Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model
Abstract
1. Introduction
1.1. Intracerebral Hemorrhage
1.2. MR Imaging Technique for ICH
1.3. Quantitative Susceptibility Mapping
1.4. Chemical Exchange Saturation Transfer
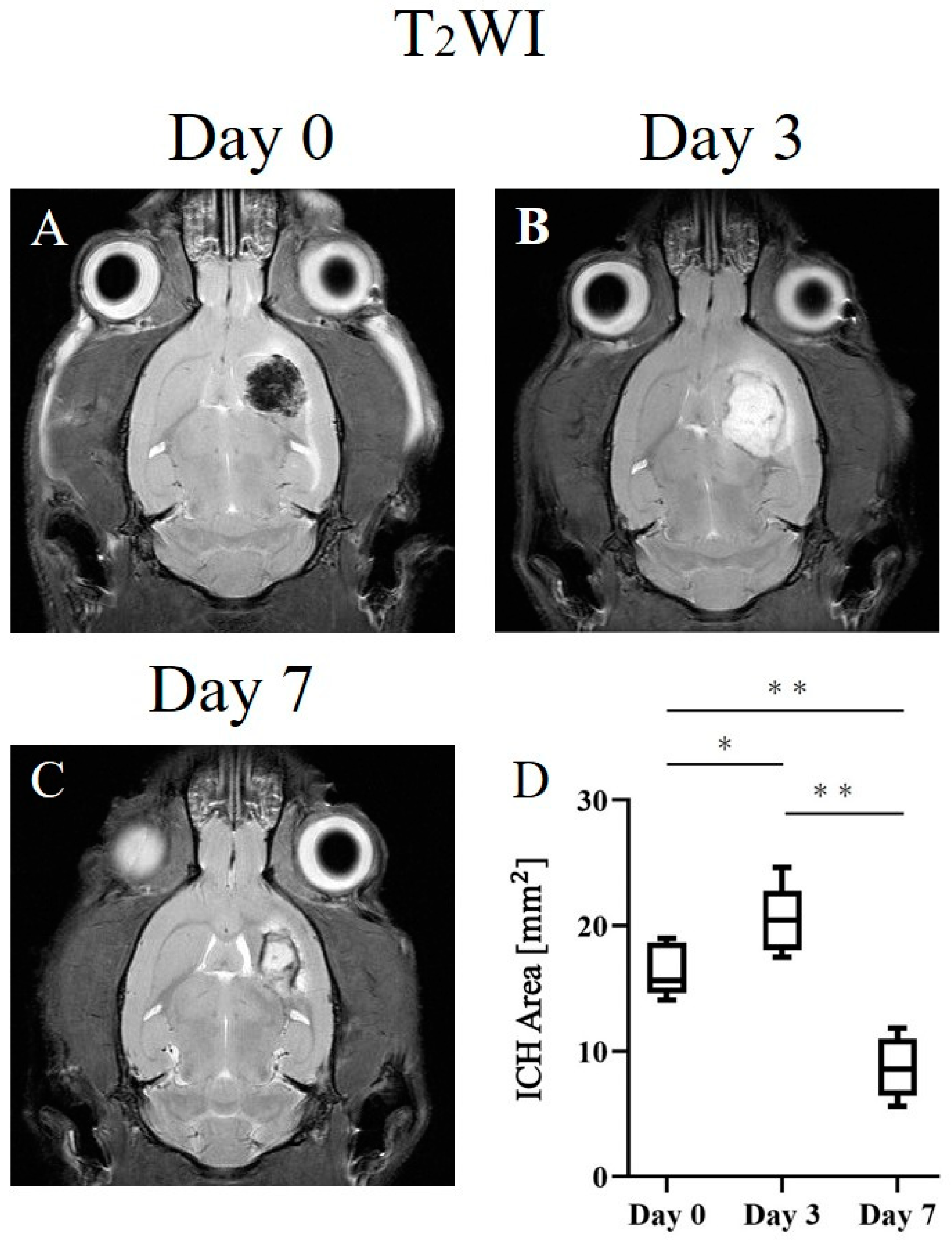
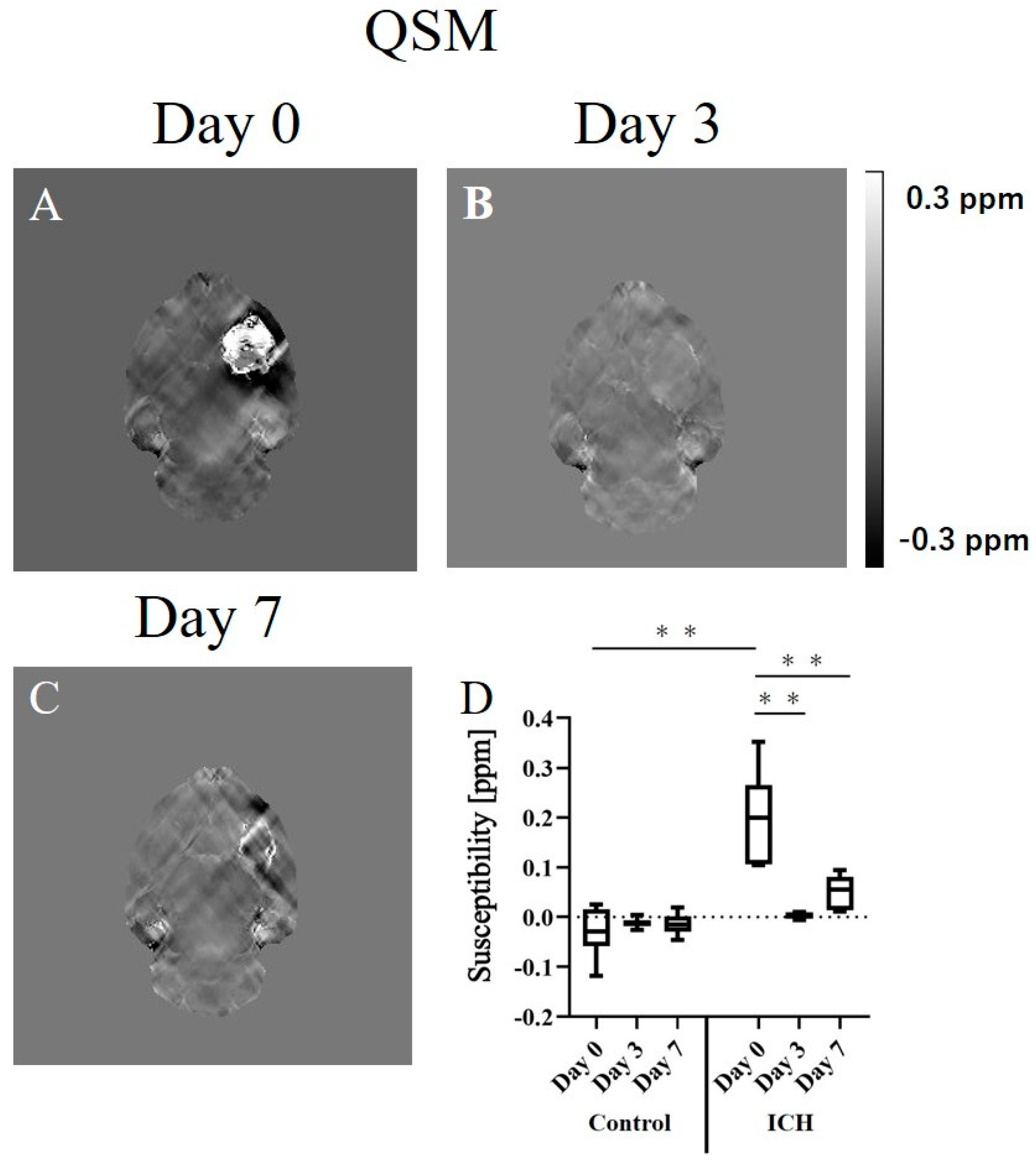
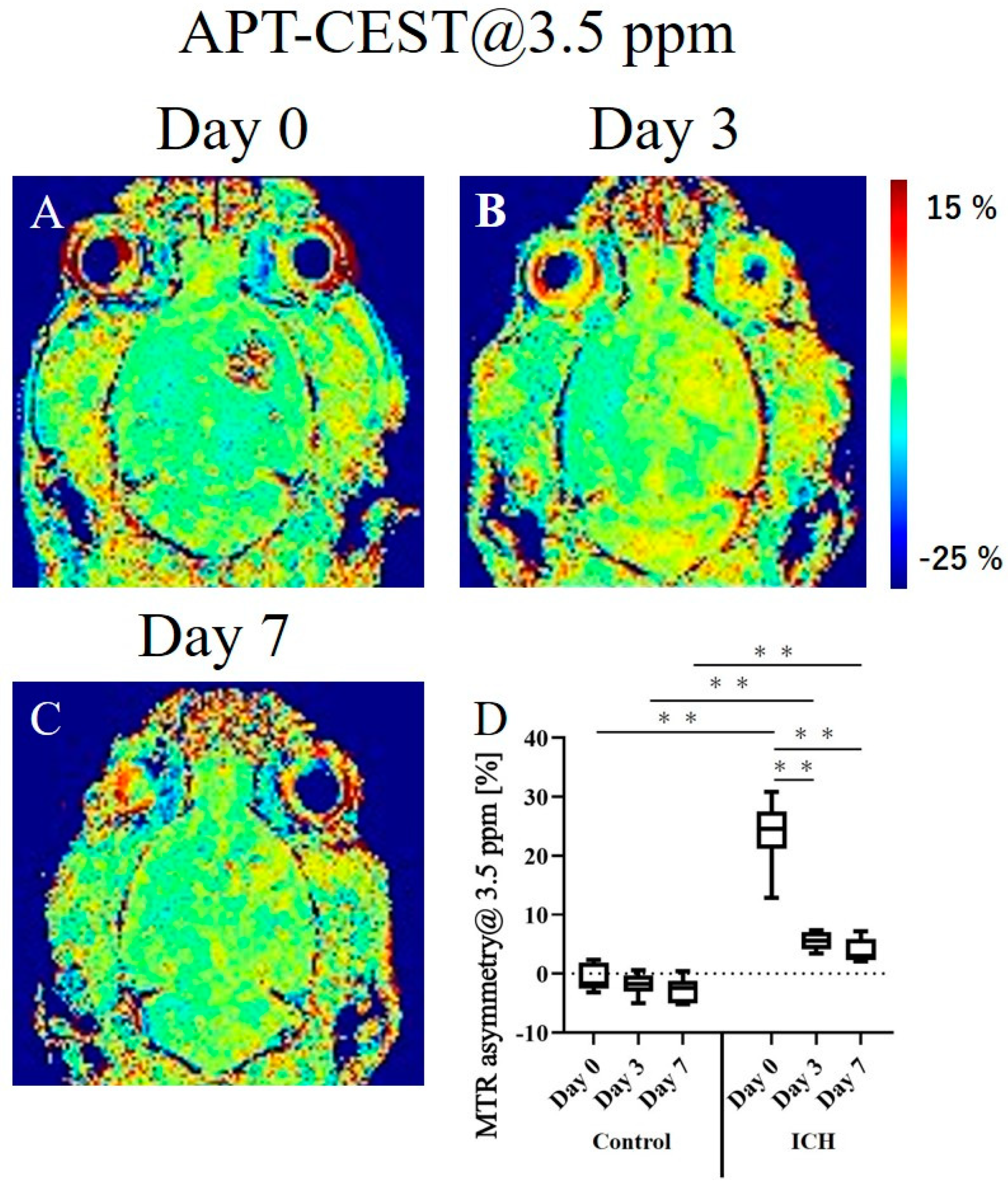
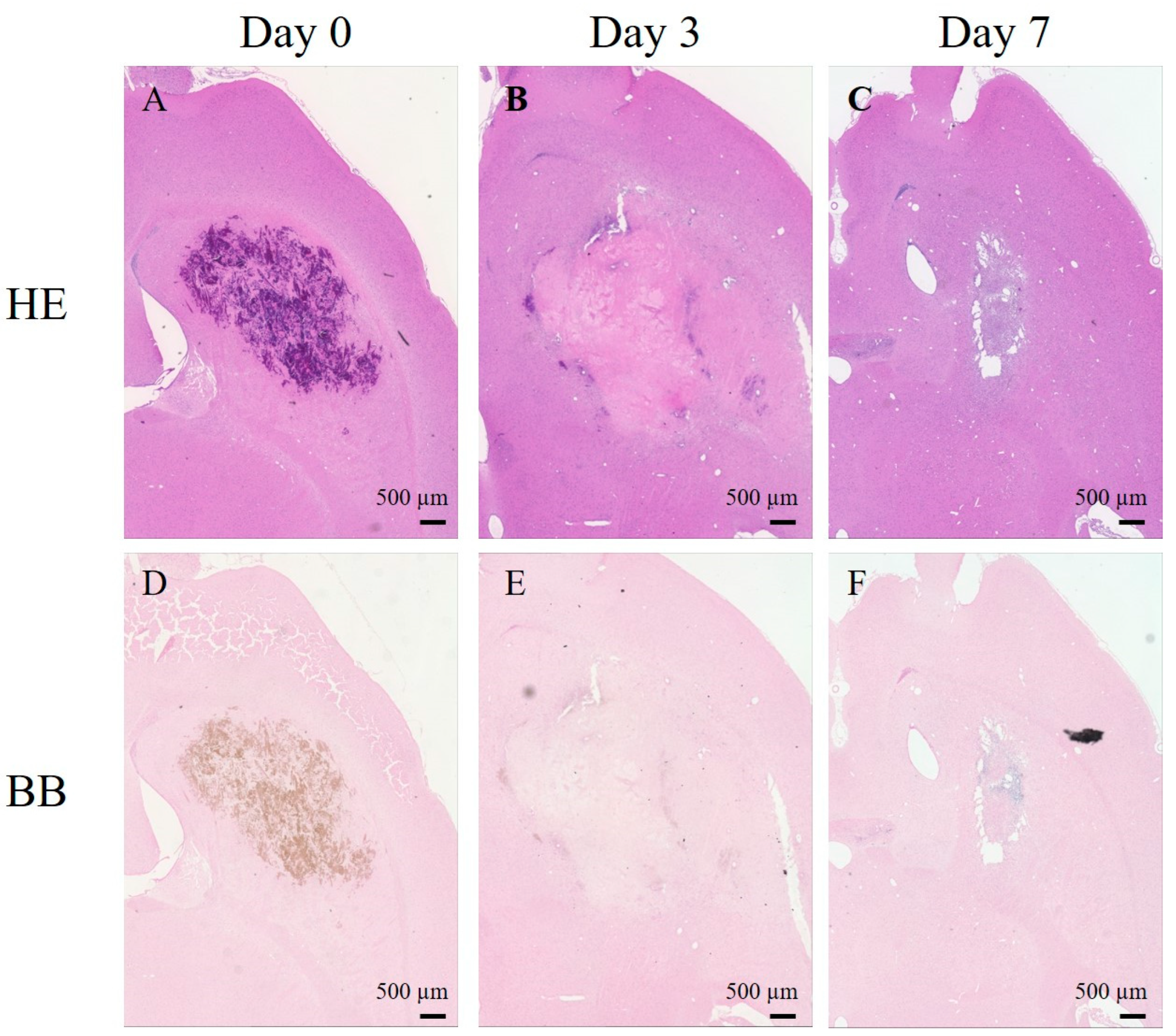
2. Results
2.1. ICH Area
2.2. Quantitative Susceptibility Mapping
2.3. APT-CEST Imaging
2.4. Histological Studies
3. Discussion
3.1. ICH Progression and Staging
3.2. ICH Area
3.3. Quantitative Susceptibility Mapping
3.4. APT-CEST Imaging
3.5. Limitations
4. Materials and Methods
4.1. Animal Preparation
4.2. MRI Examinations
4.3. Data Processing
4.4. Histopathology
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ikram, M.A.; Wieberdink, R.G.; Koudstaal, P.J. International epidemiology of intracerebral hemorrhage. Curr. Atheroscler Rep. 2012, 14, 300–306. [Google Scholar] [CrossRef]
- Sun, H.; Klahr, A.C.; Kate, M.; Gioia, L.C.; Emery, D.J.; Butcher, K.S.; Wilman, A.H. Quantitative Susceptibility Mapping for Following Intracranial Hemorrhage. Radiology 2018, 288, 830–839. [Google Scholar] [CrossRef]
- MacLellan, C.L.; Silasi, G.; Auriat, A.M.; Colbourne, F. Rodent models of intracerebral hemorrhage. Stroke 2010, 41, S95–S98. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Sun, Y.; Cronin, M.J.; He, N.; Xu, J.; Zhou, Y.; Liu, C. Quantitative susceptibility mapping (QSM) as a means to monitor cerebral hematoma treatment. J. Magn. Reson. Imaging 2018, 48, 907–915. [Google Scholar] [CrossRef]
- Bradley, W.G., Jr. MR appearance of hemorrhage in the brain. Radiology 1993, 189, 15–26. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, J.; Liu, T.; Tsiouris, A.J.; Shou, J.; Nguyen, T.; Leifer, D.; Wang, Y.; Kovanlikaya, I. Quantitative Susceptibility Mapping of Intracerebral Hemorrhages at Various Stages. J. Magn. Reson. Imaging 2016, 44, 420–425. [Google Scholar] [CrossRef]
- Kidwell, C.S.; Wintermark, M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008, 7, 256–267. [Google Scholar] [CrossRef]
- Wang, S.; Lou, M.; Liu, T.; Cui, D.; Chen, X.; Wang, Y. Hematoma volume measurement in gradient echo MRI using quantitative susceptibility mapping. Stroke 2013, 44, 2315–2317. [Google Scholar] [CrossRef]
- De, A.; Sun, H.; Emery, D.J.; Butcher, K.S.; Wilman, A.H. Quantitative susceptibility-weighted imaging in presence of strong susceptibility sources: Application to hemorrhage. Magn. Reson. Imaging 2022, 92, 224–231. [Google Scholar] [CrossRef]
- Lin, F.; Prince, M.R.; Spincemaille, P.; Wang, Y. Patents on Quantitative Susceptibility Mapping (QSM) of Tissue Magnetism. Recent Pat. Biotechnol. 2019, 13, 90–113. [Google Scholar] [CrossRef]
- Ruetten, P.P.R.; Gillard, J.H.; Graves, M.J. Introduction to Quantitative Susceptibility Mapping and Susceptibility Weighted Imaging. Br. J. Radiol. 2019, 92, 20181016. [Google Scholar] [CrossRef]
- Weerink, L.B.; Appelman, A.P.; Kloet, R.W.; Van der Hoorn, A. Susceptibility-weighted imaging in intracranial hemorrhage: Not all bleeds are black. Br. J. Radiol. 2022, 95, 20220304. [Google Scholar] [CrossRef]
- Ward, K.M.; Aletras, A.H.; Balaban, R.S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef]
- Tanoue, M.; Saito, S.; Takahashi, Y.; Araki, R.; Hashido, T.; Kioka, H.; Sakata, Y.; Yoshioka, Y. Amide proton transfer imaging of glioblastoma, neuroblastoma, and breast cancer cells on a 11.7 T magnetic resonance imaging system. Magn. Reson. Imaging 2019, 62, 181–190. [Google Scholar] [CrossRef]
- Saito, S.; Takahashi, Y.; Ohki, A.; Shintani, Y.; Higuchi, T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) with 7T MR imaging. Radiol. Phys. Technol. 2019, 12, 232–233. [Google Scholar] [CrossRef]
- Sawaya, R.; Kuribayashi, S.; Ueda, J.; Saito, S. Evaluating the Cisplatin Dose Dependence of Testicular Dysfunction Using Creatine Chemical Exchange Saturation Transfer Imaging. Diagnostics 2022, 12, 1046. [Google Scholar] [CrossRef]
- Wada, T.; Togao, O.; Tokunaga, C.; Funatsu, R.; Yamashita, Y.; Kobayashi, K.; Nakamura, Y.; Honda, H. Glycosaminoglycan chemical exchange saturation transfer in human lumbar intervertebral discs: Effect of saturation pulse and relationship with low back pain. J. Magn. Reson. Imaging 2017, 45, 863–871. [Google Scholar] [CrossRef]
- Onishi, R.; Sawaya, R.; Tsuji, K.; Arihara, N.; Ohki, A.; Ueda, J.; Hata, J.; Saito, S. Evaluation of Temozolomide Treatment for Glioblastoma Using Amide Proton Transfer Imaging and Diffusion MRI. Cancers 2022, 14, 1907. [Google Scholar] [CrossRef]
- Wang, M.; Hong, X.; Chang, C.F.; Li, Q.; Ma, B.; Zhang, H.; Xiang, S.; Heo, H.Y.; Zhang, Y.; Lee, D.H.; et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magn. Reson. Med. 2015, 74, 42–50. [Google Scholar] [CrossRef]
- Del Bigio, M.R.; Yan, H.J.; Buist, R.; Peeling, J. Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 1996, 27, 2312–2319, discussion 2319-2320. [Google Scholar] [CrossRef]
- Belayev, L.; Obenaus, A.; Zhao, W.; Saul, I.; Busto, R.; Wu, C.; Vigdorchik, A.; Lin, B.; Ginsberg, M.D. Experimental intracerebral hematoma in the rat: Characterization by sequential magnetic resonance imaging, behavior, and histopathology. Effect of albumin therapy. Brain Res. 2007, 1157, 146–155. [Google Scholar] [CrossRef]
- Liang, J.J.; Lei, L.; Zeng, Y.P.; Xiao, Z.M. High signal-intensity abnormalities in susceptibility-weighted imaging for primary intracerebral hemorrhage. Int. J. Neurosci. 2019, 129, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, W.; Tong, K.A.; Yeom, K.W.; Kuzminski, S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J. Magn. Reson. Imaging 2015, 42, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Tanaka, R.; Takeuchi, S.; Koike, T.; Minakawa, T.; Sasaki, O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J. Neurosurg. 1994, 80, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Payen, J.F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Ma, X.; Bai, Y.; Lin, Y.; Hong, X.; Liu, T.; Ma, L.; Haacke, E.M.; Zhou, J.; Wang, J.; Wang, M. Amide proton transfer magnetic resonance imaging in detecting intracranial hemorrhage at different stages: A comparative study with susceptibility weighted imaging. Sci. Rep. 2017, 7, 45696. [Google Scholar] [CrossRef]
- Jin, T.; Wang, P.; Zong, X.; Kim, S.G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn. Reson. Med. 2013, 69, 760–770. [Google Scholar] [CrossRef]
- Zhou, J.; Heo, H.Y.; Knutsson, L.; van Zijl, P.C.M.; Jiang, S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J. Magn. Reson. Imaging 2019, 50, 347–364. [Google Scholar] [CrossRef]
- Dula, A.N.; Smith, S.A.; Gore, J.C. Application of chemical exchange saturation transfer (CEST) MRI for endogenous contrast at 7 Tesla. J. Neuroimaging 2013, 23, 526–532. [Google Scholar] [CrossRef]
- Perlman, O.; Farrar, C.T.; Heo, H.Y. MR fingerprinting for semisolid magnetization transfer and chemical exchange saturation transfer quantification. NMR Biomed. 2022, e4710. [Google Scholar] [CrossRef]
- Jones, K.M.; Pollard, A.C.; Pagel, M.D. Clinical applications of chemical exchange saturation transfer (CEST) MRI. J. Magn. Reson. Imaging 2018, 47, 11–27. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Park, J.; Shin, J.I.; Kim, H.J.; Jung, D.I.; Kang, J.H.; Kim, G.; Chang, D.W.; Sur, J.H.; Yang, M.P.; et al. Temporal Evolution of MRI Characteristics in Dogs with Collagenase-Induced Intracerebral Hemorrhage. Comp. Med. 2015, 65, 517–525. [Google Scholar] [PubMed]
- Rerich, E.; Zaiss, M.; Korzowski, A.; Ladd, M.E.; Bachert, P. Relaxation-compensated CEST-MRI at 7 T for mapping of creatine content and pH--preliminary application in human muscle tissue in vivo. NMR Biomed. 2015, 28, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Windschuh, J.; Zaiss, M.; Meissner, J.E.; Paech, D.; Radbruch, A.; Ladd, M.E.; Bachert, P. Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7 T. NMR Biomed. 2015, 28, 529–537. [Google Scholar] [CrossRef]
- Chang, C.F.; Massey, J.; Osherov, A.; Angenendt da Costa, L.H.; Sansing, L.H. Bexarotene Enhances Macrophage Erythrophagocytosis and Hematoma Clearance in Experimental Intracerebral Hemorrhage. Stroke 2020, 51, 612–618. [Google Scholar] [CrossRef]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; de Rochefort, L.; Ledoux, J.; Khalidov, I.; Chen, W.; Tsiouris, A.J.; Wisnieff, C.; Spincemaille, P.; Prince, M.R.; et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012, 59, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, W.; Spincemaille, P.; Avestimehr, A.S.; Wang, Y. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE Trans. Med. Imaging 2012, 31, 816–824. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawaya, R.; Ueda, J.; Saito, S. Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model. Int. J. Mol. Sci. 2023, 24, 6627. https://doi.org/10.3390/ijms24076627
Sawaya R, Ueda J, Saito S. Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model. International Journal of Molecular Sciences. 2023; 24(7):6627. https://doi.org/10.3390/ijms24076627
Chicago/Turabian StyleSawaya, Reika, Junpei Ueda, and Shigeyoshi Saito. 2023. "Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model" International Journal of Molecular Sciences 24, no. 7: 6627. https://doi.org/10.3390/ijms24076627
APA StyleSawaya, R., Ueda, J., & Saito, S. (2023). Quantitative Susceptibility Mapping and Amide Proton Transfer-Chemical Exchange Saturation Transfer for the Evaluation of Intracerebral Hemorrhage Model. International Journal of Molecular Sciences, 24(7), 6627. https://doi.org/10.3390/ijms24076627





