Abstract
Cystic fibrosis (CF) is a serious genetic disease that leads to premature death, mainly due to impaired lung function. CF lungs are characterized by ongoing inflammation, impaired immune response, and chronic bacterial colonization. Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) are the two most predominant bacterial agents of these chronic infections. Both can colonize the lungs for years by developing host adaptation strategies. In this review, we examined the mechanisms by which SA and PA adapt to the host immune response. They are able to bypass the physical integrity of airway epithelia, evade recognition, and then modulate host immune cell proliferation. They also modulate the immune response by regulating cytokine production and by counteracting the activity of neutrophils and other immune cells. Inhibition of the immune response benefits not only the species that implements them but also other species present, and we therefore discuss how these mechanisms can promote the establishment of coinfections in CF lungs.
1. Introduction
Cystic fibrosis (CF) is a severe autosomal recessive genetic disease that affects around 70,000 patients worldwide, mostly in the Caucasian population in which the incidence reaches 1/2500 births [1]. Despite high-intensity of medical care, the mean age of death is still only 33.9 years in the U.S. in 2021 and 42.9 years in France in 2022 [2,3]. CF is caused by mutations in the gene coding for the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel located at the apical pole of epithelial cells which plays a key role in mucus homeostasis. CF is characterized by chronic inflammation of the airways due to both CFTR deficiency [4] and bacterial infection. This chronic inflammation leads to lung damage and reduced respiratory function, responsible for clinical worsening.
Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) are the most prevalent pathogens chronically colonizing CF airways [5,6]. In young CF patients, SA are the most prevalent bacteria, and then, during early adulthood, PA becomes the most prevalent bacteria which is associated with worse clinical course [7]. This kinetic is at the origin of the dogma according to which PA replaces SA. However, this hypothesis has not been confirmed by Fischer et al. [8]. It is estimated that between 8.6% to 60% of CF patients are co-colonized by the two bacteria [6,7]. In this review, we address the question of how the two major pathogens in CF can influence the host immune response and how this can promote chronic coinfections. The effects of SA and PA on host immune cells are, respectively, summarized in Table 1 and Table 2.

Table 1.
Staphylococcus aureus impacts on host immunity.

Table 2.
Pseudomonas aeruginosa impacts on host immunity.
2. Modulation of Physical Integrity of Airway Epithelium
During airway colonization and infection, the first host defense that bacteria encounter is the physical barrier constituted by the airway epithelium; in addition, airway epithelium is also responsible for mucociliary clearance. In CF patients, this mucociliary clearance is impaired due to poorly hydrated mucus, favoring bacterial adherence. PA is also able to act on this barrier by producing CFTR inhibitory factor (Cif) that induces CFTR degradation (if still present on the apical pole, which is dependent on the CF mutation [76,77]) and contributes to mucus thickening and then reinforces the impairment of mucociliary clearance [44]. PA also secrets quorum sensing (QS) molecules, such as N-3-oxododecanoyl-L-homoserine lactone (3O-C12-HSL), that induce epithelium tight junction disruption, the destruction of the adherens junction, and apoptosis in airway epithelial cells [45,78], as well as the type III secretion system (T3SS) effectors ExoS, ExoT, ExoY, and ExoU that are responsible for cytoskeleton destruction and cell retractation [78]. Additionally, ExoU is responsible for rapid eukaryotic cell death, including epithelial barriers [46]. PA also produces a protease, LasB, that contributes to the destruction of junctional proteins [78]. SA is also able to disrupt epithelial barrier via Hla production, a pore forming toxin that targets the ADAM10 receptor on epithelial cells [9]. The destruction of these cells may lead to inhibition of host immune response by reducing epithelial cell cytokine production, favoring bacterial persistence.
3. Modulation of Immune Cell Proliferation and Death
SA and PA are able to interfere with immune cells, both by activation of apoptosis or inhibition of proliferation. On the one hand, AdsA, a SA protein responsible for adenosine production by ATP, ADP, and AMP degradation, leads to deoxyadenosine formation, a metabolite responsible for caspase-3 apoptosis induction in macrophages [30,38]. AdsA, is also responsible for T cell activation and proliferation inhibition due to adenosine accumulation [15]. In parallel, staphylococcal protein A (SpA), a sortase-anchored surface protein of SA, which has high affinity for human immunoglobulins (IgA, IgD, IgG1-4, IgM and IgE), promotes B lymphocyte proliferation and their apoptotic collapse [36]. Furthermore, SA is able to kill host immune cells, such as neutrophils, by producing pore-forming toxins such as Hla, HlgAB, HlgCB, Panton–Valentine leucocidin, and other bicomponent leukocidins, leading to immune evasion [9,37]. On the other hand, PA 3O-C12-HSL and PQS molecules are able to inhibit the proliferation of peripheral blood mononuclear cells (PBMC) and mast cells [47,48], leading to reduced immune response. 3O-C12-HSL also hinders lymphocyte proliferation [54]. Likewise, ExoU, a phospholipase produced by PA, is reported to be responsible for rapid phagocyte cell death [46]; however, in a more complex model of a three-dimensional epithelial cell and macrophage co-culture, this effect was not observed [79].
In the context of CF, SA and PA often persist as biofilm which protects against antibiotics and host immune cells. Biofilms also hinder proinflammatory immune response [80]. For example PA in biofilm produces rhamnolipids when in contact with neutrophils [81,82]. These rhamnolipids induce neutrophil necrosis [66], which results in the release of proinflammatory compounds that triggers greater neutrophil recruitment, but also of DNA and actin that are used to form more biofilm [83]. In addition, Usher et al. have demonstrated that PA pyocyanin is responsible for decreased cyclic adenosine monophosphate (cAMP) concentration in neutrophils, inducing apoptosis [67]. SA in biofilms also produce toxins, such as Hla and leukicidin AB which promote immune cells death [80,84].
4. Modulation of Cytokine Levels
Once bacteria are detected by epithelial and host immune cells, pro-inflammatory cytokine secretion is triggered. PA is known as a proinflammatory cytokine inducer via its QS molecules. For example, Mayer et al. found that 3O-C12-HSL was a strong inducer of IL-6, especially in lung epithelial CF line cells (IB3-1 and CuFi) [72], confirming the role of QS molecules in triggering inflammatory response in CF patients [73]. In addition, 3O-C12-HSL is able to stimulate IL-8 production by epithelial airway cells and fibroblasts [74]. However, the effect of 3O-C12-HSL is complex as it also has an anti-inflammatory effect at elevated concentrations, decreasing IL-6, TNFα, and increasing IL-10 production by LPS-activated macrophages [52]. It also able to inhibit IL-2 produced by PBMC, TNFα produced by monocytes [47,55], and IL-12 produced by LPS-activated dendritic cells [54]. Likewise, Bortolotti et al. found that 3O-C12-HSL induces IL-10 and human leukocyte antigen-G (HLA-G), responsible for human immune response inhibition [53]. Other QS molecules, such as 4-hydroxy-2-heptilquinoline (HHQ) and 2-heptyl-3,4dihydroxyquinoline (pseudomonas quinolone signal, PQS), were found to suppress the innate immune responses in the mouse monocyte/macrophage cell line and cells in bronchoalveolar lavage via IL-6 and TNFα inhibition through the NFκB pathway [49], as well as IL-12 inhibition [54].
Thus, PA QS molecules seem to have two opposite effects on host cells: it triggers inflammatory response at low concentration, but it inhibits host immune response at high concentrations. As QS molecules were found at high concentrations in CF sputum, the anti-inflammatory effect of QS molecules is expected to play a role in host evasion and promotion of chronic infections [5]. Bedi et al. showed how PA is able to modulate host immune response by accumulation of QS molecules [85,86,87].
In addition to QS molecules, PA produces many proteins responsible for modulation of the host immunity response, such as the protease LasB that is responsible for an indirect anti-inflammatory effect by degrading pro-inflammatory cytokines such as IL-6 and IL-8 and neutrophil secreted products [50,57]. In addition to pro-inflammatory cytokine destruction, LasB was found to cleave surfactant protein A (SP-A) and D (SP-D), particularly in CF patients were SP-A and SP-B lung levels are lower [62,64]. As SP-A plays a role in opsonization and phagocytosis of numerous pathogens [63], its degradation could promote pathogens persistence inside CF lungs. As does 3O-C12-HSL, LasB may has both pro and anti-inflammatory effects. Indeed, Sun et al. have found that LasB triggers pro-IL-Iβ maturation, leading to increased IL-Iβ levels and inflammation [71]. PA also modulates host immune response via the secretion of outer membrane vesicles (OMVs) containing short interfering RNA, which led to reduction IL-8 secretion by primary human epithelial airway cells [51]. In addition, PA colonization in airways leads to PD-L1 overexpression on circulating monocytes that exhibit impaired inflammation response initiation and antigen presentation, termed endotoxin tolerance [70].
As PA, SA is also able to modulate host immunity. Chekabab et al. reported that SA reduces IL-8 production triggered by PA on immortalized airway epithelial cells (BEAS-2B) and on immortalized bronchial cells homozygous for the ΔF508 CFTR mutation CFBE41o-[16]. As IL-8 possess a predominant role in inflammation and leucocytes chemotaxis, its inhibition may be a key factor leading to chronic infections, notably in CF patients where IL-8 seems to be able to attract more leucocytes than in healthy patients [88]. IL-8 inhibition may be related to SA β-hemolysin [17] and Sae R/S two-component system (TCS), that are able to inhibit IL-8 production [18]. This reduction of IL-8 production is responsible for reduced neutrophil survival, decreased transmigration, and delayed bacterial clearance, favoring chronic infections [17,18]. SA also produces AdsA responsible for adenosine production [89], which is detected by four G-protein-coupled membrane receptors (A1, A2A, A2B, and A3), triggering anti-inflammatory signaling cascades leading to inhibition of cytokines production (Il-1a and IL-10) [15].
5. Modulation of Itaconate Immune Response
During airway infection, SA and PA induce an immunometabolic reprogramming of macrophages that results in an airway environment containing abundant immune signaling metabolites. Basically, toll-like receptor (TLR) triggering by bacteria leads to a metabolic switch from oxidative phosphorylation to glycolysis in macrophages [90]. Glycolysis, allowing more energy production, and promotes succinate, reactive oxygen species ROS, and itaconate release in the respiratory airways [91,92]. Succinate oxidation stabilizes the hypoxia-inducible transcription factor-1α (HIF-1α) that enhances IL-1β synthesis and pro-inflammatory response [90]. To limit tissue damage due to inflammation, itaconate is synthetized by myeloid cells. It suppresses succinate oxidation, leading to succinate accumulation in extracellular medium [93] (Figure 1).
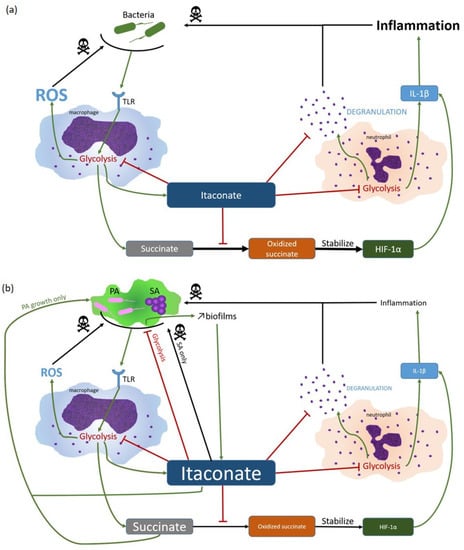
Figure 1.
Itaconate hijacking by SA and PA. (a) In normal circumstances, SA and PA are detected by TLR, leading to macrophage activation and inflammation induction via succinate oxidation. In order to modulate pro-inflammatory responses, itaconate is synthetized. Itaconate inhibits glycolysis, succinate oxidation and neutrophils degranulation, drastically reducing inflammation and protecting host cells. (b) During chronic infections, SA and PA are able to adapt and hijack host response. Despite bacterial glycolysis inhibition, itaconate induces PA growth by succinate accumulation which is used as energy source. Secondarily, itaconate leads to more EPS synthesis, enabling more biofilm formation, which in turn induces itaconate production, promoting SA PA persistency.
Itaconate has been shown to accumulate in the lungs of CF patients during both SA or PA mono- or co-infection, in function of the duration of infection/colonization [39]. It is highly expressed by myeloid cells after infection with PA [94]. Itaconate inhibits SA and PA glycolysis, leading to metabolic adaptation with increased extracellular polysaccharide (EPS) synthesis that is responsible for increased biofilm production [39,40] but which also triggers itaconate synthesis. In addition, PA isolates, after adaptation, are able to use itaconate as an energy source [39]. This use may contribute to limiting the bactericidal activity of itaconate against SA [40,95,96]. Moreover, succinate accumulation (due to presence of itaconate) induces metabolic stress in PA, increasing growth and biofilm production, both responsible for better airway colonization [97]. Ultimately, inhibition of succinate oxidation by itaconate will reduce IL-1β synthesis. In addition, itaconate decreases neutrophil degranulation and reduces inflammation by inhibiting neutrophil glycolysis through modifications of ALDOA, GAPDH, and LDHA [92,98,99,100] (Figure 1).
Thus, both SA and PA counter host immune response by hijacking itaconate metabolism, leading to bacterial host-adaptation, modifying bacterial metabolism, promoting biofilm formation, and limiting inflammation.
6. Modulation of Nutritional Immunity
Nutritional innate immunity is defined as the sequestration of essential metal ions by the host to prevent their capture by pathogens such as bacteria; without such ions, bacterial metabolism is blocked and thus the host prevents bacterial proliferation. Lactoferrin, lipocalin-2, haptoglobin, hemopexin, and calprotectin are the proteins responsible for nutritional immunity [101]. SA possesses two superoxide dismutase (SOD), SodA and SodM, essential for protection against oxidative stress, which are, respectively, manganese and manganese or iron-dependent [25,26,27]. During inflammation, host calprotectin sequesters manganese, impairing SodA activity; SodM escapes this inhibition by using iron, highlighting its importance in SA. To fight against zinc starvation, SA produces staphylopine, a metallophore enabling successful competition for zinc [41]; PA produces pseudopalin, a staphylopine analogue [102]. To counter iron starvation, SA and PA are able to modify their iron intake system; PA is able to switch from Fe3+ intake to heme intake in chronic lung infections [68,69], whereas SA prefers heme as the iron source during infection [42]. When co-cultivated in iron depleted media, PA secretes alkylhydroxyquinolones to kill SA to steal its iron [103]. However, in the presence of calprotectin, which is present at high concentrations in CF sputum and inhibits both SA and PA iron uptake [101], the anti-staphylococcal effect of PA is reduced [104]. In particular, Vermilyea et al. found that the activity of LasA and of LasB was inhibited in the presence of calprotectin [105]. Similar results were observed using mice, and in CF lung explants [104]. Thus, calprotectin tends to temper nutritive competition between SA and PA. To fight against calprotectin nutritional immunity, SA possesses two TCS: ArlRS, responsible for global virulence regulation [43], and SaeRS that is activated by calprotectin [106]. SaeRS is responsible for the regulation of more than 20 staphylococcal virulence factors, including anti-neutrophils factors [13,24]. Taken together this suggest that, in the presence of calprotectin, PA is able to modulate its anti-staphylococcal effect while SA adapts its metabolism to favor its growth in a nutriment depleted environment, thus favoring SA/PA coinfections; this may also explain why SA and PA colocalize with regions where calprotectin is highly expressed [104].
7. Evasion to Neutrophil Activities
The inflammatory response in CF airways is dominated by a massive influx of neutrophils. Neutrophil recruitment aims to regulate infection by (i) massive release of antimicrobial enzymes from granules such as myeloperoxidase, neutrophil elastase and lactoferrin, (ii) neutrophil extracellular traps (NETs), and (iii) phagocytosis. Both SA and PA are able to counteract neutrophil activities (Figure 2).
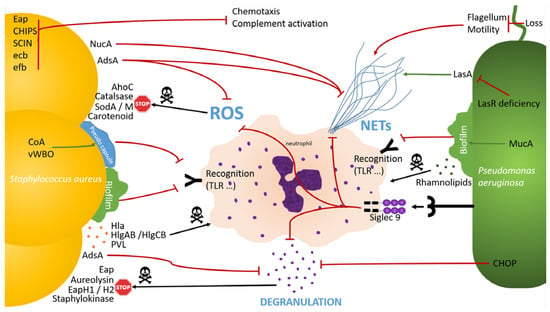
Figure 2.
SA and PA anti-neutrophils effects, phagocytosis excluded.
SA and PA are able to escape neutrophil recognition by biofilm (MucA) and pseudo capsule formation (CoA, vWBO). They are able to inhibit ROS production (Siglec 9 binding, AdsA) and to limit ROS effect (AhoC, catalase, SodA, SodM, carotenoid). SA and PA also have the ability to inhibit neutrophil degranulation (Siglec 9 binding, AdsA, CHOP) and to block neutrophils products activity (eap, aureolysin, EapH1, EapH2, staphylokinase). Finally, SA and PA are able to inhibit NETs formation (Siglec 9 binding, loss of flagellum/motility, LasR deficiency, NucA, AdsA). SA and PA may impair neutrophil response by inducing neutrophils death (γ hemolysin, PVL, rhamnolipids). Moreover, SA is able to inhibit chemotaxis and complement activation (Eap, CHIPS, SCIN, ecb, efb).
7.1. Neutrophil Recognition Evasion
To evade cellular immune responses, SA and PA are able to hide from neutrophils by producing biofilm [20], and PA mucA mutants, characterized by a high alginate production, decreased neutrophils attraction, and complement activation, are frequently isolated in CF lungs [56]. In addition to biofilm production, SA is also able to produce a pseudo capsule using its two coagulases, CoA and vWBP, impairing neutrophil access [19], and it is able to inhibit chemotaxis and complement activation with several virulence factors [extracellular adherence protein (Eap), staphylococcal complement inhibitor (SCIN), chemotaxis inhibitory protein of staphylococci (CHIPS), extracellular complement binding protein (Ecb), and extracellular fibrinogen binding protein (Efb)] leading to decreased neutrophil activity [10,11,12,13,14,20,37].
7.2. Degranulation Evasion
SA AdsA inhibits neutrophil degranulation and oxidative burst [15,21]; it also secretes three neutrophil serine protease inhibitors, namely Eap, EapH1, and EapH2, which are able to inhibit neutrophil proteases such as proteinase 3 and cathepsin G [22]. As described above, SA also secretes two super oxide dismutases, SodA and SodM, that protect itself from neutrophil-induced oxidative stress [25,26,27]. Treffon et al. found that SodM was overexpressed during CF chronic infections [26]. In addition, SA also produces a catalase, KatG, and an alkylhydroperoxide reductase, AhpC, protecting SA against hydrogen peroxide [29]. Another SA product, the carotenoid pigment, which harbors an antioxidant activity, was shown to protect SA against neutrophil killing [28]. SA is also able to protect itself against alpha-defensin via staphylokinase [24] and against the antimicrobial peptide cathelicidin LL-37 via aureolysin [23].
PA protects itself from antimicrobial peptide production via the stimulation of the UPR regulation pathway and production of CHOP as described above, although this mechanism also leads to enhanced ROS production [85]. However, PA inhibits elastase and ROS production via binding to siglec-9 at the surface of neutrophils [58]. Siglec are transmembrane proteins that bind sialylated carbohydrates on targeted cells to regulate binding, cell proliferation, cell signaling, endocytosis, and natural killer-mediated cell lysis.
7.3. NETosis Evasion
SA and PA have also developed mechanisms to counter NETosis, which is the release of NETs composed of an extracellular DNA backbone associated with antimicrobial peptides (notably calprotectin), histones and proteases by neutrophils to capture and kill bacteria [107,108]. Gray et al. found that NETosis was enhanced in CF patients, making it a critical host defense mechanism [109]. In addition to intrinsic enhanced NETosis, PA also moderately induces formation of NETs via the protease LasA [75], and more importantly by its flagellum and its motility [59]. SA also induces activation of NETs [31].
Both SA and PA have developed mechanism to escape NETosis. For instance, chronic PA strains are often LasR-deficient leading to the loss of LasA and LasB, and frequently lose its flagellum leading to reduced activation of NETs [59,60]. Another PA mechanism of NET inhibition is its binding to siglec-9 on the surface of neutrophils [58]. SA produces nuclease that is able to degrade NETs [31,32]. High nuclease-producing SA strains are selected by environments with high inflammation as seen in CF [110], and such bacteria have been reported to induce a delay in bacterial clearance and enhance mortality in an in vivo murin model of SA respiratory tract infection [32]. In addition, SA AdsA is also able to degrade NETs, and its activity is potentiated by staphylococcal nuclease [30].
8. Phagocytosis Evasion
Phagocytosis is a common strategy used by neutrophils and macrophages to eradicate bacteria. SA and PA have developed strategies to escape phagocytosis. SA AdsA is responsible for adenosine production, which downregulates phagocytosis by alveolar macrophages through A2aR/A2bR (in particular A2aR)—PKA pathways and modulation of p38 phosphorylation [33,34]. SA also produces SpA, which binds to the Fcγ domain of immunoglobulins, leading to phagocytosis inhibition [15,35]. Most SpA is cell wall-anchored, but a fraction of SpA is also secreted; Armbuster et al. demonstrated that this secreted SpA could also protect PA from phagocytosis [111]. Another SA protein, staphylococcal binder of immunoglobulin (Sbi), is also able to bind the Fcγ domain of immunoglobulins, then consumes C3 and inhibits phagocytosis [36].
In order to inhibit phagocytosis, PA is able to inject toxins inside host cells via its T3SS. Among these toxins, ExoT and ExoS, two GTPase-activating proteins, have been found to inhibit macrophage phagocytic capabilities by interfering with cytoskeletal rearrangement [46]. In addition to toxins, PA LasB is able to disarm host-protease-activated receptors 2 (PAR2), a lung inflammation regulator, inducing a reduced bacterial clearance probably through phagocytosis inhibition [65]. PA phagocytic evasion is also promoted by loss of motility, a phenotype frequently observed during chronic infections [61,112].
9. How Host Immune Response Modulation Promotes SA-PA Coinfection
As discussed above, both SA and PA possess many factors capable of modulating and inhibiting the host immune response (Table 1 and Table 2) and thus favor their persistence and the establishment of chronic infections. By different and complementary mechanisms, both species (i) inhibit the proliferation of immune cells or induce their apoptosis, (ii) inhibit the production of pro-inflammatory cytokines, and (iii) counteract the immune response by developing resistance strategies (i.e., increase in biofilm production, metabolic modification to resist oxidative stress or nutritional deficiencies, hijacking of neutrophil actions, among others). The consequent inhibition of the immune response is not specific to a species, and benefits not only the species that implements them but also the others present, potentially promoting coexistence of both pathogens in CF airways. For example, SA has been reported to demonstrate anti-inflammatory activity (reduction of IL-8 production) when co-cultivated with PA [16], suggesting cooperation between SA and PA to promote chronic infection.
Furthermore, some mechanisms developed only by one species still benefit both species. Thus, PA-induced inhibition of mucociliary clearance logically promotes SA colonization. Conversely, Wieneke et al. have found that the high nuclease producer SA were associated with PA coinfection, suggesting that nuclease, via its host immunity modulation activity is critical to promote SA-PA coinfection in CF airways [110].
The relationship between different microorganisms and the immune response may be more complex than it appears. It has been described that PA is able to highjack host immune response to outcompete other microorganisms and dominate the microenvironment. PA induces the production of sPLA2-IIA, an antimicrobial peptide, in CF epithelial cells via a T3SS-dependent process; PA inject ExoS into host cells via its T3SS, leading to Krüppel-like factor 2 (KLF2) activation, and then sPLA2-IIA production. sPLA2-IIA is found at high concentration in sputum from CF patients, sufficient to kill SA but not to kill PA [33]. Of note sPLA2-IIA reaches its highest concentration in early adulthood, when the dogma of SA colonization to PA colonization switch stands. However, SA has mechanism to resist sPLA2-IIA thanks to adenosine production that inhibits that of sPLA2-IIA [15,34], and in vivo data from a pulmonary infection of guinea pig model indicate that adenosine production decreases SA clearance from airways [34]. Therefore, this mechanism, initially expected to be responsible for SA eradication in CF airways, may finally promote SA–PA coinfections, at the expense of other microorganisms.
These different examples show how modulation of the immune response would be an actor in the establishment of coinfections, especially in CF lungs where about 40% of the patients are coinfected by these bacteria. Moreover, studies tend to show that SA/PA coinfections in CF patients lead to a less severe pulmonary condition than PA alone, suggesting that SA/PA coinfection may reduce host immune response [113,114]. However, this synergy and the idea that modulation of the immune response by one species benefits other species remains to be demonstrated by in vitro approaches.
10. Future Directions
Most of the mechanisms of immune response modulation described in this review have been explored in vitro, mainly using laboratory strains. Only a few studies have explored the behavior and impact of CF patient strains [16,26,27,31,33,40,50,56,62,69,85,97]. However, it is known that clinical strains present a significant genomic and phenotypic diversity. It would, therefore, be interesting to complete these studies using strains from CF patients. Furthermore, it has been clearly described that strains of PA [115,116,117,118,119] and SA [26,31,120,121,122,123,124] evolve during chronic infection to adapt to the lung environment and persist over time. In PA, this adaptive evolution leads to the establishment of high antibiotic resistance, increased biofilm forming capacity, slowed metabolism, and decreased virulence. In SA, this evolution is accompanied by profound metabolic changes and frequent acquisition of the small colony variant (SCV) phenotype [125], as well as improved resistance to ROS and NETs [26,31]. Thus, these evolutions may directly affect the mechanisms of immune response modulation described in this review. It would therefore be relevant to analyze longitudinally the impact of SA and PA on the host response from CF clinical strains isolated at different time points.
Finally, in this review we only looked at SA and PA interactions with the host immune system. As SA and PA are part of the CF pulmonary microbiome, we could expect that there are more interactions between the microbiome and the host immune system, which remain to be assessed.
11. Conclusions
SA and PA are able to bypass the physical integrity of airway epithelia, evade recognition, and then modulate host immune cell proliferation. They also modulate the immune response by regulating cytokine production and by counteracting the activity of neutrophils and other immune cells. Inhibition of the immune response benefits not only the species that implements them but also other species present, and therefore, it can promote the establishment of coinfections in CF lungs.
Author Contributions
A.S. and K.M. were primarily responsible for preparing the review. F.V. and A.D.-J. contributed to writing the review and editing the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the associations “Vaincre la mucoviscidose” and “Gregory Lemarchal”. Funding number are RC20190502431 and RF20220503058.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Philip Robinson from the Hospices Civils de Lyon (France) for help in manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cios, K.; Cohen, B.; Quittell, L.M.; Liu, J.; Larson, E.L. Impact of Colonizing Organism in the Respiratory Tract on the Incidence, Duration, and Time between Subsequent Hospitalizations among Patients with Cystic Fibrosis. Am. J. Infect. Control 2019, 47, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Dehillotte, C.; Lemonnier, L. Registre français de la mucoviscidose—Bilan des données 2021 Vaincre la Mucoviscidose Paris. 2022. [Google Scholar]
- Cystic Fibrosis Foundation Patient Registry. 2021 Annual Data Report Bethesda, Maryland ©2022 Cystic Fibrosis Foundation. 2022. [Google Scholar]
- Cohen, T.S.; Prince, A. Cystic Fibrosis: A Mucosal Immunodeficiency Syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Briaud, P.; Bastien, S.; Elsen, S.; Doléans-Jordheim, A.; Vandenesch, F.; Moreau, K. Trophic Cooperation Promotes Bacterial Survival of Staphylococcus Aureus and Pseudomonas Aeruginosa. ISME J. 2020, 14, 3093–3105. [Google Scholar] [CrossRef]
- Briaud, P.; Bastien, S.; Camus, L.; Boyadjian, M.; Reix, P.; Mainguy, C.; Vandenesch, F.; Doléans-Jordheim, A.; Moreau, K. Impact of Coexistence Phenotype between Staphylococcus Aureus and Pseudomonas Aeruginosa Isolates on Clinical Outcomes Among Cystic Fibrosis Patients. Front. Cell. Infect. Microbiol. 2020, 10, 266. [Google Scholar] [CrossRef]
- Hubert, D.; Réglier-Poupet, H.; Sermet-Gaudelus, I.; Ferroni, A.; Le Bourgeois, M.; Burgel, P.-R.; Serreau, R.; Dusser, D.; Poyart, C.; Coste, J. Association between Staphylococcus Aureus Alone or Combined with Pseudomonas Aeruginosa and the Clinical Condition of Patients with Cystic Fibrosis. J. Cyst. Fibros. 2013, 12, 497–503. [Google Scholar] [CrossRef]
- Fischer, A.J.; Singh, S.B.; LaMarche, M.M.; Maakestad, L.J.; Kienenberger, Z.E.; Peña, T.A.; Stoltz, D.A.; Limoli, D.H. Sustained Coinfections with Staphylococcus Aureus and Pseudomonas Aeruginosa in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 328–338. [Google Scholar] [CrossRef]
- Seilie, E.S.; Wardenburg, J.B. Staphylococcus Aureus Pore-Forming Toxins: The Interface of Pathogen and Host Complexity. Semin. Cell Dev. Biol. 2017, 72, 101–116. [Google Scholar] [CrossRef]
- Chavakis, T.; Hussain, M.; Kanse, S.M.; Peters, G.; Bretzel, R.G.; Flock, J.-I.; Herrmann, M.; Preissner, K.T. Staphylococcus Aureus Extracellular Adherence Protein Serves as Anti-Inflammatory Factor by Inhibiting the Recruitment of Host Leukocytes. Nat. Med. 2002, 8, 687–693. [Google Scholar] [CrossRef]
- de Haas, C.J.C.; Veldkamp, K.E.; Peschel, A.; Weerkamp, F.; Van Wamel, W.J.B.; Heezius, E.C.J.M.; Poppelier, M.J.J.G.; Van Kessel, K.P.M.; van Strijp, J.A.G. Chemotaxis Inhibitory Protein of Staphylococcus Aureus, a Bacterial Antiinflammatory Agent. J. Exp. Med. 2004, 199, 687–695. [Google Scholar] [CrossRef]
- Rooijakkers, S.H.M.; Ruyken, M.; Van Roon, J.; Van Kessel, K.P.M.; Van Strijp, J.A.G.; Van Wamel, W.J.B. Early Expression of SCIN and CHIPS Drives Instant Immune Evasion by Staphylococcus Aureus. Cell. Microbiol. 2006, 8, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, S.H.M.; Ruyken, M.; Roos, A.; Daha, M.R.; Presanis, J.S.; Sim, R.B.; van Wamel, W.J.B.; van Kessel, K.P.M.; van Strijp, J.A.G. Immune Evasion by a Staphylococcal Complement Inhibitor That Acts on C3 Convertases. Nat. Immunol. 2005, 6, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Jongerius, I.; von Köckritz-Blickwede, M.; Horsburgh, M.J.; Ruyken, M.; Nizet, V.; Rooijakkers, S.H.M. Staphylococcus Aureus Virulence Is Enhanced by Secreted Factors That Block Innate Immune Defenses. J. Innate Immun. 2012, 4, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal Manipulation of Host Immune Responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef]
- Chekabab, S.M.; Silverman, R.J.; Lafayette, S.L.; Luo, Y.; Rousseau, S.; Nguyen, D. Staphylococcus Aureus Inhibits IL-8 Responses Induced by Pseudomonas Aeruginosa in Airway Epithelial Cells. PLoS ONE 2015, 10, e0137753. [Google Scholar] [CrossRef]
- Tajima, A.; Iwase, T.; Shinji, H.; Seki, K.; Mizunoe, Y. Inhibition of Endothelial Interleukin-8 Production and Neutrophil Transmigration by Staphylococcus Aureus Beta-Hemolysin. Infect. Immun. 2009, 77, 327–334. [Google Scholar] [CrossRef]
- Zurek, O.W.; Pallister, K.B.; Voyich, J.M. Staphylococcus Aureus Inhibits Neutrophil-Derived IL-8 to Promote Cell Death. J. Infect. Dis. 2015, 212, 934–938. [Google Scholar] [CrossRef]
- Guggenberger, C.; Wolz, C.; Morrissey, J.A.; Heesemann, J. Two Distinct Coagulase-Dependent Barriers Protect Staphylococcus Aureus from Neutrophils in a Three Dimensional in Vitro Infection Model. PLoS Pathog. 2012, 8, e1002434. [Google Scholar] [CrossRef]
- Parker, D.; Ahn, D.; Cohen, T.; Prince, A. Innate Immune Signaling Activated by MDR Bacteria in the Airway. Physiol. Rev. 2016, 96, 19–53. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Kern, J.W.; Missiakas, D.M.; Schneewind, O. Staphylococcus Aureus Synthesizes Adenosine to Escape Host Immune Responses. J. Exp. Med. 2009, 206, 2417–2427. [Google Scholar] [CrossRef]
- Stapels, D.A.C.; Ramyar, K.X.; Bischoff, M.; von Köckritz-Blickwede, M.; Milder, F.J.; Ruyken, M.; Eisenbeis, J.; McWhorter, W.J.; Herrmann, M.; van Kessel, K.P.M.; et al. Staphylococcus Aureus Secretes a Unique Class of Neutrophil Serine Protease Inhibitors. Proc. Natl. Acad. Sci. USA 2014, 111, 13187–13192. [Google Scholar] [CrossRef] [PubMed]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of Human Antimicrobial Peptide LL-37 by Staphylococcus Aureus-Derived Proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Bokarewa, M.; Foster, T.; Mitchell, J.; Higgins, J.; Tarkowski, A. Staphylococcus Aureus Resists Human Defensins by Production of Staphylokinase, a Novel Bacterial Evasion Mechanism. J. Immunol. 2004, 172, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.M.; Barwinska-Sendra, A.; Tarrant, E.; Skaar, E.P.; Waldron, K.J.; Kehl-Fie, T.E. A Superoxide Dismutase Capable of Functioning with Iron or Manganese Promotes the Resistance of Staphylococcus Aureus to Calprotectin and Nutritional Immunity. PLoS Pathog. 2017, 13, e1006125. [Google Scholar] [CrossRef]
- Treffon, J.; Block, D.; Moche, M.; Reiss, S.; Fuchs, S.; Engelmann, S.; Becher, D.; Langhanki, L.; Mellmann, A.; Peters, G.; et al. Adaptation of Staphylococcus Aureus to Airway Environments in Patients with Cystic Fibrosis by Upregulation of Superoxide Dismutase M and Iron-Scavenging Proteins. J. Infect. Dis. 2018, 217, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Treffon, J.; Chaves-Moreno, D.; Niemann, S.; Pieper, D.H.; Vogl, T.; Roth, J.; Kahl, B.C. Importance of Superoxide Dismutases A and M for Protection of Staphylococcus Aureus in the Oxidative Stressful Environment of Cystic Fibrosis Airways. Cell. Microbiol. 2020, 22, e13158. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus Aureus Golden Pigment Impairs Neutrophil Killing and Promotes Virulence through Its Antioxidant Activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Cosgrove, K.; Coutts, G.; Jonsson, I.-M.; Tarkowski, A.; Kokai-Kun, J.F.; Mond, J.J.; Foster, S.J. Catalase (KatA) and Alkyl Hydroperoxide Reductase (AhpC) Have Compensatory Roles in Peroxide Stress Resistance and Are Required for Survival, Persistence, and Nasal Colonization in Staphylococcus Aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus Aureus Degrades Neutrophil Extracellular Traps to Promote Immune Cell Death. Science 2013, 342, 863–866. [Google Scholar] [CrossRef]
- Herzog, S.; Dach, F.; de Buhr, N.; Niemann, S.; Schlagowski, J.; Chaves-Moreno, D.; Neumann, C.; Goretzko, J.; Schwierzeck, V.; Mellmann, A.; et al. High Nuclease Activity of Long Persisting Staphylococcus Aureus Isolates within the Airways of Cystic Fibrosis Patients Protects against NET-Mediated Killing. Front. Immunol. 2019, 10, 2552. [Google Scholar] [CrossRef]
- Berends, E.T.M.; Horswill, A.R.; Haste, N.M.; Monestier, M.; Nizet, V.; von Köckritz-Blickwede, M. Nuclease Expression by Staphylococcus Aureus Facilitates Escape from Neutrophil Extracellular Traps. J. Innate Immun. 2010, 2, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Pernet, E.; Guillemot, L.; Burgel, P.-R.; Martin, C.; Lambeau, G.; Sermet-Gaudelus, I.; Sands, D.; Leduc, D.; Morand, P.C.; Jeammet, L.; et al. Pseudomonas Aeruginosa Eradicates Staphylococcus Aureus by Manipulating the Host Immunity. Nat. Commun. 2014, 5, 5105. [Google Scholar] [CrossRef] [PubMed]
- Pernet, E.; Brunet, J.; Guillemot, L.; Chignard, M.; Touqui, L.; Wu, Y. Staphylococcus Aureus Adenosine Inhibits SPLA2-IIA–Mediated Host Killing in the Airways. J. Immunol. 2015, 194, 5312–5319. [Google Scholar] [CrossRef] [PubMed]
- Falugi, F.; Kim, H.K.; Missiakas, D.M.; Schneewind, O. Role of Protein A in the Evasion of Host Adaptive Immune Responses by Staphylococcus Aureus. mBio 2013, 4, e00575-13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent Infections and Immune Evasion Strategies of Staphylococcus Aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar] [CrossRef]
- Foster, T.J. Immune Evasion by Staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Winstel, V.; Schneewind, O.; Missiakas, D. Staphylococcus Aureus Exploits the Host Apoptotic Pathway to Persist during Infection. mBio 2019, 10, e02270-19. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Liimatta, K.; Lung, T.W.F.; Fields, B.; Ahn, D.; Chen, D.; Lozano, C.; Sáenz, Y.; Uhlemann, A.-C.; Kahl, B.C.; et al. Pseudomonas Aeruginosa Utilizes Host-Derived Itaconate to Redirect Its Metabolism to Promote Biofilm Formation. Cell Metab. 2020, 31, 1091–1106.e6. [Google Scholar] [CrossRef]
- Tomlinson, K.L.; Lung, T.W.F.; Dach, F.; Annavajhala, M.K.; Gabryszewski, S.J.; Groves, R.A.; Drikic, M.; Francoeur, N.J.; Sridhar, S.H.; Smith, M.L.; et al. Staphylococcus Aureus Induces an Itaconate-Dominated Immunometabolic Response That Drives Biofilm Formation. Nat. Commun. 2021, 12, 1399. [Google Scholar] [CrossRef]
- Grim, K.P.; San Francisco, B.; Radin, J.N.; Brazel, E.B.; Kelliher, J.L.; Párraga Solórzano, P.K.; Kim, P.C.; McDevitt, C.A.; Kehl-Fie, T.E. The Metallophore Staphylopine Enables Staphylococcus Aureus to Compete with the Host for Zinc and Overcome Nutritional Immunity. mBio 2017, 8, e01281-17. [Google Scholar] [CrossRef]
- Skaar, E.P.; Humayun, M.; Bae, T.; DeBord, K.L.; Schneewind, O. Iron-Source Preference of Staphylococcus Aureus Infections. Science 2004, 305, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Radin, J.N.; Kelliher, J.L.; Solórzano, P.K.P.; Kehl-Fie, T.E. The Two-Component System ArlRS and Alterations in Metabolism Enable Staphylococcus Aureus to Resist Calprotectin-Induced Manganese Starvation. PLoS Pathog. 2016, 12, e1006040. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A. Effects of Pseudomonas Aeruginosa on CFTR Chloride Secretion and the Host Immune Response. Am. J. Physiol.-Cell Physiol. 2017, 312, C357–C366. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, C.; Ravishankar, B.; Patanwala, M.; Shuai, S.; Fu, Z.; Illek, B.; Fischer, H.; Machen, T.E. Thapsigargin Blocks Pseudomonas Aeruginosa Homoserine Lactone-Induced Apoptosis in Airway Epithelia. Am. J. Physiol.-Cell Physiol. 2014, 306, C844–C855. [Google Scholar] [CrossRef]
- Hauser, A.R. The Type III Secretion System of Pseudomonas Aeruginosa: Infection by Injection. Nat. Rev. Microbiol. 2009, 7, 654–665. [Google Scholar] [CrossRef]
- Hooi, D.S.W.; Bycroft, B.W.; Chhabra, S.R.; Williams, P.; Pritchard, D.I. Differential Immune Modulatory Activity of Pseudomonas Aeruginosa Quorum-Sensing Signal Molecules. Infect Immun. 2004, 72, 8. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Ye, L.; Mao, Y.; Xie, X.; Xia, C.; Chen, J.; Lu, Z.; Song, J. Influence of Pseudomonas Aeruginosa Quorum Sensing Signal Molecule N-(3-Oxododecanoyl) Homoserine Lactone on Mast Cells. Med. Microbiol. Immunol. 2009, 198, 113–121. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.U.; Koh, B.H.; Hwang, S.S.; Kim, S.-H.; Lépine, F.; Cho, Y.-H.; Lee, G.R. HHQ and PQS, Two Pseudomonas Aeruginosa Quorum-Sensing Molecules, down-Regulate the Innate Immune Responses through the Nuclear Factor-ΚB Pathway. Immunology 2010, 129, 578–588. [Google Scholar] [CrossRef]
- LaFayette, S.L.; Houle, D.; Beaudoin, T.; Wojewodka, G.; Radzioch, D.; Hoffman, L.R.; Burns, J.L.; Dandekar, A.A.; Smalley, N.E.; Chandler, J.R.; et al. Cystic Fibrosis–Adapted Pseudomonas Aeruginosa Quorum Sensing LasR Mutants Cause Hyperinflammatory Responses. Sci. Adv. 2015, 1, e1500199. [Google Scholar] [CrossRef]
- Koeppen, K.; Hampton, T.H.; Jarek, M.; Scharfe, M.; Gerber, S.A.; Mielcarz, D.W.; Demers, E.G.; Dolben, E.L.; Hammond, J.H.; Hogan, D.A.; et al. A Novel Mechanism of Host-Pathogen Interaction through SRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016, 12, e1005672. [Google Scholar] [CrossRef]
- Glucksam-Galnoy, Y.; Sananes, R.; Silberstein, N.; Krief, P.; Kravchenko, V.V.; Meijler, M.M.; Zor, T. The Bacterial Quorum-Sensing Signal Molecule N -3-Oxo-Dodecanoyl-l-Homoserine Lactone Reciprocally Modulates Pro- and Anti-Inflammatory Cytokines in Activated Macrophages. J. Immunol. 2013, 191, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; LeMaoult, J.; Trapella, C.; Di Luca, D.; Carosella, E.D.; Rizzo, R. Pseudomonas Aeruginosa Quorum Sensing Molecule N-(3-Oxododecanoyl)-l-Homoserine-Lactone Induces HLA-G Expression in Human Immune Cells. Infect. Immun. 2015, 83, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Skindersoe, M.E.; Zeuthen, L.H.; Brix, S.; Fink, L.N.; Lazenby, J.; Whittall, C.; Williams, P.; Diggle, S.P.; Froekiaer, H.; Cooley, M.; et al. Pseudomonas Aeruginosa Quorum-Sensing Signal Molecules Interfere with Dendritic Cell-Induced T-Cell Proliferation. FEMS Immunol. Med. Microbiol. 2009, 55, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Telford, G.; Wheeler, D.; Williams, P.; Tomkins, P.T.; Appleby, P.; Sewell, H.; Stewart, G.S.A.B.; Bycroft, B.W.; Pritchard, D.I. The Pseudomonas Aeruginosa Quorum-Sensing Signal Molecule N-(3-Oxododecanoyl)-l-Homoserine Lactone Has Immunomodulatory Activity. Infect. Immun. 1998, 66, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.S.; Kharazmi, A.; Espersen, F.; Høiby, N. Pseudomonas Aeruginosa Alginate in Cystic Fibrosis Sputum and the Inflammatory Response. Infect. Immun. 1990, 58, 3363–3368. [Google Scholar] [CrossRef] [PubMed]
- Skopelja, S.; Hamilton, B.J.; Jones, J.D.; Yang, M.-L.; Mamula, M.; Ashare, A.; Gifford, A.H.; Rigby, W.F.C. The Role for Neutrophil Extracellular Traps in Cystic Fibrosis Autoimmunity. JCI Insight 2016, 1, e88912. [Google Scholar] [CrossRef]
- Khatua, B.; Bhattacharya, K.; Mandal, C. Sialoglycoproteins Adsorbed by Pseudomonas Aeruginosa Facilitate Their Survival by Impeding Neutrophil Extracellular Trap through Siglec-9. J. Leukoc. Biol. 2012, 91, 641–655. [Google Scholar] [CrossRef]
- Floyd, M.; Winn, M.; Cullen, C.; Sil, P.; Chassaing, B.; Yoo, D.; Gewirtz, A.T.; Goldberg, J.B.; McCarter, L.L.; Rada, B. Swimming Motility Mediates the Formation of Neutrophil Extracellular Traps Induced by Flagellated Pseudomonas Aeruginosa. PLoS Pathog. 2016, 12, e1005987. [Google Scholar] [CrossRef]
- Skopelja-Gardner, S.; Theprungsirikul, J.; Lewis, K.A.; Hammond, J.H.; Carlson, K.M.; Hazlett, H.F.; Nymon, A.; Nguyen, D.; Berwin, B.L.; Hogan, D.A.; et al. Regulation of Pseudomonas Aeruginosa-Mediated Neutrophil Extracellular Traps. Front. Immunol. 2019, 10, 1670. [Google Scholar] [CrossRef]
- Lovewell, R.R.; Collins, R.M.; Acker, J.L.; O’Toole, G.A.; Wargo, M.J.; Berwin, B. Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion. PLoS Pathog. 2011, 7, e1002253. [Google Scholar] [CrossRef]
- Mariencheck, W.I.; Alcorn, J.F.; Palmer, S.M.; Wright, J.R. Pseudomonas Aeruginosa Elastase Degrades Surfactant Proteins A and D. Am. J. Respir. Cell Mol. Biol. 2003, 28, 528–537. [Google Scholar] [CrossRef]
- Kuang, Z.; Hao, Y.; Walling, B.E.; Jeffries, J.L.; Ohman, D.E.; Lau, G.W. Pseudomonas Aeruginosa Elastase Provides an Escape from Phagocytosis by Degrading the Pulmonary Surfactant Protein-A. PLoS ONE 2011, 6, e27091. [Google Scholar] [CrossRef]
- Alcorn, J.F.; Wright, J.R. Degradation of Pulmonary Surfactant Protein D by Pseudomonas Aeruginosa Elastase Abrogates Innate Immune Function. J. Biol. Chem. 2004, 279, 30871–30879. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.J.; Martin, R.; Plumb, J.D.; Vachon, E.; Cameron, C.M.; Danesh, A.; Kelvin, D.J.; Ruf, W.; Downey, G.P. Role of PAR2 in Murine Pulmonary Pseudomonal Infection. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L368–L377. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.Ø.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid Necrotic Killing of Polymorphonuclear Leukocytes Is Caused by Quorum-Sensing-Controlled Production of Rhamnolipid by Pseudomonas Aeruginosa. Microbiology 2007, 153, 1329–1338. [Google Scholar] [CrossRef]
- Usher, L.R.; Lawson, R.A.; Geary, I.; Taylor, C.J.; Bingle, C.D.; Taylor, G.W.; Whyte, M.K.B. Induction of Neutrophil Apoptosis by the Pseudomonas Aeruginosa Exotoxin Pyocyanin: A Potential Mechanism of Persistent Infection. J. Immunol. 2002, 168, 1861–1868. [Google Scholar] [CrossRef]
- Reinhart, A.A.; Oglesby-Sherrouse, A.G. Regulation of Pseudomonas Aeruginosa Virulence by Distinct Iron Sources. Genes 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; O’Neill, M.J.; Watts, A.M.; Robson, C.L.; Lamont, I.L.; Wilks, A.; Oglesby-Sherrouse, A.G. Adaptation of Iron Homeostasis Pathways by a Pseudomonas Aeruginosa Pyoverdine Mutant in the Cystic Fibrosis Lung. J. Bacteriol. 2014, 196, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Ortiz, J.; Llanos-González, E.; Toledano, V.; del Campo, R.; Cubillos-Zapata, C.; Lozano-Rodríguez, R.; Ismail, A.; Prados, C.; Gómez-Campelo, P.; Aguirre, L.A.; et al. Pseudomonas Aeruginosa Colonization Causes PD-L1 Overexpression on Monocytes, Impairing the Adaptive Immune Response in Patients with Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, 630–635. [Google Scholar] [CrossRef]
- Sun, J.; LaRock, D.L.; Skowronski, E.A.; Kimmey, J.M.; Olson, J.; Jiang, Z.; O’Donoghue, A.J.; Nizet, V.; LaRock, C.N. The Pseudomonas Aeruginosa Protease LasB Directly Activates IL-1β. EBioMedicine 2020, 60, 102984. [Google Scholar] [CrossRef]
- Mayer, M.L.; Sheridan, J.A.; Blohmke, C.J.; Turvey, S.E.; Hancock, R.E.W. The Pseudomonas Aeruginosa Autoinducer 3O-C12 Homoserine Lactone Provokes Hyperinflammatory Responses from Cystic Fibrosis Airway Epithelial Cells. PLoS ONE 2011, 6, e16246. [Google Scholar] [CrossRef] [PubMed]
- Shiner, E.K.; Terentyev, D.; Bryan, A.; Sennoune, S.; Martinez-Zaguilan, R.; Li, G.; Gyorke, S.; Williams, S.C.; Rumbaugh, K.P. Pseudomonas Aeruginosa Autoinducer Modulates Host Cell Responses through Calcium Signalling. Cell. Microbiol. 2006, 8, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Fedyk, E.R.; Springer, T.A.; Mukaida, N.; Iglewski, B.H.; Phipps, R.P. IL-8 Production in Human Lung Fibroblasts and Epithelial Cells Activated by the Pseudomonas Autoinducer N-3-Oxododecanoyl Homoserine Lactone Is Transcriptionally Regulated by NF-ΚB and Activator Protein-2. J. Immunol. 2001, 167, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Gambello, M.J.; Kaye, S.; Iglewski, B.H. LasR of Pseudomonas Aeruginosa Is a Transcriptional Activator of the Alkaline Protease Gene (Apr) and an Enhancer of Exotoxin a Expression. Infect. Immun. 1993, 61, 1180–1184. [Google Scholar] [CrossRef]
- Férec, C. La mucoviscidose: Du gène à la thérapeutique. Médecine/Sciences 2021, 37, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.J.; Smith, A.E. Molecular Mechanisms of CFTR Chloride Channel Dysfunction in Cystic Fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef]
- Zheng, M.; Sun, S.; Zhou, J.; Liu, M. Virulence Factors Impair Epithelial Junctions during Bacterial Infection. J. Clin. Lab. Anal. 2021, 35, e23627. [Google Scholar] [CrossRef]
- Crabbé, A.; Sarker, S.F.; Van Houdt, R.; Ott, C.M.; Leys, N.; Cornelis, P.; Nickerson, C.A. Alveolar Epithelium Protects Macrophages from Quorum Sensing-Induced Cytotoxicity in a Three-Dimensional Co-Culture Model. Cell. Microbiol. 2011, 13, 469–481. [Google Scholar] [CrossRef]
- Yamada, K.J.; Kielian, T. Biofilm-Leukocyte Cross-Talk: Impact on Immune Polarization and Immunometabolism. J. Innate Immun. 2019, 11, 280–288. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T.; Jensen, P.O.; Phipps, R.K.; Moser, C.; Christophersen, L.; Christensen, L.D.; van Gennip, M.; Parsek, M.; Hoiby, N.; et al. Pseudomonas Aeruginosa Recognizes and Responds Aggressively to the Presence of Polymorphonuclear Leukocytes. Microbiology 2009, 155, 3500–3508. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T.; Givskov, M.; Alhede, M. Pseudomonas Aeruginosa Biofilms. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 86, pp. 1–40. ISBN 978-0-12-800262-9. [Google Scholar]
- Walker, T.S.; Tomlin, K.L.; Worthen, G.S.; Poch, K.R.; Lieber, J.G.; Saavedra, M.T.; Fessler, M.B.; Malcolm, K.C.; Vasil, M.L.; Nick, J.A. Enhanced Pseudomonas Aeruginosa Biofilm Development Mediated by Human Neutrophils. Infect. Immun. 2005, 73, 3693–3701. [Google Scholar] [CrossRef] [PubMed]
- Scherr, T.D.; Hanke, M.L.; Huang, O.; James, D.B.A.; Horswill, A.R.; Bayles, K.W.; Fey, P.D.; Torres, V.J.; Kielian, T. Staphylococcus Aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. mBio 2015, 6, e01021-15. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; Lin, K.-C.; Maurice, N.M.; Yuan, Z.; Bijli, K.; Koval, M.; Hart, C.M.; Goldberg, J.B.; Stecenko, A.; Sadikot, R.T. UPR Modulation of Host Immunity by Pseudomonas Aeruginosa in Cystic Fibrosis. Clin. Sci. 2020, 134, 1911–1934. [Google Scholar] [CrossRef]
- Bedi, B.; Maurice, N.M.; Ciavatta, V.T.; Lynn, K.S.; Yuan, Z.; Molina, S.A.; Joo, M.; Tyor, W.R.; Goldberg, J.B.; Koval, M.; et al. Peroxisome Proliferator-Activated Receptor-γ Agonists Attenuate Biofilm Formation by Pseudomonas Aeruginosa. FASEB J. 2017, 31, 3608–3621. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; Yuan, Z.; Joo, M.; Zughaier, S.M.; Goldberg, J.B.; Arbiser, J.L.; Hart, C.M.; Sadikot, R.T. Enhanced Clearance of Pseudomonas Aeruginosa by Peroxisome Proliferator-Activated Receptor Gamma. Infect. Immun. 2016, 84, 1975–1985. [Google Scholar] [CrossRef]
- Al Alam, D.; Deslee, G.; Tournois, C.; Lamkhioued, B.; Lebargy, F.; Merten, M.; Belaaouaj, A.; Guenounou, M.; Gangloff, S.C. Impaired Interleukin-8 Chemokine Secretion by Staphylococcus Aureus–Activated Epithelium and T-Cell Chemotaxis in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2010, 42, 644–650. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Schneewind, O.; Missiakas, D.M. Enzymatic Properties of Staphylococcus Aureus Adenosine Synthase (AdsA). BMC Biochem. 2011, 12, 56. [Google Scholar] [CrossRef]
- Tannahill, G.; Curtis, A.; Adamik, J.; Palsson-McDermott, E.; McGettrick, A.; Goel, G.; Frezza, C.; Bernard, N.; Kelly, B.; Foley, N.; et al. Succinate Is a Danger Signal That Induces IL-1β via HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A.J. Succinate: A Metabolic Signal in Inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Artyomov, M.N. Itaconate: The Poster Child of Metabolic Reprogramming in Macrophage Function. Nat. Rev. Immunol. 2019, 19, 273–281. [Google Scholar] [CrossRef]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef]
- D’Arpa, P.; Karna, S.L.R.; Chen, T.; Leung, K.P. Pseudomonas Aeruginosa Transcriptome Adaptations from Colonization to Biofilm Infection of Skin Wounds. Sci. Rep. 2021, 11, 20632. [Google Scholar] [CrossRef] [PubMed]
- Price, J.V.; Russo, D.; Ji, D.X.; Chavez, R.A.; DiPeso, L.; Lee, A.Y.-F.; Coers, J.; Vance, R.E. IRG1 and Inducible Nitric Oxide Synthase Act Redundantly with Other Interferon-Gamma-Induced Factors to Restrict Intracellular Replication of Legionella Pneumophila. mBio 2019, 10, e02629-19. [Google Scholar] [CrossRef] [PubMed]
- Naujoks, J.; Tabeling, C.; Dill, B.D.; Hoffmann, C.; Brown, A.S.; Kunze, M.; Kempa, S.; Peter, A.; Mollenkopf, H.-J.; Dorhoi, A.; et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016, 12, e1005408. [Google Scholar] [CrossRef]
- Riquelme, S.A.; Lozano, C.; Moustafa, A.M.; Liimatta, K.; Tomlinson, K.L.; Britto, C.; Khanal, S.; Gill, S.K.; Narechania, A.; Azcona-Gutiérrez, J.M.; et al. CFTR-PTEN–Dependent Mitochondrial Metabolic Dysfunction Promotes Pseudomonas Aeruginosa Airway Infection. Sci. Transl. Med. 2019, 11, eaav4634. [Google Scholar] [CrossRef]
- Liao, S.-T.; Han, C.; Xu, D.-Q.; Fu, X.-W.; Wang, J.-S.; Kong, L.-Y. 4-Octyl Itaconate Inhibits Aerobic Glycolysis by Targeting GAPDH to Exert Anti-Inflammatory Effects. Nat. Commun. 2019, 10, 5091. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Qin, K.; Zhang, Y.; Jia, W.; Chen, Y.; Cheng, B.; Peng, L.; Chen, N.; Liu, Y.; Zhou, W.; et al. S-Glycosylation-Based Cysteine Profiling Reveals Regulation of Glycolysis by Itaconate. Nat. Chem. Biol. 2019, 15, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate Is an Anti-Inflammatory Metabolite That Activates Nrf2 via Alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Zygiel, E.M.; Obisesan, A.O.; Nelson, C.E.; Oglesby, A.G.; Nolan, E.M. Heme Protects Pseudomonas Aeruginosa and Staphylococcus Aureus from Calprotectin-Induced Iron Starvation. J. Biol. Chem. 2021, 296, 100160. [Google Scholar] [CrossRef]
- Lhospice, S.; Gomez, N.O.; Ouerdane, L.; Brutesco, C.; Ghssein, G.; Hajjar, C.; Liratni, A.; Wang, S.; Richaud, P.; Bleves, S.; et al. Pseudomonas Aeruginosa Zinc Uptake in Chelating Environment Is Primarily Mediated by the Metallophore Pseudopaline. Sci. Rep. 2017, 7, 17132. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Jones, J.W.; Ruge, M.A.; Kane, M.A.; Oglesby-Sherrouse, A.G. Iron Depletion Enhances Production of Antimicrobials by Pseudomonas Aeruginosa. J. Bacteriol. 2015, 197, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, C.A.; Moore, J.L.; Noto, M.J.; Zhang, Y.; Singleton, M.D.; Prentice, B.M.; Gilston, B.A.; Doster, R.S.; Gaddy, J.A.; Chazin, W.J.; et al. The Innate Immune Protein Calprotectin Promotes Pseudomonas Aeruginosa and Staphylococcus Aureus Interaction. Nat. Commun. 2016, 7, 11951. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, D.M.; Crocker, A.W.; Gifford, A.H.; Hogan, D.A. Calprotectin-Mediated Zinc Chelation Inhibits Pseudomonas Aeruginosa Protease Activity in Cystic Fibrosis Sputum. J. Bacteriol. 2021, 203, 17. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jeong, D.-W.; Liu, Q.; Yeo, W.-S.; Vogl, T.; Skaar, E.P.; Chazin, W.J.; Bae, T. Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus Aureus Infections. PLoS Pathog. 2015, 11, e1005026. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida Albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Gray, R.D.; Hardisty, G.; Regan, K.H.; Smith, M.; Robb, C.T.; Duffin, R.; Mackellar, A.; Felton, J.M.; Paemka, L.; McCullagh, B.N.; et al. Delayed Neutrophil Apoptosis Enhances NET Formation in Cystic Fibrosis. Thorax 2018, 73, 134–144. [Google Scholar] [CrossRef]
- Wieneke, M.K.; Dach, F.; Neumann, C.; Görlich, D.; Kaese, L.; Thißen, T.; Dübbers, A.; Kessler, C.; Große-Onnebrink, J.; Küster, P.; et al. Association of Diverse Staphylococcus Aureus Populations with Pseudomonas Aeruginosa Coinfection and Inflammation in Cystic Fibrosis Airway Infection. mSphere 2021, 6, e00358-21. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Wolter, D.J.; Mishra, M.; Hayden, H.S.; Radey, M.C.; Merrihew, G.; MacCoss, M.J.; Burns, J.; Wozniak, D.J.; Parsek, M.R.; et al. Staphylococcus Aureus Protein A Mediates Interspecies Interactions at the Cell Surface of Pseudomonas Aeruginosa. mBio 2016, 7, e00538-16. [Google Scholar] [CrossRef]
- Amiel, E.; Lovewell, R.R.; O’Toole, G.A.; Hogan, D.A.; Berwin, B. Pseudomonas Aeruginosa Evasion of Phagocytosis Is Mediated by Loss of Swimming Motility and Is Independent of Flagellum Expression. Infect. Immun. 2010, 78, 2937–2945. [Google Scholar] [CrossRef]
- Schwerdt, M.; Neumann, C.; Schwartbeck, B.; Kampmeier, S.; Herzog, S.; Görlich, D.; Dübbers, A.; Große-Onnebrink, J.; Kessler, C.; Küster, P.; et al. Staphylococcus Aureus in the Airways of Cystic Fibrosis Patients—A Retrospective Long-Term Study. Int. J. Med. Microbiol. 2018, 308, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Camus, L.; Vandenesch, F.; Moreau, K. From Genotype to Phenotype: Adaptations of Pseudomonas Aeruginosa to the Cystic Fibrosis Environment. Microb. Genom. 2021, 7, mgen000513. [Google Scholar] [CrossRef] [PubMed]
- Markussen, T.; Marvig, R.L.; Gómez-Lozano, M.; Aanæs, K.; Burleigh, A.E.; Høiby, N.; Johansen, H.K.; Molin, S.; Jelsbak, L. Environmental Heterogeneity Drives within-Host Diversification and Evolution of Pseudomonas Aeruginosa. mBio 2014, 5, e01592-14. [Google Scholar] [CrossRef] [PubMed]
- Marvig, R.L.; Sommer, L.M.; Molin, S.; Johansen, H.K. Convergent Evolution and Adaptation of Pseudomonas Aeruginosa within Patients with Cystic Fibrosis. Nat. Genet. 2015, 47, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D’Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic Adaptation by Pseudomonas Aeruginosa to the Airways of Cystic Fibrosis Patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492. [Google Scholar] [CrossRef]
- Klockgether, J.; Cramer, N.; Fischer, S.; Wiehlmann, L.; Tümmler, B. Long-Term Microevolution of Pseudomonas Aeruginosa Differs between Mildly and Severely Affected Cystic Fibrosis Lungs. Am. J. Respir. Cell Mol. Biol. 2018, 59, 11. [Google Scholar] [CrossRef]
- Westphal, C.; Görlich, D.; Kampmeier, S.; Herzog, S.; Braun, N.; Hitschke, C.; Mellmann, A.; Peters, G.; Kahl, B.C. Antibiotic Treatment and Age Are Associated with Staphylococcus Aureus Carriage Profiles during Persistence in the Airways of Cystic Fibrosis Patients. Front. Microbiol. 2020, 11, 230. [Google Scholar] [CrossRef]
- Camus, L.; Briaud, P.; Vandenesch, F.; Moreau, K. How Bacterial Adaptation to Cystic Fibrosis Environment Shapes Interactions between Pseudomonas Aeruginosa and Staphylococcus Aureus. Front. Microbiol. 2021, 12, 617784. [Google Scholar] [CrossRef]
- Lennartz, F.E.; Schwartbeck, B.; Dübbers, A.; Große-Onnebrink, J.; Kessler, C.; Küster, P.; Schültingkemper, H.; Peters, G.; Kahl, B.C. The Prevalence of Staphylococcus Aureus with Mucoid Phenotype in the Airways of Patients with Cystic Fibrosis—A Prospective Study. Int. J. Med. Microbiol. 2019, 309, 283–287. [Google Scholar] [CrossRef]
- Tan, X.; Coureuil, M.; Ramond, E.; Euphrasie, D.; Dupuis, M.; Tros, F.; Meyer, J.; Nemazanyy, I.; Chhuon, C.; Guerrera, I.C.; et al. Chronic Staphylococcus Aureus Lung Infection Correlates with Proteogenomic and Metabolic Adaptations Leading to an Increased Intracellular Persistence. Clin. Infect. Dis. 2019, 69, 1937–1945. [Google Scholar] [CrossRef]
- Chatterjee, I.; Kriegeskorte, A.; Fischer, A.; Deiwick, S.; Theimann, N.; Proctor, R.A.; Peters, G.; Herrmann, M.; Kahl, B.C. In Vivo Mutations of Thymidylate Synthase (Encoded by ThyA) Are Responsible for Thymidine Dependency in Clinical Small-Colony Variants of Staphylococcus Aureus. J. Bacteriol. 2008, 190, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).