Stearoyl-CoA Desaturases1 Accelerates Non-Small Cell Lung Cancer Metastasis by Promoting Aromatase Expression to Improve Estrogen Synthesis
Abstract
1. Introduction
2. Results
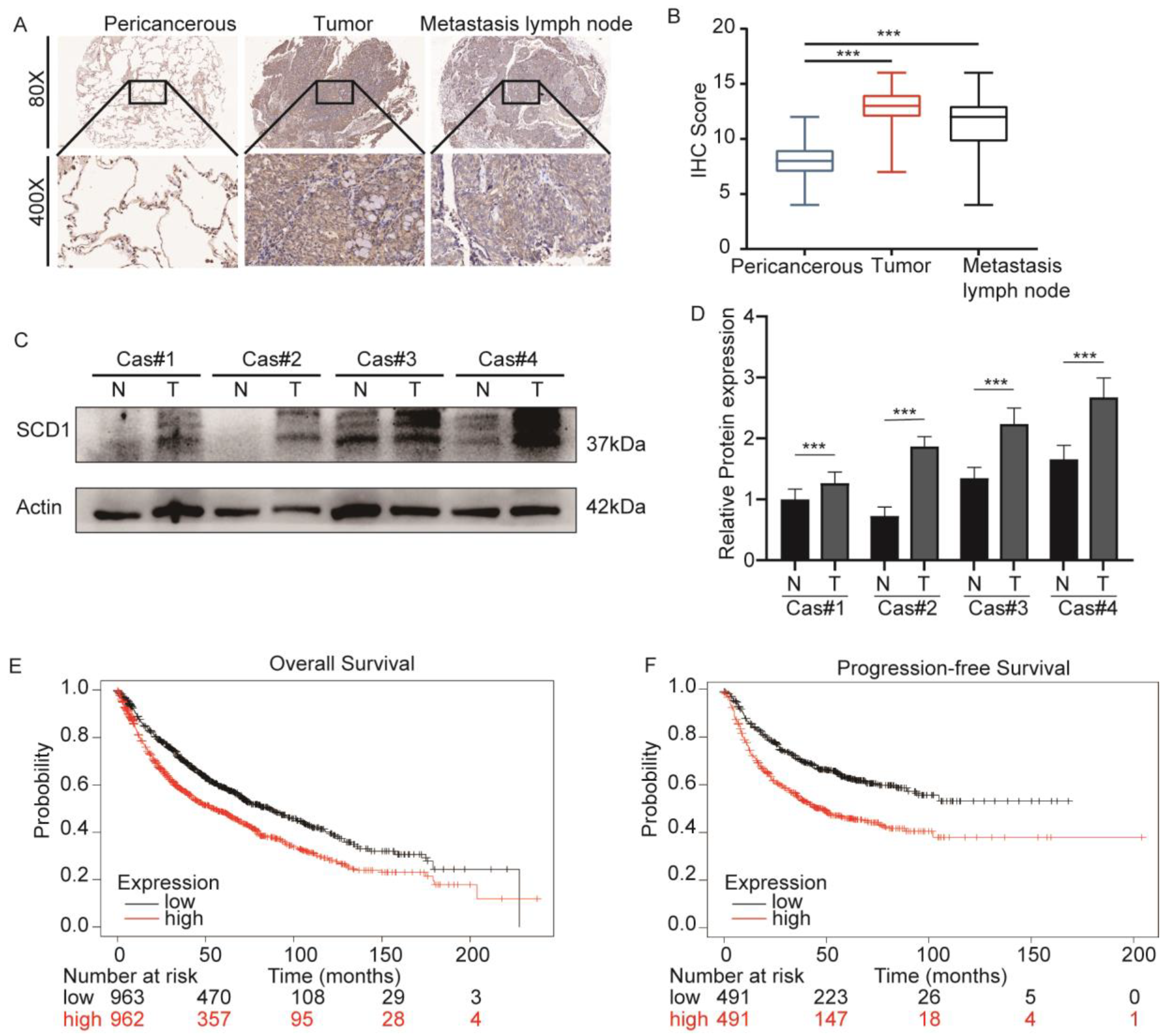
2.1. SCD1 Is Upregulated in Lung Cancer and Associated with a Poor Prognosis
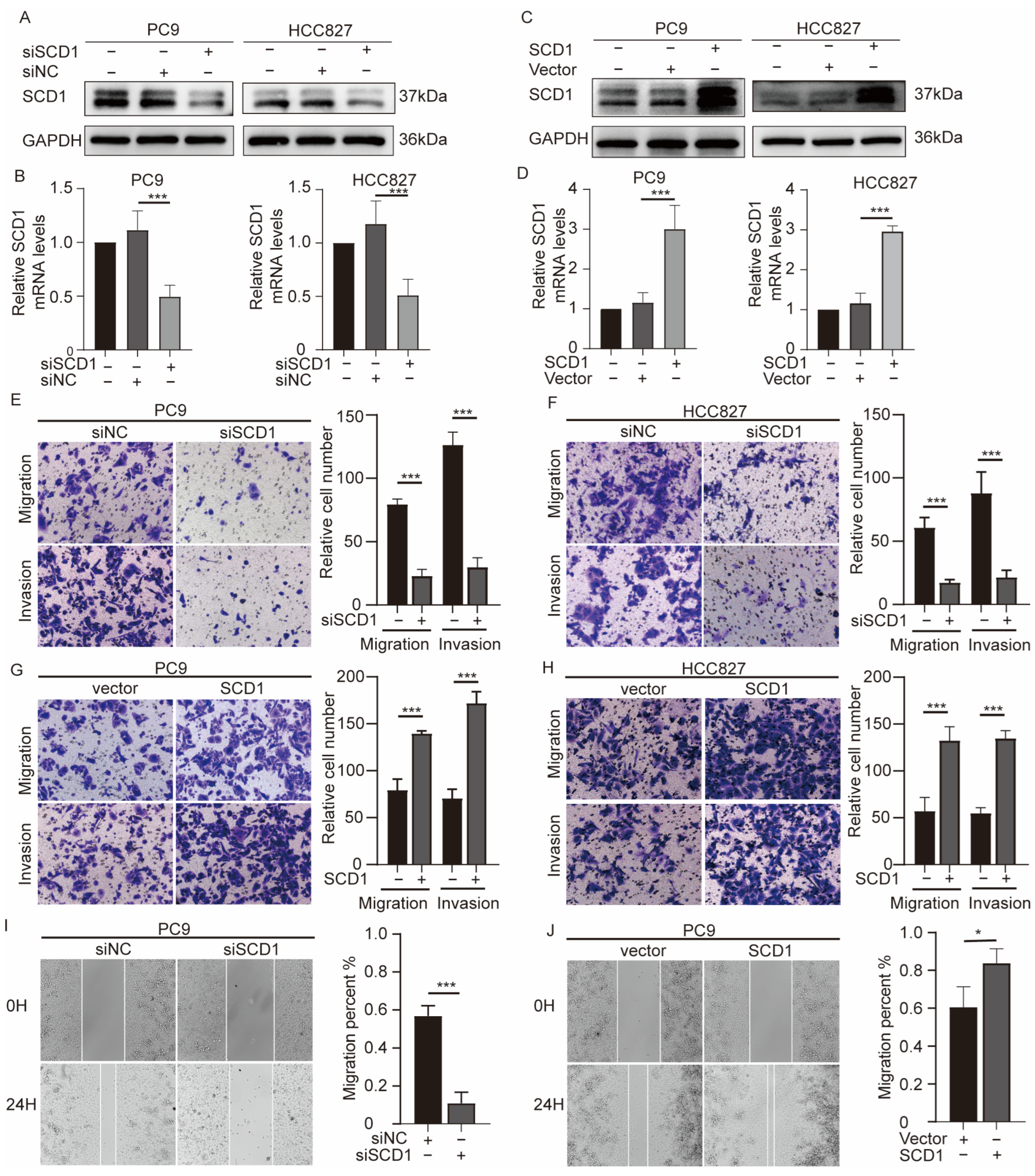
2.2. SCD1 Promotes Migration and Invasion of NSCLC Cells
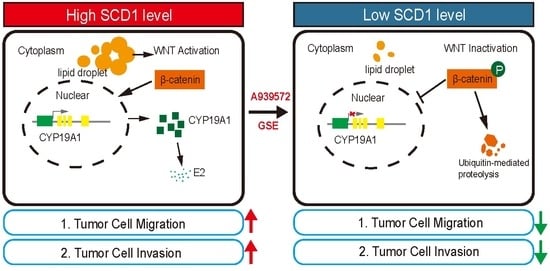
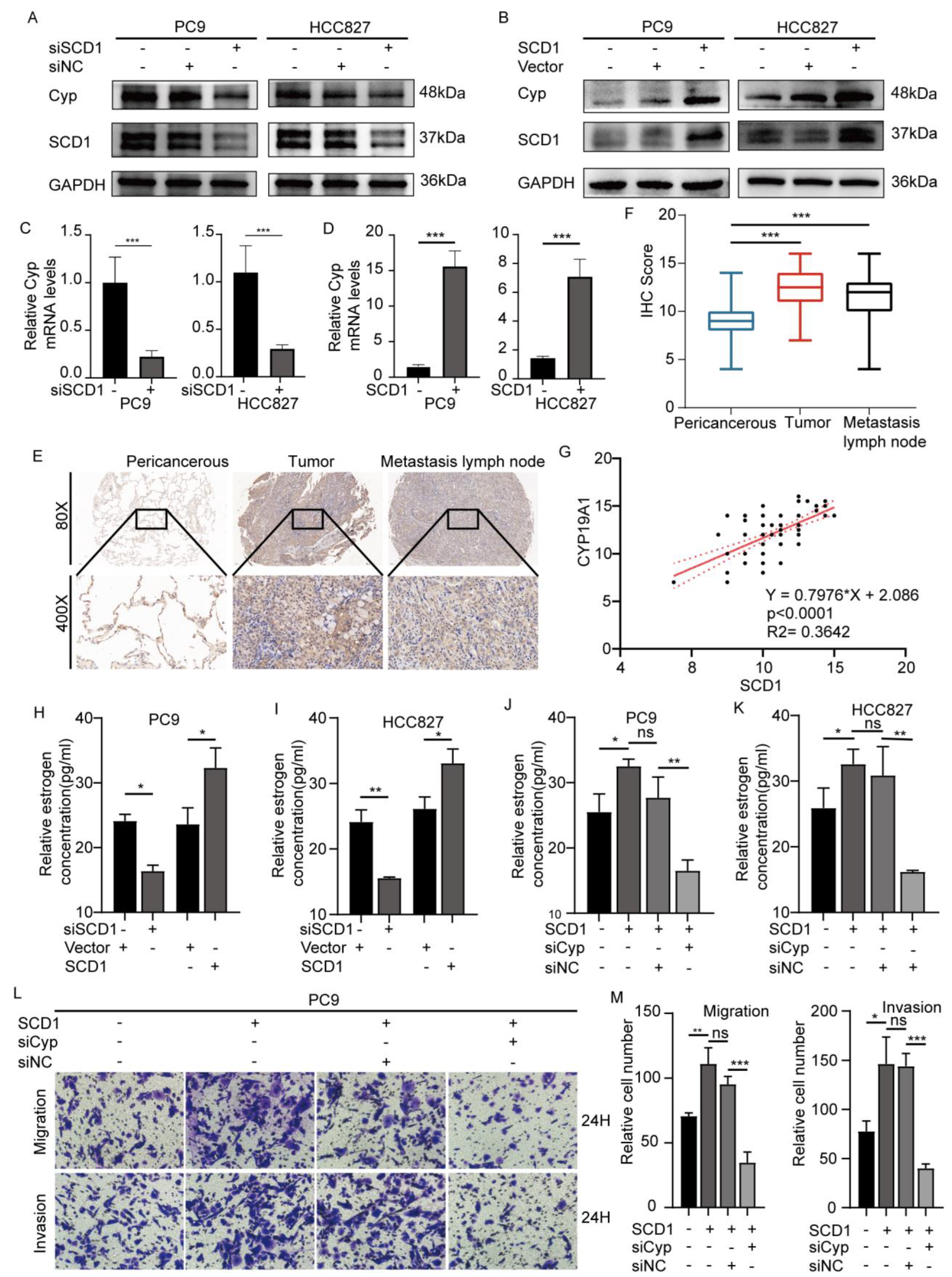
2.3. SCD1 Promotes Tumor Invasion and Metastasis via CYP19A1 Upregulation and Enhanced Estrogen Biosynthesis
2.4. SCD1 Promotes CYP19A1 Expression through the Wnt/β-Catenin Signaling Pathway
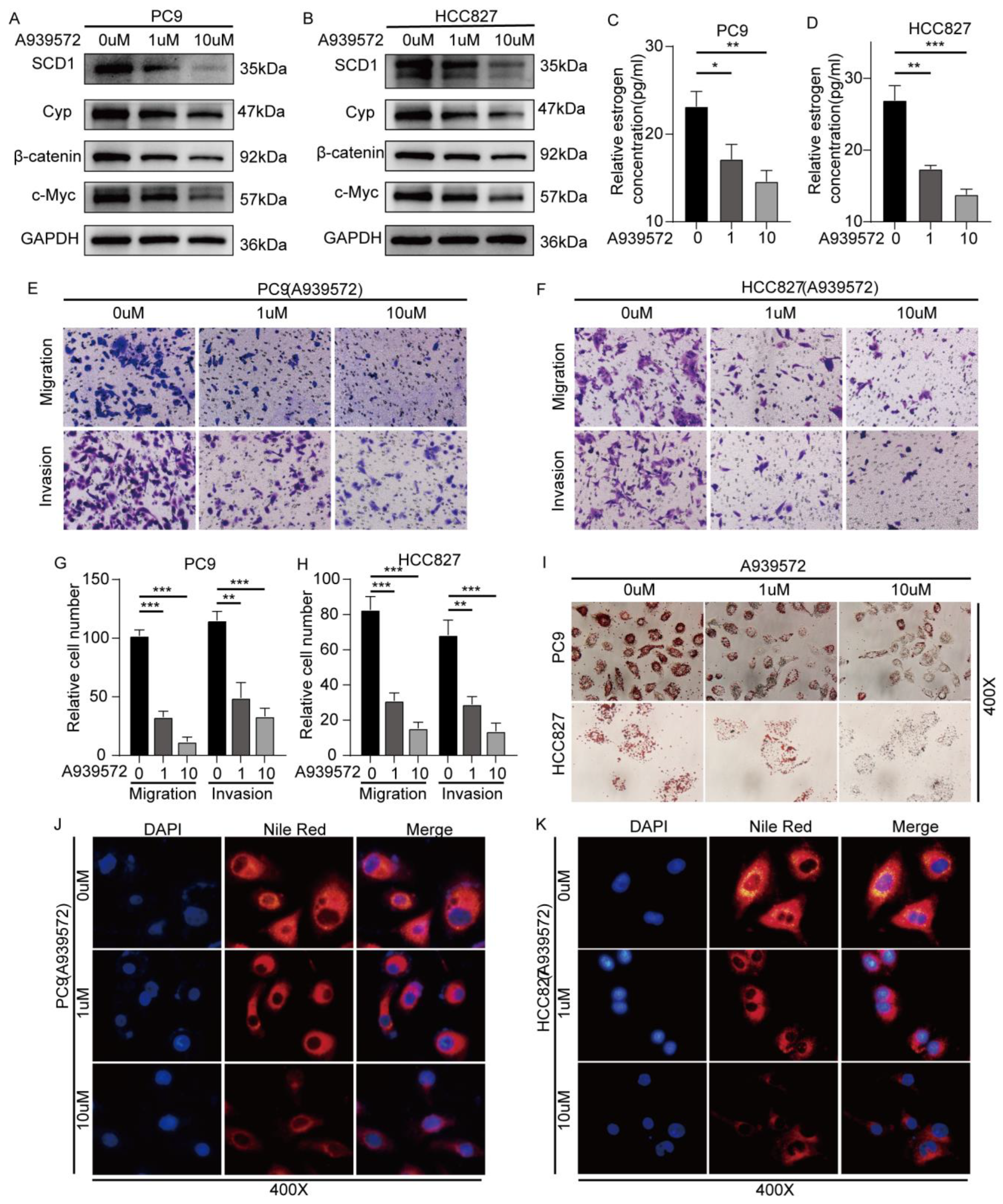
2.5. SCD1 Inhibitor A939572 Effectively Inhibits the β-Catenin/CYP19A1 Axis and Estrogen Biogenesis
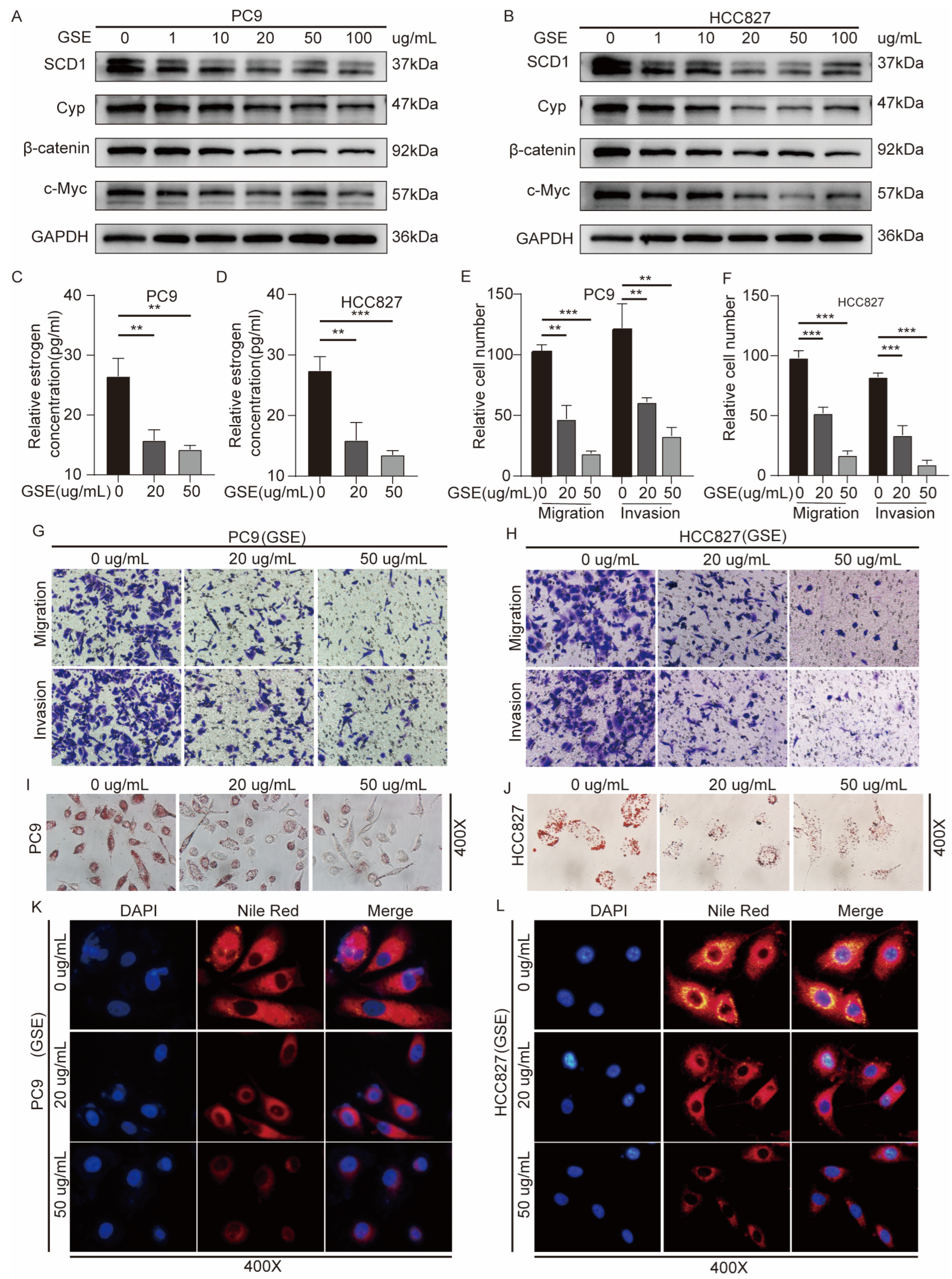
2.6. Grape Seed Extract Is a Potential Inhibitor of SCD1 and Inhibits Lipid Accumulation and Estrogen Biosynthesis
2.7. In Vivo shSCD1 Inhibits Tumor Growth and Metastasis
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Cell Lines and Cell Culture
4.3. Cell Treatment and Cell Morphological Observation
4.4. Oil Red O Staining
4.5. Nile Red Staining
4.6. IHC
4.7. Coimmunoprecipitation (Co-IP) and Western Blotting
4.8. Immunofluorescence (IF) Staining of Cultured Cells
4.9. RNA Extraction and qRT-PCR
4.10. Wound Healing Assays
4.11. Transwell Assays
4.12. Cell Transfections
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Tumor Formation Assay
4.15. In Vivo Cancer Metastasis Assay
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume Ct Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-Associated Exosomes Promote Lung Cancer Metastasis through Multiple Mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid Metabolism in Cancer: New Perspectives and Emerging Mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Karagiota, A.; Chachami, G.; Paraskeva, E. Lipid Metabolism in Cancer: The Role of Acylglycerolphosphate Acyltransferases (Agpats). Cancers 2022, 14, 228. [Google Scholar] [CrossRef]
- Bian, X.L.; Liu, R.; Meng, Y.; Xing, D.M.; Xu, D.Q.; Lu, Z.M. Cancer Focus Lipid Metabolism and Cancer. J. Exp. Med. 2021, 218, e20201606. [Google Scholar] [CrossRef]
- Iwamoto, H.; Abe, M.; Yang, Y.; Cui, D.; Seki, T.; Nakamura, M.; Hosaka, K.; Lim, S.; Wu, J.; He, X.; et al. Cancer Lipid Metabolism Confers Antiangiogenic Drug Resistance. Cell Metab. 2018, 28, 104. [Google Scholar] [CrossRef]
- Li, H.; Feng, Z.; He, M.L. Lipid Metabolism Alteration Contributes to and Maintains the Properties of Cancer Stem Cells. Theranostics 2020, 10, 7053–7069. [Google Scholar] [CrossRef]
- Schwartsburd, P. Lipid Droplets: Could They Be Involved in Cancer Growth and Cancer-Microenvironment Communications? Cancer Commun. 2022, 42, 83–87. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid Metabolism Reprogramming and Its Potential Targets in Cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/Beta-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Gu, X.; Sun, R.; Chen, L.; Chu, S.; Doll, M.A.; Li, X.; Feng, W.; Siskind, L.; McClain, C.J.; Deng, Z. Neutral Ceramidase Mediates Nonalcoholic Steatohepatitis by Regulating Monounsaturated Fatty Acids and Gut Iga(+) B Cells. Hepatology 2021, 73, 901–919. [Google Scholar] [CrossRef]
- Ran, H.; Zhu, Y.; Deng, R.; Zhang, Q.; Liu, X.; Feng, M.; Zhong, J.; Lin, S.; Tong, X.; Su, Q. Stearoyl-Coa Desaturase-1 Promotes Colorectal Cancer Metastasis in Response to Glucose by Suppressing Pten. J. Exp. Clin. Cancer Res. 2018, 37, 54. [Google Scholar] [CrossRef]
- Li, J.; Condello, S.; Thomes-Pepin, J.; Ma, X.; Xia, Y.; Hurley, T.D.; Matei, D.; Cheng, J.-X. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem. Cell 2017, 20, 303–314.e5. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhang, Y.; Yuan, P.; Liu, J.; Ding, L.; Ye, Q. Mir-4310 Regulates Hepatocellular Carcinoma Growth and Metastasis through Lipid Synthesis. Cancer Lett. 2021, 519, 161–171. [Google Scholar] [CrossRef]
- Jin, X.; Demere, Z.; Nair, K.; Ali, A.; Ferraro, G.B.; Natoli, T.; Deik, A.; Petronio, L.; Tang, A.A.; Zhu, C.; et al. A Metastasis Map of Human Cancer Cell Lines (Vol 588, Pg 331, 2020). Nature 2021, 599, E7. [Google Scholar] [CrossRef]
- Noto, A.; Raffa, S.; De Vitis, C.; Roscilli, G.; Malpicci, D.; Coluccia, P.; Di Napoli, A.; Ricci, A.; Giovagnoli, M.R.; Aurisicchio, L.; et al. Stearoyl-Coa Desaturase-1 Is a Key Factor for Lung Cancer-Initiating Cells. Cell Death Dis. 2013, 4, e947. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Li, D.; Wei, X.; Jia, Y.; Zhang, Z.; Ai, B.; Cao, X.; Guo, T.; Liao, Y. Co-Administration of 20(S)-Protopanaxatriol (G-Ppt) and Egfr-Tki Overcomes Egfr-Tki Resistance by Decreasing Scd1 Induced Lipid Accumulation in Non-Small Cell Lung Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 129. [Google Scholar] [CrossRef]
- Noto, A.; De Vitis, C.; Pisanu, M.E.; Roscilli, G.; Ricci, G.; Catizone, A.; Sorrentino, G.; Chianese, G.; Taglialatela-Scafati, O.; Trisciuoglio, D.; et al. Stearoyl-Coa-Desaturase 1 Regulates Lung Cancer Stemness Via Stabilization and Nuclear Localization of Yap/Taz (Vol 36, Pg 4573, 2017). Oncogene 2017, 36, 4671–4672. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Noto, A.; De Vitis, C.; Morrone, S.; Scognamiglio, G.; Botti, G.; Venuta, F.; Diso, D.; Jakopin, Z.; Padula, F.; et al. Blockade of Stearoyl-Coa-Desaturase 1 Activity Reverts Resistance to Cisplatin in Lung Cancer Stem Cells. Cancer Lett. 2017, 406, 93–104. [Google Scholar] [CrossRef]
- Burns, T.F.; Stabile, L.P. Targeting the Estrogen Pathway for the Treatment and Prevention of Lung Cancer. Lung Cancer Manag. 2014, 3, 43–52. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic Control of Mitochondrial Function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Fan, S.; Liao, Y.; Qiu, W.; Huang, Q.; Xiao, H.; Liu, C.; Li, D.; Cao, X.; Li, L.; Liang, H.; et al. Estrogen Promotes the Metastasis of Nonsmall Cell Lung Cancer Via Estrogen Receptor Beta by Upregulation of Tolllike Receptor 4 and Activation of the Myd88/Nfkappab/Mmp2 Pathway. Oncol. Rep. 2020, 43, 2105–2119. [Google Scholar] [CrossRef]
- Gerard, C.; Brown, K.A. Obesity and Breast Cancer—Role of Estrogens and the Molecular Underpinnings of Aromatase Regulation in Breast Adipose Tissue. Mol. Cell. Endocrinol. 2018, 466, 15–30. [Google Scholar] [CrossRef]
- Miki, Y.; Suzuki, T.; Abe, K.; Suzuki, S.; Niikawa, H.; Iida, S.; Hata, S.; Akahira, J.-I.; Mori, K.; Evans, D.B.; et al. Intratumoral Localization of Aromatase and Interaction between Stromal and Parenchymal Cells in the Non-Small Cell Lung Carcinoma Microenvironment. Cancer Res. 2010, 70, 6659–6669. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, Z.; Liao, Y.; Liu, C.; Fan, S.; Wei, X.; Ai, B.; Xiong, J. 17beta-Estradiol Upregulates Il6 Expression through the Erbeta Pathway to Promote Lung Adenocarcinoma Progression. J. Exp. Clin. Cancer Res. 2018, 37, 133. [Google Scholar] [CrossRef]
- Gyorffy, B.; Surowiak, P.; Budczies, J.; Lanczky, A. Online Survival Analysis Software to Assess the Prognostic Value of Biomarkers Using Transcriptomic Data in Non-Small-Cell Lung Cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Xiong, Z.; Xu, T.; Liu, J.; Liu, Y.; Chen, J.; Shi, J.; Shou, Y.; Yue, C.; et al. Cenpa Promotes Clear Cell Renal Cell Carcinoma Progression and Metastasis Via Wnt/Beta-Catenin Signaling Pathway. J. Transl. Med. 2021, 19, 417. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/Beta-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Silvan, J.; Gutierrez-Docio, A.; Guerrero-Hurtado, E.; Domingo-Serrano, L.; Blanco-Suarez, A.; Prodanov, M.; Alarcon-Cavero, T.; Martinez-Rodriguez, A. Pre-Treatment with Grape Seed Extract Reduces Inflammatory Response and Oxidative Stress Induced by Helicobacter Pylori Infection in Human Gastric Epithelial Cells. Antioxidants 2021, 10, 943. [Google Scholar] [CrossRef]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Kumar, S.R.; Thomas, S.; Kalarikkal, N. Grape Seed Extract-Soluplus Dispersion and Its Antioxidant Activity. Drug Dev. Ind. Pharm. 2020, 46, 1219–1229. [Google Scholar] [CrossRef]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.L.; Chen, S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef]
- Salvador, M.M.; de Cedrón, M.G.; Rubio, J.M.; Martínez, S.F.; Martínez, R.S.; Casado, E.; de Molina, A.R.; Sereno, M. Lipid Metabolism and Lung Cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Eltayeb, K.; La Monica, S.; Tiseo, M.; Alfieri, R.; Fumarola, C. Reprogramming of Lipid Metabolism in Lung Cancer: An Overview with Focus on Egfr-Mutated Non-Small Cell Lung Cancer. Cells 2022, 11, 413. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Sun, X.; Peng, X.; Cao, Y.; Zhou, Y.; Sun, Y. Adnp Promotes Neural Differentiation by Modulating Wnt/Beta-Catenin Signaling. Nat. Commun. 2020, 11, 2984. [Google Scholar] [CrossRef]
- Ren, Z.; van Andel, H.; de Lau, W.; Hartholt, R.B.; Maurice, M.M.; Clevers, H.; Kersten, M.J.; Spaargaren, M.; Pals, S.T. Syndecan-1 Promotes Wnt/Beta-Catenin Signaling in Multiple Myeloma by Presenting Wnts and R-Spondins. Blood 2018, 131, 982–994. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/Beta-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Fatima, I.; Barman, S.; Rai, R.; Thiel, K.W.W.; Chandra, V. Targeting Wnt Signaling in Endometrial Cancer. Cancers 2021, 13, 2351. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Tang, S.-C.; Wang, J.; Wang, H.; Wang, P.; Du, N.; Qin, S.; Li, G.; Xu, S.; et al. Let-7c Blocks Estrogen-Activated Wnt Signaling in Induction of Self-Renewal of Breast Cancer Stem Cells. Cancer Gene Ther. 2016, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Pasini, E.; Pastrello, C.; Angeli, M.; Baciu, C.; Abovsky, M.; Coffee, A.; Adeyi, O.; Kotlyar, M.; Jurisica, I. Estrogen Receptor 1 Inhibition of Wnt/Beta-Catenin Signaling Contributes to Sex Differences in Hepatocarcinogenesis. Front. Oncol. 2021, 11, 777834. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Gu, L.; Lin, Q.; Ma, Y.; Liu, C.; Pei, X.; Li, P.A.; Yang, Y. The Immp2l Mutation Causes Ovarian Aging through Ros-Wnt/Beta-Catenin-Estrogen Pathway: Preventive Effect of Melatonin. Endocrinology 2020, 161, bqaa119. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H.S. Reactive Oxygen Species-Induced Changes in Glucose and Lipid Metabolism Contribute to the Accumulation of Cholesterol in the Liver During Aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, C.; Yuan, Y.; Jin, S.; Zhao, J.; Zhang, W.; Liang, H.; Chen, X.; Zhang, B. Foxm1-Mediated Activation of Phospholipase D1 Promotes Lipid Droplet Accumulation and Reduces Ros to Support Paclitaxel Resistance in Metastatic Cancer Cells. Free Radic. Biol. Med. 2022, 179, 213–228. [Google Scholar] [CrossRef]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on Mcf-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef]
- Wang, L.; Zhan, J.; Huang, W. Grape Seed Proanthocyanidins Induce Apoptosis and Cell Cycle Arrest of Hepg2 Cells Accompanied by Induction of the Mapk Pathway and Nag-1. Antioxidants 2020, 9, 1200. [Google Scholar] [CrossRef]
- Verma, M.K.; Miki, Y.; Abe, K.; Nagasaki, S.; Niikawa, H.; Suzuki, S.; Kondo, T.; Sasano, H. Co-Expression of Estrogen Receptor Beta and Aromatase in Japanese Lung Cancer Patients: Gender-Dependent Clinical Outcome. Life Sci. 2012, 91, 800–808. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Norris, J.D. Connections and Regulation of the Human Estrogen Receptor. Science 2002, 296, 1642–1644. [Google Scholar] [CrossRef]
- Wohlhieter, C.A.; Richards, A.L.; Uddin, F.; Hulton, C.H.; Quintanal-Villalonga, A.; Martin, A.; de Stanchina, E.; Bhanot, U.; Asher, M.; Shah, N.S.; et al. Concurrent Mutations in Stk11 and Keap1 Promote Ferroptosis Protection and Scd1 Dependence in Lung Cancer. Cell Rep. 2020, 33, 108444. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Zhao, X.; Jiang, H.; Wu, X.; Wang, B.; Zhou, M.; Tian, M.; Shi, B.; Wang, H.; et al. Egfr Modulates Monounsaturated Fatty Acid Synthesis through Phosphorylation of Scd1 in Lung Cancer. Mol. Cancer 2017, 16, 127. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic Activation of Pi3k-Akt-Mtor Signaling Suppresses Ferroptosis Via Srebp-Mediated Lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Katoh, Y.; Yaguchi, T.; Kubo, A.; Iwata, T.; Morii, K.; Kato, D.; Ohta, S.; Satomi, R.; Yamamoto, Y.; Oyamada, Y.; et al. Inhibition of Stearoyl-Coa Desaturase 1 (Scd1) Enhances the Antitumor T Cell Response through Regulating Beta-Catenin Signaling in Cancer Cells and Er Stress in T Cells and Synergizes with Anti-Pd-1 Antibody. J. Immunother. Cancer 2022, 10, e004616. [Google Scholar] [CrossRef]
- She, K.; Fang, S.; Du, W.; Fan, X.; He, J.; Pan, H.; Huang, L.; He, P.; Huang, J. Scd1 Is Required for Egfr-Targeting Cancer Therapy of Lung Cancer Via Re-Activation of Egfr/Pi3k/Akt Signals. Cancer Cell Int. 2019, 19, 103. [Google Scholar] [CrossRef]
- Brasacchio, D.; Alsop, A.E.; Noori, T.; Lufti, M.; Iyer, S.; Simpson, K.J.; Bird, P.I.; Kluck, R.M.; Johnstone, R.W.; Trapani, J.A. Epigenetic Control of Mitochondrial Cell Death through Pacs1-Mediated Regulation of Bax/Bak Oligomerization. Cell Death Differ. 2017, 24, 961–970. [Google Scholar] [CrossRef]


| No. of Patients (%) | SCD1 Expression | χ2 | p-Value | CYP19A1 Expression | χ2 | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| High | Low | High | Low | ||||||
| All | 96 | 83 | 13 | 71 | 25 | ||||
| Genders | |||||||||
| Females | 23 | 17 | 6 | 4.066 | 0.044 | 17 | 6 | 0.000 | 0.996 |
| Males | 73 | 66 | 7 | 54 | 19 | ||||
| Age | |||||||||
| <60 | 58 | 50 | 8 | 0.008 | 0.929 | 41 | 17 | 0.813 | 0.367 |
| ≥60 | 38 | 33 | 5 | 30 | 8 | ||||
| T stage | |||||||||
| 1–2 | 64 | 57 | 7 | 0.429 | 0.513 | 45 | 19 | 1.325 | 0.249 |
| 3–4 | 32 | 27 | 5 | 26 | 6 | ||||
| N stage | |||||||||
| N0–N1 | 38 | 33 | 5 | 2.796 | 0.094 | 25 | 13 | 2.179 | 0.139 |
| N2–N3 | 58 | 42 | 16 | 46 | 12 | ||||
| TNM stage | |||||||||
| I–II | 27 | 25 | 2 | 1.207 | 0.271 | 18 | 9 | 1.812 | 0.178 |
| III–IV | 69 | 58 | 11 | 55 | 14 | ||||
| Tumor histology | |||||||||
| SQC | 44 | 39 | 5 | 0.676 | 0.411 | 30 | 14 | 2.013 | 0.155 |
| ADC | 52 | 43 | 9 | 42 | 10 | ||||
| Tumor differentiation | |||||||||
| Well/moderate | 69 | 60 | 9 | 0.066 | 0.796 | 52 | 17 | 0.251 | 0.616 |
| Poor | 27 | 24 | 3 | 19 | 8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, Y.; Meng, W.; Zhao, R.; Lin, W.; Xiao, H.; Liao, Y. Stearoyl-CoA Desaturases1 Accelerates Non-Small Cell Lung Cancer Metastasis by Promoting Aromatase Expression to Improve Estrogen Synthesis. Int. J. Mol. Sci. 2023, 24, 6826. https://doi.org/10.3390/ijms24076826
Chen J, Wang Y, Meng W, Zhao R, Lin W, Xiao H, Liao Y. Stearoyl-CoA Desaturases1 Accelerates Non-Small Cell Lung Cancer Metastasis by Promoting Aromatase Expression to Improve Estrogen Synthesis. International Journal of Molecular Sciences. 2023; 24(7):6826. https://doi.org/10.3390/ijms24076826
Chicago/Turabian StyleChen, Jiaping, Yangwei Wang, Wangyang Meng, Rong Zhao, Wei Lin, Han Xiao, and Yongde Liao. 2023. "Stearoyl-CoA Desaturases1 Accelerates Non-Small Cell Lung Cancer Metastasis by Promoting Aromatase Expression to Improve Estrogen Synthesis" International Journal of Molecular Sciences 24, no. 7: 6826. https://doi.org/10.3390/ijms24076826
APA StyleChen, J., Wang, Y., Meng, W., Zhao, R., Lin, W., Xiao, H., & Liao, Y. (2023). Stearoyl-CoA Desaturases1 Accelerates Non-Small Cell Lung Cancer Metastasis by Promoting Aromatase Expression to Improve Estrogen Synthesis. International Journal of Molecular Sciences, 24(7), 6826. https://doi.org/10.3390/ijms24076826