Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration
Abstract
1. Introduction
2. Molecular Effectors and Mechanisms Involved in Exercise-Related Benefits
2.1. Mechanical Signals Transduction
2.1.1. Mechanical Signals to Cellular Signals Transduction
2.1.2. Cell-Cell Mechanotransduction
2.2. The Activation of Sequential Signaling Cascades
2.3. Exercise Mimics
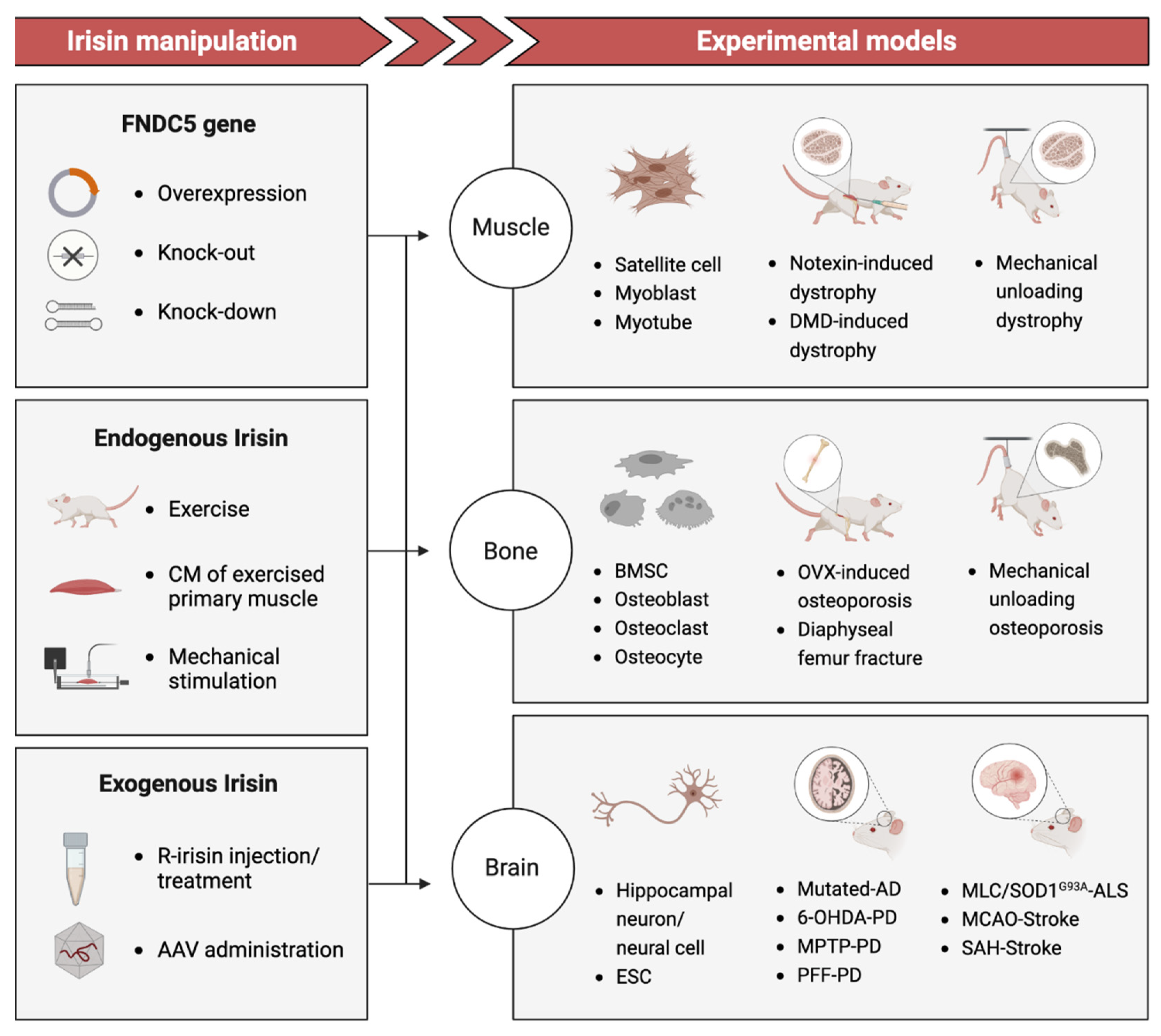
3. Current Evidence: Irisin as a Potential Link to Degenerative Disorders
3.1. Muscle Atrophy
3.1.1. Clinical Studies
3.1.2. Basic Studies
3.2. Osteoporosis
3.2.1. Clinical Studies
3.2.2. Basic Studies
3.3. Neurodegenerative Disease and Injury
3.3.1. Clinical Studies
3.3.2. Basic Studies
4. Is Irisin an Oracle to Tissue Repair/Regeneration?
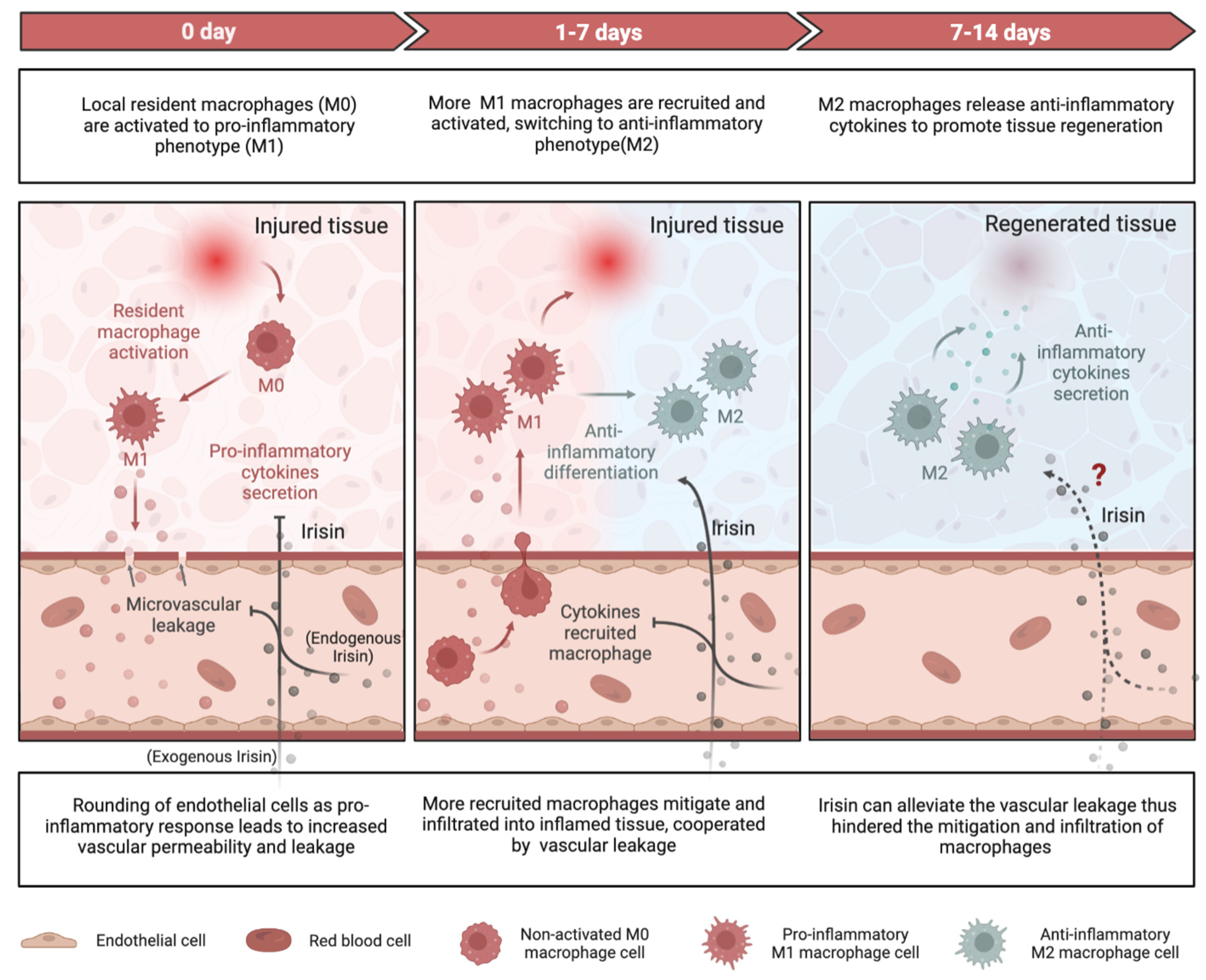
4.1. Role of Irisin in Regulating Inflammatory Responses
4.1.1. Pro-Inflammation and Anti-Inflammation
4.1.2. Macrophage Function
4.1.3. Vascular Permeability
4.2. Role of Irisin in Coordinating Proliferation, Differentiation and Apoptosis
4.2.1. Myogenesis
4.2.2. Osteogenesis
4.2.3. Neurogenesis
5. Conclusions and Further Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silverman, M.N.; Deuster, P.A. Biological Mechanisms Underlying the Role of Physical Fitness in Health and Resilience. Interface Focus 2014, 4, 20140040. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Polk, J.D. Linking Brains and Brawn: Exercise and the Evolution of Human Neurobiology. Proc. R. Soc. 2013, 280, 20122250. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Z.; No, M.H.; Heo, J.W.; Park, D.H.; Kang, J.H.; Kim, S.H.; Kwak, H.B. Role of Exercise in Age-Related Sarcopenia. J. Exerc. Rehabil. 2018, 14, 551. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for Preventing and Treating Osteoporosis in Postmenopausal Women. Cochrane Database Syst. Rev. 2011, CD000333. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, T.; Chu, J.M.T.; Chen, Y.; Dunnett, S.; Ho, Y.S.; Wong, G.T.C.; Chang, R.C.C. The Beneficial Effects of Physical Exercise in the Brain and Related Pathophysiological Mechanisms in Neurodegenerative Diseases. Lab. Investig. 2019 997 2019, 99, 943–957. [Google Scholar] [CrossRef]
- Takada, S.; Okita, K.; Suga, T.; Omokawa, M.; Kadoguchi, T.; Sato, T.; Takahashi, M.; Yokota, T.; Hirabayashi, K.; Morita, N.; et al. Low-Intensity Exercise Can Increase Muscle Mass and Strength Proportionally to Enhanced Metabolic Stress under Ischemic Conditions. J. Appl. Physiol. 2012, 113, 199–205. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, G. How Does Exercise Affect Bone Development during Growth? Sports Med. 2006, 36, 561–569. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of Obesity, Diabetes and Exercise on Fndc5 Gene Expression and Irisin Release in Human Skeletal Muscle and Adipose Tissue: In Vivo and in Vitro Studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436. [Google Scholar] [CrossRef]
- Tu, T.; Peng, J.; Jiang, Y. FNDC5/Irisin: A New Protagonist in Acute Brain Injury. Stem Cells Dev. 2020, 29, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; He, Z.; Du, J.; Xu, M.; Cui, J.; Han, X.; Tong, D.; Li, H.; Yan, M.; Yu, Z. Irisin Promotes Fracture Healing by Improving Osteogenesis and Angiogenesis. J. Orthop. Transl. 2022, 37, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin Is a Pro-Myogenic Factor That Induces Skeletal Muscle Hypertrophy and Rescues Denervation-Induced Atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Ghahrizjani, F.; Ghaedi, K.; Salamian, A.; Tanhaei, S.; Shoaraye Nejati, A.; Salehi, H.; Nabiuni, M.; Baharvand, H.; Nasr-Esfahani, M.H. Enhanced Expression of FNDC5 in Human Embryonic Stem Cell-Derived Neural Cells along with Relevant Embryonic Neural Tissues. Gene 2015, 557, 123–129. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J. Irisin Directly Stimulates Osteoclastogenesis and Bone Resorption in Vitro and in Vivo. eLife 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Reza, M.M.; Sim, C.M.; Subramaniyam, N.; Ge, X.; Sharma, M.; Kambadur, R.; McFarlane, C. Irisin Treatment Improves Healing of Dystrophic Skeletal Muscle. Oncotarget 2017, 8, 98553. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin. Cell 2019, 178, 507. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-Linked FNDC5/Irisin Rescues Synaptic Plasticity and Memory Defects in Alzheimer’s Models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, Z.; Marandi, S.M.; Alaei, H.; Esfarjani, F. The Effect of Preventive Exercise on the Neuroprotection in 6-Hydroxydopamine-Lesioned Rat Brain. Appl. Physiol. Nutr. Metab. 2019, 44, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Zarbakhsh, S.; Safari, M.; Aldaghi, M.R.; Sameni, H.R.; Ghahari, L.; Khaleghi Lagmouj, Y.; Rahimi Jaberi, K.; Parsaie, H. Irisin Protects the Substantia Nigra Dopaminergic Neurons in the Rat Model of Parkinson’s Disease. Iran. J. Basic Med. Sci. 2019, 22, 722. [Google Scholar] [CrossRef] [PubMed]
- Mudò, G.; Mäkelä, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic Expression and Activation of PGC-1α Protect Dopaminergic Neurons in the MPTP Mouse Model of Parkinson’s Disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef]
- Kam, T.I.; Park, H.; Chou, S.C.; Van Vranken, J.G.; Mittenbuhler, M.J.; Kim, H.; Mu, A.; Choi, Y.R.; Biswas, D.; Wang, J.; et al. Amelioration of Pathologic α-Synuclein-Induced Parkinson’s Disease by Irisin. Proc. Natl. Acad. Sci. USA 2022, 119, e2204835119. [Google Scholar] [CrossRef]
- Camerino, G.M.; Fonzino, A.; Conte, E.; De Bellis, M.; Mele, A.; Liantonio, A.; Tricarico, D.; Tarantino, N.; Dobrowolny, G.; Musarò, A.; et al. Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in Search of New Therapeutic Options. Sci. Rep. 2019, 9, 3185. [Google Scholar] [CrossRef]
- Asadi, Y.; Gorjipour, F.; Behrouzifar, S.; Vakili, A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018, 43, 1549–1560. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The Novel Exercise-Induced Hormone Irisin Protects against Neuronal Injury via Activation of the Akt and ERK1/2 Signaling Pathways and Contributes to the Neuroprotection of Physical Exercise in Cerebral Ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef]
- Tu, T.; Yin, S.; Pang, J.; Zhang, X.; Zhang, L.; Zhang, Y.; Xie, Y.; Guo, K.; Chen, L.; Peng, J.; et al. Irisin Contributes to Neuroprotection by Promoting Mitochondrial Biogenesis After Experimental Subarachnoid Hemorrhage. Front. Aging Neurosci. 2021, 13, 640215. [Google Scholar] [CrossRef]
- Dunn, S.L.; Olmedo, M.L.; Dunn, S.L.; Olmedo, M.L. Mechanotransduction: Relevance to Physical Therapist Practice—Understanding Our Ability to Affect Genetic Expression Through Mechanical Forces. Phys. Ther. 2016, 96, 712–721. [Google Scholar] [CrossRef]
- Khan, K.M.; Scott, A. Mechanotherapy: How Physical Therapists’ Prescription of Exercise Promotes Tissue Repair. Br. J. Sports Med. 2009, 43, 247. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by Mechanical Signals Promotes Bone Anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, F.; Hayashi, M.; Mouri, Y.; Nakamura, S.; Adachi, T.; Nakashima, T. Mechanotransduction via the Piezo1-Akt Pathway Underlies Sost Suppression in Osteocytes. Biochem. Biophys. Res. Commun. 2020, 521, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo Type Mechanosensitive Ion Channel Component 1 Functions as a Regulator of the Cell Fate Determination of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 17696. [Google Scholar] [CrossRef]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The Mechanosensitive Piezo1 Channel Is Required for Bone Formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical Sensing Protein PIEZO1 Regulates Bone Homeostasis via Osteoblast-Osteoclast Crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Song, J.; Liu, L.; Lv, L.; Hu, S.; Tariq, A.; Wang, W.; Dang, X. Fluid Shear Stress Induces Runx-2 Expression via Upregulation of PIEZO1 in MC3T3-E1 Cells. Cell Biol. Int. 2020, 44, 1491–1502. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 Mediate Mechanotransduction Essential for Bone Formation through Concerted Activation of NFAT-YAP1-ß-Catenin. eLife 2020, 9, e52779. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Hara, Y.; Okuda, M.; Itoh, K.; Nishioka, R.; Shiomi, A.; Nagao, K.; Mori, M.; Mori, Y.; Ikenouchi, J.; et al. Cell Surface Flip-Flop of Phosphatidylserine Is Critical for PIEZO1-Mediated Myotube Formation. Nat. Commun. 2018, 9, 2049. [Google Scholar] [CrossRef]
- Hirata, Y.; Nomura, K.; Kato, D.; Tachibana, Y.; Niikura, T.; Uchiyama, K.; Hosooka, T.; Fukui, T.; Oe, K.; Kuroda, R.; et al. A Piezo1/KLF15/IL-6 Axis Mediates Immobilization-Induced Muscle Atrophy. J. Clin. Investig. 2022, 132, 1–13. [Google Scholar] [CrossRef]
- Hughes, D.E.; Salter, D.M.; Dedhar, S.; Simpson, R. Integrin Expression in Human Bone. J. Bone Miner. Res. 1993, 8, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Litzenberger, J.B.; Tang, W.J.; Castillo, A.B.; Jacobs, C.R. Deletion of Β1 Integrins from Cortical Osteocytes Reduces Load-Induced Bone Formation. Cell. Mol. Bioeng. 2009, 2, 416–424. [Google Scholar] [CrossRef]
- Miyauchi, A.; Gotoh, M.; Kamioka, H.; Notoya, K.; Sekiya, H.; Takagi, Y.; Yoshimoto, Y.; Ishikawa, H.; Chihara, K.; Takano-Yamamoto, T.; et al. AlphaVbeta3 Integrin Ligands Enhance Volume-Sensitive Calcium Influx in Mechanically Stretched Osteocytes. J. Bone Miner. Metab. 2006, 24, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, M.B.; Shim, J.H.; Bok, S.; Kim, J.M. The Extracellular Signal-Regulated Kinase Mitogen-Activated Protein Kinase Pathway in Osteoblasts. J. Bone Metab. 2022, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.C.; Feng, X. PYK2 and FAK in Osteoclasts. Front. Biosci. 2003, 8, d1219–d1226. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Kazi, A.A.; Lang, C.H.; Timmons, J.A.; Pierre, P.; Wilkinson, D.J.; Smith, K.; Szewczyk, N.J.; Atherton, P.J. Focal Adhesion Kinase Is Required for IGF-I-Mediated Growth of Skeletal Muscle Cells via a TSC2/MTOR/S6K1-Associated Pathway. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E183–E193. [Google Scholar] [CrossRef]
- Watkins, M.; Grimston, S.K.; Norris, J.Y.; Guillotin, B.; Shaw, A.; Beniash, E.; Civitelli, R. Osteoblast Connexin43 Modulates Skeletal Architecture by Regulating Both Arms of Bone Remodeling. Mol. Biol. Cell 2011, 22, 1240. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Laird, D.W.; Amedee, J. Role of Connexins and Pannexins during Ontogeny, Regeneration, and Pathologies of Bone. BMC Cell Biol. 2016, 17, 29–38. [Google Scholar] [CrossRef]
- Gioftsidi, S.; Relaix, F.; Mourikis, P. The Notch Signaling Network in Muscle Stem Cells during Development, Homeostasis, and Disease. Skelet. Muscle 2022, 12, 9. [Google Scholar] [CrossRef]
- Arthur, S.T.; Cooley, I.D. The Effect of Physiological Stimuli on Sarcopenia; Impact of Notch and Wnt Signaling on Impaired Aged Skeletal Muscle Repair. Int. J. Biol. Sci. 2012, 8, 731. [Google Scholar] [CrossRef]
- Qin, Y.X.; Hu, M. Mechanotransduction in Musculoskeletal Tissue Regeneration: Effects of Fluid Flow, Loading, and Cellular-Molecular Pathways. Biomed Res. Int. 2014, 2014, 863421. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef]
- Astudillo, P. Extracellular Matrix Stiffness and Wnt/β-Catenin Signaling in Physiology and Disease. Biochem. Soc. Trans. 2020, 48, 1187–1198. [Google Scholar] [CrossRef]
- Robling, A.G.; Niziolek, P.J.; Baldridge, L.A.; Condon, K.W.; Allen, M.R.; Alam, I.; Mantila, S.M.; Gluhak-Heinrich, J.; Bellido, T.M.; Harris, S.E.; et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. J. Biol. Chem. 2008, 283, 5866–5875. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin Mediates Bone Response to Mechanical Unloading Through Antagonizing Wnt/β-Catenin Signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Witcher, P.C.; Miner, S.E.; Horan, D.J.; Bullock, W.A.; Lim, K.E.; Kang, K.S.; Adaniya, A.L.; Ross, R.D.; Loots, G.G.; Robling, A.G. Sclerostin Neutralization Unleashes the Osteoanabolic Effects of Dkk1 Inhibition. JCI Insight 2018, 3, e98673. [Google Scholar] [CrossRef]
- Sawakami, K.; Robling, A.G.; Ai, M.; Pitner, N.D.; Liu, D.; Warden, S.J.; Li, J.; Maye, P.; Rowe, D.W.; Duncan, R.L.; et al. The Wnt Co-Receptor LRP5 Is Essential for Skeletal Mechanotransduction but Not for the Anabolic Bone Response to Parathyroid Hormone Treatment. J. Biol. Chem. 2006, 281, 23698–23711. [Google Scholar] [CrossRef]
- Little, R.D.; Carulli, J.P.; Del Mastro, R.G.; Dupuis, J.; Osborne, M.; Folz, C.; Manning, S.P.; Swain, P.M.; Zhao, S.C.; Eustace, B.; et al. A Mutation in the LDL Receptor-Related Protein 5 Gene Results in the Autosomal Dominant High-Bone-Mass Trait. Am. J. Hum. Genet. 2002, 70, 11–19. [Google Scholar] [CrossRef]
- Niziolek, P.J.; Warman, M.L.; Robling, A.G. Mechanotransduction in Bone Tissue: The A214V and G171V Mutations in Lrp5 Enhance Load-Induced Osteogenesis in a Surface-Selective Manner. Bone 2012, 51, 459–465. [Google Scholar] [CrossRef]
- Zhong, Z.; Zeng, X.L.; Ni, J.H.; Huang, X.F. Comparison of the Biological Response of Osteoblasts after Tension and Compression. Eur. J. Orthod. 2013, 35, 59–65. [Google Scholar] [CrossRef]
- Da, Y.; Mou, Y.; Wang, M.; Yuan, X.; Yan, F.; Lan, W.; Zhang, F. Mechanical Stress Promotes Biological Functions of C2C12 Myoblasts by Activating PI3K/AKT/MTOR Signaling Pathway. Mol. Med. Rep. 2020, 21, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chi, R.; Liu, G.; Tian, W.; Zhang, J.; Zhang, R. Aerobic Exercise Regulates Apoptosis through the PI3K/Akt/GSK-3 β Signaling Pathway to Improve Cognitive Impairment in Alzheimer’s Disease Mice. Neural Plast. 2022, 2022, 1500710. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.H.; Lee, C.H.; Seo, M.K.; Cho, H.Y.; Lee, J.G.; Lee, B.J.; Park, S.W.; Kim, Y.H. Effect of Treadmill Exercise on the BDNF-Mediated Pathway in the Hippocampus of Stressed Rats. Neurosci. Res. 2013, 76, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.V.; Chang, L.W.; Brixius, K.; Wickström, S.A.; Montanez, E.; Thievessen, I.; Schwander, M.; Müller, U.; Bloch, W.; Mayer, U.; et al. Integrin-Linked Kinase Stabilizes Myotendinous Junctions and Protects Muscle from Stress-Induced Damage. J. Cell Biol. 2008, 180, 1037. [Google Scholar] [CrossRef]
- Xia, H.; Nho, R.S.; Kahm, J.; Kleidon, J.; Henke, C.A. Focal Adhesion Kinase Is Upstream of Phosphatidylinositol 3-Kinase/Akt in Regulating Fibroblast Survival in Response to Contraction of Type I Collagen Matrices via a Beta 1 Integrin Viability Signaling Pathway. J. Biol. Chem. 2004, 279, 33024–33034. [Google Scholar] [CrossRef]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The Role of Hormones in Muscle Hypertrophy. Phys. Sportsmed. 2018, 46, 129–134. [Google Scholar] [CrossRef]
- Koo, J.H.; Kwon, I.S.; Kang, E.B.; Lee, C.K.; Lee, N.H.; Kwon, M.G.; Cho, I.H.; Cho, J. yong Neuroprotective Effects of Treadmill Exercise on BDNF and PI3-K/Akt Signaling Pathway in the Cortex of Transgenic Mice Model of Alzheimer’s Disease. J. Exerc. Nutr. Biochem. 2013, 17, 151. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Chen, S.; Zhang, W.; Chen, Y.; Yang, Y. Moderate Exercise Has Beneficial Effects on Mouse Ischemic Stroke by Enhancing the Functions of Circulating Endothelial Progenitor Cell-Derived Exosomes. Exp. Neurol. 2020, 330, 113325. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579. [Google Scholar] [CrossRef]
- Gubert, C.; Hannan, A.J. Exercise Mimetics: Harnessing the Therapeutic Effects of Physical Activity. Nat. Rev. Drug Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Jung, S.Y.; Kim, D.Y.; Chung, Y.R.; Choi, H.H.; Jeon, J.W.; Han, J.H. PI3K-Akt-Wnt Pathway Is Implicated in Exercise-Induced Improvement of Short-Term Memory in Cerebral Palsy Rats. Int. Neurourol. J. 2018, 22, S156. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, W.; Lin, G.; Cheng, J.; Zeng, Y.; Shi, Y. Physical Exercise Promotes Proliferation and Differentiation of Endogenous Neural Stem Cells via ERK in Rats with Cerebral Infarction. Mol. Med. Rep. 2018, 18, 1455. [Google Scholar] [CrossRef]
- Hoffman, L.; Jensen, C.C.; Yoshigi, M.; Beckerle, M. Mechanical Signals Activate P38 MAPK Pathway-Dependent Reinforcement of Actin via Mechanosensitive HspB1. Mol. Biol. Cell 2017, 28, 2661. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Gardiner, P.F. Insight into Skeletal Muscle Mechanotransduction: MAPK Activation Is Quantitatively Related to Tension. J. Appl. Physiol. 2001, 91, 693–702. [Google Scholar] [CrossRef]
- Aharonov, A.; Shakked, A.; Umansky, K.B.; Savidor, A.; Genzelinakh, A.; Kain, D.; Lendengolts, D.; Revach, O.Y.; Morikawa, Y.; Dong, J.; et al. ERBB2 Drives YAP Activation and EMT-like Processes during Cardiac Regeneration. Nat. Cell Biol. 2020, 22, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Valladares-Ide, D.; Peñailillo, L.; Collao, N.; Marambio, H.; Deldicque, L.; Zbinden-Foncea, H. Activation of Protein Synthesis, Regeneration, and MAPK Signaling Pathways Following Repeated Bouts of Eccentric Cycling. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1131–E1139. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise Stimulates Pgc-1α Transcription in Skeletal Muscle through Activation of the P38 MAPK Pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Koulmann, N.; Richard-Bulteau, H.; Crassous, B.; Serrurier, B.; Pasdeloup, M.; Bigard, X.; Banzet, S. Physical Exercise during Muscle Regeneration Improves Recovery of the Slow/Oxidative Phenotype. Muscle Nerve 2017, 55, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1α Regulation by Exercise Training and Its Influences on Muscle Function and Insulin Sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists Are Exercise Mimetics. Cell 2008, 134, 405–415. [Google Scholar] [CrossRef]
- Inoue, A.; Cheng, X.W.; Huang, Z.; Hu, L.; Kikuchi, R.; Jiang, H.; Piao, L.; Sasaki, T.; Itakura, K.; Wu, H.; et al. Exercise Restores Muscle Stem Cell Mobilization, Regenerative Capacity and Muscle Metabolic Alterations via Adiponectin/AdipoR1 Activation in SAMP10 Mice. J. Cachexia. Sarcopenia Muscle 2017, 8, 370–385. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. Lausanne 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.S.; Kim, N.; Kong, I.D. Circulating Irisin Levels as a Predictive Biomarker for Sarcopenia: A Cross-Sectional Community-Based Study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Zerlotin, R.; Oranger, A.; Pignataro, P.; Dicarlo, M.; Maselli, F.; Mori, G.; Colucci, S.C.; Grano, M.; Colaianni, G. Irisin and Secondary Osteoporosis in Humans. Int. J. Mol. Sci. 2022, 23, 690. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2022, 33, 2049. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kwon, K.S. Pharmacological Interventions for Treatment of Sarcopenia: Current Status of Drug Development for Sarcopenia. Ann. Geriatr. Med. Res. 2019, 23, 98. [Google Scholar] [CrossRef]
- Kumar, S. Overcoming Gaps in the Treatment of Neurodegenerative Disease. eBioMedicine 2020, 60, 103088. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina Kaunas 2019, 55, 485. [Google Scholar] [CrossRef] [PubMed]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and Cellular Mechanisms of Skeletal Muscle Atrophy: An Update. J. Cachexia. Sarcopenia Muscle 2012, 3, 163. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance Exercise Training Increases the Expression of Irisin Concomitant with Improvement of Muscle Function in Aging Mice and Humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The Novel Myokine Irisin: Clinical Implications and Potential Role as a Biomarker for Sarcopenia in postmenopausal women. endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.A.; Nam, B.Y.; Park, S.; Lee, S.H.; Ryu, H.J.; Kwon, Y.E.; Kim, Y.L.; Park, K.S.; Oh, H.J.; et al. Irisin, a Novel Myokine Is an Independent Predictor for Sarcopenia and Carotid Atherosclerosis in Dialysis Patients. Atherosclerosis 2015, 242, 476–482. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, S.; Park, J.W.; Lee, N.S.; Hwang, S.Y.; Huh, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Youn, B.S.; et al. Implication of Circulating Irisin Levels with Brown Adipose Tissue and Sarcopenia in Humans. J. Clin. Endocrinol. Metab. 2014, 99, 2778–2785. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jang, I.Y.; Jung, H.W.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Lee, E.; Kim, B.J. Serum Irisin Level Is Independent of Sarcopenia and Related Muscle Parameters in Older Adults. Exp. Gerontol. 2022, 162, 111744. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Wanders, K.; Zammit, P.S. Myogenin Is an Essential Regulator of Adult Myofibre Growth and Muscle Stem Cell Homeostasis. eLife 2020, 9, 1–23. [Google Scholar] [CrossRef]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 Isoforms on Muscle Growth and Sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Ríos, R.; Carneiro, I.; Arce, V.M.; Devesa, J. Myostatin Is an Inhibitor of Myogenic Differentiation. Am. J. Physiol. Cell Physiol. 2002, 282, C993–C999. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin Stimulates Muscle Growth-Related Genes and Regulates Adipocyte Differentiation and Metabolism in Humans. Int. J. Obes. Lond 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin Knockout Drives Browning of White Adipose Tissue through Activating the AMPK-PGC1α-Fndc5 Pathway in Muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, A.A. Aging of the Human Neuromuscular System. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Kang, H.; Lin, C.Y.; Fan, Y. Applying Exercise-Mimetic Engineered Skeletal Muscle Model to Interrogate the Adaptive Response of Irisin to Mechanical Force. iScience 2022, 25, 104135. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef]
- Reppe, S.; Lien, T.G.; Hsu, Y.-H.; Gautvik, V.T.; Olstad, O.K.; Yu, R.; Bakke, H.G.; Lyle, R.; Kringen, M.K.; Glad, I.K.; et al. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef]
- Rozenberg, S.; Bruyère, O.; Bergmann, P.; Cavalier, E.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Lapauw, B.; Laurent, M.R.; De Schepper, J.; et al. How to Manage Osteoporosis before the Age of 50. Maturitas 2020, 138, 14–25. [Google Scholar] [CrossRef]
- Roos, P.M. Osteoporosis in Neurodegeneration. J. Trace Elem. Med. Biol. 2014, 28, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T. Osteoporosis and Cancer. Curr. Osteoporos. Rep. 2013, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Stavnichuk, M.; Mikolajewicz, N.; Corlett, T.; Morris, M.; Komarova, S.V. A Systematic Review and Meta-Analysis of Bone Loss in Space Travelers. Npj Microgravity 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92. [Google Scholar]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive Exercise in Astronauts on Prolonged Spaceflights Provides Partial Protection against Spaceflight-Induced Bone Loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating Irisin Is Associated with Osteoporotic Fractures in Postmenopausal Women with Low Bone Mass but Is Not Affected by Either Teriparatide or Denosumab Treatment for 3 Months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef]
- Roomi, A.B.; Nori, W.; Hamed, R.M. Lower Serum Irisin Levels Are Associated with Increased Osteoporosis and Oxidative Stress in Postmenopausal. Reports Biochem. Mol. Biol. 2021, 10, 13. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.J.; Guo, W.C.; Yang, J. Low Serum Concentrations of Irisin Are Associated with Increased Risk of Hip Fracture in Chinese Older Women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin Levels Are Lower in Young Amenorrheic Athletes Compared with Eumenorrheic Athletes and Non-Athletes and Are Associated with Bone Density and Strength Estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin Is Associated with Osteoporotic Fractures Independently of Bone Mineral Density, Body Composition or Daily Physical Activity. Clin. Endocrinol. (Oxf) 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Serbest, S.; Tiftikçi, U.; Tosun, H.B.; Kısa, Ü. The Irisin Hormone Profile and Expression in Human Bone Tissue in the Bone Healing Process in Patients. Med. Sci. Monit. 2017, 23, 4278. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The Decisive Early Phase of Bone Regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An Overview of the Regulation of Bone Remodelling at the Cellular Level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The Effect of Irisin on Bone Cells in Vivo and in Vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yinrg, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. Dis. Model. Mech. 2017, 10, 499. [Google Scholar] [CrossRef]
- Miller, J.H.; Das, V. Potential for Treatment of Neurodegenerative Diseases with Natural Products or Synthetic Compounds That Stabilize Microtubules. Curr. Pharm. Des. 2020, 26, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhu, J.Y.; Liu, X.D.; Luo, M.Y.; Xu, N.J. Roles of Physical Exercise in Neurodegeneration: Reversal of Epigenetic Clock. Transl. Neurodegener. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Whitty, E.; Mansour, H.; Aguirre, E.; Palomo, M.; Charlesworth, G.; Ramjee, S.; Poppe, M.; Brodaty, H.; Kales, H.C.; Morgan-Trimmer, S.; et al. Efficacy of Lifestyle and Psychosocial Interventions in Reducing Cognitive Decline in Older People: Systematic Review. Ageing Res. Rev. 2020, 62, 101113. [Google Scholar] [CrossRef] [PubMed]
- Küster, O.C.; Fissler, P.; Laptinskaya, D.; Thurm, F.; Scharpf, A.; Woll, A.; Kolassa, S.; Kramer, A.F.; Elbert, T.; von Arnim, C.A.F.; et al. Cognitive Change Is More Positively Associated with an Active Lifestyle than with Training Interventions in Older Adults at Risk of Dementia: A Controlled Interventional Clinical Trial. BMC Psychiatry 2016, 16, 315. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.F.; Erickson, K.I. Capitalizing on Cortical Plasticity: Influence of Physical Activity on Cognition and Brain Function. Trends Cogn. Sci. 2007, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kwak, S.; Ha, J.; Oh, D.J.; Kim, M.; Cho, S.Y.; Kim, H.; Lee, J.Y.; Kim, E. Loss of Association between Plasma Irisin Levels and Cognition in Alzheimer’s Disease. Psychoneuroendocrinology 2022, 136, 105624. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C. Circulating Levels of Irisin in Obese Individuals at Genetic Risk for Alzheimer’s Disease: Correlations with Amyloid-β, Metabolic, and Neurocognitive Indices. Behav. Brain Res. 2021, 400, 113013. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, S.; Parone, P.A.; Lopes, V.S.; Lillo, C.; McAlonis-Downes, M.; Lee, S.K.; Vetto, A.P.; Petrosyan, S.; Marsala, M.; Murphy, A.N.; et al. Elevated PGC-1α Activity Sustains Mitochondrial Biogenesis and Muscle Function without Extending Survival in a Mouse Model of Inherited ALS. Cell Metab. 2012, 15, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.; Lang, K.; Buck, E.; Higelin, J.; Barteczko, L.; Pasquarelli, N.; Sprissler, J.; Lucas, T.; Holzmann, K.; Demestre, M.; et al. ALS-Causing Mutations Differentially Affect PGC-1α Expression and Function in the Brain vs. Peripheral Tissues. Neurobiol. Dis. 2017, 97, 36–45. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Tremolizzo, L.; Ruscica, M.; Macchi, C.; Riva, N.; Weydt, P.; Corradi, E.; Magni, P.; Sansone, V. Serum Irisin Is Upregulated in Patients Affected by Amyotrophic Lateral Sclerosis and Correlates with Functional and Metabolic Status. J. Neurol. 2018, 265, 3001–3008. [Google Scholar] [CrossRef]
- Tu, W.J.; Qiu, H.C.; Cao, J.L.; Liu, Q.; Zeng, X.W.; Zhao, J.Z. Decreased Concentration of Irisin Is Associated with Poor Functional Outcome in Ischemic Stroke. Neurotherapeutics 2018, 15, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, Z.; Ke, J.; Wang, Y.; Wu, H. Exercise-Linked Irisin Prevents Mortality and Enhances Cognition in a Mice Model of Cerebral Ischemia by Regulating Klotho Expression. Oxid. Med. Cell. Longev. 2021, 2021, 1697070. [Google Scholar] [CrossRef]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent. Neuroscience 2013, 240, 155. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise Hormone Irisin Is a Critical Regulator of Cognitive Function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kuzuya, A.; Tanigawa, K.; Araki, M.; Kawai, R.; Ma, B.; Sasakura, Y.; Maesako, M.; Tashiro, Y.; Miyamoto, M.; et al. Fibronectin Type III Domain-Containing Protein 5 Interacts with APP and Decreases Amyloid β Production in Alzheimer’s Disease. Mol. Brain 2018, 11, 61. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The Effects of Acute and Chronic Exercise on PGC-1α, Irisin and Browning of Subcutaneous Adipose Tissue in Humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Belviranlı, M.; Okudan, N. Exercise Training Protects Against Aging-Induced Cognitive Dysfunction via Activation of the Hippocampal PGC-1α/FNDC5/BDNF Pathway. NeuroMolecular Med. 2018, 20, 386–400. [Google Scholar] [CrossRef]
- Turner, G.M.; McMullan, C.; Aiyegbusi, O.L.; Bem, D.; Marshall, T.; Calvert, M.; Mant, J.; Belli, A. Stroke Risk Following Traumatic Brain Injury: Systematic Review and Meta-Analysis. Int. J. Stroke 2021, 16, 370. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative Neural Stem Cells Have High Endogenous ROS Levels That Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Peng, J.; Deng, X.; Huang, W.; Yu, J.H.; Wang, J.X.; Wang, J.P.; Yang, S.B.; Liu, X.; Wang, L.; Zhang, Y.; et al. Irisin Protects against Neuronal Injury Induced by Oxygen-Glucose Deprivation in Part Depends on the Inhibition of ROS-NLRP3 Inflammatory Signaling Pathway. Mol. Immunol. 2017, 91, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Tissue Repair. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1517–1538. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and Metabolism in Tissue Repair and Regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Stramer, B.M.; Mori, R.; Martin, P. The Inflammation-Fibrosis Link? A Jekyll and Hyde Role for Blood Cells during Wound Repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.; Rohde, T.; Asp, S.; Schjerling, P.; Pedersen, B.K. Pro- and Anti-Inflammatory Cytokine Balance in Strenuous Exercise in Humans. J. Physiol. 1999, 515, 287. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.; Bilski, J.; Pochec, E.; Brzozowski, T. New Insight Into the Direct Anti-Inflammatory Activity of a Myokine:Irisin against pro-Inflammatory Activation of Adipocytes. J. Physiol. Pharmacol. 2017, 5, 243–251. [Google Scholar]
- Li, Q.; Zhang, M.; Zhao, Y.; Dong, M. Irisin Protects Against LPS-Stressed Cardiac Damage Through Inhibiting Inflammation, Apoptosis, and Pyroptosis. Shock 2021, 56, 1009–1018. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin Alleviates LPS-Induced Liver Injury and Inflammation through Inhibition of NLRP3 Inflammasome and NF-ΚB Signaling. J. Recept. Signal Transduct. 2020, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Li, Q.; Zhong, J.; Xia, F.; Zheng, S.; Lu, J.; Deng, Y.; Hu, Y. Combination of Melatonin and Irisin Ameliorates Lipopolysaccharide-Induced Cardiac Dysfunction through Suppressing the Mst1-JNK Pathways. J. Cell. Physiol. 2020, 235, 6647–6659. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, M.; Tan, J.; Pei, X.; Lu, C.; Xin, Y.; Deng, S.; Zhao, F.; Gao, Y.; Gong, Y. Irisin Ameliorates Neuroinflammation and Neuronal Apoptosis through Integrin αVβ5/AMPK Signaling Pathway after Intracerebral Hemorrhage in Mice. J. Neuroinflammation 2022, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Yu, Q.; Li, G.; Li, J.; Sun, L.; Yang, Y.; Tao, L. Irisin Protects Cerebral Neurons from Hypoxia/Reoxygenation via Suppression of Apoptosis and Expression of Pro-Inflammatory Cytokines. Neuroimmunomodulation 2022, 29, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I. Irisin Acts as a Regulator of Macrophages Host Defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk between Muscle and Adipose Tissues. Int. J. Obes. (Lond) 2016, 40, 434. [Google Scholar] [CrossRef]
- Xiong, X.Q.; Geng, Z.; Zhou, B.; Zhang, F.; Han, Y.; Zhou, Y.B.; Wang, J.J.; Gao, X.Y.; Chen, Q.; Li, Y.H.; et al. FNDC5 Attenuates Adipose Tissue Inflammation and Insulin Resistance via AMPK-Mediated Macrophage Polarization in Obesity. Metabolism 2018, 83, 31–41. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise Hormone Irisin Mitigates Endothelial Barrier Dysfunction and Microvascular Leakage–Related Diseases. JCI Insight 2020, 5, e136277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Q.; Wu, J.; Zhou, X.; Weng, J.; Xu, J.; Wang, W.; Huang, Q.; Guo, X. Role of Src in Vascular Hyperpermeability Induced by Advanced Glycation End Products. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin αVβ5 Receptor. J. Cell. Mol. Med. 2020, 24, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.N.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G.S. In Vivo Satellite Cell Activation via Myf5 and MyoD in Regenerating Mouse Skeletal Muscle. J. Cell Sci. 1999, 112, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of Satellite Cell Function in Muscle Regeneration and Its Disruption in Ageing. Nat. Rev. Mol. Cell Biol. 2021, 23, 204–226. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Hill, M.; Wernig, A.; Goldspink, G. Muscle Satellite (Stem) Cell Activation during Local Tissue Injury and Repair. J. Anat. 2003, 203, 89. [Google Scholar] [CrossRef]
- Park, K.; Ju, W.C.; Yeo, J.H.; Kim, J.Y.; Seo, H.S.; Uchida, Y.; Cho, Y. Increased OPG/RANKL Ratio in the Conditioned Medium of Soybean-Treated Osteoblasts Suppresses RANKL-Induced Osteoclast Differentiation. Int. J. Mol. Med. 2014, 33, 178–184. [Google Scholar] [CrossRef]
- Colucci, S.C.; Buccoliero, C.; Sanesi, L.; Errede, M.; Colaianni, G.; Annese, T.; Khan, M.P.; Zerlotin, R.; Dicarlo, M.; Schipani, E.; et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. Int. J. Mol. Sci. 2021, 22, 10863. [Google Scholar] [CrossRef]
- Ostadsharif, M.; Ghaedi, K.; Hossein Nasr-Esfahani, M.; Mojbafan, M.; Tanhaie, S.; Karbalaie, K.; Baharvand, H. The Expression of Peroxisomal Protein Transcripts Increased by Retinoic Acid during Neural Differentiation. Differentiation 2011, 81, 127–132. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 Knockdown Significantly Decreased Neural Differentiation Rate of Mouse Embryonic Stem Cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Wang, X.; Wang, Z.Y.; Wang, Y.Z.; Chen, L.W.; Luo, Z.J. Brain-Derived Neurotrophic Factor Stimulates Proliferation and Differentiation of Neural Stem Cells, Possibly by Triggering the Wnt/β-Catenin Signaling Pathway. J. Neurosci. Res. 2013, 91, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined Adult Neurogenesis and BDNF Mimic Exercise Effects on Cognition in an Alzheimer’s Mouse Model. Science 2018, 361, eaan8821. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zou, D.; Yi, L.; Chen, M.; Gao, Y.; Zhou, R.; Zhang, Q.; Zhou, Y.; Zhu, J.; Chen, K.; et al. Quercetin Ameliorates Hypobaric Hypoxia-Induced Memory Impairment through Mitochondrial and Neuron Function Adaptation via the PGC-1α Pathway. Restor. Neurol. Neurosci. 2015, 33, 143–157. [Google Scholar] [CrossRef]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological Concentrations of Irisin Increase Cell Proliferation without Influencing Markers of Neurite Outgrowth and Synaptogenesis in Mouse H19-7 Hippocampal Cell Lines. Metabolism 2013, 62, 1131–1136. [Google Scholar] [CrossRef]
- Xia, D.Y.; Huang, X.; Bi, C.F.; Mao, L.L.; Peng, L.J.; Qian, H.R. PGC-1α or FNDC5 Is Involved in Modulating the Effects of Aβ1-42 Oligomers on Suppressing the Expression of BDNF, a Beneficial Factor for Inhibiting Neuronal Apoptosis, Aβ Deposition and Cognitive Decline of APP/PS1 Tg Mice. Front. Aging Neurosci. 2017, 9, 65. [Google Scholar] [CrossRef]
- Huang, S.H.; Yang, S.M.; Lo, J.J.; Wu, S.H.; Tai, M.H. Irisin Gene Delivery Ameliorates Burn-Induced Sensory and Motor Neuropathy. Int. J. Mol. Sci. 2020, 21, 7798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, L.; Kang, H.; Lin, C.-Y.; Fan, Y. Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 6551. https://doi.org/10.3390/ijms24076551
Zhang Y, Wang L, Kang H, Lin C-Y, Fan Y. Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. International Journal of Molecular Sciences. 2023; 24(7):6551. https://doi.org/10.3390/ijms24076551
Chicago/Turabian StyleZhang, Yuwei, Lizhen Wang, Hongyan Kang, Chia-Ying Lin, and Yubo Fan. 2023. "Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration" International Journal of Molecular Sciences 24, no. 7: 6551. https://doi.org/10.3390/ijms24076551
APA StyleZhang, Y., Wang, L., Kang, H., Lin, C.-Y., & Fan, Y. (2023). Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration. International Journal of Molecular Sciences, 24(7), 6551. https://doi.org/10.3390/ijms24076551







