Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities
Abstract
1. Introduction
2. Multiple Sclerosis
- 1-
- Early traditional injectable DMTs, such as interferon beta (IFNβ-1a and -1b), that seem to act by suppressing T cell activity, inducing IL-10 production, and altering the differentiation of CD4+ T cells toward a Th2 phenotype [13]; and glatiramer acetate (GA), that targets myelin-specific autoantibodies in order to reduce autoreactivity and promote a predominant Th2 phenotype [13].
- 2-
- Oral medications, represented by sphingosine 1 phosphate (s1p) receptor modulators such as Fingolimod, alter the immune migration by binding S1P receptors on lymphocytes, inducing sequestration of circulating mature lymphocytes [9], and Teriflunomide, which acts by effecting rapidly dividing lymphocytes [13]. More recently, Cladribine is a nucleoside analogue that inhibits DNA synthesis and DNA chain termination with cytotoxic activity towards lymphocytes and monocytes [9]. Among the fumaric acid derivates, dimethyl fumarate (DMF) suppresses Th1/Th17 inflammatory responses and promotes a Th2-type response. It is also reported that DMF reduces IL-17 production by CD4 T cells [20].
- 3-
- Infusion and injectable DMTs are a group of monoclonal antibodies that are comprised of Natalizumab, which prevents lymphocyte adhesion and migration from the peripheral vascular bed to the CNS; Alemtuzumab, which selectively binds the CD52 protein, inducing the clearance of T and B cells and increasing the secretion of brain-derived neurotrophic factor (BDNF) [9,13]; Rituximab, which targets CD20; and Ocrelizumab, which induces an antibody-dependent cytolysis and complement-mediated lysis of B cells [9]. Recently, Ofatumumab, another monoclonal antibody that also binds to CD20+ B cells, resulting in B cell depletion, has been approved and is currently administered to MS patients [13].
3. Parkinson’s Disease
4. Alzheimer’s Disease
5. Amyotrophic Lateral Sclerosis
6. Spinal Muscular Atrophy
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV-9 | Adeno-associated virus |
| Ab | Antibodies |
| AChE | Acetylcholinesterase enzyme |
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic lateral sclerosis |
| APCs | Antigen-presenting cells |
| ApoE4 | Apolipoprotein E4 |
| Aβ | Amyloid-β plaques |
| AURKC | Aurora kinase C |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body Mass Index |
| CCNF | Cyclin F |
| CD3+ | Cluster of differentiation 3+ |
| CD4+ | Cluster of differentiation 4+ |
| CD8+ | Cluster of differentiation 8+ |
| CD14+ | Cluster of differentiation 14+ |
| CD20 | Cluster of differentiation 20 |
| CD52 | Clusters of differentiation 52 |
| CD206 | Cluster of differentiation 206 |
| CHCHD10 | Coiled-coil-helix-coiled-coil-helix domain containing 10 |
| CLDN16 | Claudin-16 |
| CNS | Central nervous system |
| COMT | Catechol-O-methyltransferase |
| CSF | Cerebrospinal fluid |
| CYP450 | Cytochrome P450 |
| DMF | Dimethyl Fumarate |
| DMTS | Disease modifying therapies |
| EBV | Epstein Barr virus |
| ESR1 | Estrogen Receptor 1 |
| ESR2 | Estrogen Receptor 2 |
| fALS | Familial amyotrophic lateral sclerosis |
| FTD | Frontotemporal dementia |
| FUS | Fused in sarcoma |
| GA | Glutiramer Acetate |
| GM-1 | Monosialotetrahexosylganglioside |
| GRN | Granulin precursor |
| HLA-DRB1*1501 | Human leucocyte Antigen, class II, DR beta 1*1501 |
| HFSRE | Hammersmith Functional Rating Scale Expanded |
| IFNβ | Interferon beta |
| IFNγ | Interferon gamma |
| IGF-2 | Insulin Like Growth Factor 2 |
| IL-1 | Interleukin-1 |
| IL-1β | Interleukin-1β |
| IL-2 | Interleukin-2 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| IL-18 | Interleukin-18 |
| IL-21 | Interleukin-21 |
| IL-27 | Interleukin-27 |
| Kdm6a | Lysine demethylase 6A |
| LOY | Loss of chromosome Y |
| LPS | Lipopolysaccharide |
| LRRK2 | Leucine-rich repeat kinase 2 |
| LV | Levodopa |
| M1 | Microglia 1 |
| M2 | Microglia 2 |
| MAO-B | Monoamine oxidase B |
| MATR3 | Matrin 3 |
| MGMT | O-6-Methylguanine-DNA Methyltransferase |
| MHC | Major Histocompatibility Complex |
| miRNA | MicroRNA |
| MRI | Magnetic Resonance Imaging |
| MS | Multiple Sclerosis |
| nAbs | Natural Antibodies |
| NAIP | Neuronal apoptosis inhibitory protein |
| NDs | Neurodegenerative Diseases |
| NEK1 | NIMA Related Kinase 1 |
| NF-kβ | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NH-F | Neurofilament subunit |
| NK | Natural killer |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxyde |
| NRF1 | Nuclear respiratory factors 1 |
| NRF2 | Nuclear respiratory factors 2 |
| OSTN | Osteocrin |
| PCDH11X | Protocadherin 11 X-Linked |
| PD | Parkinson’s Disease |
| PET | Positron emission tomography |
| PGC-1α | Peroxisome proliferator-activated receptor-gamma coactivator α |
| PGR | Progesteron receptor |
| PINK-1 | PTEN induced kinase 1 |
| PLS1,3 | Plastin 1,3 |
| PP | Primary progressives |
| PPMS | Primary progressives multiple sclerosis |
| PRKN | Parkin RBR E3 Ubiquitin Protein Ligase |
| ROS | Reactive oxygen species |
| RR | Relapsing-remitting |
| RRMS | Relapsing-remitting multiple sclerosis |
| S1p | Sphingosine 1 Phosphate |
| sALS | Sporadic Amyotrophic lateral sclerosis |
| SERPINA1 | Serpin family member 1 |
| SMA | Spinal muscular atrophy |
| SMN1 | Survival motor neuron 1 |
| SMN2 | Survival motor neuron 2 |
| SNCA | Synuclein alpha |
| SNP | Single Nucleotide Polymorphism |
| SNpc | Substantia nigra pars compacta |
| SOD1 | Superoxide dismutase type 1 |
| SPMS | Secondary progressive multiple sclerosis |
| STN DBS | Subthalamic nucleus deep brain stimulation |
| ST2 | Suppression Of Tumorigenicity 2 |
| TARDBP | Transactive response DNA binding protein |
| TBK1 | Tank binding kinase 1 |
| TFAM | Transcription factor A mitochondrial |
| Th1 | T Lymphocytes helper 1 |
| Th2 | T Lymphocytes helper 2 |
| Th17 | T Lymphocytes helper 17 |
| TLR2 | Toll like receptor 2 |
| TLR4 | Toll like receptor 4 |
| TNFα | Tumor necrosis factor alpha |
| TREM2 | Triggering Receptor Expressed on Myeloid Cells 2 |
| TUBA4A | Tubulin Alpha 4a |
| UBA1 | Ubiquitin Like Modifier Activating Enzyme 1 |
| USP9X | Ubiquitin Specific Peptidase 9 X-Linked |
| USP11 | Ubiquitin-specific peptidase 11 |
References
- Raine, C.S. Inflammation in Alzheimer’s Disease: A View from the Periphery. Neurobiol. Aging 2000, 21, 437–440, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of Inflammatory Processes to Alzheimer’s Disease: Molecular Mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Butnaru, D.; Chapman, J. The Impact of Self-Replicating Proteins on Inflammation, Autoimmunity and Neurodegeneration—An Untraveled Path. Autoimmun. Rev. 2019, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T Cells from Patients with Parkinson’s Disease Recognize α-Synuclein Peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The Relation between Inflammation and Neurodegeneration in Multiple Sclerosis Brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef]
- Milo, R.; Korczyn, A.D.; Manouchehri, N.; Stüve, O. The Temporal and Causal Relationship between Inflammation and Neurodegeneration in Multiple Sclerosis. Mult. Scler. 2020, 26, 876–886. [Google Scholar] [CrossRef]
- Doust, Y.V.; King, A.E.; Ziebell, J.M. Implications for Microglial Sex Differences in Tau-Related Neurodegenerative Diseases. Neurobiol. Aging 2021, 105, 340–348. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender Differences in Autoimmune Disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Wei, W.; Ma, D.; Li, L.; Zhang, L. Progress in the Application of Drugs for the Treatment of Multiple Sclerosis. Front. Pharmacol. 2021, 12, 724718. [Google Scholar] [CrossRef]
- Coyle, P.K. What Can We Learn from Sex Differences in MS? J. Pers. Med. 2021, 11, 1006. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Yang, J.H.; Rempe, T.; Whitmire, N.; Dunn-Pirio, A.; Graves, J.S. Therapeutic Advances in Multiple Sclerosis. Front. Neurol. 2022, 13, 1111. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Gold, S.M. Sex-Related Factors in Multiple Sclerosis Susceptibility and Progression. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef]
- Gold, S.M.; Willing, A.; Leypoldt, F.; Paul, F.; Friese, M.A. Sex Differences in Autoimmune Disorders of the Central Nervous System. Semin. Immunopathol. 2019, 41, 177–188. [Google Scholar] [CrossRef]
- Nytrova, P.; Dolezal, O. Sex Bias in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: How It Influences Clinical Course, MRI Parameters and Prognosis. Front. Immunol. 2022, 13, 933415. [Google Scholar] [CrossRef]
- Lopez-Lee, C.; Kodama, L.; Gan, L. Sex Differences in Neurodegeneration: The Role of the Immune System in Humans. Biol. Psychiatry 2021, 91, P72–P80. [Google Scholar] [CrossRef]
- Angeloni, B.; Bigi, R.; Bellucci, G.; Mechelli, R.; Ballerini, C.; Romano, C.; Morena, E.; Pellicciari, G.; Reniè, R.; Rinaldi, V.; et al. A Case of Double Standard: Sex Differences in Multiple Sclerosis Risk Factors. Int. J. Mol. Sci. 2021, 22, 3696. [Google Scholar] [CrossRef]
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex Affects Immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef]
- Ryan, L.; Mills, K.H.G. Sex Differences Regulate Immune Responses in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Eur. J. Immunol. 2022, 52, 24–33. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Ciesielska, A.; Gromadzka, G.; Kurkowska-Jastrzebska, I. Estrogen and Cytokines Production—The Possible Cause of Gender Differences in Neurological Diseases. Curr. Pharm. Des. 2005, 11, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, S.M.; Qin, H.; Hendrickson, R.C.; Muwanguzi, J.E.; Lefkowitz, E.J.; Kennedy, R.E.; Yan, Z.; Yacoubian, T.A.; Benveniste, E.N.; West, A.B.; et al. Sex-Based Differences in the Activation of Peripheral Blood Monocytes in Early Parkinson Disease. npj Park. Dis. 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Bilbo, S.D. Sex Differences in Neurodevelopmental and Neurodegenerative Disorders: Focus on Microglial Function and Neuroinflammation during Development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef]
- Zalewska, T.; Pawelec, P.; Ziabska, K.; Ziemka-Nalecz, M. Sexual Dimorphism in Neurodegenerative Diseases and in Brain Ischemia. Biomolecules 2022, 13, 26. [Google Scholar] [CrossRef]
- Frost, G.R.; Jonas, L.A.; Li, Y.-M. Friend, Foe or Both? Immune Activity in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 337. [Google Scholar] [CrossRef]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-Cell Transcriptomic Analysis of Alzheimer’s Disease. Nature 2019, 570, 332–337. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.-V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial Metabolism Is a Pivotal Factor in Sexual Dimorphism in Alzheimer’s Disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.-T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Santiago, J.A.; Quinn, J.P.; Potashkin, J.A. Network Analysis Identifies Sex-Specific Gene Expression Changes in Blood of Amyotrophic Lateral Sclerosis Patients. Int. J. Mol. Sci. 2021, 22, 7150. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Prim. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Ralli, M.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Amyotrophic Lateral Sclerosis: Autoimmune Pathogenic Mechanisms, Clinical Features, and Therapeutic Perspectives. Isr. Med. Assoc. J. 2019, 21, 438–443. [Google Scholar]
- Wang, M.; Liu, Z.; Du, J.; Yuan, Y.; Jiao, B.; Zhang, X.; Hou, X.; Shen, L.; Guo, J.; Jiang, H.; et al. Evaluation of Peripheral Immune Activation in Amyotrophic Lateral Sclerosis. Front. Neurol. 2021, 12, 628710. [Google Scholar] [CrossRef]
- Singh, N.N.; Hoffman, S.; Reddi, P.P.; Singh, R.N. Spinal Muscular Atrophy: Broad Disease Spectrum and Sex-Specific Phenotypes. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166063. [Google Scholar] [CrossRef]
- Deguise, M.; Kothary, R. New Insights into SMA Pathogenesis: Immune Dysfunction and Neuroinflammation. Ann. Clin. Transl. Neurol. 2017, 4, 522–530. [Google Scholar] [CrossRef]
- Nuzzo, T.; Russo, R.; Errico, F.; D’Amico, A.; Tewelde, A.G.; Valletta, M.; Hassan, A.; Tosi, M.; Panicucci, C.; Bruno, C.; et al. Nusinersen Mitigates Neuroinflammation in Severe Spinal Muscular Atrophy Patients. Commun. Med. 2023, 3, 28. [Google Scholar] [CrossRef]
- Trojano, M.; Pellegrini, F.; Paolicelli, D.; Fuiani, A.; Zimatore, G.B.; Tortorella, C.; Simone, I.L.; Patti, F.; Ghezzi, A.; Portaccio, E.; et al. Post-Marketing of Disease Modifying Drugs in Multiple Sclerosis: An Exploratory Analysis of Gender Effect in Interferon Beta Treatment. J. Neurol. Sci. 2009, 286, 109–113. [Google Scholar] [CrossRef]
- Manni, A.; Direnzo, V.; Iaffaldano, A.; Di Lecce, V.; Tortorella, C.; Zoccolella, S.; Iaffaldano, P.; Trojano, M.; Paolicelli, D. Gender Differences in Safety Issues during Fingolimod Therapy: Evidence from a Real-Life Relapsing Multiple Sclerosis Cohort. Brain Behav. 2017, 7, e00804. [Google Scholar] [CrossRef]
- Ganguly, U.; Singh, S.; Pal, S.; Prasad, S.; Agrawal, B.K.; Saini, R.V.; Chakrabarti, S. Alpha-Synuclein as a Biomarker of Parkinson’s Disease: Good, but Not Good Enough. Front. Aging Neurosci. 2021, 13, 702639. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Le, W. Biomarkers for Parkinson’s Disease: How Good Are They? Neurosci. Bull. 2019, 36, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Cicognola, C.; Eusebi, P.; Chiasserini, D. Value of Cerebrospinal Fluid α-Synuclein Species as Biomarker in Parkinson’s Diagnosis and Prognosis. Biomark. Med. 2016, 10, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation—An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Bonam, S.R.; Muller, S. Parkinson’s Disease Is an Autoimmune Disease: A Reappraisal. Autoimmun. Rev. 2020, 19, 102684. [Google Scholar] [CrossRef] [PubMed]
- Shalash, A.; Salama, M.; Makar, M.; Roushdy, T.; Elrassas, H.H.; Mohamed, W.; El-Balkimy, M.; Abou Donia, M. Elevated Serum α-Synuclein Autoantibodies in Patients with Parkinson’s Disease Relative to Alzheimer’s Disease and Controls. Front. Neurol. 2017, 8, 720. [Google Scholar] [CrossRef]
- Jiang, T.; Li, G.; Xu, J.; Gao, S.; Chen, X. The Challenge of the Pathogenesis of Parkinson’s Disease: Is Autoimmunity the Culprit? Front. Immunol. 2018, 9, 2047. [Google Scholar] [CrossRef]
- Georgiev, D.; Hamberg, K.; Hariz, M.; Forsgren, L.; Hariz, G.-M. Gender Differences in Parkinson’s Disease: A Clinical Perspective. Acta Neurol. Scand. 2017, 136, 570–584. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Kim, R.; Lee, J.; Kim, Y.; Kim, A.; Jang, M.; Kim, H.-J.; Jeon, B.; Kang, U.J.; Fahn, S. Presynaptic Striatal Dopaminergic Depletion Predicts the Later Development of Freezing of Gait in de Novo Parkinson’s Disease: An Analysis of the PPMI Cohort. Park. Relat. Disord. 2018, 51, 49–54. [Google Scholar] [CrossRef]
- Ou, R.; Liu, H.; Hou, Y.; Song, W.; Cao, B.; Wei, Q.; Yuan, X.; Chen, Y.; Zhao, B.; Shang, H. Predictors of Camptocormia in Patients with Parkinson’s Disease: A Prospective Study from Southwest China. Park. Relat. Disord. 2018, 52, 69–75. [Google Scholar] [CrossRef]
- Silverdale, M.A.; Kobylecki, C.; Kass-Iliyya, L.; Martinez-Martin, P.; Lawton, M.; Cotterill, S.; Chaudhuri, K.R.; Morris, H.; Baig, F.; Williams, N.; et al. A Detailed Clinical Study of Pain in 1957 Participants with Early/Moderate Parkinson’s Disease. Park. Relat. Disord. 2018, 56, 27–32. [Google Scholar] [CrossRef]
- Buhmann, C.; Dogac, S.; Vettorazzi, E.; Hidding, U.; Gerloff, C.; Jürgens, T.P. The Impact of Parkinson Disease on Patients’ Sexuality and Relationship. J. Neural. Transm. 2017, 124, 983–996. [Google Scholar] [CrossRef]
- Millet, X.; Raoux, N.; Le Carret, N.; Bouisson, J.; Dartigues, J.-F.; Amieva, H. Gender-Related Differences in Visuospatial Memory Persist in Alzheimer’s Disease. Arch. Clin. Neuropsychol. 2009, 24, 783–789. [Google Scholar] [CrossRef]
- Curtis, A.F.; Masellis, M.; Camicioli, R.; Davidson, H.; Tierney, M.C. Cognitive Profile of Non-Demented Parkinson’s Disease: Meta-Analysis of Domain and Sex-Specific Deficits. Park. Relat. Disord. 2019, 60, 32–42. [Google Scholar] [CrossRef]
- Reekes, T.H.; Higginson, C.I.; Ledbetter, C.R.; Sathivadivel, N.; Zweig, R.M.; Disbrow, E.A. Sex Specific Cognitive Differences in Parkinson Disease. NPJ Park. Dis. 2020, 6, 7. [Google Scholar] [CrossRef]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Piscopo, P.; Bellenghi, M.; Manzini, V.; Crestini, A.; Pontecorvi, G.; Corbo, M.; Ortona, E.; Carè, A.; Confaloni, A. A Sex Perspective in Neurodegenerative Diseases: MicroRNAs as Possible Peripheral Biomarkers. Int. J. Mol. Sci. 2021, 22, 4423. [Google Scholar] [CrossRef]
- Russillo, M.C.; Andreozzi, V.; Erro, R.; Picillo, M.; Amboni, M.; Cuoco, S.; Barone, P.; Pellecchia, M.T. Sex Differences in Parkinson’s Disease: From Bench to Bedside. Brain Sci. 2022, 12, 917. [Google Scholar] [CrossRef]
- Conti, V.; Izzo, V.; Russillo, M.C.; Picillo, M.; Amboni, M.; Scaglione, C.L.M.; Nicoletti, A.; Cani, I.; Cicero, C.E.; De Bellis, E.; et al. Gender Differences in Levodopa Pharmacokinetics in Levodopa-Naïve Patients with Parkinson’s Disease. Front. Med. 2022, 9, 909936. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.G.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of Gastrointestinal Tract Variability on Oral Drug Absorption and Pharmacokinetics: An UNGAP Review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Meoni, S.; Macerollo, A.; Moro, E. Sex Differences in Movement Disorders. Nat. Rev. Neurol. 2020, 16, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.F.; Dos Santos, E.U.D.; de Lima, G.D.C.; Dos Anjos, R.S.G.; da Silva, R.C.; Asano, A.G.C.; Asano, N.M.J.; Crovella, S.; de Souza, P.R.E. MAO-B and COMT Genetic Variations Associated with Levodopa Treatment Response in Patients with Parkinson’s Disease. J. Clin. Pharm. 2018, 58, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Philipe de Souza Ferreira, L.; André da Silva, R.; Marques Mesquita da Costa, M.; Moraes de Paiva Roda, V.; Vizcaino, S.; Janisset, N.R.L.L.; Ramos Vieira, R.; Marcos Sanches, J.; Maria Soares Junior, J.; de Jesus Simões, M. Sex Differences in Parkinson’s Disease: An Emerging Health Question. Clinics 2022, 77, 100121. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Picillo, M.; Russillo, M.C.; De Pandis, M.F.; Bonizzoni, E.; Marjanovic, I.; Cattaneo, C. Efficacy of Safinamide and Gender Differences during Routine Clinical Practice. Front. Neurol. 2021, 12, 756304. [Google Scholar] [CrossRef]
- Jurado-Coronel, J.C.; Cabezas, R.; Ávila Rodríguez, M.F.; Echeverria, V.; García-Segura, L.M.; Barreto, G.E. Sex Differences in Parkinson’s Disease: Features on Clinical Symptoms, Treatment Outcome, Sexual Hormones and Genetics. Front. Neuroendocr. 2018, 50, 18–30. [Google Scholar] [CrossRef]
- Weaver, D.F. Alzheimer’s Disease as an Innate Autoimmune Disease (AD2): A New Molecular Paradigm. Alzheimers Dement. 2022, 19, 1086–1098. [Google Scholar] [CrossRef]
- Meier-Stephenson, F.S.; Meier-Stephenson, V.C.; Carter, M.D.; Meek, A.R.; Wang, Y.; Pan, L.; Chen, Q.; Jacobo, S.; Wu, F.; Lu, E.; et al. Alzheimer’s Disease as an Autoimmune Disorder of Innate Immunity Endogenously Modulated by Tryptophan Metabolites. Alzheimers Dement. Transl. Res. Clin. Interv. 2022, 8, e12283. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Biechele, G.; Franzmeier, N.; Blume, T.; Ewers, M.; Luque, J.M.; Eckenweber, F.; Sacher, C.; Beyer, L.; Ruch-Rubinstein, F.; Lindner, S.; et al. Glial Activation Is Moderated by Sex in Response to Amyloidosis but Not to Tau Pathology in Mouse Models of Neurodegenerative Diseases. J. Neuroinflamm. 2020, 17, 374. [Google Scholar] [CrossRef]
- Davis, E.J.; Broestl, L.; Abdulai-Saiku, S.; Worden, K.; Bonham, L.W.; Miñones-Moyano, E.; Moreno, A.J.; Wang, D.; Chang, K.; Williams, G.; et al. A Second X Chromosome Contributes to Resilience in a Mouse Model of Alzheimer’s Disease. Sci. Transl. Med. 2020, 12, eaaz5677. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s Disease: Recent Treatment Strategies. Eur. J. Pharm. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Canevelli, M.; Quarata, F.; Remiddi, F.; Lucchini, F.; Lacorte, E.; Vanacore, N.; Bruno, G.; Cesari, M. Sex and Gender Differences in the Treatment of Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. Pharmacol. Res. 2017, 115, 218–223. [Google Scholar] [CrossRef]
- Buckley, R.F.; Mormino, E.C.; Rabin, J.S.; Hohman, T.J.; Landau, S.; Hanseeuw, B.J.; Jacobs, H.I.L.; Papp, K.V.; Amariglio, R.E.; Properzi, M.J.; et al. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol. 2019, 76, 542–551. [Google Scholar] [CrossRef]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering Sex and Gender in Alzheimer Disease and Other Dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, M.B.; Zhang, L.; Zhang, B.; Cai, D. Sex Differences in Alzheimer’s Disease: Insights from the Multiomics Landscape. Biol. Psychiatry 2022, 91, 61–71. [Google Scholar] [CrossRef]
- Carrasquillo, M.M.; Zou, F.; Pankratz, V.S.; Wilcox, S.L.; Ma, L.; Walker, L.P.; Younkin, S.G.; Younkin, C.S.; Younkin, L.H.; Bisceglio, G.D.; et al. Genetic Variation in PCDH11X Is Associated with Susceptibility to Late-Onset Alzheimer’s Disease. Nat. Genet. 2009, 41, 192–198. [Google Scholar] [CrossRef]
- Dumanski, J.P.; Lambert, J.-C.; Rasi, C.; Giedraitis, V.; Davies, H.; Grenier-Boley, B.; Lindgren, C.M.; Campion, D.; Dufouil, C.; Pasquier, F.; et al. Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am. J. Hum. Genet. 2016, 98, 1208–1219. [Google Scholar] [CrossRef]
- Chung, J.; Das, A.; Sun, X.; Sobreira, D.R.; Leung, Y.Y.; Igartua, C.; Mozaffari, S.; Chou, Y.-F.; Thiagalingam, S.; Mez, J.; et al. Genome-Wide Association and Multi-Omics Studies Identify MGMT as a Novel Risk Gene for Alzheimer’s Disease among Women. Alzheimers Dement. 2022, 19, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, X.; Wong, H.Y.; Ouyang, L.; Ip, F.C.; Chau, V.M.; Lau, S.F.; Wu, W.; Wong, D.Y.; Seo, H.; et al. An IL1RL1 Genetic Variant Lowers Soluble ST2 Levels and the Risk Effects of APOE-Ε4 in Female Patients with Alzheimer’s Disease. Nat. Aging 2022, 2, 616–634. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Chaput, D.; Shin, M.-K.; Koh, Y.; Gan, L.; Pieper, A.A.; Woo, J.-A.A.; Kang, D.E. X-Linked Ubiquitin-Specific Peptidase 11 Increases Tauopathy Vulnerability in Women. Cell 2022, 185, 3913–3930.e19. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Solsberg, C.W.; White, C.C.; Miñones-Moyano, E.; Sirota, M.; Chibnik, L.; Bennett, D.A.; De Jager, P.L.; Yokoyama, J.S.; Dubal, D.B. Sex-Specific Association of the X Chromosome with Cognitive Change and Tau Pathology in Aging and Alzheimer Disease. JAMA Neurol. 2021, 78, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Nagata, K.; Nonaka, T.; Tarutani, A.; Imamura, T.; Hashimoto, T.; Bannai, T.; Koshi-Mano, K.; Tsuchida, T.; Ohtomo, R.; et al. Neuron-Specific Methylome Analysis Reveals Epigenetic Regulation and Tau-Related Dysfunction of BRCA1 in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2017, 114, E9645–E9654. [Google Scholar] [CrossRef]
- Coales, I.; Tsartsalis, S.; Fancy, N.; Weinert, M.; Clode, D.; Owen, D.; Matthews, P.M. Alzheimer’s Disease-Related Transcriptional Sex Differences in Myeloid Cells. J. Neuroinflamm. 2022, 19, 247. [Google Scholar] [CrossRef]
- Baron, S.; Ulstein, I.; Werheid, K. Psychosocial Interventions in Alzheimer’s Disease and Amnestic Mild Cognitive Impairment: Evidence for Gender Bias in Clinical Trials. Aging Ment. Health 2015, 19, 290–305. [Google Scholar] [CrossRef]
- Corbi, G.; Gambassi, G.; Pagano, G.; Russomanno, G.; Conti, V.; Rengo, G.; Leosco, D.; Bernabei, R.; Filippelli, A.; Ferrara, N. Impact of an Innovative Educational Strategy on Medication Appropriate Use and Length of Stay in Elderly Patients. Medicine 2015, 94, e918. [Google Scholar] [CrossRef]
- Chia, R.; Chiò, A.; Traynor, B.J. Novel Genes Associated with Amyotrophic Lateral Sclerosis: Diagnostic and Clinical Implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The Key Cytokine in Neurodegenerative Diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. Effects of Gender in Amyotrophic Lateral Sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef]
- Palmieri, A.; Mento, G.; Calvo, V.; Querin, G.; D’Ascenzo, C.; Volpato, C.; Kleinbub, J.R.; Bisiacchi, P.S.; Sorarù, G. Female Gender Doubles Executive Dysfunction Risk in ALS: A Case-Control Study in 165 Patients. J. Neurol. Neurosurg. Psychiatry 2015, 86, 574–579. [Google Scholar] [CrossRef]
- Bede, P.; Elamin, M.; Byrne, S.; Hardiman, O. Sexual Dimorphism in ALS: Exploring Gender-Specific Neuroimaging Signatures. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 235–243. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.-Y.; Cho, K.-S.; Kim, H.N.; Koh, J.-Y. Autophagy Activation and Neuroprotection by Progesterone in the G93A-SOD1 Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. Neurobiol. Dis. 2013, 59, 80–85. [Google Scholar] [CrossRef]
- Pegoraro, V.; Merico, A.; Angelini, C. Micro-RNAs in ALS Muscle: Differences in Gender, Age at Onset and Disease Duration. J. Neurol. Sci. 2017, 380, 58–63. [Google Scholar] [CrossRef]
- Bellingham, M.C. A Review of the Neural Mechanisms of Action and Clinical Efficiency of Riluzole in Treating Amyotrophic Lateral Sclerosis: What Have We Learned in the Last Decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Maggi, L.; Bello, L.; Bonanno, S.; Govoni, A.; Caponnetto, C.; Passamano, L.; Grandis, M.; Trojsi, F.; Cerri, F.; Gardani, A.; et al. Adults with Spinal Muscular Atrophy: A Large-Scale Natural History Study Shows Gender Effect on Disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1253–1261. [Google Scholar] [CrossRef]
- Jablonka, S.; Hennlein, L.; Sendtner, M. Therapy Development for Spinal Muscular Atrophy: Perspectives for Muscular Dystrophies and Neurodegenerative Disorders. Neurol. Res. Pract. 2022, 4, 2. [Google Scholar] [CrossRef]
- Abati, E.; Citterio, G.; Bresolin, N.; Comi, G.P.; Corti, S. Glial Cells Involvement in Spinal Muscular Atrophy: Could SMA Be a Neuroinflammatory Disease? Neurobiol. Dis. 2020, 140, 104870. [Google Scholar] [CrossRef]
- Bonanno, S.; Cavalcante, P.; Salvi, E.; Giagnorio, E.; Malacarne, C.; Cattaneo, M.; Andreetta, F.; Venerando, A.; Pensato, V.; Gellera, C.; et al. Identification of a Cytokine Profile in Serum and Cerebrospinal Fluid of Pediatric and Adult Spinal Muscular Atrophy Patients and Its Modulation upon Nusinersen Treatment. Front. Cell. Neurosci. 2022, 16, 982760. [Google Scholar] [CrossRef]
- Kuru, S.; Sakai, M.; Konagaya, M.; Yoshida, M.; Hashizume, Y.; Saito, K. An Autopsy Case of Spinal Muscular Atrophy Type III (Kugelberg-Welander Disease). Neuropathology 2009, 29, 63–67. [Google Scholar] [CrossRef] [PubMed]
- McGivern, J.V.; Patitucci, T.N.; Nord, J.A.; Barabas, M.-E.A.; Stucky, C.L.; Ebert, A.D. Spinal Muscular Atrophy Astrocytes Exhibit Abnormal Calcium Regulation and Reduced Growth Factor Production. GLIA 2013, 61, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.K.Y.; Lin, M.-Y.; Zingg, B.; Feng, Z.; Ko, C.-P. Synaptic Defects in the Spinal and Neuromuscular Circuitry in a Mouse Model of Spinal Muscular Atrophy. PLoS ONE 2010, 5, e15457. [Google Scholar] [CrossRef] [PubMed]
- Tarabal, O.; Caraballo-Miralles, V.; Cardona-Rossinyol, A.; Correa, F.J.; Olmos, G.; Lladó, J.; Esquerda, J.E.; Calderó, J. Mechanisms Involved in Spinal Cord Central Synapse Loss in a Mouse Model of Spinal Muscular Atrophy. J. Neuropathol. Exp. Neurol. 2014, 73, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Ar Rochmah, M.; Shima, A.; Harahap, N.I.F.; Niba, E.T.E.; Morisada, N.; Yanagisawa, S.; Saito, T.; Kaneko, K.; Saito, K.; Morioka, I.; et al. Gender Effects on the Clinical Phenotype in Japanese Patients with Spinal Muscular Atrophy. Kobe J. Med. Sci. 2017, 63, E41–E44. [Google Scholar]
- De Amicis, R.; Baranello, G.; Foppiani, A.; Leone, A.; Battezzati, A.; Bedogni, G.; Ravella, S.; Giaquinto, E.; Mastella, C.; Agosto, C.; et al. Growth Patterns in Children with Spinal Muscular Atrophy. Orphanet J. Rare Dis. 2021, 16, 375. [Google Scholar] [CrossRef]
- Gaignard, P.; Savouroux, S.; Liere, P.; Pianos, A.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice. Endocrinology 2015, 156, 2893–2904. [Google Scholar] [CrossRef]
- Deguise, M.-O.; De Repentigny, Y.; Tierney, A.; Beauvais, A.; Michaud, J.; Chehade, L.; Thabet, M.; Paul, B.; Reilly, A.; Gagnon, S.; et al. Motor Transmission Defects with Sex Differences in a New Mouse Model of Mild Spinal Muscular Atrophy. EBioMedicine 2020, 55, 102750. [Google Scholar] [CrossRef]
- Mosher, K.I.; Wyss-Coray, T. Microglial Dysfunction in Brain Aging and Alzheimer’s Disease. Biochem. Pharm. 2014, 88, 594–604. [Google Scholar] [CrossRef]
- Czeh, M.; Gressens, P.; Kaindl, A.M. The Yin and Yang of Microglia. Dev. Neurosci. 2011, 33, 199–209. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s Disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Baidya, F.; Bohra, M.; Datta, A.; Sarmah, D.; Shah, B.; Jagtap, P.; Raut, S.; Sarkar, A.; Singh, U.; Kalia, K.; et al. Neuroimmune Crosstalk and Evolving Pharmacotherapies in Neurodegenerative Diseases. Immunology 2021, 162, 160–178. [Google Scholar] [CrossRef]
- Kodama, L.; Gan, L. Do Microglial Sex Differences Contribute to Sex Differences in Neurodegenerative Diseases? Trends Mol. Med. 2019, 25, 741–749. [Google Scholar] [CrossRef]
- Meibohm, B.; Beierle, I.; Derendorf, H. How Important Are Gender Differences in Pharmacokinetics? Clin. Pharm. 2002, 41, 329–342. [Google Scholar] [CrossRef]
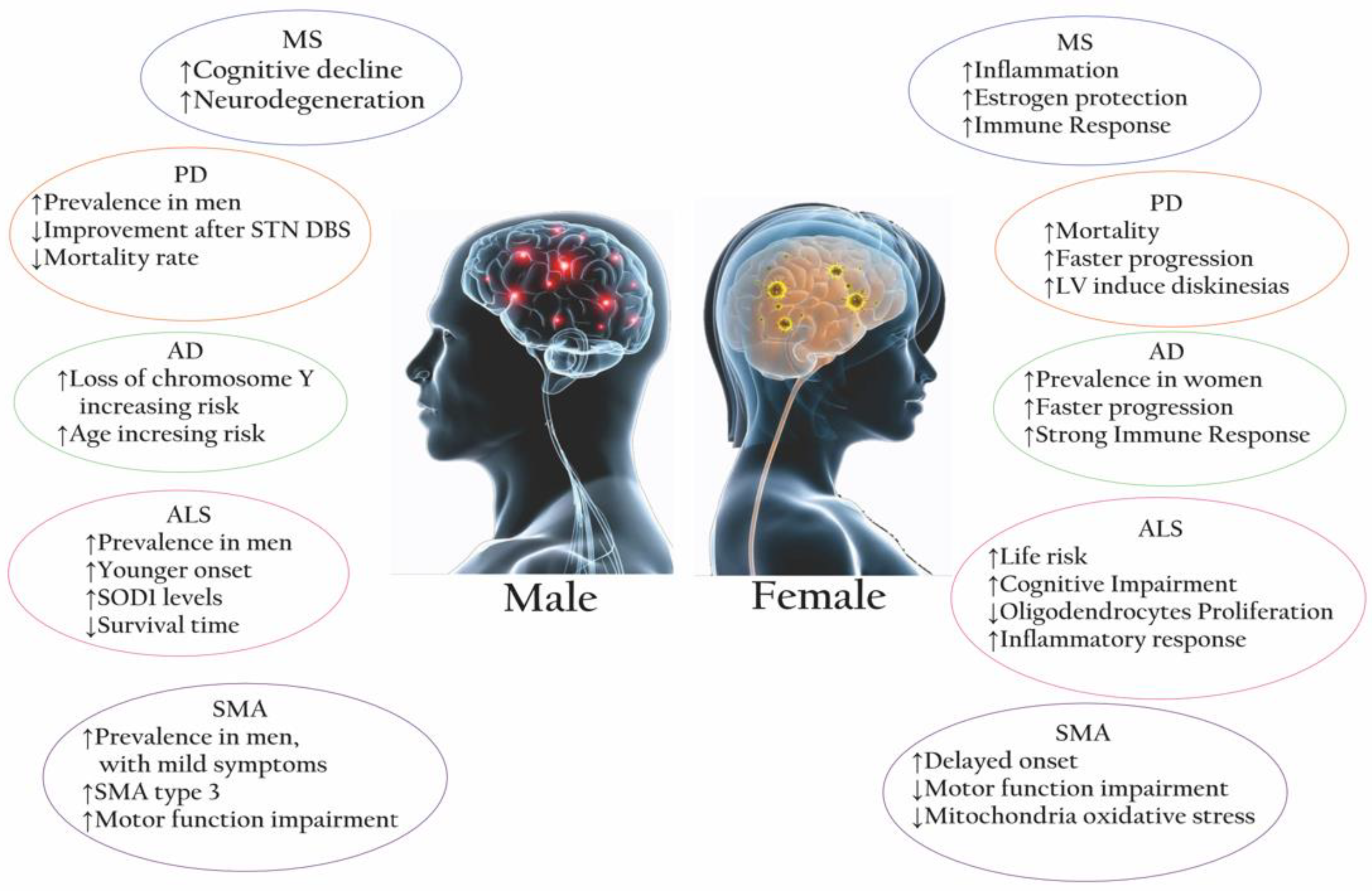
| NDs | Female/Male Ratio | Main IMMUNE FEATURES (In Vivo/Vitro) | ||
|---|---|---|---|---|
| Female | Male | In Common | ||
| MS | 3:1 [10] | Higher neutrophils/macrophages activity [11] Higher CD4+ T cell, CD4+/CD8+ ratio [11] APCs are more competent [11] Higher PGR expression in microglia [17] Higher expression of IL-21, IL-27, and IL-18 [18] Notable Treg, TH1/TH2 variability [18,19]. (Mice) Higher Th1 cytokine production [20] | Higher NK cells [11] Higher CD8+ Tcell [11] Higher CD3+ and TNFα [21] Higher IL-1β and TNF [17] APCs secrete IL-10 [21] (Mice) Higher lymphocyte infiltration [20] | M1 in early MS shifts to M2 in later stages [22] Patients with more severe disease have higher proportions of lesions with foamy microglia/macrophages [17] TNFα is increased by macrophages/microglia during the early development of sclerotic plaques [21] |
| PD | 1:1.5 [8] | Strong activation of peripheral monocytes. Consistent signature of changes in inflammatory signals (e.g., natural killer cell-mediated cytotoxicity pathway, APC, cytokine-cytokine receptor pathway) [23] (Mice): enhanced expression of IP-10 in astrocytes [24] | (Mice): enhanced expression of IL-1, IL-2, IL-6, and TNF-α in astrocytes [24] | The cytokine inflammatory signature and a-synuclein-specific T-cell reactivity are intense in the preclinical/early stage but may wane in more advanced PD [23] |
| AD | 2:1 [25] | Increased inflammatory cytokines, chemokines, and gliosis [11,26] Activation of neurons in the active phase [27] (Mice): rod-shaped microglia with compromised phagocytic capacity [28] and an early state of alertness to inflammatory stimuli with accumulated Ab [29] | Activation of olygodendrocytes in the active phase (Mice): amoeboid microglia with greater phagocytic capacity [28] | Neuron-glia interactions: microglia adopt and activate an inflammatory phenotype by shifting to glycolysis [28] |
| ALS | 1:1.2–1.5 [30] | Stronger immune response (STAT3 activation) [31] Higher expression of inflammatory genes in macrophages and innate immune cells [17] | Increased T cells, macrophages, IL-17A [32], IL-1β, TNFα, ROS, and NO [33] Lower levels of CD4+/higher levels of CD8+ [33] Release of proinflammatory cytokines by TLR2, TLR4, CD14 [34] Higher expression of IL2, IL5, IL6, IL8 [31], IL-13 [33] Higher levels of IL-7, IL−6, TNFa, TNF-R1, TNF-R2 [35] Microglia shift from the M1 to the M2 phenotype as soon as the disease progresses [22] | |
| SMA | 1:2 [36] | (Mouse): lower oxidative stress in muscular mitochondria, milder involvement of inflammatory pathways [36] | (Mouse): higher male susceptibility to the cumulative effects of oxidative stress [36] | T-cell alterations to an abnormal neuroinflammatory response and disease exacerbation [37] Increased astrogliosis [20] Increased levels of proinflammatory cytokines and neurotrophic factors in the CSF of active SMA1 patients [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, A.; Antonacci, Y.; Liguori, M. Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 6354. https://doi.org/10.3390/ijms24076354
Bianco A, Antonacci Y, Liguori M. Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. International Journal of Molecular Sciences. 2023; 24(7):6354. https://doi.org/10.3390/ijms24076354
Chicago/Turabian StyleBianco, Annalisa, Ylenia Antonacci, and Maria Liguori. 2023. "Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities" International Journal of Molecular Sciences 24, no. 7: 6354. https://doi.org/10.3390/ijms24076354
APA StyleBianco, A., Antonacci, Y., & Liguori, M. (2023). Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. International Journal of Molecular Sciences, 24(7), 6354. https://doi.org/10.3390/ijms24076354






