15, 1451–1457. [Google Scholar] [CrossRef]
- Amaddeo, A.; De Sanctis, L.; Arroyo, J.O.; Khirani, S.; Bahi-Buisson, N.; Fauroux, B. Polysomnographic findings in Rett syndrome. Eur. J. Paediatr. Neuro. 2019, 23, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Esposito, M.; D’Aniello, A.; Rippa, C.D.; Precenzano, F.; Pascotto, A.; Bravaccio, C.; Elia, M. Polysomnographic findings in Rett syndrome: A case-control study. Sleep Breath. 2013, 17, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hagebeuk, E.E.O.; Bijlmer, R.P.G.M.; Koelman, J.H.T.M.; Poll-The, B.T. Respiratory Disturbances in Rett Syndrome: Don’t Forget to Evaluate Upper Airway Obstruction. J. Child. Neurol. 2012, 27, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, S.; Signorini, C.; Boasiako, L.; Scandurra, V.; Hayek, J.; Ciccoli, L.; Rossi, M.; Canitano, R.; De Felice, C. Breathing Abnormalities During Sleep and Wakefulness in Rett Syndrome: Clinical Relevance and Paradoxical Relationship With Circulating Pro-oxidant Markers. Front. Neurol. 2022, 13, 833239. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Zhan, W.-Z.; Simmon, V.F.; Vanderklish, P.W.; Sarraf, S.T.; Sieck, G.C. Novel Regenerative Drug, SPG302 Promotes Functional Recovery of Diaphragm Muscle Activity After Cervical Spinal Cord Injury. J. Physiol. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, M.J.; Brandenburg, J.E.; Zhan, W.Z.; Sieck, G.C. Diaphragm Muscle Function in a Mouse Model of Early Onset Spasticity. J. Appl. Physiol. 2022, 133, 60–68. [Google Scholar] [CrossRef]
- Khurram, O.U.; Fogarty, M.J.; Sarrafian, T.L.; Bhatt, A.; Mantilla, C.B.; Sieck, G.C. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol. Rep. 2018, 6, e13786. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, N.; Xing, H.; Zhong, W.; Arrowood, C.; Johnson, C.M.; Jiang, C. In vivo evidence for the cellular basis of central hypoventilation of Rett syndrome and pharmacological correction in the rat model. J. Cell Physiol. 2021, 236, 8082–8098. [Google Scholar] [CrossRef]
- Medrihan, L.; Tantalaki, E.; Aramuni, G.; Sargsyan, V.; Dudanova, I.; Missler, M.; Zhang, W. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J. Neurophysiol. 2008, 99, 112–121. [Google Scholar] [CrossRef]
- Xing, H.; Cui, N.; Johnson, C.M.; Faisthalab, Z.; Jiang, C. Dual synaptic inhibitions of brainstem neurons by GABA and glycine with impact on Rett syndrome. J. Cell Physiol. 2021, 236, 3615–3628. [Google Scholar] [CrossRef]
- Chen, C.Y.; Di Lucente, J.; Lin, Y.C.; Lien, C.C.; Rogawski, M.A.; Maezawa, I.; Jin, L.W. Defective GABAergic neurotransmission in the nucleus tractus solitarius in Mecp2-null mice, a model of Rett syndrome. Neurobiol. Dis. 2018, 109, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Abdala, A.P.; Toward, M.A.; Dutschmann, M.; Bissonnette, J.M.; Paton, J.F. Deficiency of GABAergic synaptic inhibition in the Kolliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. J. Physiol. 2016, 594, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.R.; Zhu, Y.; Jacono, F.J.; Katz, D.M.; Galan, R.F.; Dick, T.E. Decreased Hering-Breuer input-output entrainment in a mouse model of Rett syndrome. Front. Neural Circuits 2013, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Dutschmann, M.; Herbert, H. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 2006, 24, 1071–1084. [Google Scholar] [CrossRef]
- Stettner, G.M.; Huppke, P.; Brendel, C.; Richter, D.W.; Gartner, J.; Dutschmann, M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J. Physiol. 2007, 579, 863–876. [Google Scholar] [CrossRef]
- Didden, R.; Korzilius, H.; Smeets, E.; Green, V.A.; Lang, R.; Lancioni, G.E.; Curfs, L.M. Communication in Individuals with Rett Syndrome: An Assessment of Forms and Functions. J. Dev. Phys. Disabil. 2010, 22, 105–118. [Google Scholar] [CrossRef]
- Mezzedimi, C.; Livi, W.; De Felice, C.; Cocca, S. Dysphagia in Rett Syndrome: A Descriptive Study. Ann. Otol. Rhinol. Laryngol. 2017, 126, 640–645. [Google Scholar] [CrossRef]
- Johnson, C.M.; Cui, N.; Xing, H.; Wu, Y.; Jiang, C. The antitussive cloperastine improves breathing abnormalities in a Rett Syndrome mouse model by blocking presynaptic GIRK channels and enhancing GABA release. Neuropharmacology 2020, 176, 108214. [Google Scholar] [CrossRef]
- Abdala, A.P.; Dutschmann, M.; Bissonnette, J.M.; Paton, J.F. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 18208–18213. [Google Scholar] [CrossRef]
- Martin, B.J.; Corlew, M.M.; Wood, H.; Olson, D.; Golopol, L.A.; Wingo, M.; Kirmani, N. The association of swallowing dysfunction and aspiration pneumonia. Dysphagia 1994, 9, 1–6. [Google Scholar] [CrossRef]
- MacKay, J.; Leonard, H.; Wong, K.; Wilson, A.; Downs, J. Respiratory morbidity in Rett syndrome: An observational study. Dev. Med. Child. Neurol. 2018, 60, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Laurvick, C.L.; De Klerk, N.; Bower, C.; Christodoulou, J.; Ravine, D.; Ellaway, C.; Williamson, S.; Leonard, H. Rett syndrome in Australia: A review of the epidemiology. J. Pediatr. 2006, 148, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Wong, K.; Jacoby, P.; Downs, J.; Leonard, H. Twenty years of surveillance in Rett syndrome: What does this tell us? Orphanet J. Rare Dis. 2014, 9, 87. [Google Scholar] [CrossRef]
- Hubner, C.A.; Stein, V.; Hermans-Borgmeyer, I.; Meyer, T.; Ballanyi, K.; Jentsch, T.J. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 2001, 30, 515–524. [Google Scholar] [CrossRef]
- Tornberg, J.; Voikar, V.; Savilahti, H.; Rauvala, H.; Airaksinen, M.S. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur. J. Neurosci. 2005, 21, 1327–1337. [Google Scholar] [CrossRef]
- Simonnet, C.; Sinha, M.; Goutierre, M.; Moutkine, I.; Daumas, S.; Poncer, J.C. Silencing KCC2 in mouse dorsal hippocampus compromises spatial and contextual memory. Neuropsychopharmacology, 2022; in press. [Google Scholar] [CrossRef]
- Gogliotti, R.G.; Fisher, N.M.; Stansley, B.J.; Jones, C.K.; Lindsley, C.W.; Conn, P.J.; Niswender, C.M. Total RNA Sequencing of Rett Syndrome Autopsy Samples Identifies the M(4) Muscarinic Receptor as a Novel Therapeutic Target. J. Pharmacol. Exp. Ther. 2018, 365, 291–300. [Google Scholar] [CrossRef]
- Tang, X.; Kim, J.; Zhou, L.; Wengert, E.; Zhang, L.; Wu, Z.; Carromeu, C.; Muotri, A.R.; Marchetto, M.C.; Gage, F.H.; et al. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Rikhye, R.V.; Breton-Provencher, V.; Tang, X.; Li, C.; Li, K.; Runyan, C.A.; Fu, Z.; Jaenisch, R.; Sur, M. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E7287–E7296. [Google Scholar] [CrossRef]
- Yeo, M.; Berglund, K.; Augustine, G.; Liedtke, W. Novel repression of Kcc2 transcription by REST-RE-1 controls developmental switch in neuronal chloride. J. Neurosci. 2009, 29, 14652–14662. [Google Scholar] [CrossRef]
- Chang, Q.A.; Khare, G.; Dani, V.; Nelson, S.; Jaenisch, R. The disease progression mutant mice is affected of Mecp2 by the level of BDNF expression. Neuron 2006, 49, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.N.; Ho, H.Y.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C.; et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52, 255–269. [Google Scholar] [CrossRef]
- Ogier, M.; Wang, H.; Hong, E.; Wang, Q.; Greenberg, M.E.; Katz, D.M. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 2007, 27, 10912–10917. [Google Scholar] [CrossRef]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell. Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef]
- Gigliucci, V.; Teutsch, J.; Woodbury-Smith, M.; Luoni, M.; Busnelli, M.; Chini, B.; Banerjee, A. Region-Specific KCC2 Rescue by rhIGF-1 and Oxytocin in a Mouse Model of Rett Syndrome. Cereb. Cortex 2022, 32, 2885–2894. [Google Scholar] [CrossRef]
- Tang, X.; Drotar, J.; Li, K.; Clairmont, C.D.; Brumm, A.S.; Sullins, A.J.; Wu, H.; Liu, X.S.; Wang, J.; Gray, N.S.; et al. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci. Transl. Med. 2019, 11, eaau0164. [Google Scholar] [CrossRef]
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child. Neurol. 2007, 109, 8–14, Erratum in Dev. Med. Child. Neurol. 2007, 49, 480. [Google Scholar]
- Yeargin-Allsopp, M.; Van Naarden Braun, K.; Doernberg, N.S.; Benedict, R.E.; Kirby, R.S.; Durkin, M.S. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: A multisite collaboration. Pediatrics 2008, 121, 547–554. [Google Scholar] [CrossRef]
- Paneth, N.; Hong, T.; Korzeniewski, S. The descriptive epidemiology of cerebral palsy. Clin. Perinatol. 2006, 33, 251–267. [Google Scholar] [CrossRef]
- Bekteshi, S.; Monbaliu, E.; McIntyre, S.; Saloojee, G.; Hilberink, S.R.; Tatishvili, N.; Dan, B. Towards functional improvement of motor disorders associated with cerebral palsy. Lancet Neurol. 2023, 22, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Van Naarden Braun, K.; Doernberg, N.S.; Maenner, M.J.; Arneson, C.L.; Durkin, M.S.; Benedict, R.E.; Kirby, R.S.; Wingate, M.S.; Fitzgerald, R.; et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev. Med. Child. Neurol. 2014, 56, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.E.; Fogarty, M.J.; Sieck, G.C. Chapter 15—The spa transgenic mouse model of hypertonia and use for studying cerebral palsy. In Handbook of Animal Models in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 183–192. [Google Scholar]
- Back, S.A. Cerebral white and gray matter injury in newborns: New insights into pathophysiology and management. Clin. Perinatol. 2014, 41, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Riddle, A.; Dean, J.; Hohimer, A.R. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics 2012, 9, 359–370. [Google Scholar] [CrossRef]
- Bax, M.; Tydeman, C.; Flodmark, O. Clinical and MRI correlates of cerebral palsy: The European Cerebral Palsy Study. J. Am. Med. Assoc. 2006, 296, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwinckel, J.; Schang, A.L.; Sigaut, S.; Chhor, V.; Degos, V.; Hagberg, H.; Baud, O.; Fleiss, B.; Gressens, P. Brain damage of the preterm infant: New insights into the role of inflammation. Biochem. Soc. Trans. 2014, 42, 557–563. [Google Scholar] [CrossRef]
- Ellenberg, J.H.; Nelson, K.B. The association of cerebral palsy with birth asphyxia: A definitional quagmire. Dev. Med. Child. Neurol. 2013, 55, 210–216. [Google Scholar] [CrossRef]
- Nelson, K.B. Causative factors in cerebral palsy. Clin. Obstet. Gynecol. 2008, 51, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Woodward, L.J.; Anderson, P.J.; Austin, N.C.; Howard, K.; Inder, T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. New Engl. J. Med. 2006, 355, 685–694. [Google Scholar] [CrossRef]
- Katz, R.T.; Rymer, W.Z. Spastic hypertonia: Mechanisms and measurement. Arch. Phys. Med. Rehabil. 1989, 70, 144–155. [Google Scholar]
- McGuire, J.; Rymer, W.Z. Spasticity: Mechanisms and Management. In Medical Management of Long-Term Disability; Green, D., Ed.; Butterworth-Heinemann: Newton, MA, USA, 1996. [Google Scholar]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Shevell, M.; Dagenais, L.; Oskoui, M. The epidemiology of cerebral palsy: New perspectives from a Canadian registry. Semin. Pediatr. Neurol. 2013, 20, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W. Pathophysiology of spasticity and clinical experience with baclofen. In Spasticity: Disordered Motor Control; Feldman, R.G., Young, R.R., Koella, W.P., Eds.; Year Book Medical Publishers: Chicago, IL, USA, 1980; pp. 185–220. [Google Scholar]
- Sanger, T.D.; Delgado, M.R.; Gaebler-Spira, D.; Hallett, M.; Mink, J.W.; Task Force on Childhood Motor, D. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 2003, 111, e89–e97. [Google Scholar] [CrossRef] [PubMed]
- Haberfehlner, H.; Jaspers, R.T.; Rutz, E.; Harlaar, J.; van der Sluijs, J.A.; Witbreuk, M.M.; van Hutten, K.; Romkes, J.; Freslier, M.; Brunner, R.; et al. Outcome of medial hamstring lengthening in children with spastic paresis: A biomechanical and morphological observational study. PLoS ONE 2018, 13, e0192573. [Google Scholar] [CrossRef]
- Walker, H.K. Deep Tendon Reflexes. In The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Nielsen, J.B.; Crone, C.; Hultborn, H. The spinal pathophysiology of spasticity—From a basic science point of view. Acta Physiol. 2007, 189, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Stein, V.; Hermans-Borgmeyer, I.; Jentsch, T.J.; Hubner, C.A. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004, 468, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stil, A.; Liabeuf, S.; Jean-Xavier, C.; Brocard, C.; Viemari, J.C.; Vinay, L. Developmental up-regulation of the potassium-chloride cotransporter type 2 in the rat lumbar spinal cord. Neuroscience 2009, 164, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.K.; Rosenbaum, P.; Paneth, N.; Dan, B.; Lin, J.P.; Damiano, D.L.; Becher, J.G.; Gaebler-Spira, D.; Colver, A.; Reddihough, D.S.; et al. Cerebral palsy. Nat. Rev. Dis. Prim. 2016, 2, 15082. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.E.; Fogarty, M.J.; Sieck, G.C. Why individuals with cerebral palsy are at higher risk for respiratory complications from COVID-19. J. Pediatr. Rehabil. Med. 2020, 13, 317–327. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Lee, H.Y. Differences of respiratory function in children with spastic diplegic and hemiplegic cerebral palsy, compared with normally developed children. J. Pediatr. Rehabil. Med. 2013, 6, 113–117. [Google Scholar] [CrossRef]
- Lee, J.D.; Park, H.-J.; Park, E.S.; Oh, M.-K.; Park, B.; Rha, D.-W.; Cho, S.-R.; Kim, E.Y.; Park, J.Y.; Kim, C.H.; et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain 2011, 134, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Ferriero, D.M.; Vannucci, S.J.; Hagberg, H. Models of cerebral palsy: Which ones are best? J. Child. Neurol. 2005, 20, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Fowler, E.; Brandenburg, J.E.; Levey, E.; Mcintyre, S.; Sukal-Moulton, T.; Ramey, S.L.; Rose, J.; Sienko, S.; Stashinko, E.; et al. A common data language for clinical research studies: The National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 1.0 recommendations. Dev. Med. Child. Neurol. 2018, 60, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Hamimi, S.; Robinson, S.; Jantzie, L.L. Chapter 16—Transient occlusion of uterine arteries and intra-amniotic injection of lipopolysaccharide in rats as a model of cerebral palsy. In Handbook of Animal Models in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 193–204. [Google Scholar]
- Cavarsan, C.F.; Gorassini, M.A.; Quinlan, K.A. Animal models of developmental motor disorders: Parallels to human motor dysfunction in cerebral palsy. J. Neurophysiol. 2019, 122, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.E.; Fogarty, M.J.; Sieck, G.C. Growth and survival characteristics of spa mice. Anim. Model Exp. Med. 2020, 3, 319–324. [Google Scholar] [CrossRef]
- Hutton, J.L. Cerebral palsy life expectancy. Clin. Perinatol. 2006, 33, 545–555. [Google Scholar] [CrossRef]
- Durufle-Tapin, A.; Colin, A.; Nicolas, B.; Lebreton, C.; Dauvergne, F.; Gallien, P. Analysis of the medical causes of death in cerebral palsy. Ann. Phys. Rehabil. Med. 2014, 57, 24–37. [Google Scholar] [CrossRef]
- Jantzie, L.L.; Corbett, C.J.; Berglass, J.; Firl, D.J.; Flores, J.; Mannix, R.; Robinson, S. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J. Neuroinflammation 2014, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.S. Phenotypic heterogeneity and disease course in three murine strains with mutations in genes encoding for alpha 1 and beta glycine receptor subunits. Mov. Disord. 1997, 12, 221–228. [Google Scholar] [CrossRef]
- Chai, C.K. Hereditary Spasticity in Mice. J. Hered. 1961, 52, 241–243. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Fogarty, M.J.; Brown, A.D.; Sieck, G.C. Phrenic motor neuron loss in an animal model of early onset hypertonia. J. Neurophysiol. 2020, 123, 1682–1690. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Gransee, H.M.; Fogarty, M.J.; Sieck, G.C. Differences in Lumbar Motor Neuron Pruning in an Animal Model of Early Onset Spasticity. J. Neurophysiol. 2018, 120, 601–609. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Brandenburg, J.E.; Sieck, G.C. Diaphragm Neuromuscular Transmission Failure in a Mouse Model of an Early-Onset Neuromotor Disorder. J. Appl. Physiol. 2021, 130, 708–720. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Sieck, G.C.; Brandenburg, J.E. Impaired neuromuscular transmission of the tibialis anterior in a rodent model of hypertonia. J. Neurophysiol. 2020, 123, 1864–1869. [Google Scholar] [CrossRef]
- Rivares, C.; Vignaud, A.; Noort, W.; Koopmans, B.; Loos, M.; Kalinichev, M.; Jaspers, R.T. Glycine receptor subunit-beta-deficiency in a mouse model of spasticity results in attenuated physical performance, growth, and muscle strength. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R368–R388. [Google Scholar] [CrossRef]
- Petroff, O.A.C.; Rothman, D.L.; Behar, K.L.; Mattson, R.H. Low brain GABA level is associated with poor seizure control. Ann. Neurol. 1996, 40, 908–911. [Google Scholar] [CrossRef]
- Wood, J.H.; Hare, T.A.; Glaeser, B.S.; Ballenger, J.C.; Post, R.M. Low cerebrospinal fluid gamma-aminobutyric acid content in seizure patients. Neurology 1979, 29, 1203–1208. [Google Scholar] [CrossRef]
- Cediel, M.L.; Stawarski, M.; Blanc, X.; Noskova, L.; Magner, M.; Platzer, K.; Gburek-Augustat, J.; Baldridge, D.; Constantino, J.N.; Ranza, E.; et al. GABBR1 monoallelic de novo variants linked to neurodevelopmental delay and epilepsy. Am. J. Hum. Genet. 2022, 109, 1885–1893. [Google Scholar] [CrossRef]
- Maillard, P.Y.; Baer, S.; Schaefer, E.; Desnous, B.; Villeneuve, N.; Lepine, A.; Fabre, A.; Lacoste, C.; El Chehadeh, S.; Piton, A.; et al. Molecular and clinical descriptions of patients with GABA(A) receptor gene variants (GABRA1, GABRB2, GABRB3, GABRG2): A cohort study, review of literature, and genotype-phenotype correlation. Epilepsia 2022, 63, 2519–2533. [Google Scholar] [CrossRef]
- Vogel, F.D.; Krenn, M.; Westphal, D.S.; Graf, E.; Wagner, M.; Leiz, S.; Koniuszewski, F.; Auge-Stock, M.; Kramer, G.; Scholze, P.; et al. A de novo missense variant in GABRA4 alters receptor function in an epileptic and neurodevelopmental phenotype. Epilepsia 2022, 63, e35–e41. [Google Scholar] [CrossRef]
- Bernardino, I.; Dionisio, A.; Violante, I.R.; Monteiro, R.; Castelo-Branco, M. Motor Cortex Excitation/Inhibition Imbalance in Young Adults With Autism Spectrum Disorder: A MRS-TMS Approach. Front. Psychiatry 2022, 13, 860448. [Google Scholar] [CrossRef]
- Eisenberg, C.; Subramanian, D.; Afrasiabi, M.; Ziobro, P.; DeLucia, J.; Hirschberg, P.R.; Shiflett, M.W.; Santhakumar, V.; Tran, T.S. Reduced hippocampal inhibition and enhanced autism-epilepsy comorbidity in mice lacking neuropilin 2. Transl. Psychiatry 2021, 11, 537. [Google Scholar] [CrossRef]
- Fontes-Dutra, M.; Righes Marafiga, J.; Santos-Terra, J.; Deckmann, I.; Brum Schwingel, G.; Rabelo, B.; Kazmierzak de Moraes, R.; Rockenbach, M.; Vendramin Pasquetti, M.; Gottfried, C.; et al. GABAergic synaptic transmission and cortical oscillation patterns in the primary somatosensory area of a valproic acid rat model of autism spectrum disorder. Eur. J. Neurosci. 2023, 57, 527–546. [Google Scholar] [CrossRef]
- Chen, L.; Wan, L.; Wu, Z.; Ren, W.; Huang, Y.; Qian, B.; Wang, Y. KCC2 downregulation facilitates epileptic seizures. Sci. Rep. 2017, 7, 156. [Google Scholar] [CrossRef]
- He, Q.; Nomura, T.; Xu, J.; Contractor, A. The Developmental Switch in GABA Polarity Is Delayed in Fragile X Mice. J. Neurosci. 2014, 34, 446–450. [Google Scholar] [CrossRef]
- Pisella, L.I.; Gaiarsa, J.L.; Diabira, D.; Zhang, J.; Khalilov, I.; Duan, J.; Kahle, K.T.; Medina, I. Impaired regulation of KCC2 phosphorylation leads to neuronal network dysfunction and neurodevelopmental pathology. Sci. Signal. 2019, 12, eaay0300. [Google Scholar] [CrossRef]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef]
- Yuan, H.B.; Cheng, L.Y.; Yin, F.; Zhang, G.X.; Peng, J.; Kang, M.X.; Xu, Y.M.; Chen, R.L.; Wang, L. Levels of amino acids in cerebral spinal fluid in children with cerebral palsy. Zhongguo Dang Dai Er Ke Za Zhi 2008, 10, 475–477. [Google Scholar]
- Lee, J.D.; Park, H.J.; Park, E.S.; Kim, D.G.; Rha, D.W.; Kim, E.Y.; Kim, D.I.; Kim, J.J.; Yun, M.; Ryu, Y.H.; et al. Assessment of regional GABA(A) receptor binding using 18F-fluoroflumazenil positron emission tomography in spastic type cerebral palsy. Neuroimage 2007, 34, 19–25. [Google Scholar] [CrossRef]
- Achache, V.; Roche, N.; Lamy, J.C.; Boakye, M.; Lackmy, A.; Gastal, A.; Quentin, V.; Katz, R. Transmission within several spinal pathways in adults with cerebral palsy. Brain 2010, 133, 1470–1483. [Google Scholar] [CrossRef]
- Leonard, C.T.; Moritani, T.; Hirschfeld, H.; Forssberg, H. Deficits in Reciprocal Inhibition of Children with Cerebral-Palsy as Revealed by H-Reflex Testing. Dev. Med. Child. Neurol. 1990, 32, 974–984. [Google Scholar] [CrossRef]
- Leonard, C.T.; Sandholdt, D.Y.; McMillan, J.A.; Queen, S. Short- and long-latency contributions to reciprocal inhibition during various levels of muscle contraction of individuals with cerebral palsy. J. Child. Neurol. 2006, 21, 240–246. [Google Scholar] [CrossRef]
- Frisk, R.F.; Lorentzen, J.; Nielsen, J.B. Contribution of corticospinal drive to ankle plantar flexor muscle activation during gait in adults with cerebral palsy. Exp. Brain Res. 2019, 237, 1457–1467. [Google Scholar] [CrossRef]
- Heinen, F.; Kirschner, J.; Fietzek, U.; Glocker, F.X.; Mall, V.; Korinthenberg, R. Absence of transcallosal inhibition in adolescents with diplegic cerebral palsy. Muscle Nerve 1999, 22, 255–257. [Google Scholar] [CrossRef]
- Condliffe, E.G.; Jeffery, D.T.; Emery, D.J.; Gorassini, M.A. Spinal inhibition and motor function in adults with spastic cerebral palsy. J. Physiol. 2016, 594, 2691–2705. [Google Scholar] [CrossRef]
- Reilly, M.; Liuzzo, K.; Blackmer, A.B. Pharmacological Management of Spasticity in Children With Cerebral Palsy. J Pediatr Health Care 2020, 34, 495–509. [Google Scholar] [CrossRef]
- Davidoff, R.A. Antispasticity drugs: Mechanisms of action. Ann. Neurol. 1985, 17, 107–116. [Google Scholar] [CrossRef]
- de Beaurepaire, R. A Review of the Potential Mechanisms of Action of Baclofen in Alcohol Use Disorder. Front. Psychiatry 2018, 9, 506. [Google Scholar] [CrossRef]
- Gerrow, K.; Triller, A. GABA(A) receptor subunit composition and competition at synapses are tuned by GABA(B) receptor activity. Mol. Cell. Neurosci. 2014, 60, 97–107. [Google Scholar] [CrossRef]
- Connelly, W.M.; Fyson, S.J.; Errington, A.C.; McCafferty, C.P.; Cope, D.W.; Di Giovanni, G.; Crunelli, V. GABAB Receptors Regulate Extrasynaptic GABAA Receptors. J. Neurosci. 2013, 33, 3780–3785. [Google Scholar] [CrossRef]
- Penn, R.D.; Kroin, J.S. Continuous intrathecal baclofen for severe spasticity. Lancet 1985, 2, 125–127. [Google Scholar] [CrossRef]
- Brandenburg, J.E. Is baclofen the least worst option for spasticity management in children? J. Pediatr. Rehabil. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.K.; Lee, K.C.; Cho, H.E.; Chae, M.; Chang, J.W.; Chang, W.S.; Cho, S.R. Outcomes of intrathecal baclofen therapy in patients with cerebral palsy and acquired brain injury. Medicine 2017, 96, e7472. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.; Abraham, M.E.; Gold, J.; Patel, N.A.; Gendreau, J.L.; Herschman, Y.; Mammis, A. Intrathecal baclofen, selective dorsal rhizotomy, and extracorporeal shockwave therapy for the treatment of spasticity in cerebral palsy: A systematic review. Neurosurg. Rev. 2021, 44, 3209–3228. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, J.J.; Ferguson, C.; Jester, K.; Firestone, L.L.; Homanics, G.E. Mice with glycine receptor subunit mutations are both sensitive and resistant to volatile anesthetics. Anesth. Analg. 2002, 95, 578–582. [Google Scholar] [CrossRef]
- Stern, P.; Bokonjic, R. Glycine therapy in 7 cases of spasticity. A pilot study. Pharmacology 1974, 12, 117–119. [Google Scholar] [CrossRef]
- Nutt, D.J.; Malizia, A.L. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Brit. J. Psychiat. 2001, 179, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.S. The use of diazepam in childhood cerebral palsy. Report of a small study including electromyographic observations. Ann. Phys. Med. 1964, 7, 16–24. [Google Scholar] [CrossRef]
- Engle, H.A. The effect of diazepam (Valium) in children with cerebral palsy: A double-blind study. Dev. Med. Child. Neurol. 1966, 8, 661–667. [Google Scholar] [CrossRef]
- Mathew, A.; Mathew, M.C. Bedtime diazepam enhances well-being in children with spastic cerebral palsy. Pediatr. Rehabil. 2005, 8, 63–66. [Google Scholar] [CrossRef]
- Tickner, N.; Apps, J.R.; Keady, S.; Sutcliffe, A.G. An overview of drug therapies used in the treatment of dystonia and spasticity in children. Arch. Dis. Child. Educ. Pract. Ed. 2012, 97, 230–235. [Google Scholar] [CrossRef]
- O’Brien, C.P. Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 2005, 66 (Suppl. S2), 28–33. [Google Scholar]
- Panteliadis, C.; Panteliadis, P.; Vassilyadi, F. Hallmarks in the history of cerebral palsy: From antiquity to mid-20th century. Brain Dev. 2013, 35, 285–292. [Google Scholar] [CrossRef]
- Christensen, E.; Melchior, J.C. Cerebral Palsy A Clinical and Neuropathological Study; Butterworth-Heinemann: Newton, MA, USA, 1967. [Google Scholar]
- Marciniak, C.; Li, X.; Zhou, P. An examination of motor unit number index in adults with cerebral palsy. J. Electromyogr. Kinesiol. 2015, 25, 444–450. [Google Scholar] [CrossRef]
- Trevarrow, M.P.; Baker, S.E.; Wilson, T.W.; Kurz, M.J. Microstructural changes in the spinal cord of adults with cerebral palsy. Dev. Med. Child. Neurol. 2021, 63, 998–1003. [Google Scholar] [CrossRef]
- Trevarrow, M.P.; Reelfs, A.; Baker, S.E.; Hoffman, R.M.; Wilson, T.W.; Kurz, M.J. Spinal cord microstructural changes are connected with the aberrant sensorimotor cortical oscillatory activity in adults with cerebral palsy. Sci. Rep. 2022, 12, 4807. [Google Scholar] [CrossRef]
- Drobyshevsky, A.; Quinlan, K.A. Spinal cord injury in hypertonic newborns after antenatal hypoxia-ischemia in a rabbit model of cerebral palsy. Exp. Neurol. 2017, 293, 13–26. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Mantilla, C.B.; Zhan, W.Z.; Smithson, K.G.; Sieck, G.C. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 2000, 89, 563–572. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Mu, E.W.H.; Lavidis, N.A.; Noakes, P.G.; Bellingham, M.C. Motor Areas Show Altered Dendritic Structure in an Amyotrophic Lateral Sclerosis Mouse Model. Front. Neurosci. 2017, 11, 609. [Google Scholar] [CrossRef]
- Kanjhan, R.; Fogarty, M.J.; Noakes, P.G.; Bellingham, M.C. Developmental changes in the morphology of mouse hypoglossal motor neurons. Brain Struct. Funct. 2016, 221, 3755–3786. [Google Scholar] [CrossRef]
- Williams, P.A.; Bellinger, D.L.; Wilson, C.G. Changes in the Morphology of Hypoglossal Motor Neurons in the Brainstem of Developing Rats. Anat. Rec. 2019, 302, 869–892. [Google Scholar] [CrossRef]
- Korfage, J.A.M.; Van Wessel, T.; Langenbach, G.E.J.; Van Eijden, T.M.G.J. Heterogeneous postnatal transitions in myosin heavy chain isoforms within the rabbit temporalis muscle. Anat. Rec. Part A 2006, 288a, 1095–1104. [Google Scholar] [CrossRef]
- Korfage, J.A.; van Wessel, T.; Langenbach, G.E.; Ay, F.; van Eijden, T.M. Postnatal transitions in myosin heavy chain isoforms of the rabbit superficial masseter and digastric muscle. J. Anat. 2006, 208, 743–751. [Google Scholar] [CrossRef]
- Korfage, J.A.; Helmers, R.; Matignon Mde, G.; van Wessel, T.; Langenbach, G.E.; van Eijden, T.M. Postnatal development of fiber type composition in rabbit jaw and leg muscles. Cells Tissues Organs 2009, 190, 42–52. [Google Scholar] [CrossRef]
- Saito, K.; Morita, T.; Takasu, H.; Kuroki, K.; Fujiwara, T.; Hiraba, K.; Goto, S. Histochemical study of rabbit medial pterygoid muscle during postnatal development. Odontology 2017, 105, 141–149. [Google Scholar] [CrossRef]
- Dohare, P.; Zia, M.T.; Ahmed, E.; Ahmed, A.; Yadala, V.; Schober, A.L.; Ortega, J.A.; Kayton, R.; Ungvari, Z.; Mongin, A.A.; et al. AMPA-Kainate Receptor Inhibition Promotes Neurologic Recovery in Premature Rabbits with Intraventricular Hemorrhage. J. Neurosci. 2016, 36, 3363–3377. [Google Scholar] [CrossRef]
- Palazon-Garcia, R.; Benavente-Valdepenas, A.M. Botulinum Toxin: From Poison to Possible Treatment for Spasticity in Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 4886. [Google Scholar] [CrossRef]
- Kaya Keles, C.S.; Ates, F. Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles. Toxins 2022, 14, 772. [Google Scholar] [CrossRef]
- Klein, C.; Gouron, R.; Barbier, V. Effects of botulinum toxin injections in the upper limbs of children with cerebral palsy: A systematic review of the literature. Orthop. Traumatol. Surg. Res. 2023, 103578. [Google Scholar] [CrossRef] [PubMed]
- Koman, L.A.; Mooney, J.F., 3rd; Smith, B.P.; Goodman, A.; Mulvaney, T. Management of spasticity in cerebral palsy with botulinum-A toxin: Report of a preliminary, randomized, double-blind trial. J. Pediatr. Orthop. 1994, 14, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Britt, B.A. Dantrolene. Can. Anaesth. Soc. J. 1984, 31, 61–75. [Google Scholar] [CrossRef]
- Pinder, R.M.; Brogden, R.N.; Speight, T.M.; Avery, G.S. Dantrolene sodium: A review of its pharmacological properties and therapeutic efficacy in spasticity. Drugs 1977, 13, 3–23. [Google Scholar] [CrossRef]
- Ward, A.; Chaffman, M.O.; Sorkin, E.M. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs 1986, 32, 130–168. [Google Scholar] [CrossRef] [PubMed]
- Wiley, M.E.; Damiano, D.L. Lower-extremity strength profiles in spastic cerebral palsy. Dev. Med. Child Neurol. 1998, 40, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Armand, S.; De Coulon, G.; Sarah, R.D.D.S.; Maffiuletti, N.A.; Kayser, B.; Place, N. Plantar flexor muscle weakness and fatigue in spastic cerebral palsy patients. Res. Dev. Disabil. 2017, 61, 66–76. [Google Scholar] [CrossRef]
- Eken, M.M.; Braendvik, S.M.; Bardal, E.M.; Houdijk, H.; Dallmeijer, A.J.; Roeleveld, K. Lower limb muscle fatigue during walking in children with cerebral palsy. Dev. Med. Child. Neurol. 2019, 61, 212–218. [Google Scholar] [CrossRef]
- Eek, M.N.; Beckung, E. Walking ability is related to muscle strength in children with cerebral palsy. Gait Posture 2008, 28, 366–371. [Google Scholar] [CrossRef]
- Eken, M.M.; Dallmeijer, A.J.; Houdijk, H.; Doorenbosch, C.A. Muscle fatigue during repetitive voluntary contractions: A comparison between children with cerebral palsy, typically developing children and young healthy adults. Gait Posture 2013, 38, 962–967. [Google Scholar] [CrossRef]
- Puce, L.; Pallecchi, I.; Chamari, K.; Marinelli, L.; Innocenti, T.; Pedrini, R.; Mori, L.; Trompetto, C. Systematic Review of Fatigue in Individuals With Cerebral Palsy. Front. Hum. Neurosci. 2021, 15, 598800. [Google Scholar] [CrossRef]
- Wass, C.T.; Warner, M.E.; Worrell, G.A.; Castagno, J.A.; Howe, M.; Kerber, K.A.; Palzkill, J.M.; Schroeder, D.R.; Cascino, G.D. Effect of general anesthesia in patients with cerebral palsy at the turn of the new millennium: A population-based study evaluating perioperative outcome and brief overview of anesthetic implications of this coexisting disease. J. Child Neurol. 2012, 27, 859–866. [Google Scholar] [CrossRef]
- Honan, I.; Finch-Edmondson, M.; Imms, C.; Novak, I.; Hogan, A.; Clough, S.; Bonyhady, B.; McIntyre, S.; Elliott, C.; Wong, S.; et al. Is the search for cerebral palsy ‘cures’ a reasonable and appropriate goal in the 2020s? Dev. Med. Child Neurol. 2022, 64, 49–55. [Google Scholar] [CrossRef]
- Turner, L. Hope, hype, cures, and persons with cerebral palsy. Dev. Med. Child. Neurol. 2022, 64, 8. [Google Scholar] [CrossRef]
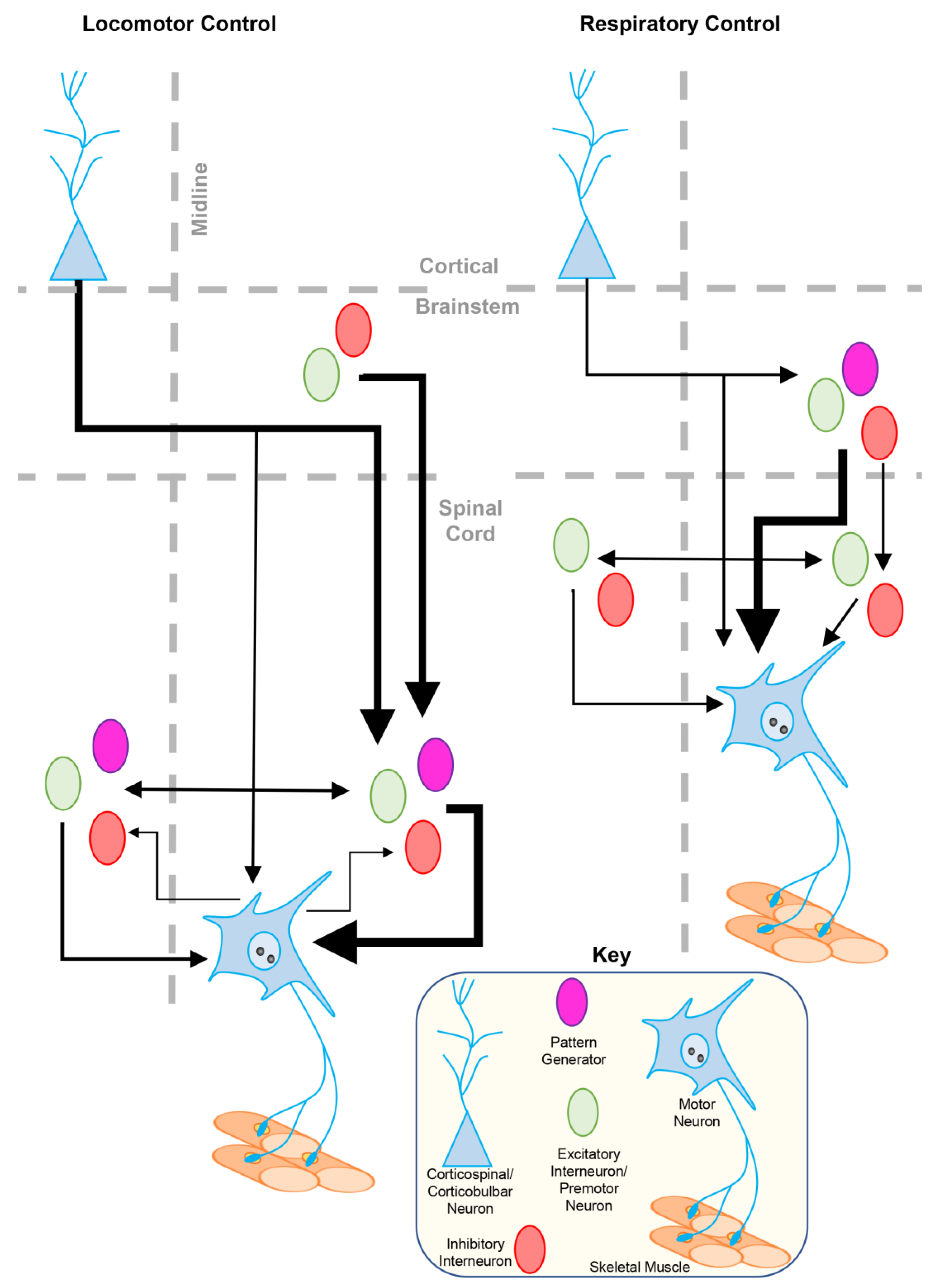
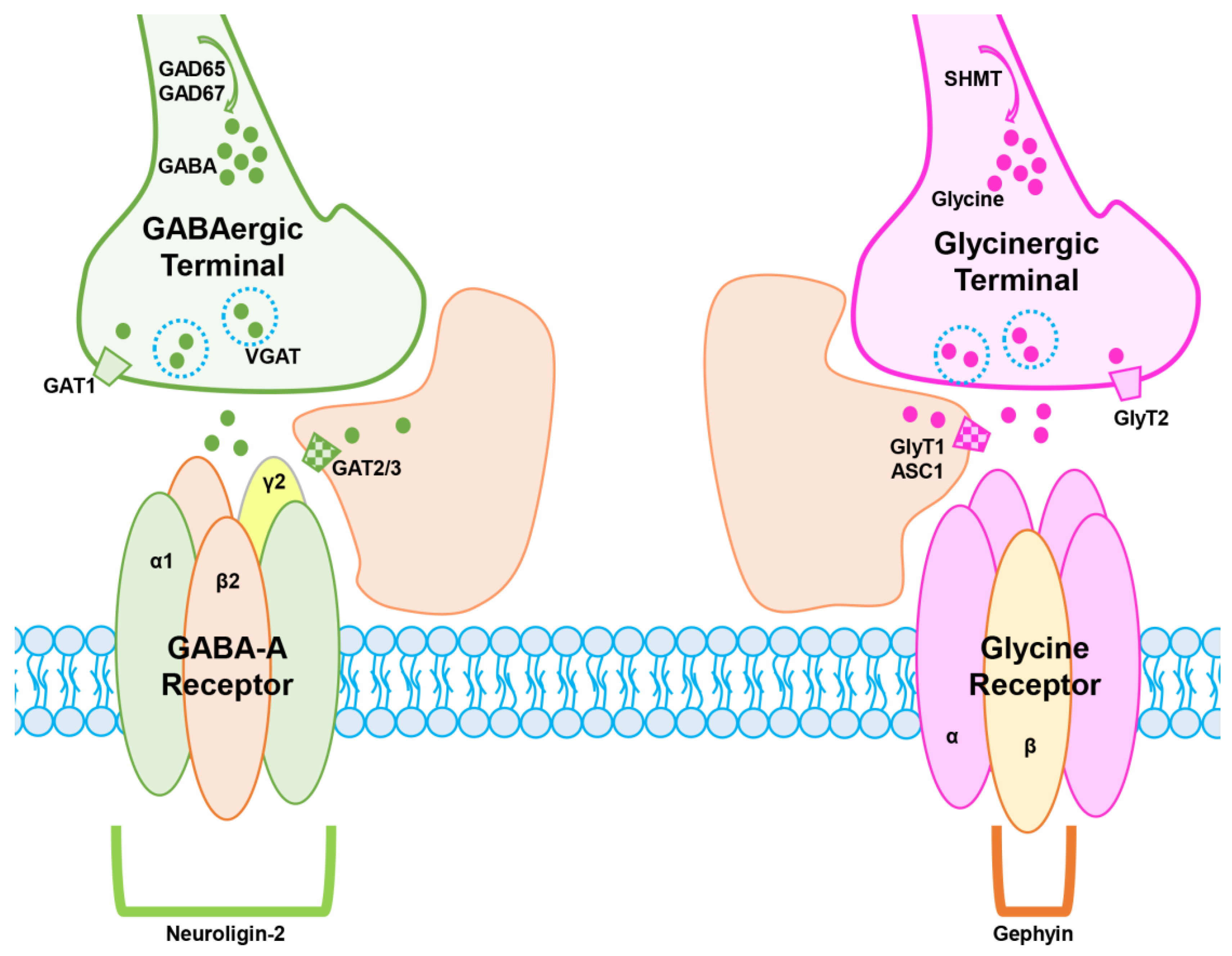
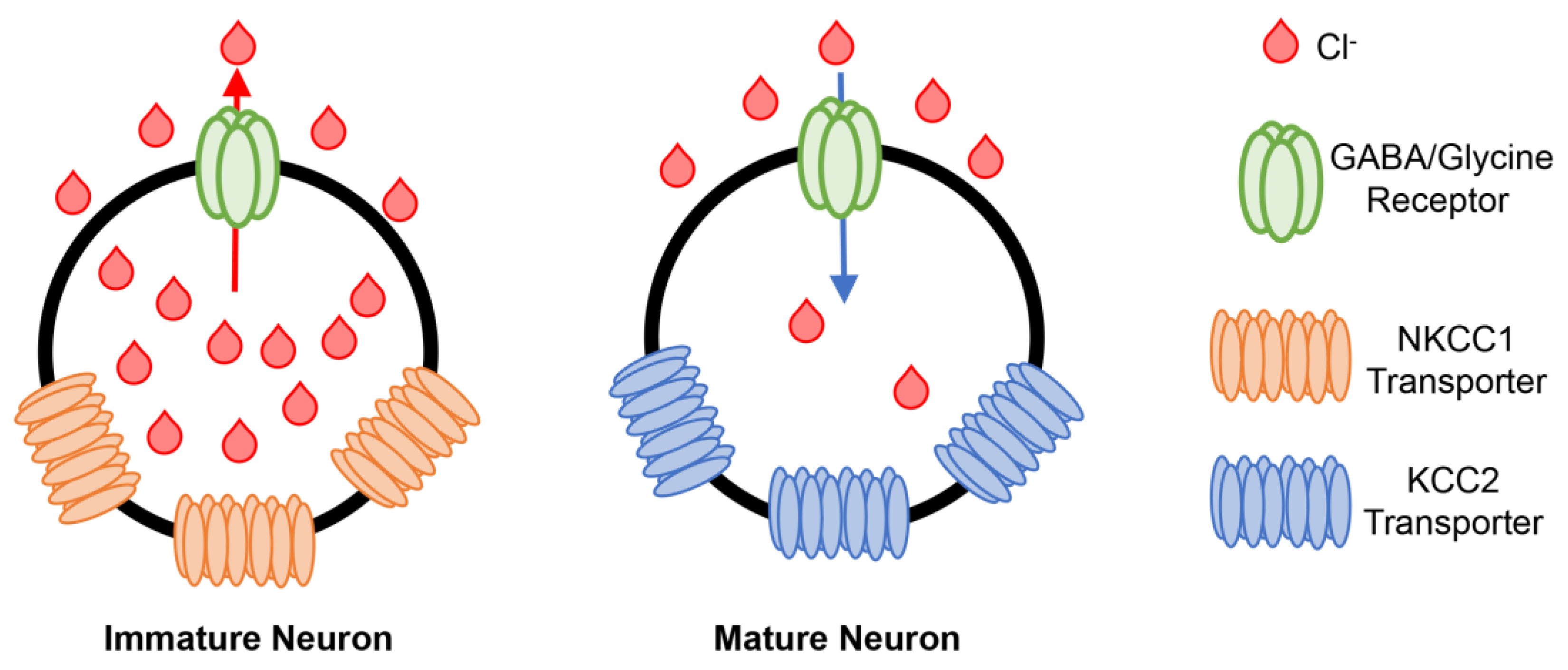
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogarty, M.J. Inhibitory Synaptic Influences on Developmental Motor Disorders. Int. J. Mol. Sci. 2023, 24, 6962. https://doi.org/10.3390/ijms24086962
Fogarty MJ. Inhibitory Synaptic Influences on Developmental Motor Disorders. International Journal of Molecular Sciences. 2023; 24(8):6962. https://doi.org/10.3390/ijms24086962
Chicago/Turabian StyleFogarty, Matthew J. 2023. "Inhibitory Synaptic Influences on Developmental Motor Disorders" International Journal of Molecular Sciences 24, no. 8: 6962. https://doi.org/10.3390/ijms24086962
APA StyleFogarty, M. J. (2023). Inhibitory Synaptic Influences on Developmental Motor Disorders. International Journal of Molecular Sciences, 24(8), 6962. https://doi.org/10.3390/ijms24086962



