Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-ε-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering
Abstract
1. Introduction
2. Results
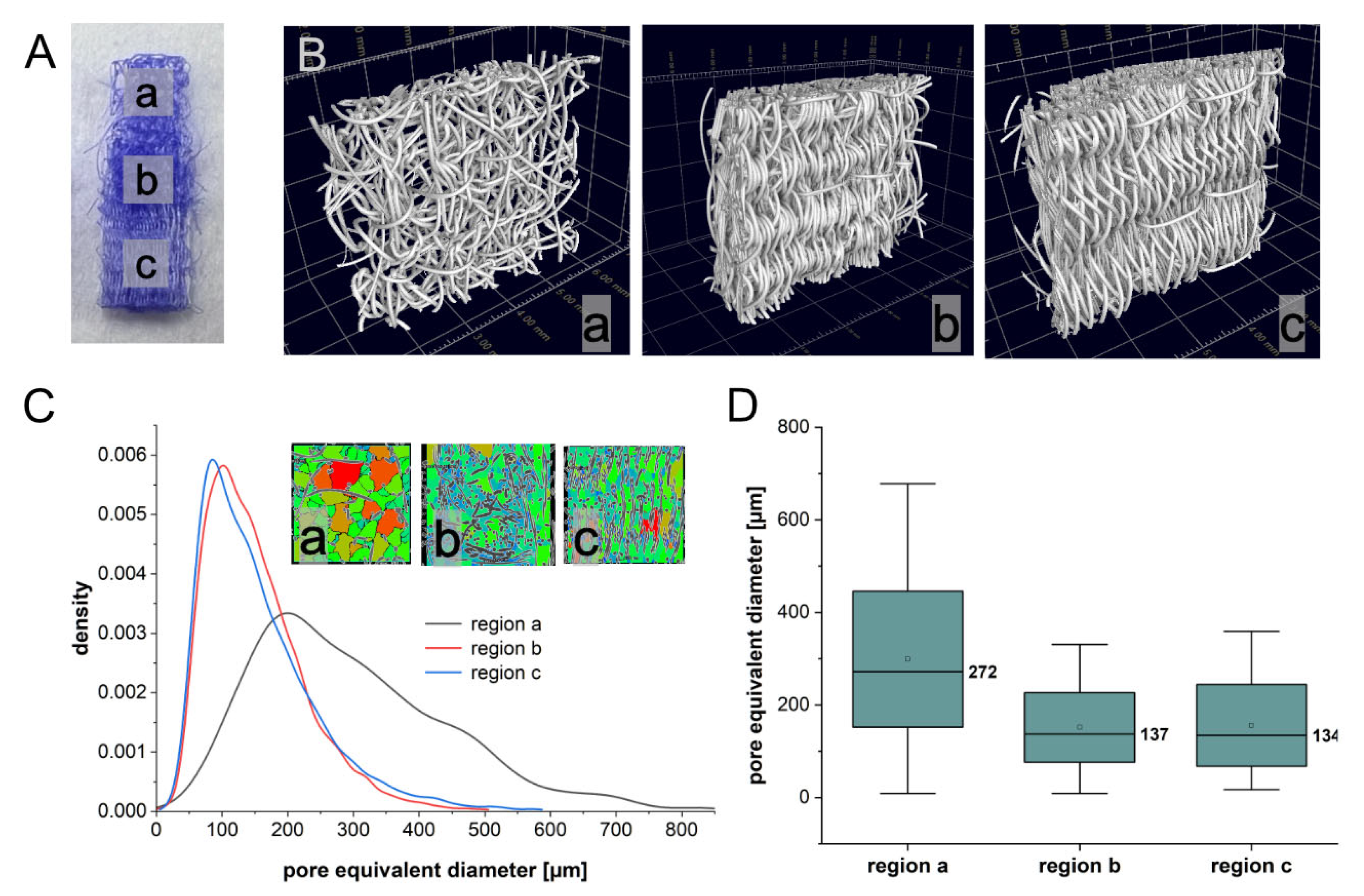
2.1. Porosity of Triphasic Embroidered Scaffolds
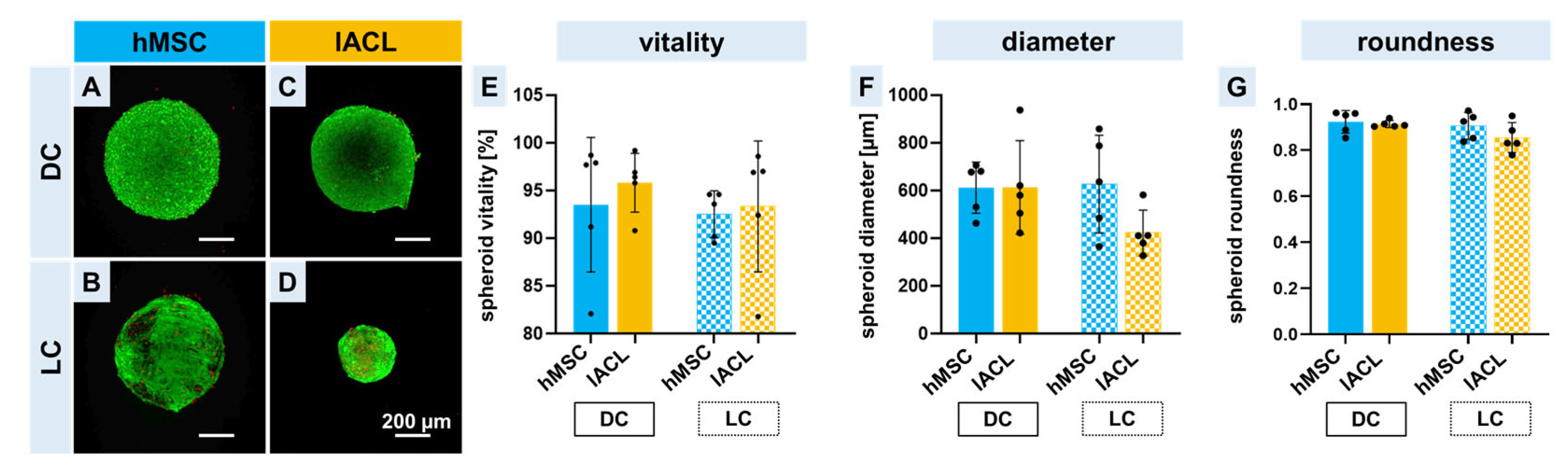
2.2. Spheroid Formation, Vitality and Changes in Size and Shape
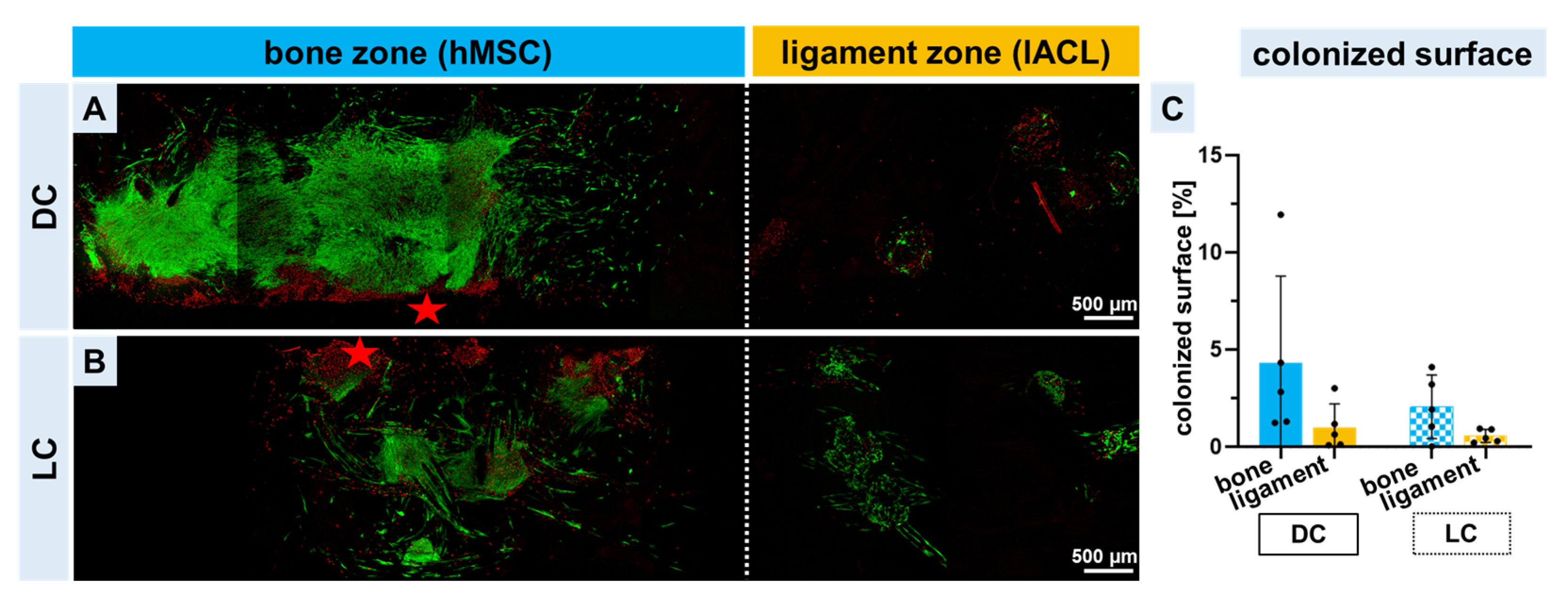
2.3. Vitality of Co-Colonized Scaffolds
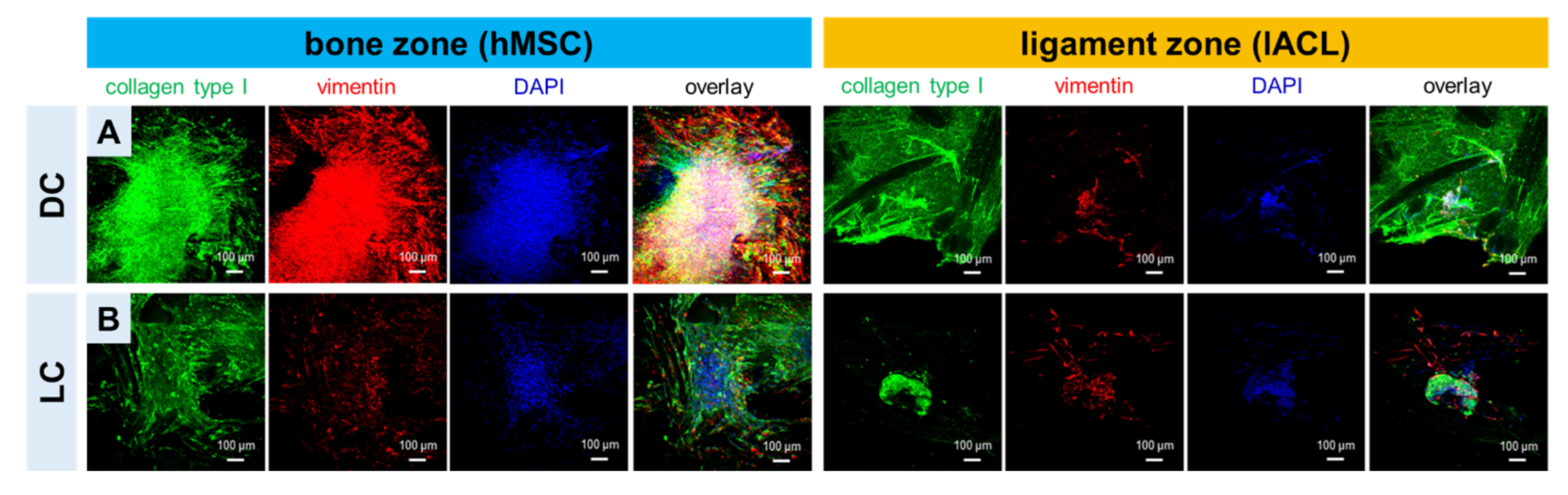
2.4. Expression of Collagen Type I and Vimentin of hMSCs and lACLs in Bone and Ligament Scaffold Zones and Cell Proliferation
2.5. DNA and Sulphated Glycosaminoglycan Contents in Bone and Ligament Scaffold Zones
2.6. Relative Gene Expression of Zonal Specific Extracellular Matrix Components and Transcription Factors
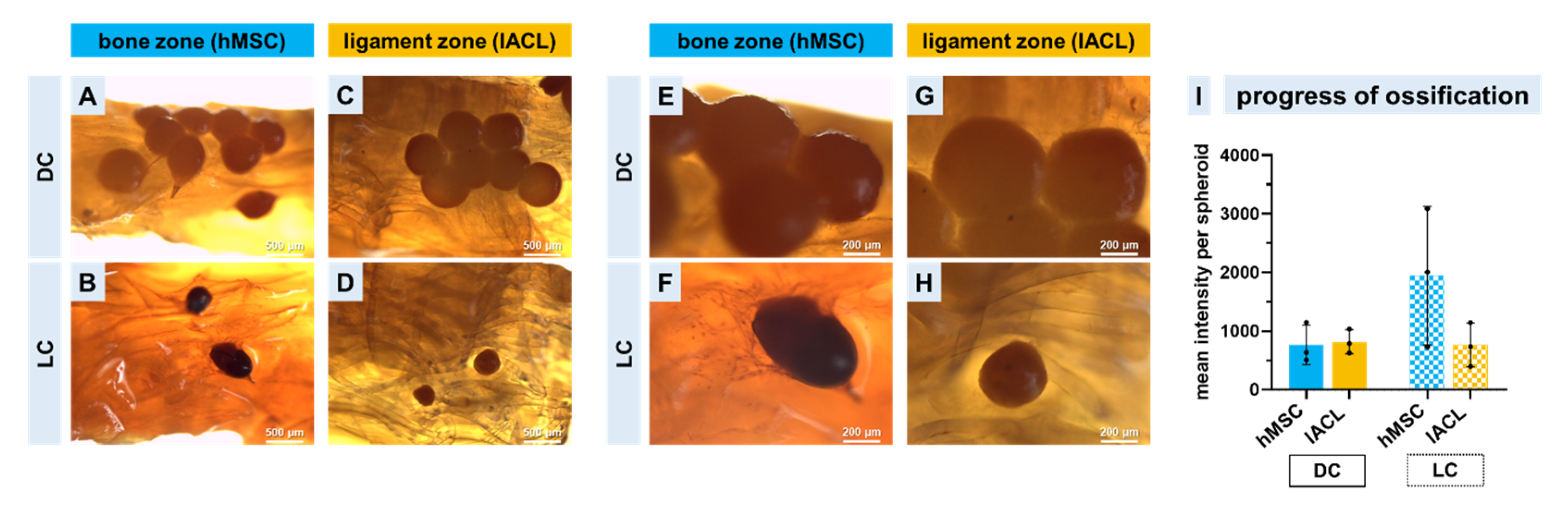
2.7. Progression of Calcium Deposition
3. Discussion
4. Materials and Methods
4.1. Preparation of Embroidered P(LA-CL)/PLA Triphasic Scaffolds
4.2. Determination of the Porosity Using Computed Tomography
4.3. Functionalization of Embroidered Scaffolds
4.4. hMSC Isolation
4.5. lACL Isolation
4.6. Spheroid Preparation and Long-Time Pre-Cultivation
4.7. Triphasic Scaffold Colonization
4.8. Cell Survival
4.9. Immunocytochemistry
4.10. Quantitative Measurement of Total DNA and sGAG Content
4.11. RNA Isolation
4.12. Relative Gene Expression
4.13. Alizarin Red Staining
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulmer, M.G.; Imhoff, A.B. Bandverletzungen am Kniegelenk. Orthop. Unf. up2date 2006, 1, 303–324. [Google Scholar] [CrossRef]
- Gianotti, S.M.; Marshall, S.W.; Hume, P.A.; Bunt, L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A national population-based study. J. Sci. Med. Sport 2009, 12, 622–627. [Google Scholar] [CrossRef]
- Beaulieu, M.L.; Carey, G.E.; Schlecht, S.H.; Wojtys, E.M.; Ashton-Miller, J.A. On the heterogeneity of the femoral enthesis of the human ACL: Microscopic anatomy and clinical implications. J. Exp. Orthop. 2016, 3, 14. [Google Scholar] [CrossRef]
- Zhao, L.; Thambyah, A.; Broom, N.D. A multi-scale structural study of the porcine anterior cruciate ligament tibial enthesis. J. Anat. 2014, 224, 624–633. [Google Scholar] [CrossRef]
- Schneider, H. Structure of tendon attachments. Z. Anat. Entwickl. 1956, 119, 431–456. [Google Scholar] [CrossRef]
- Schlecht, S.H.; Martin, C.T.; Ochocki, D.N.; Nolan, B.T.; Wojtys, E.M.; Ashton-Miller, J.A. Morphology of Mouse Anterior Cruciate Ligament-Complex Changes Following Exercise During Pubertal Growth. J. Orthop. Res. 2019, 37, 1910–1919. [Google Scholar] [CrossRef]
- Roffino, S.; Camy, C.; Foucault-Bertaud, A.; Lamy, E.; Pithioux, M.; Chopard, A. Negative impact of disuse and unloading on tendon enthesis structure and function. Life Sci. Space Res. 2021, 29, 46–52. [Google Scholar] [CrossRef]
- Benjamin, M.; Kumai, T.; Milz, S.; Boszczyk, B.M.; Boszczyk, A.A.; Ralphs, J.R. The skeletal attachment of tendons—tendon ‘entheses’. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 931–945. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion—What development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010, 10, 35–45. [Google Scholar]
- Xu, K.; Kuntz, L.A.; Foehr, P.; Kuempel, K.; Wagner, A.; Tuebel, J.; Deimling, C.V.; Burgkart, R.H. Efficient decellularization for tissue engineering of the tendon-bone interface with preservation of biomechanics. PLoS ONE 2017, 12, e0171577. [Google Scholar] [CrossRef]
- Fan, L.; Xu, B.; Liu, N.; Wang, L. Histopathological changes in patellar tendon enthesis of rabbit induced by electrical stimulation intensity. J. Orthop. Sci. 2019, 25, 344–348. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.G.; Delgado-Calcares, M.; Stange, R.; Wildemann, B.; Docheva, D. Tendon healing: A concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon. Bone Jt. Res. 2022, 11, 561–574. [Google Scholar] [CrossRef]
- Paschos, N.K.; Howell, S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016, 1, 398–408. [Google Scholar] [CrossRef]
- Chen, T.; Wang, H.; Warren, R.; Maher, S. Loss of ACL function leads to alterations in tibial plateau common dynamic contact stress profiles. J. Biomech. 2017, 61, 275–279. [Google Scholar] [CrossRef]
- Balke, M.; Metzlaff, S.; Faber, S.; Niethammer, T.; Roessler, P.P.; Henkelmann, R.; Diermeier, T.; Kurme, A.; Winkler, P.W.; Colcuc, S.; et al. Posterior meniscus root tears. Orthopade 2021, 50, 1039–1050. [Google Scholar] [CrossRef]
- Fowler, P.J. Bone injuries associated with anterior cruciate ligament disruption. Arthrosc. J. Arthrosc. Relat. Surg. 1994, 10, 453–460. [Google Scholar] [CrossRef]
- Heilmeier, U.; Mamoto, K.; Amano, K.; Eck, B.; Tanaka, M.; Bullen, J.; Schwaiger, B.; Huebner, J.; Stabler, T.; Kraus, V.; et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthr. Cartil. 2019, 28, 82–91. [Google Scholar] [CrossRef]
- Willinger, L.; Balendra, G.; Pai, V.; Lee, J.; Mitchell, A.; Jones, M.; Williams, A. High incidence of superficial and deep medial collateral ligament injuries in ‘isolated’ anterior cruciate ligament ruptures: A long overlooked injury. Knee Surgery, Sports Traumatol. Arthrosc. 2021, 30, 167–175. [Google Scholar] [CrossRef]
- Thaunat, M.; Fayard, J.M.; Sonnery-Cottet, B. Hamstring tendons or bone-patellar tendon-bone graft for anterior cruciate ligament reconstruction? Orthop. Traumatol. Surg. Res. 2018, 105, S89–S94. [Google Scholar] [CrossRef]
- Jia, Z.-Y.; Zhang, C.; Cao, S.-Q.; Xue, C.-C.; Liu, T.-Z.; Huang, X.; Xu, W.-D. Comparison of artificial graft versus autograft in anterior cruciate ligament reconstruction: A meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Bakirci, E.; Guenat, O.; Ahmad, S.; Gantenbein, B. Tissue engineering approaches for the repair and regeneration of the anterior cruciate ligament: Towards 3D bioprinted ACL-on-chip. Eur. Cells Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, G.; Placella, G.; Sebastiani, E.; Tei, M.M.; Speziali, A.; Manfreda, F. ACL Reconstruction: Choosing the Graft. Joints 2013, 1, 18–24. [Google Scholar] [PubMed]
- Legnani, C.; Ventura, A.; Terzaghi, C.; Borgo, E.; Albisetti, W. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int. Orthop. 2010, 34, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, S.J.; Devitt, B.M.; Porter, T.; Hartwig, T.; Klemm, H.; Hookway, S.; Norsworthy, C.J. Primary ACL reconstruction using the LARS device is associated with a high failure rate at minimum of 6-year follow-up. Knee Surgery Sports Traumatol. Arthrosc. 2019, 27, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Sun, Y.; Lin, J.; Liu, S.; Chen, J. A Synthetic Graft With Multilayered Co-Electrospinning Nanoscaffolds for Bridging Massive Rotator Cuff Tear in a Rat Model. Am. J. Sports Med. 2020, 48, 1826–1836. [Google Scholar] [CrossRef]
- No, Y.J.; Castilho, M.; Ramaswamy, Y.; Zreiqat, H. Role of Biomaterials and Controlled Architecture on Tendon/Ligament Repair and Regeneration. Adv. Mater. 2019, 32, e1904511. [Google Scholar] [CrossRef]
- Tits, A.; Plougonven, E.; Blouin, S.; Hartmann, M.A.; Kaux, J.-F.; Drion, P.; Fernandez, J.; van Lenthe, G.H.; Ruffoni, D. Local anisotropy in mineralized fibrocartilage and subchondral bone beneath the tendon-bone interface. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef]
- Han, S.; Kim, J.; Lee, G.; Kim, D. Mechanical Properties of Materials for Stem Cell Differentiation. Adv. Biosyst. 2020, 4, e2000247. [Google Scholar] [CrossRef]
- Karamuk, E.; Mayer, J.; Wintermantel, E. Sticktechnologie für medizinische Textilien und Tissue Engineering. In Medizintechnik Life Science Engineering: Interdisziplinarität Biokompatibilität Technologien Implantate Diagnostik Werkstoffe Business; Springer: Berlin/Heidelberg, Germany, 2008; pp. 777–786. [Google Scholar]
- Kajave, N.S.; Schmitt, T.; Patrawalla, N.Y.; Kishore, V. Design–Build–Validate Strategy to 3D Print Bioglass Gradients for Anterior Cruciate Ligament Enthesis Reconstruction. Tissue Eng. Part C Methods 2022, 28, 158–167. [Google Scholar] [CrossRef]
- Bai, L.; Han, Q.; Meng, Z.; Chen, B.; Qu, X.; Xu, M.; Su, Y.; Qiu, Z.; Xue, Y.; He, J.; et al. Bioprinted living tissue constructs with layer-specific, growth factor-loaded microspheres for improved enthesis healing of a rotator cuff. Acta Biomater. 2022, 154, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Criscenti, G.; Longoni, A.; Di Luca, A.; De Maria, C.; Van Blitterswijk, C.A.; Vozzi, G.; Moroni, L. Triphasic scaffolds for the regeneration of the bone–ligament interface. Biofabrication 2016, 8, 015009. [Google Scholar] [CrossRef] [PubMed]
- Olvera, D.; Sathy, B.N.; Kelly, D.J. Spatial Presentation of Tissue-Specific Extracellular Matrix Components along Electrospun Scaffolds for Tissue Engineering the Bone–Ligament Interface. ACS Biomater. Sci. Eng. 2020, 6, 5145–5161. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Dorthé, E.; Williams, A.; D’Lima, D.D. Nanofiber Scaffolds by Electrospinning for Rotator Cuff Tissue Engineering. Chonnam Med. J. 2021, 57, 13–26. [Google Scholar] [CrossRef]
- Walters, V.I.; Kwansa, A.L.; Freeman, J.W. Design and Analysis of Braid-Twist Collagen Scaffolds. Connect. Tissue Res. 2011, 53, 255–266. [Google Scholar] [CrossRef]
- Hahn, J.; Bittrich, L.; Breier, A.; Spickenheuer, A. Stress adapted embroidered meshes with a graded pattern design for abdominal wall hernia repair. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 062005. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Hahn, J.; Schulze-Tanzil, G.; Schröpfer, M.; Meyer, M.; Gögele, C.; Hoyer, M.; Spickenheuer, A.; Heinrich, G.; Breier, A. Viscoelastic Behavior of Embroidered Scaffolds for ACL Tissue Engineering Made of PLA and P(LA-CL) After In Vitro Degradation. Int. J. Mol. Sci. 2019, 20, 4655. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Kokozidou, M.; Gögele, C.; Pirrung, F.; Hammer, N.; Werner, C.; Kohl, B.; Hahn, J.; Breier, A.; Schröpfer, M.; Meyer, M.; et al. In vivo ligamentogenesis in embroidered poly(lactic-co-ε-caprolactone) / polylactic acid scaffolds functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams. Histochem. Cell. Biol. 2022, 1–18. [Google Scholar] [CrossRef]
- Schroepfer, M.; Junghans, F.; Voigt, D.; Meyer, M.; Breier, A.; Schulze-Tanzil, G.; Prade, I. Gas-Phase Fluorination on PLA Improves Cell Adhesion and Spreading. ACS Omega 2020, 5, 5498–5507. [Google Scholar] [CrossRef]
- Gögele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schröpfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-ε-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, S.; Mochizuki, Y.; Nagata, Y.; Deie, M.; Ochi, M. Tendon-Bone Insertion Repair and Regeneration Using Polyglycolic Acid Sheet in the Rabbit Rotator Cuff Injury Model. Am. J. Sports Med. 2008, 36, 1298–1309. [Google Scholar] [CrossRef]
- Fan, J.; Sun, L.; Chen, X.; Qu, L.; Li, H.; Liu, X.; Zhang, Y.; Cheng, P.; Fan, H. Implementation of a stratified approach and gene immobilization to enhance the osseointegration of a silk-based ligament graft. J. Mater. Chem. B 2017, 5, 7035–7050. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Luo, H.; Pan, J.; Cui, W.; Cheng, B.; Zhao, S.; Chen, G. Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair. J. Shoulder Elb. Surg. 2020, 30, 544–553. [Google Scholar] [CrossRef] [PubMed]
- He, S.-K.; Ning, L.-J.; Yao, X.; Hu, R.-N.; Cui, J.; Zhang, Y.; Ding, W.; Luo, J.-C.; Qin, T.-W. Hierarchically Demineralized Cortical Bone Combined With Stem Cell–Derived Extracellular Matrix for Regeneration of the Tendon-Bone Interface. Am. J. Sports Med. 2021, 49, 1323–1332. [Google Scholar] [CrossRef]
- Hoyer, M.; Meier, C.; Kohl, B.; Lohan, A.; Kokozidou, M.; Schulze-Tanzil, G. Histological and biochemical characteristics of the rabbit anterior cruciate ligament in comparison to potential autografts. Histol. Histopathol. 2016, 31, 867–877. [Google Scholar] [CrossRef]
- Nelson, I.R.; Chen, J.; Love, R.; Davis, B.R.; Maletis, G.B.; Funahashi, T.T. A comparison of revision and rerupture rates of ACL reconstruction between autografts and allografts in the skeletally immature. Knee Surgery Sports Traumatol. Arthrosc. 2016, 24, 773–779. [Google Scholar] [CrossRef]
- Tellado, S.F.; Bonani, W.; Balmayor, E.R.; Foehr, P.; Motta, A.; Migliaresi, C.; Van Griensven, M. Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 859–872. [Google Scholar] [CrossRef]
- Silva, C.J.P.; Müller, S.A.; Quirk, N.; Poh, P.S.P.; Mayer, C.; Motta, A.; Migliaresi, C.; Coenen, M.J.; Evans, C.H.; Balmayor, E.R.; et al. Enthesis Healing Is Dependent on Scaffold Interphase Morphology—Results from a Rodent Patellar Model. Cells 2022, 11, 1752. [Google Scholar] [CrossRef]
- Chen, C.; Shi, Q.; Li, M.; Chen, Y.; Zhang, T.; Xu, Y.; Liao, Y.; Ding, S.; Wang, Z.; Li, X.; et al. Engineering an enthesis-like graft for rotator cuff repair: An approach to fabricate highly biomimetic scaffold capable of zone-specifically releasing stem cell differentiation inducers. Bioact. Mater. 2022, 16, 451–471. [Google Scholar] [CrossRef]
- Nowlin, J.; A Bismi, M.; Delpech, B.; Dumas, P.; Zhou, Y.; Tan, G.Z. Engineering the hard–soft tissue interface with random-to-aligned nanofiber scaffolds. Nanobiomedicine 2018, 5, 1849543518803538. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; Bottino, M.C. Advanced biomaterials for periodontal tissue regeneration. Genesis 2022, 60, e23501. [Google Scholar] [CrossRef]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P. Multiphasic Scaffolds for Periodontal Tissue Engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Macovei, G.; Stoleriu, S.; Martu, M.-A.; Luchian, I.; Kappenberg-Nitescu, D.-C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Guo, J.L.; Melchiorri, A.; Mikos, A.G. Three-dimensional printing of multilayered tissue engineering scaffolds. Mater. Today 2018, 21, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Gögele, C.; Konrad, J.; Hahn, J.; Breier, A.; Schröpfer, M.; Meyer, M.; Merkel, R.; Hoffmann, B.; Schulze-Tanzil, G. Maintenance of Ligament Homeostasis of Spheroid-Colonized Embroidered and Functionalized Scaffolds after 3D Stretch. Int. J. Mol. Sci. 2021, 22, 8204. [Google Scholar] [CrossRef]
- Kruize, C.P.; Panahkhahi, S.; Putra, N.E.; Diaz-Payno, P.; van Osch, G.; Zadpoor, A.A.; Mirzaali, M.J. Biomimetic Approaches for the Design and Fabrication of Bone-to-Soft Tissue Interfaces. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Frenkel, D.; Ginsbury, E.; Sharabi, M. The Mechanics of Bioinspired Stiff-to-Compliant Multi-Material 3D-Printed Interfaces. Biomimetics 2022, 7, 170. [Google Scholar] [CrossRef]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Spalazzi, J.P.; Dagher, E.; Doty, S.B.; Guo, X.E.; Rodeo, S.A.; Lu, H.H. In vivo evaluation of a multiphased scaffold designed for orthopaedic interface tissue engineering and soft tissue-to-bone integration. J. Biomed. Mater. Res. Part A 2008, 86A, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barajaa, M.A.; Nair, L.S.; Laurencin, C.T. Bioinspired Scaffold Designs for Regenerating Musculoskeletal Tissue Interfaces. Regen. Eng. Transl. Med. 2019, 6, 451–483. [Google Scholar] [CrossRef]
- Iannucci, L.E.; Boys, A.J.; McCorry, M.C.; Estroff, L.A.; Bonassar, L.J. Cellular and Chemical Gradients to Engineer the Meniscus-to-Bone Insertion. Adv. Healthc. Mater. 2018, 8, e1800806. [Google Scholar] [CrossRef]
- Cooper, J.O.; Bumgardner, J.D.; Cole, J.A.; Smith, R.A.; Haggard, W.O. Co-cultured tissue-specific scaffolds for tendon/bone interface engineering. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhou, Y.; Wang, S.; Wu, Y. Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J. Cell. Mol. Med. 2014, 18, 2009–2019. [Google Scholar] [CrossRef]
- Lauschke, V.M.; Hendriks, D.F.; Bell, C.C.; Andersson, T.B.; Ingelman-Sundberg, M. Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Chem. Res. Toxicol. 2016, 29, 1936–1955. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Zhao, D.; Li, Y.; Cheong, S.S.; Han, D.; Li, Q. Three-dimensional printed multiphasic scaffolds with stratified cell-laden gelatin methacrylate hydrogels for biomimetic tendon-to-bone interface engineering. J. Orthop. Transl. 2020, 23, 89–100. [Google Scholar] [CrossRef]
- Lui, H.; Bindra, R.; Baldwin, J.; Ivanovski, S.; Vaquette, C. Additively Manufactured Multiphasic Bone–Ligament–Bone Scaffold for Scapholunate Interosseous Ligament Reconstruction. Adv. Healthc. Mater. 2019, 8, e1900133. [Google Scholar] [CrossRef]
- Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, G.C.J.; Cui, X.; Durham, M.; Veenendaal, L.; Schon, B.S.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Probing Multicellular Tissue Fusion of Cocultured Spheroids—A 3D-Bioassembly Model. Adv. Sci. 2021, 8, e2103320. [Google Scholar] [CrossRef]
- Hsieh, H.-Y.; Yao, C.-C.; Hsu, L.-F.; Tsai, L.-H.; Jeng, J.-H.; Young, T.-H.; Chen, Y.-J. Biological properties of human periodontal ligament cell spheroids cultivated on chitosan and polyvinyl alcohol membranes. J. Formos. Med. Assoc. 2022, 121, 2191–2202. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, Y.; Zelzer, E.; Leong, K.W.; Thomopoulos, S. A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell 2022, 29, 1669–1684.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kulkarni, A.; Soraru, G.D.; Pearce, J.M.; Motta, A. 3D Printed SiOC(N) Ceramic Scaffolds for Bone Tissue Regeneration: Improved Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 13676. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhang, Q.; Zhu, Y.; Wang, S.; Lv, J.; Sun, J.; Qiu, P.; Fan, S.; Jin, K.; Chen, L.; et al. Preparation of Decellularized Triphasic Hierarchical Bone-Fibrocartilage-Tendon Composite Extracellular Matrix for Enthesis Regeneration. Adv. Healthc. Mater. 2019, 8, e1900831. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zou, J.; Wang, W.; Yang, A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol. Med. Rep. 2020, 23, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wu, B.; Fan, S.; Liu, Y.; Ma, X.; Fu, X. SNHG14 induces osteogenic differentiation of human stromal (mesenchymal) stem cells in vitro by downregulating miR-2861. BMC Musculoskelet. Disord. 2020, 21, 525. [Google Scholar] [CrossRef]
- Haddad-Weber, M.; Prager, P.; Kunz, M.; Seefried, L.; Jakob, F.; Murray, M.M.; Evans, C.H.; Nöth, U.; Steinert, A.F. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy 2010, 12, 505–513. [Google Scholar] [CrossRef]
- Su, X.; Wang, J.; Kang, H.; Bao, G.; Liu, L. Effects of dynamic radial tensile stress on fibrocartilage differentiation of bone marrow mesenchymal stem cells. Biomed. Eng. Online 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Z.; Jiang, D.; Ding, J.-X.; Wang, S.-J.; Zhang, L.; Zhang, J.-Y.; Qi, Y.-S.; Chen, X.-S.; Yu, J.-K. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 2016, 43, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Twomey-Kozak, J.; Jayasuriya, C.T. Meniscus Repair and Regeneration: A Systematic Review from a Basic and Translational Science Perspective. Clin. Sports Med. 2020, 39, 125–163. [Google Scholar] [CrossRef]
- Gomes, M.E.; Holtorf, H.L.; Reis, R.L.; Mikos, A.G. Influence of the Porosity of Starch-Based Fiber Mesh Scaffolds on the Proliferation and Osteogenic Differentiation of Bone Marrow Stromal Cells Cultured in a Flow Perfusion Bioreactor. Tissue Eng. 2006, 12, 801–809. [Google Scholar] [CrossRef]
- Yao, Y.-T.; Yang, Y.; Ye, Q.; Cao, S.-S.; Zhang, X.-P.; Zhao, K.; Jian, Y. Effects of pore size and porosity on cytocompatibility and osteogenic differentiation of porous titanium. J. Mater. Sci. Mater. Med. 2021, 32, 72. [Google Scholar] [CrossRef]
- Kuo, C.K.; Tuan, R.S. Mechanoactive Tenogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2008, 14, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Zahn, I.; Braun, T.; Gögele, C.; Schulze-Tanzil, G. Minispheroids as a Tool for Ligament Tissue Engineering: Do the Self-Assembly Techniques and Spheroid Dimensions Influence the Cruciate Ligamentocyte Phenotype? Int. J. Mol. Sci. 2021, 22, 11011. [Google Scholar] [CrossRef]
- Hoyer, M.; Meier, C.; Breier, A.; Hahner, J.; Heinrich, G.; Drechsel, N.; Meyer, M.; Rentsch, C.; Garbe, L.-A.; Ertel, W.; et al. In vitro characterization of self-assembled anterior cruciate ligament cell spheroids for ligament tissue engineering. Histochem. Cell Biol. 2014, 143, 289–300. [Google Scholar] [CrossRef]
- Hua, R.; Ni, Q.; Eliason, T.D.; Han, Y.; Gu, S.; Nicolella, D.P.; Wang, X.; Jiang, J.X. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020, 94, 95–109. [Google Scholar] [CrossRef]
- Halper, J. Proteoglycans and Diseases of Soft Tissues. Adv. Exp. Med. Biol. 2013, 802, 49–58. [Google Scholar] [CrossRef]
- Spalazzi, J.P.; Doty, S.B.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Development of Controlled Matrix Heterogeneity on a Triphasic Scaffold for Orthopedic Interface Tissue Engineering. Tissue Eng. 2006, 12, 3497–3508. [Google Scholar] [CrossRef]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Coffman, J.A. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol. Int. 2003, 27, 315–324. [Google Scholar] [CrossRef]
- Li, W.-J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef]
- Calejo, I.; Costa-Almeida, R.; Gonçalves, A.I.; Berdecka, D.; Reis, R.L.; Gomes, M.E. Bi-directional modulation of cellular interactions in an in vitro co-culture model of tendon-to-bone interface. Cell Prolif. 2018, 51, e12493. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2005, 17, 319–336. [Google Scholar] [CrossRef]
- Broek, D.L.; Madri, J.; Eikenberry, E.F.; Brodsky, B. Characterization of the tissue form of type V collagen from chick bone. J. Biol. Chem. 1985, 260, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Guille, M.M.G.; Mosser, G.; Helary, C.; Eglin, D. Bone matrix like assemblies of collagen: From liquid crystals to gels and biomimetic materials. Micron 2005, 36, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Soraya, Z.; Ghollasi, M.; Halabian, R.; Eftekhari, E.; Tabasi, A.; Salimi, A. Donepezil hydrochloride as a novel inducer for osteogenic differentiation of mesenchymal stem cells on PLLA scaffolds in vitro. Biotechnol. J. 2021, 16, 2100112. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Tillmann, B. Anatomy and function of the anterior cruciate ligament. Orthopade 2002, 31, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Tendon Injury and Tendinopathy: Healing and repair. J. Bone Jt. Surg. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Ito, Y.; Toriuchi, N.; Yoshitaka, T.; Ueno-Kudoh, H.; Sato, T.; Yokoyama, S.; Nishida, K.; Akimoto, T.; Takahashi, M.; Miyaki, S.; et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 10538–10542. [Google Scholar] [CrossRef]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]


| Target | Primary Antibody | Dilution | Secondary Antibody | Dilution |
|---|---|---|---|---|
| Collagen type I | Goat anti-human, Biozol | 1:30 | Alexa Fluor 488, Donkey-anti-goat, Invitrogen AG | 1:200 |
| Vimentin | Monoclonal mouse-anti-vimentin, Dako | 1:40 | Indocarbocyanine Cy3, Donkey-anti-mouse, Invitrogen AG | 1:200 |
| F-Actin | Phalloidin-Alexa-Fluor 488, Abcam | 1:100 | ||
| Ki-67 | Mouse-anti-human, Merck | 1:30 | Donkey-anti-mouse Cy3, Invitrogen | 1:200 |
| Gene Symbol | Species | Gene Name | Amplicon Length | Assay ID | Efficacy |
|---|---|---|---|---|---|
| COL1A1 | Homo sapiens | Collagen type I alpha 1 | 66 | Hs00164004_m1 | 2.06 |
| COL10A1 | Homo sapiens | Collagen type X alpha 1 | 76 | Hs00166657_m1 | 2.15 |
| RUNX2 | Homo sapiens | Runt-related protein | 116 | Hs00231692_m1 | 1.94 |
| BAC | Homo sapiens | Beta actin | 171 | Hs99999903_m1 | 1.89 |
| COL1A1 | Oryctolagus cuniculus | Collagen type I alpha 1 | 70 | Oc03396073_g1 | 1.94 |
| TNC | Oryctolagus cuniculus | Tenascin C | 61 | Oc06726696_m1 | 1.83 |
| MKX | Oryctolagus cuniculus | Mohawk homeobox | 60 | Oc06754037_m1 | 1.83 |
| GAPDH | Oryctolagus cuniculus | glyceraldehyde 3-phosphate dehydrogenase | 82 | Oc03823402_g1 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gögele, C.; Vogt, J.; Hahn, J.; Breier, A.; Bernhardt, R.; Meyer, M.; Schröpfer, M.; Schäfer-Eckart, K.; Schulze-Tanzil, G. Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-ε-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 6714. https://doi.org/10.3390/ijms24076714
Gögele C, Vogt J, Hahn J, Breier A, Bernhardt R, Meyer M, Schröpfer M, Schäfer-Eckart K, Schulze-Tanzil G. Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-ε-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(7):6714. https://doi.org/10.3390/ijms24076714
Chicago/Turabian StyleGögele, Clemens, Julia Vogt, Judith Hahn, Annette Breier, Ricardo Bernhardt, Michael Meyer, Michaela Schröpfer, Kerstin Schäfer-Eckart, and Gundula Schulze-Tanzil. 2023. "Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-ε-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering" International Journal of Molecular Sciences 24, no. 7: 6714. https://doi.org/10.3390/ijms24076714
APA StyleGögele, C., Vogt, J., Hahn, J., Breier, A., Bernhardt, R., Meyer, M., Schröpfer, M., Schäfer-Eckart, K., & Schulze-Tanzil, G. (2023). Co-Culture of Mesenchymal Stem Cells and Ligamentocytes on Triphasic Embroidered Poly(L-lactide-co-ε-caprolactone) and Polylactic Acid Scaffolds for Anterior Cruciate Ligament Enthesis Tissue Engineering. International Journal of Molecular Sciences, 24(7), 6714. https://doi.org/10.3390/ijms24076714









