3D Bioprinting for Next-Generation Personalized Medicine
Abstract
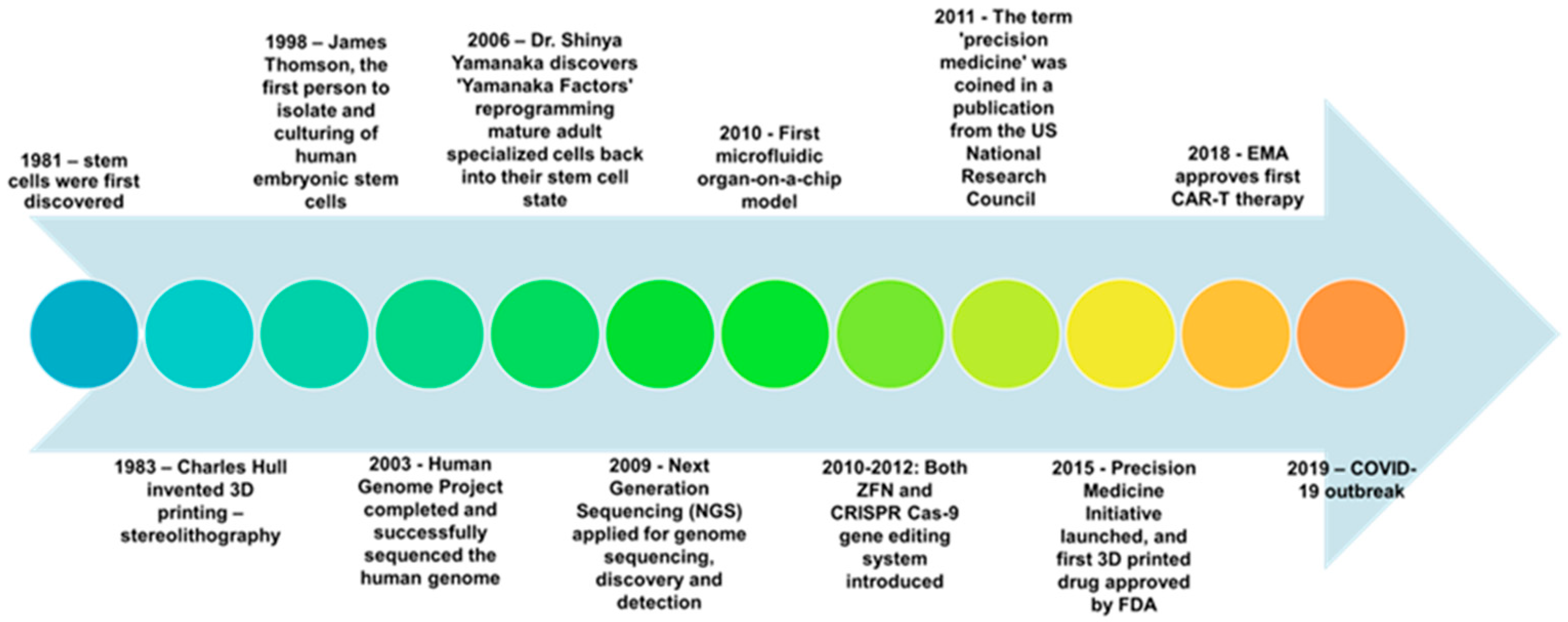
1. Introduction
2. Bioprinting: Methods and Materials
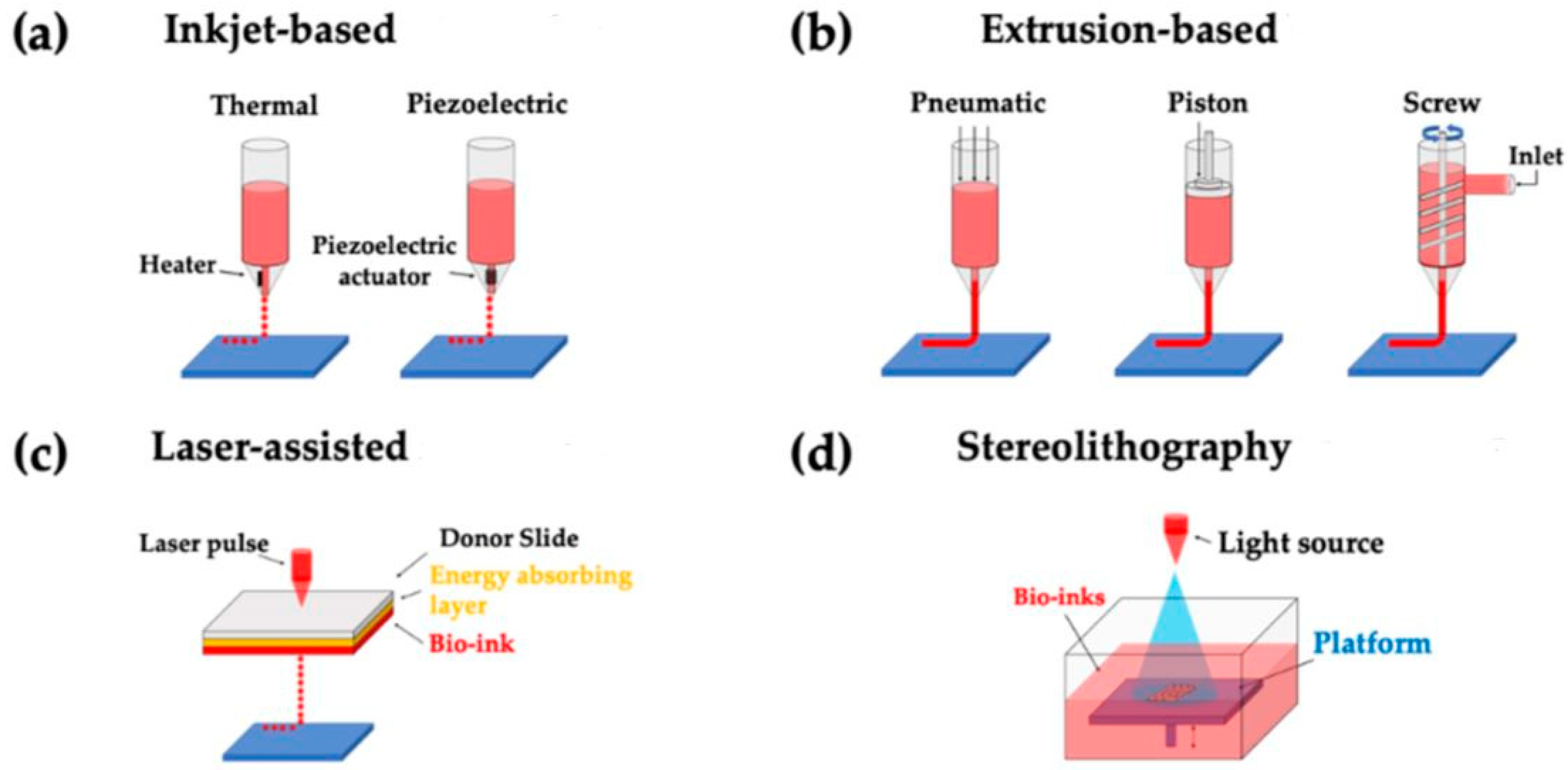
2.1. Bioprinting Technology
2.1.1. Inkjet Printing
2.1.2. Extrusion Printing
2.1.3. Laser-Assisted Printing
2.1.4. Stereolithography
| Bioprinting Method | Key Aspects | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Inkjet | First bioprinting technology that has a bio-ink cartridge. Minimum droplet volume of 20 nL |
|
| [13,14,15,16] |
| Extrusion | A modification of inkjet-based bioprinting that prints a cylindrical stream onto a printing surface in a continuous line |
|
| [22,23,32] |
| Laser-assisted | Propels bio-ink onto the printing surface |
|
| [11,20,21,33] |
| Stereolithography | Uses UV light to solidify bio-ink layer-by-layer |
|
| [24,25,27,34] |
2.2. Cell Source and Bio-Inks
3. Applications in the Discovery of Personalized Medicine
3.1. Printing of Stem-Cell Differentiated Organs for Tissue Regeneration
3.1.1. Bone
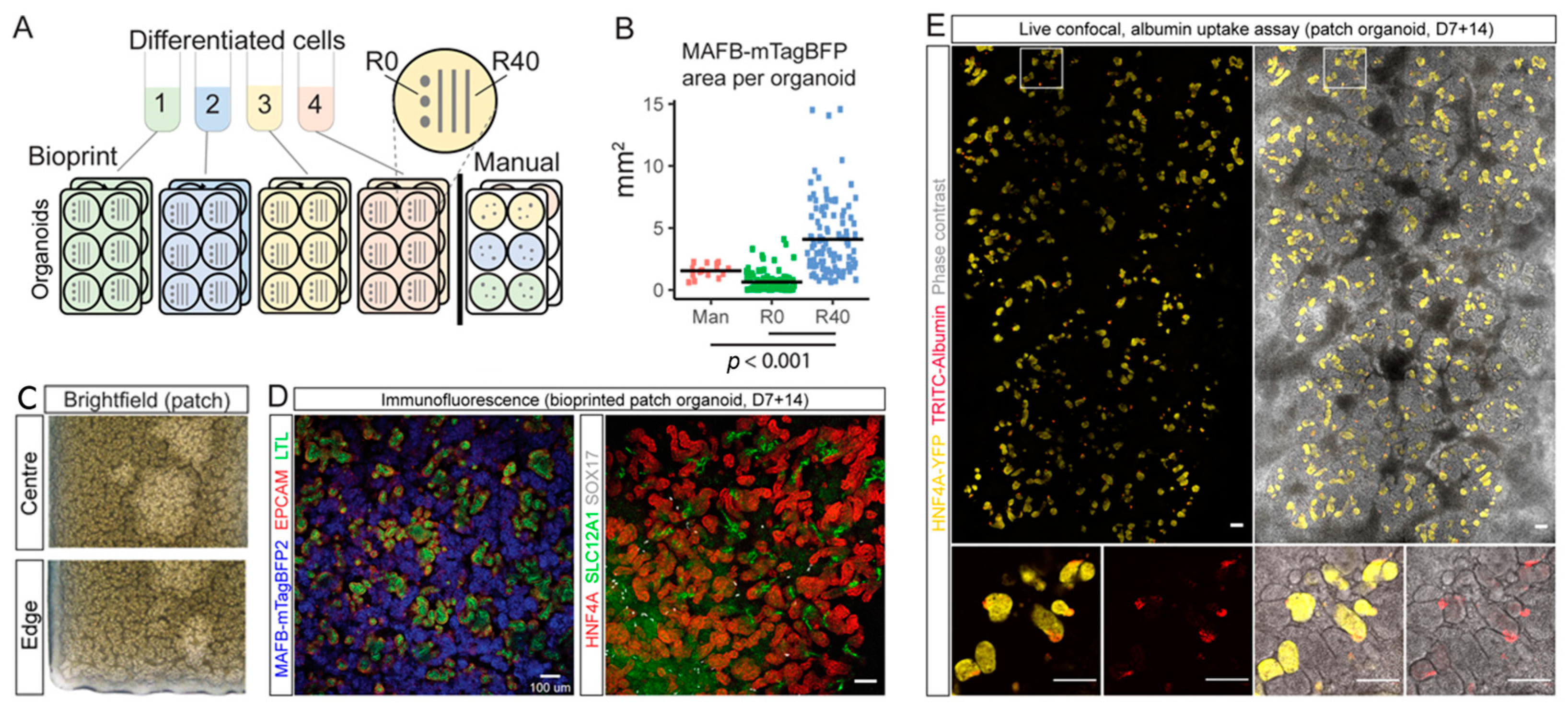
3.1.2. Kidney
| Target Tissue | Bioprinting Method | Cell Type | Biomaterial | Cellular Response | References |
|---|---|---|---|---|---|
| Bone | Commercial fused-filament fabrication 3D printer (DeltaWASP 2040; CSP srl, Massa Lombarda, Italy) | Human gingival mesenchymal stem cells (hGMSCs) | Poly(lactide) (PLA), extracellular vesicles (EVs), polyethyleneimine (PEI)-engineered EVs (PEI-EVs) | (1) Both 3D-PLA + EVs + hGMSCs and 3D-PLA + PEI-EVs + hGMSCs showed no cytotoxicity (2) Better osteogenic properties were observed in 3D-PLA + PEI-EVs + hGMSCs. New bone nodules and blood vessels were observed in calvariae after in vivo implantation in rats subjected to cortical calvaria bone tissue damage. | [62] |
| 3D Cloning FDM printer (Microbras, Brazil), PLA white commercial filament (1.75 mm in diameter, produced by E-Sun, China) | Porcine bone marrow stem cells (MSCs) | Poly(lactic acid) (PLA), polydopamine (PDA), type-I colla-gen (COL I) | PDA combined with COL coating increased cell adhesion and the metabolic activity of MSCs in the early stage (<7 days) of cell culture and facilitated the deposition of extracellular matrix by day 14, and produced much higher amounts of alkaline phosphatase than un-coated PLA by day 21. | ||
| Kidney and Liver | Extrusion bioprinting (Cellink INKREDIBLE + 3D bioprinter) | iPS-derived parenchymal (hepatocyte-like) cells, iPS-derived hepato-cyte-like cells spheroids | Matrigel | Liver constructs from 3D printing with hepatic spheroids showed prolonged survival, reduced cell death, increased urea production, and prolonged secretion of albumin and A1AT, as compared to printed constructs using single-cell dispersion. | [66] |
| Extrusion bioprinting (Novogen 3D bio-printer) | iPSC | STEMdiff APEL and TESR-E6 medium | Bioprinted line conformation increased nephron numbers, as measured by an increase in MAFB+ glomerular area, as compared to manual organoids. | [68] | |
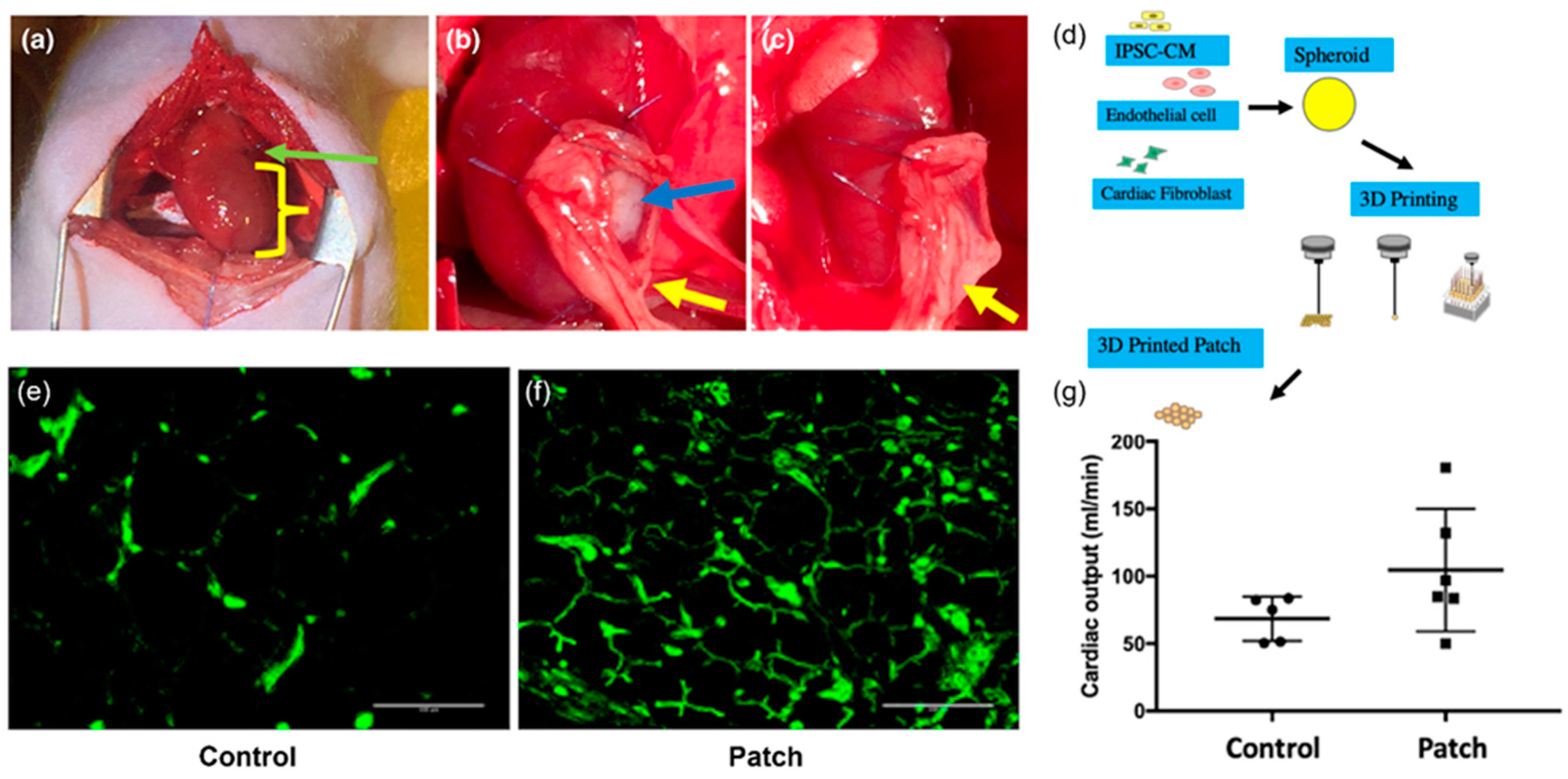
| Heart | Spheroid bioprinting with microfluidic-chip-based 3D cell-culturing system (Regenova, Cyfuse Bio-medical K.K., Tokyo, Japan) | Human-induced pluripotent stem-cell-derived cardiomyocytes (hiPSC-CMs), human adult ventricular cardiac fibroblasts (FBs), and human umbilical vein endothelial cells (ECs) | Free of biomaterials | In vivo implantation of the 3D-bioprinted cardiac patches onto nude rat hearts showed viable cells in the patch along with erythrocytes (evidence of vascularization), and the presence of human nucleic acid (HNA)-positive cells in rat myocardium (evidence of engraftment). | [69] |
| Spheroid bioprinting with microfluidic-chip-based 3D cell-culturing system (Regenova, Cyfuse Biomedical K.K., Tokyo, Japan) | Human IPSC-derived cardiomyocytes, fibroblasts, and endothelial cells | Free of biomaterials | In vivo implantation of the bioprinted cardiac patches onto rat myocardial infarction model showed lower scar area, higher vessel count, and higher cardiac output than the control group without the implantation. The survival rates were 100% and 83% in the experimental and the control groups, respectively, after 4 weeks of surgery | [70] | |
| Nerve | Micro-extrusion bioprinting | Frontal cortical human neural stem cells (hNSCs) | Polysaccharides alginate (Al), carboxymethyl-chitosan (CMC), and agarose (Ag) | Co-printing of cells with bio-ink allowed the formation of a porous 3D-scaffold encapsulation of stem cells for in situ expansion and differentiation. Differentiated neurons formed synaptic contacts and showed spontaneous calcium spikes and bicuculline-induced bursting activity. | [71] |
| Extrusion bioprinting | Cortical neurons and glial cells de-rived from human iPSCs | Matrigel and alginate | Long-term survival of neurons, up to 70 days post-printing, was observed. Functional analysis showed calcium activity and a small degree of synchronous activity. | [49] | |
| Lab-on-a-printer (LOP) technology (Aspect Biosystems’ RX1 printer) | hiPSC-derived neural progenitor cells | Fibrinogen base with alginate, cross-linked with a mixture of chitosan, calcium chloride, thrombin, and genipin | Cell viability was 91.65 ± 6.85% by day 6 of the culture period, and 64.12 ± 21.27% by day 15. The printed neural tissues showed neurite extension and the expression of neuronal marker TUJ1 and nucleated cell marker | [72,73] | |
| Extrusion bioprinting | Induced pluripotent stem cell (iPSC)-derived spinal neuronal progenitor cells (sNPCs) and oligodendrocyte progenitor cells (OPCs) | Matrigel | Cell viability was >75% for both iPSC-derived sNPCs and OPCs printed in 50% Matrigel after 4 days in culture. The bioprinted sNPCs differentiated and showed progressive axon propagation in the micro-scale scaffold channels. Functionality was verified by cellular response signaling molecules, potassium and glutamate. | [54] | |
| Pancreas | Micro-extrusion bioprinting | Human umbilical vein endothelial cells | Pancreatic tissue-derived dECM (pdECM) | PdECM increased the insulin secretion over the conventionally applied biomaterials, alginate and collagen. Co-culturing with human umbilical vein-derived endothelial cells decreased the central necrosis of islets. Culturing in both 3D gels (without printing) and the printed construct showed similar viability on days 1 and 5. | [74] |
| Cornea | Laser-assisted bioprinting (LaBP) | Human embryonic stem-cell-derived limbal epithelial stem cells (hESC-LESC), human adipose-tissue-derived stem cells (hASCs) | Recombinant human laminin and human sourced collagen I | The printed hESC-LESCs retained an epithelium-like structure and showed apical expression of CK3 and basal expression of the progenitor markers. After 7 days in vivo transplantation in the porcine organ, the 3D-bioprinted stromal structures showed interaction and attachment to the host tissue. | [75] |
3.1.3. Heart
3.1.4. Neurons and Central Nervous System
3.1.5. Others Approaches for Pancreatic and Corneal Applications
3.2. Printing of In Vitro Models for Drug Development
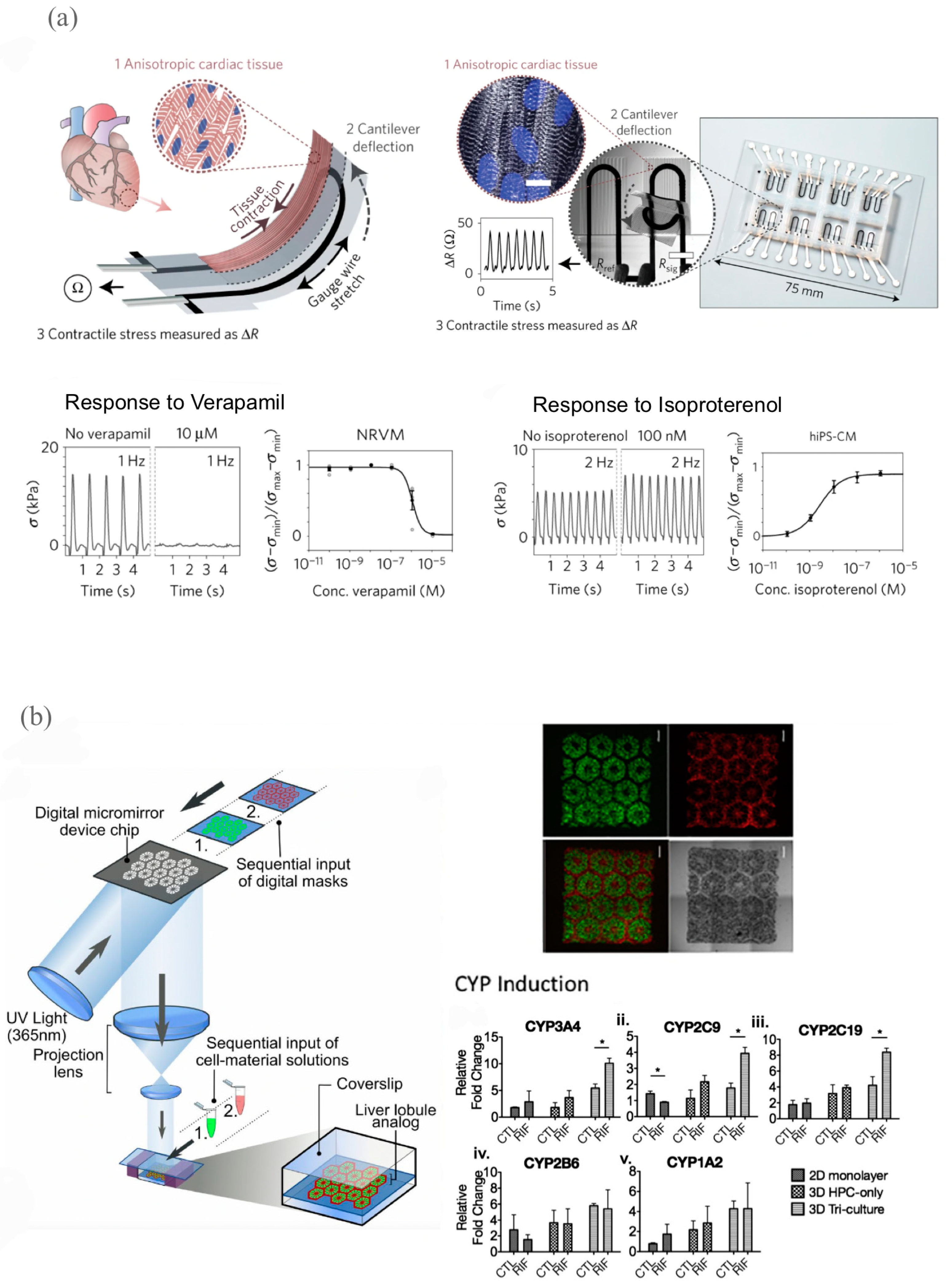
3.2.1. Cardiovascular Models for Drug Development
3.2.2. Liver Models for Drug Development
3.2.3. Kidney Models for Drug Development
3.2.4. Brain Models for Drug Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorshkov, K.; Chen, C.Z.; Marshall, R.E.; Mihatov, N.; Choi, Y.; Nguyen, D.-T.; Southall, N.; Chen, K.; Park, J.K.; Zheng, W. Advancing Precision Medicine with Personalized Drug Screening. Drug Discov. Today 2019, 24, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nieuwenhuis, L.M.; Keating, B.J.; Festen, E.A.M.; de Meijer, V.E. The Impact of Donor and Recipient Genetic Variation on Outcomes After Solid Organ Transplantation: A Scoping Review and Future Perspectives. Transplantation 2022, 106, 1548–1557. [Google Scholar] [CrossRef]
- Mathur, S.; Sutton, J. Personalized Medicine Could Transform Healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Robertson, J.A. Human Embryonic Stem Cell Research: Ethical and Legal Issues. Nat. Rev. Genet. 2001, 2, 74–78. [Google Scholar] [CrossRef]
- Reddy, C.V.; Balamuralidhara, V.; Venkatesh, M.P.; Pramod Kumar, T.M. First FDA Approved 3D Printed Drug Paved New Path for Increased Precision in Patient Care. Appl. Clin. Res. Clin. Trials Regul. Aff. 2020, 7, 93–103. [Google Scholar] [CrossRef]
- Overby, C.L.; Tarczy-Hornoch, P. Personalized Medicine: Challenges and Opportunities for Translational Bioinformatics. Pers. Med. 2013, 10, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, S.; Cheng, S.; Jin, Y.; Zhang, N.; Wang, Y. Application of Ovarian Cancer Organoids in Precision Medicine: Key Challenges and Current Opportunities. Front. Cell Dev. Biol. 2021, 9, 701429. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Powell, R.T.; Gottlieb, A. Molecular Pathways Enhance Drug Response Prediction Using Transfer Learning from Cell Lines to Tumors and Patient-Derived Xenografts. Sci. Rep. 2022, 12, 16109. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; Soker, S.; Skardal, A. 3D Bioprinting for High-Throughput Screening: Drug Screening, Disease Modeling, and Precision Medicine Applications. Appl. Phys. Rev. 2019, 6, 011302. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shao, G.; Li, L. Micro/Nano Functional Devices Fabricated by Additive Manufacturing. Prog. Mater. Sci. 2023, 131, 101020. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of Cell Viability and Functionality in Vessel-like Bioprintable Cell-Laden Tubular Channels. J. Biomech. Eng. 2013, 135, 091011. [Google Scholar] [CrossRef]
- Ng, W.L.; Huang, X.; Shkolnikov, V.; Goh, G.L.; Suntornnond, R.; Yeong, W.Y. Controlling Droplet Impact Velocity and Droplet Volume: Key Factors to Achieving High Cell Viability in Sub-Nanoliter Droplet-Based Bioprinting. Int. J. Bioprint. 2021, 8, 424. [Google Scholar] [CrossRef]
- Cui, X.; Gao, G.; Qiu, Y. Accelerated Myotube Formation Using Bioprinting Technology for Biosensor Applications. Biotechnol. Lett. 2013, 35, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Jammalamadaka, U.; Tappa, K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent Advances in Bioprinting Techniques: Approaches, Applications and Future Prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell Damage Evaluation of Thermal Inkjet Printed Chinese Hamster Ovary Cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Xia, Z.; Jin, S.; Ye, K. Tissue and Organ 3D Bioprinting. SLAS Technol. 2018, 23, 301–314. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current Advances and Future Perspectives in Extrusion-Based Bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.T.; Dang, T.T.; Hwang, C.H.; Back, S.H.; Koo, K. Coaxial Printing of Double-Layered and Free-Standing Blood Vessel Analogues without Ultraviolet Illumination for High-Volume Vascularised Tissue. Biofabrication 2020, 12, 045033. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Duong, V.T.; Hwang, C.H.; Koo, K. Angiogenesis in Free-Standing Two-Vasculature-Embedded Scaffold Extruded by Two-Core Laminar Flow Device. Int. J. Bioprint. 2022, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Koch, L.; Brandt, O.; Deiwick, A.; Chichkov, B. Laser-Assisted Bioprinting at Different Wavelengths and Pulse Durations with a Metal Dynamic Release Layer: A Parametric Study. Int. J. Bioprint. 2017, 3, 96. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, X.; Dai, R.; Holzman, J.F.; Kim, K. An Ultrafast Hydrogel Photocrosslinking Method for Direct Laser Bioprinting. RSC Adv. 2016, 6, 21099–21104. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.-C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of Complex Porous Tissue Engineering Scaffolds Using 3D Projection Stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Weng, W.; Cheng, K.; Wang, Q. Visible-Light-Responsive Surfaces for Efficient, Noninvasive Cell Sheet Harvesting. ACS Appl. Mater. Interfaces 2017, 9, 28250–28259. [Google Scholar] [CrossRef]
- Hoffmann, A.; Leonards, H.; Tobies, N.; Pongratz, L.; Kreuels, K.; Kreimendahl, F.; Apel, C.; Wehner, M.; Nottrodt, N. New Stereolithographic Resin Providing Functional Surfaces for Biocompatible Three-Dimensional Printing. J. Tissue Eng. 2017, 8, 2041731417744485. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, S.M.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective Bioprinting Resolution in Tissue Model Fabrication. Lab. Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Yu, Y. Bioprinting Toward Organ Fabrication: Challenges and Future Trends. IEEE Trans. Biomed. Eng. 2013, 60, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks. Biofabrication 2015, 7, 045009. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a Clay Based Bioink for 3D Cell Printing for Skeletal Application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kim, B.-S.; Gao, G. Advanced Strategies for 3D Bioprinting of Tissue and Organ Analogs Using Alginate Hydrogel Bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Narayanan, J.; Liu, X.; Chong, T.; Chen, S.; Chung, T.-S. Topology Evolution and Gelation Mechanism of Agarose Gel. Available online: https://pubs.acs.org/doi/pdf/10.1021/jp044473u (accessed on 23 February 2023).
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Leone, L.M.; Kaufman, L.J. Elastic Moduli of Collagen Gels Can Be Predicted from Two-Dimensional Confocal Microscopy. Biophys. J. 2009, 97, 2051–2060. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Rosendahl, J.; Catalán, J. Nanocelluloses—Nanotoxicology, Safety Aspects and 3D Bioprinting. In Nanotoxicology in Safety Assessment of Nanomaterials; Louro, H., Silva, M.J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; pp. 155–177. ISBN 978-3-030-88071-2. [Google Scholar]
- Bandyopadhyay, A.; Mandal, B.B.; Bhardwaj, N. 3D Bioprinting of Photo-Crosslinkable Silk Methacrylate (SilMA)-Polyethylene Glycol Diacrylate (PEGDA) Bioink for Cartilage Tissue Engineering. J. Biomed. Mater. Res. A 2022, 110, 884–898. [Google Scholar] [CrossRef]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-Write Bioprinting Three-Dimensional Biohybrid Systems for Future Regenerative Therapies. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98B, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D Bioprinting Using Stem Cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef]
- Skeldon, G.; Lucendo-Villarin, B.; Shu, W. Three-Dimensional Bioprinting of Stem-Cell Derived Tissues for Human Regenerative Medicine. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170224. [Google Scholar] [CrossRef]
- Yamanaka, S. Strategies and New Developments in the Generation of Patient-Specific Pluripotent Stem Cells. Cell Stem Cell 2007, 1, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser Bioprinting of Human Induced Pluripotent Stem Cells-the Effect of Printing and Biomaterials on Cell Survival, Pluripotency, and Differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef] [PubMed]
- Salaris, F.; Colosi, C.; Brighi, C.; Soloperto, A.; de Turris, V.; Benedetti, M.C.; Ghirga, S.; Rosito, M.; Di Angelantonio, S.; Rosa, A. 3D Bioprinted Human Cortical Neural Constructs Derived from Induced Pluripotent Stem Cells. J. Clin. Med. 2019, 8, 1595. [Google Scholar] [CrossRef] [PubMed]
- Chikae, S.; Kubota, A.; Nakamura, H.; Oda, A.; Yamanaka, A.; Akagi, T.; Akashi, M. Three-Dimensional Bioprinting Human Cardiac Tissue Chips of Using a Painting Needle Method. Biotechnol. Bioeng. 2019, 116, 3136–3142. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative Study of Gelatin Methacrylate Hydrogels from Different Sources for Biofabrication Applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Sakthivel, K.; Kumar, H.; Mohamed, M.G.A.; Talebjedi, B.; Shim, J.; Najjaran, H.; Hoorfar, M.; Kim, K. High Throughput Screening of Cell Mechanical Response Using a Stretchable 3D Cellular Microarray Platform. Small 2020, 16, 2000941. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; Tomaskovic-Crook, E. Bioprinting 3D Human Induced Pluripotent Stem Cell Constructs for Multilineage Tissue Engineering and Modeling. In 3D Bioprinting: Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2140, pp. 251–258. [Google Scholar] [CrossRef]
- Cofiño, C.; Perez-Amodio, S.; Semino, C.E.; Engel, E.; Mateos-Timoneda, M.A. Development of a Self-Assembled Peptide/Methylcellulose-Based Bioink for 3D Bioprinting. Macromol. Mater. Eng. 2019, 304, 1900353. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, J.-S.; Chun, W.; Kim, G.H. An Innovative Collagen-Based Cell-Printing Method for Obtaining Human Adipose Stem Cell-Laden Structures Consisting of Core-Sheath Structures for Tissue Engineering. Biomacromolecules 2016, 17, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing Three-Dimensional Tissue Analogues with Decellularized Extracellular Matrix Bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.-H. 3D Bioprinting of Mechanically Tuned Bioinks Derived from Cardiac Decellularized Extracellular Matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H.; et al. 3D Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Sun, L.; Wang, J. Progress and Prospect of Technical and Regulatory Challenges on Tissue-Engineered Cartilage as Therapeutic Combination Product. Bioact. Mater. 2023, 20, 501–518. [Google Scholar] [CrossRef]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-Dimensional Printed PLA Scaffold and Human Gingival Stem Cell-Derived Extracellular Vesicles: A New Tool for Bone Defect Repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; Thiré, R.M.d.S.M. Evaluation of Bone Marrow Stem Cell Response to PLA Scaffolds Manufactured by 3D Printing and Coated with Polydopamine and Type I Collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef]
- Montserrat, N.; Garreta, E.; Izpisua Belmonte, J.C. Regenerative Strategies for Kidney Engineering. FEBS J. 2016, 283, 3303–3324. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Luu, N.-T.; Alwahsh, S.M.; Ginai, M.; Alhaque, S.; Dong, H.; Tomaz, R.A.; Vernay, B.; Vigneswara, V.; Hallett, J.M.; et al. 3D Human Liver Tissue from Pluripotent Stem Cells Displays Stable Phenotype in Vitro and Supports Compromised Liver Function in Vivo. Arch. Toxicol. 2018, 92, 3117–3129. [Google Scholar] [CrossRef] [PubMed]
- Goulart, E.; de Caires-Junior, L.C.; Telles-Silva, K.A.; Araujo, B.H.S.; Rocco, S.A.; Sforca, M.; de Sousa, I.L.; Kobayashi, G.S.; Musso, C.M.; Assoni, A.F.; et al. 3D Bioprinting of Liver Spheroids Derived from Human Induced Pluripotent Stem Cells Sustain Liver Function and Viability in Vitro. Biofabrication 2019, 12, 015010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Kilian, K.A. The Effect of Mesenchymal Stem Cell Shape on the Maintenance of Multipotency. Biomaterials 2013, 34, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.T.; Vanslambrouck, J.M.; Higgins, J.W.; Chambon, A.; Bishard, K.; Arndt, D.; Er, P.X.; Wilson, S.B.; Howden, S.E.; Tan, K.S.; et al. Cellular Extrusion Bioprinting Improves Kidney Organoid Reproducibility and Conformation. Nat. Mater. 2021, 20, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Fukunishi, T.; Zhang, H.; Huang, C.Y.; Nashed, A.; Blazeski, A.; DiSilvestre, D.; Vricella, L.; Conte, J.; Tung, L.; et al. Biomaterial-Free Three-Dimensional Bioprinting of Cardiac Tissue Using Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. Sci. Rep. 2017, 7, 4566. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac Regeneration Using Human-Induced Pluripotent Stem Cell-Derived Biomaterial-Free 3D-Bioprinted Cardiac Patch in Vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Abelseth, E.; Abelseth, L.; De la Vega, L.; Beyer, S.T.; Wadsworth, S.J.; Willerth, S.M. 3D Printing of Neural Tissues Derived from Human Induced Pluripotent Stem Cells Using a Fibrin-Based Bioink. ACS Biomater. Sci. Eng. 2019, 5, 234–243. [Google Scholar] [CrossRef]
- De la Vega, L.; Rosas Gómez, D.A.; Abelseth, E.; Abelseth, L.; Allisson da Silva, V.; Willerth, S.M. 3D Bioprinting Human Induced Pluripotent Stem Cell-Derived Neural Tissues Using a Novel Lab-on-a-Printer Technology. Appl. Sci. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Kim, J.; Shim, I.K.; Hwang, D.G.; Lee, Y.N.; Kim, M.; Kim, H.; Kim, S.-W.; Lee, S.; Kim, S.C.; Cho, D.-W.; et al. 3D Cell Printing of Islet-Laden Pancreatic Tissue-Derived Extracellular Matrix Bioink Constructs for Enhancing Pancreatic Functions. J. Mater. Chem. B 2019, 7, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human Stem Cell Based Corneal Tissue Mimicking Structures Using Laser-Assisted 3D Bioprinting and Functional Bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generating Kidney Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Hughson, M.; Farris, A.B.; Douglas-Denton, R.; Hoy, W.E.; Bertram, J.F. Glomerular Number and Size in Autopsy Kidneys: The Relationship to Birth Weight. Kidney Int. 2003, 63, 2113–2122. [Google Scholar] [CrossRef]
- Hale, L.J.; Howden, S.E.; Phipson, B.; Lonsdale, A.; Er, P.X.; Ghobrial, I.; Hosawi, S.; Wilson, S.; Lawlor, K.T.; Khan, S.; et al. 3D Organoid-Derived Human Glomeruli for Personalised Podocyte Disease Modelling and Drug Screening. Nat. Commun. 2018, 9, 5167. [Google Scholar] [CrossRef]
- Vanslambrouck, J.M.; Wilson, S.B.; Tan, K.S.; Soo, J.Y.-C.; Scurr, M.; Spijker, H.S.; Starks, L.T.; Neilson, A.; Cui, X.; Jain, S.; et al. A Toolbox to Characterize Human Induced Pluripotent Stem Cell-Derived Kidney Cell Types and Organoids. J. Am. Soc. Nephrol. JASN 2019, 30, 1811–1823. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D Bioprinting of Neural Stem Cell-Laden Thermoresponsive Biodegradable Polyurethane Hydrogel and Potential in Central Nervous System Repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Drug Screening—Latest Research and News|Nature. Available online: https://www.nature.com/subjects/drug-screening (accessed on 21 December 2022).
- Ma, X.; Liu, J.; Zhu, W.; Tang, M.; Lawrence, N.; Yu, C.; Gou, M.; Chen, S. 3D Bioprinting of Functional Tissue Models for Personalized Drug Screening and in Vitro Disease Modeling. Adv. Drug Deliv. Rev. 2018, 132, 235–251. [Google Scholar] [CrossRef]
- Mullard, A. New Drugs Cost US$2.6 Billion to Develop. Nat. Rev. Drug Discov. 2014, 13, 877. [Google Scholar] [CrossRef]
- Ribas, J.; Sadeghi, H.; Manbachi, A.; Leijten, J.; Brinegar, K.; Zhang, Y.S.; Ferreira, L.; Khademhosseini, A. Cardiovascular Organ-on-a-Chip Platforms for Drug Discovery and Development. Appl. Vitro Toxicol. 2016, 2, 82–96. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.-J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented Cardiac Microphysiological Devices via Multimaterial Three-Dimensional Printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically Patterned Biomimetic Human IPSC-Derived Hepatic Model via Rapid 3D Bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Liu Tsang, V.; Chen, A.A.; Cho, L.M.; Jadin, K.D.; Sah, R.L.; DeLong, S.; West, J.L.; Bhatia, S.N. Fabrication of 3D Hepatic Tissues by Additive Photopatterning of Cellular Hydrogels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Khetani, S.R.; Bhatia, S.N. Microscale Culture of Human Liver Cells for Drug Development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Hewitt, N.J.; Lechón, M.J.G.; Houston, J.B.; Hallifax, D.; Brown, H.S.; Maurel, P.; Kenna, J.G.; Gustavsson, L.; Lohmann, C.; Skonberg, C.; et al. Primary Hepatocytes: Current Understanding of the Regulation of Metabolic Enzymes and Transporter Proteins, and Pharmaceutical Practice for the Use of Hepatocytes in Metabolism, Enzyme Induction, Transporter, Clearance, and Hepatotoxicity Studies. Drug Metab. Rev. 2007, 39, 159–234. [Google Scholar] [CrossRef]
- Fransen, M.F.J.; Addario, G.; Bouten, C.V.C.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of Kidney in Vitro Models: Cells, Biomaterials, and Manufacturing Techniques. Essays Biochem. 2021, 65, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Chuah, J.K.C.; Zink, D. Stem Cell-Derived Kidney Cells and Organoids: Recent Breakthroughs and Emerging Applications. Biotechnol. Adv. 2017, 35, 150–167. [Google Scholar] [CrossRef]
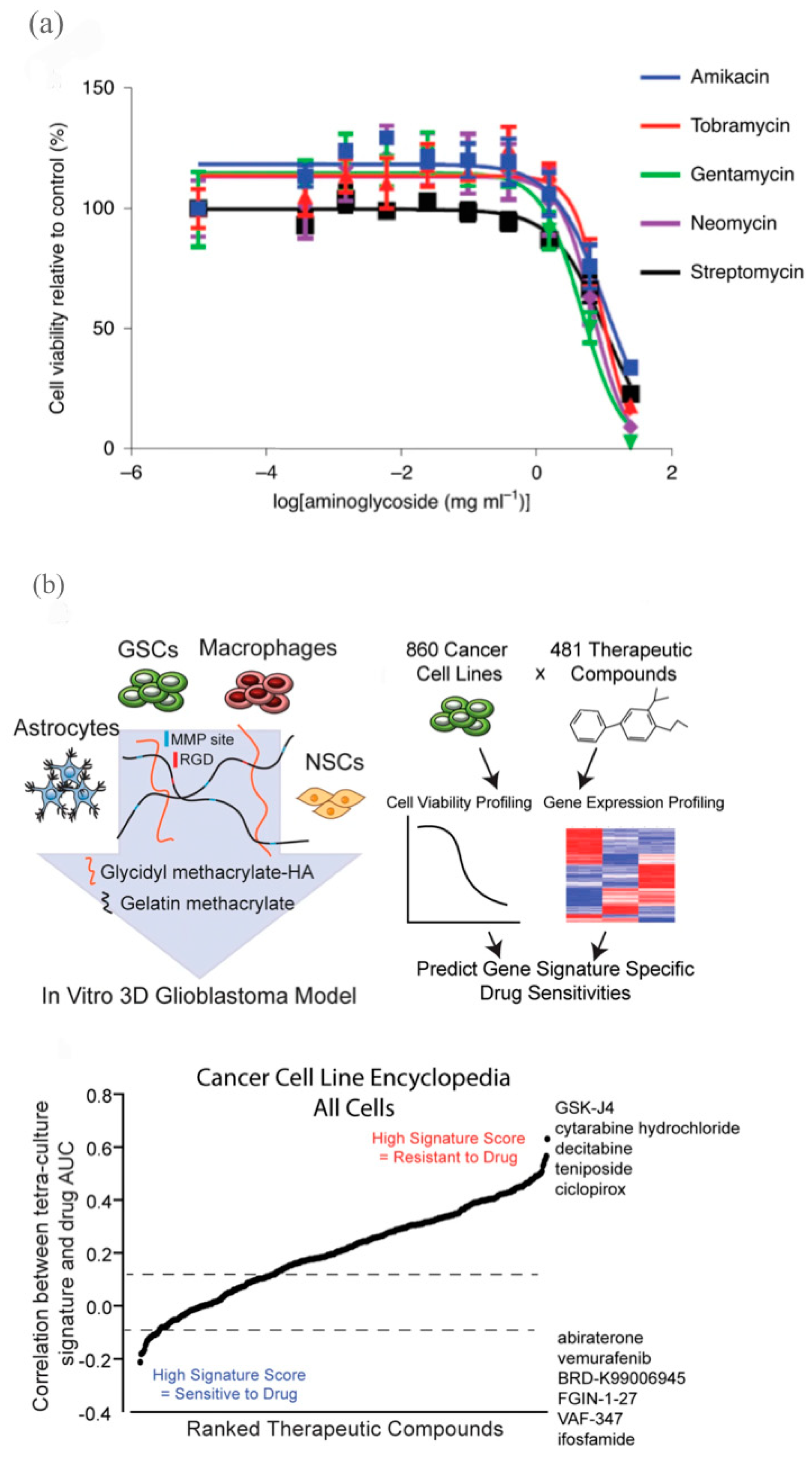
- Tang, M.; Xie, Q.; Gimple, R.C.; Zhong, Z.; Tam, T.; Tian, J.; Kidwell, R.L.; Wu, Q.; Prager, B.C.; Qiu, Z.; et al. Three-Dimensional Bioprinted Glioblastoma Microenvironments Model Cellular Dependencies and Immune Interactions. Cell Res. 2020, 30, 833–853. [Google Scholar] [CrossRef]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High Cell Density and High-Resolution 3D Bioprinting for Fabricating Vascularized Tissues. Sci. Adv. 2023, 9, eade7923. [Google Scholar] [CrossRef]

| Bio-ink Material | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Alginate | Natural negatively charged polysaccharides from brown algae |
|
| [14,35,36,37] |
| Agarose | Polysaccharide obtained from seaweed | High cell viability | Poor support and limited cell growth | [35,38] |
| Collagen | Structural protein in the extracellular matrix | Easily obtainable from skin and connective tissues of organisms Relatively strong 3D structures |
| [39,40] |
| Nanocellulose | Cellulose that can be derived from biomass, bacteria, and marine sources |
| May not be an accurate model for human cells as we do not produce cellulase to be biodegraded | [41] |
| PEGDA | Synthetic polymer used for hydrogel fabrication and UV curing |
| Material can be brittle and rigid | [42] |
| Pluronic® | Synthetic polymer-poloxamer |
| Biocompatibility is not sufficient for long-term cell survival | [43,44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, E.H.Y.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. https://doi.org/10.3390/ijms24076357
Lam EHY, Yu F, Zhu S, Wang Z. 3D Bioprinting for Next-Generation Personalized Medicine. International Journal of Molecular Sciences. 2023; 24(7):6357. https://doi.org/10.3390/ijms24076357
Chicago/Turabian StyleLam, Ethan Hau Yin, Fengqing Yu, Sabrina Zhu, and Zongjie Wang. 2023. "3D Bioprinting for Next-Generation Personalized Medicine" International Journal of Molecular Sciences 24, no. 7: 6357. https://doi.org/10.3390/ijms24076357
APA StyleLam, E. H. Y., Yu, F., Zhu, S., & Wang, Z. (2023). 3D Bioprinting for Next-Generation Personalized Medicine. International Journal of Molecular Sciences, 24(7), 6357. https://doi.org/10.3390/ijms24076357








