Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation
Abstract
1. Introduction
2. Results
2.1. Urinary Biomarkers of Exposure
2.2. Blood Biomarkers of Exposure
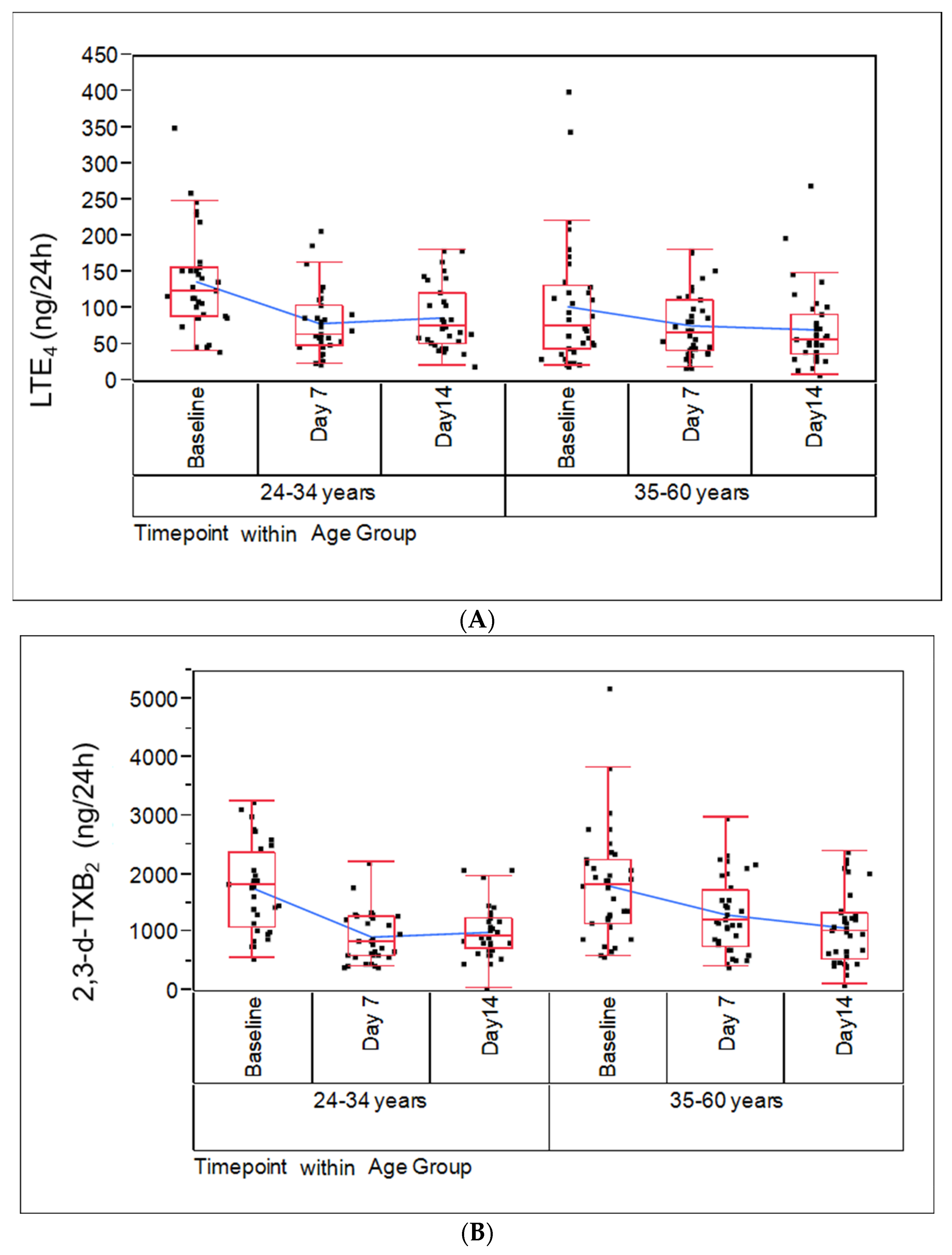
2.3. Biomarkers of Potential Harm
2.4. Hematological Biomarkers
2.5. Physiological Biomarkers of Potential Harm
3. Discussion
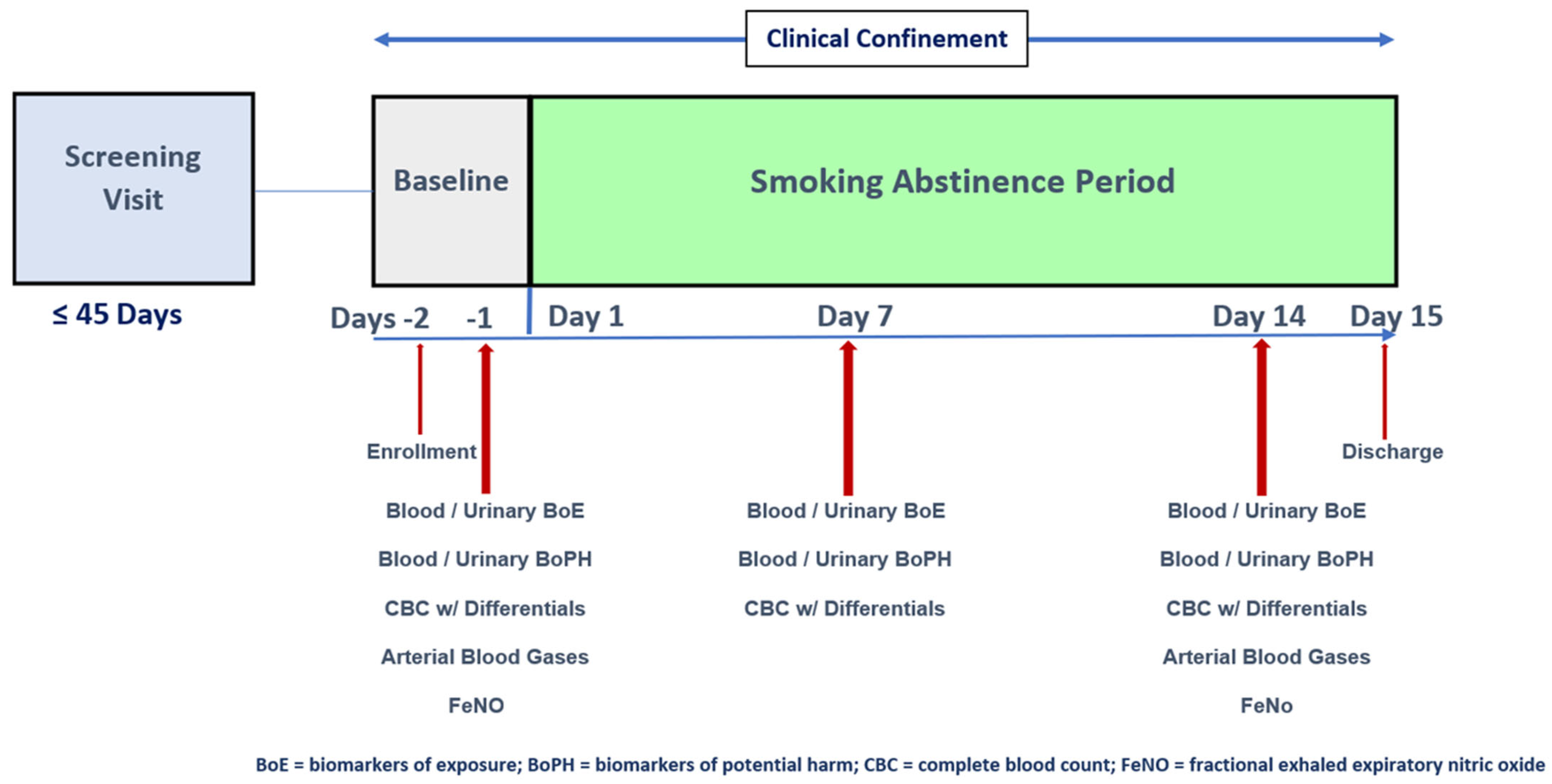
4. Materials and Methods
4.1. Ethical Conduct
4.2. Study Design
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. In A report of the Surgeon General; Centers for Disease Control and Prevention Office on Smoking and Health: Atlanta, GA, USA, 2010. [Google Scholar]
- U.S. Department of Health and Human Services. Smoking Cessation: A Report of the Surgeon General; U.S. Department of Health and Human Services, National Center for Chronic Disease Centers for Disease Control and Prevention and Office on Smoking and Health Prevention and Health Promotion, Eds.; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2020.
- U.S. Congress. Family Smoking Prevention and Tobacco Control and Federal Retirement Reform. Public Law 2009:111–31; HHS, Ed.; 2009. Available online: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-table-contents (accessed on 28 February 2023).
- Food and Drug Administration. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. U.S. Department of Health and Human Services, Ed.; Office of the Federal Register: Washington, DC, USA, 2012; Volume 77, pp. 20034–20037.
- Institute of Medicine Committee to Assess the Science Base for Tobacco Harm Reduction. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction; Stratton, K., Shetty, P., Wallace, R., Bondurant, S., Eds.; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Chang, C.M.; Cheng, Y.C.; Cho, T.M.; Mishina, E.V.; Del Valle-Pinero, A.Y.; van Bemmel, D.M.; Hatsukami, D.K. Biomarkers of Potential Harm: Summary of an FDA-Sponsored Public Workshop. Nicotine Tob. Res. 2019, 21, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Edwards, S.H.; Arab, A.; Del Valle-Pinero, A.Y.; Yang, L.; Hatsukami, D.K. Biomarkers of Tobacco Exposure: Summary of an FDA-Sponsored Public Workshop. Cancer Epidemiol. Biomark. Prev. 2017, 26, 291–302. [Google Scholar] [CrossRef] [PubMed]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/pdf/Bookshelf_NBK326791.pdf (accessed on 25 November 2020).
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The key contribution of platelet and vascular arachidonic acid metabolism to the pathophysiology of atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef]
- Patrono, C.; Rocca, B. Measurement of Thromboxane Biosynthesis in Health and Disease. Front. Pharm. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, A.; Pouly, S.; de La Bourdonnaye, G.; Ng, W.T.; Baker, G.; Lüdicke, F. Influence of smoking and smoking cessation on levels of urinary 11-dehydro thromboxane B(2). Toxicol. Rep. 2018, 5, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Frost-Pineda, K.; Liang, Q.; Liu, J.; Rimmer, L.; Jin, Y.; Feng, S.; Kapur, S.; Mendes, P.; Roethig, H.; Sarkar, M. Biomarkers of potential harm among adult smokers and nonsmokers in the total exposure study. Nicotine Tob. Res. 2011, 13, 182–193. [Google Scholar] [CrossRef]
- Lowe, F.J.; Gregg, E.O.; McEwan, M. Evaluation of biomarkers of exposure and potential harm in smokers, former smokers and never-smokers. Clin. Chem. Lab. Med. 2009, 47, 311–320. [Google Scholar] [CrossRef]
- Prasad, G.L.; Jones, B.A.; Chen, P.; Gregg, E.O. A cross-sectional study of biomarkers of exposure and effect in smokers and moist snuff consumers. Clin. Chem. Lab. Med. 2016, 54, 633–642. [Google Scholar] [CrossRef]
- Morita, H.; Ikeda, H.; Haramaki, N.; Eguchi, H.; Imaizumi, T. Only two-week smoking cessation improves platelet aggregability and intraplatelet redox imbalance of long-term smokers. J. Am. Coll. Cardiol. 2005, 45, 589–594. [Google Scholar] [CrossRef]
- Rångemark, C.; Ciabattoni, G.; Wennmalm, A. Excretion of thromboxane metabolites in healthy women after cessation of smoking. Arterioscler. Thromb. A J. Vasc. Biol. 1993, 13, 777–782. [Google Scholar] [CrossRef]
- Brownawell, A.M. Biological Efects Assessment in the Evaluation of Potential Reduced-Risk Tobacco Products; The Life Sciences Research Office: Bethesda, MD, USA, 2007; p. 242. [Google Scholar]
- Hatsukami, D.K.; Joseph, A.M.; Lesage, M.; Jensen, J.; Murphy, S.E.; Pentel, P.R.; Kotlyar, M.; Borgida, E.; Le, C.; Hecht, S.S. Developing the science base for reducing tobacco harm. Nicotine Tob. Res. 2007, 9 (Suppl. S4), S537–S553. [Google Scholar] [CrossRef]
- Bohadana, A.B.; Nilsson, F.; Westin, A.; Martinet, N.; Martinet, Y. Smoking cessation—But not smoking reduction--improves the annual decline in FEV1 in occupationally exposed workers. Respir. Med. 2006, 100, 1423–1430. [Google Scholar] [CrossRef]
- Pride, N.B. Smoking cessation: Effects on symptoms, spirometry and future trends in COPD. Thorax 2001, 56 (Suppl. S2), ii7–ii10. [Google Scholar] [PubMed]
- Kanobe, M.N.; Jones, B.A.; Nelson, P.; Brown, B.G.; Chen, P.; Makena, P.; Schmidt, E.; Darnell, J.; Caraway, J.W.; Prasad, G.L.; et al. Part three: A randomized study to assess biomarker changes in cigarette smokers switched to Vuse Solo or Abstinence. Sci. Rep. 2022, 12, 20658. [Google Scholar] [CrossRef] [PubMed]
- Makena, P.; Liu, G.; Chen, P.; Yates, C.R.; Prasad, G.L. Urinary Leukotriene E4 and 2,3-Dinor Thromboxane B2 are Biomarkers of Potential Harm in Short-term Tobacco Switching Studies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Carmella, S.G.; Chen, M.; Jensen, J.A.; Wilkens, L.R.; Le Marchand, L.; Hatsukami, D.K.; Murphy, S.E.; Hecht, S.S. Urinary Cyanoethyl Mercapturic Acid, a Biomarker of the Smoke Toxicant Acrylonitrile, Clearly Distinguishes Smokers From Nonsmokers. Nicotine Tob. Res. 2020, 22, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.; Cheung, F.; Errington, G.; Sterz, K.; Scherer, G. Urinary excretion of the acrylonitrile metabolite 2-cyanoethylmercapturic acid is correlated with a variety of biomarkers of tobacco smoke exposure and consumption. Biomarkers 2011, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.M.; Çolak, Y.; Ellervik, C.; Hasselbalch, H.C.; Bojesen, S.E.; Nordestgaard, B.G. Smoking and Increased White and Red Blood Cells. Arter. Thromb. Vasc. Biol. 2019, 39, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.; McEwan, M.; Eldridge, A.C.; Fearon, I.M.; Sherwood, N.; Bowen, E.; McDermott, S.; Holmes, E.; Hedge, A.; Hossack, S.; et al. Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to Tobacco Heating Products: A Randomized, Controlled Study in Healthy Japanese Subjects. Nicotine Tob. Res. 2019, 21, 1220–1227. [Google Scholar] [CrossRef]
- Krautter, G.R.; Chen, P.X.; Borgerding, M.F. Consumption patterns and biomarkers of exposure in cigarette smokers switched to Snus, various dissolvable tobacco products, Dual use, or tobacco abstinence. Regul. Toxicol. Pharm. 2015, 71, 186–197. [Google Scholar] [CrossRef]
- Theophilus, E.H.; Coggins, C.R.; Chen, P.; Schmidt, E.; Borgerding, M.F. Magnitudes of biomarker reductions in response to controlled reductions in cigarettes smoked per day: A one-week clinical confinement study. Regul. Toxicol. Pharm. 2015, 71, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Bosilkovska, M.; de La Bourdonnaye, G.; Blanc, N.; Haziza, C. Reduced levels of biomarkers of exposure in smokers switching to the Carbon-Heated Tobacco Product 1.0: A controlled, randomized, open-label 5-day exposure trial. Sci. Rep. 2020, 10, 19227. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, A.; Pouly, S.; de La Bourdonnaye, G.; Baker, G.; Lüdicke, F. Influence of smoking on levels of urinary 8-iso Prostaglandin F2α. Toxicol. Rep. 2019, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- McElroy, J.P.; Carmella, S.G.; Heskin, A.K.; Tang, M.K.; Murphy, S.E.; Reisinger, S.A.; Jensen, J.A.; Hatsukami, D.K.; Hecht, S.S.; Shields, P.G. Effects of cessation of cigarette smoking on eicosanoid biomarkers of inflammation and oxidative damage. PLoS ONE 2019, 14, e0218386. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kodali, M.; Tello-Montoliu, A.; Angiolillo, D.J. Role of platelets and antiplatelet therapy in cardiovascular disease. J. Atheroscler. Thromb. 2011, 18, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, G.; Tartarone, A.; Lerose, R.; Lalinga, A.V.; Capobianco, A.M. Cardiovascular risk of smoking and benefits of smoking cessation. J. Thorac. Dis. 2020, 12, 3866–3876. [Google Scholar] [CrossRef]
- Kito, Y.; Iida, M.; Tanabe, K.; Onuma, T.; Tsujimoto, M.; Nagase, K.; Tokuda, H.; Iwama, T.; Kozawa, O.; Iida, H. Smoking cessation affects human platelet activation induced by collagen. Exp. Ther. Med. 2019, 18, 3809–3816. [Google Scholar] [CrossRef]
- Bain, B.J.; Rothwell, M.; Feher, M.D.; Robinson, R.; Brown, J.; Sever, P.S. Acute changes in haematological parameters on cessation of smoking. J. R. Soc. Med. 1992, 85, 80–82. [Google Scholar] [CrossRef]
- Roethig, H.J.; Koval, T.; Muhammad-Kah, R.; Jin, Y.; Mendes, P.; Unverdorben, M. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul. Toxicol. Pharm. 2010, 57, 333–337. [Google Scholar] [CrossRef]
- Hardie, J.A.; Vollmer, W.M.; Buist, A.S.; Ellingsen, I.; Mørkve, O. Reference values for arterial blood gases in the elderly. Chest 2004, 125, 2053–2060. [Google Scholar] [CrossRef]
- Fricker, M.; Goggins, B.J.; Mateer, S.; Jones, B.; Kim, R.Y.; Gellatly, S.L.; Jarnicki, A.G.; Powell, N.; Oliver, B.G.; Radford-Smith, G.; et al. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. J. Clin. Investig. 2018, 3, e94040. [Google Scholar] [CrossRef] [PubMed]
- Högman, M.; Holmkvist, T.; Wålinder, R.; Meriläinen, P.; Lúdvíksdóttir, D.; Håkansson, L.; Hedenström, H. Increased nitric oxide elimination from the airways after smoking cessation. Clin. Sci. 2002, 103, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.A.; Robbins, R.A.; Yates, D.; Keatings, V.; Barnes, P.J. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 1995, 152, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Damiani, G.; Carpagnano, G.E.; Olivini, A.; Radaeli, A.; Ragnoli, B.; Foschino, M.P.; Olivieri, M. Values in Elderly People for Exhaled Nitric Oxide Study. Rejuvenation Res. 2016, 19, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Strzelak, A.; Ratajczak, A.; Adamiec, A.; Feleszko, W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. Int. J. Environ. Res. Public Health 2018, 15, 1033. [Google Scholar] [CrossRef]
- Oliveri, D.; Liang, Q.; Sarkar, M. Real-World Evidence of Differences in Biomarkers of Exposure to Select Harmful and Potentially Harmful Constituents and Biomarkers of Potential Harm Between Adult E-Vapor Users and Adult Cigarette Smokers. Nicotine Tob. Res. 2020, 22, 1114–1122. [Google Scholar] [CrossRef]
- Sakaguchi, C.; Nagata, Y.; Kikuchi, A.; Takeshige, Y.; Minami, N. Differences in Levels of Biomarkers of Potential Harm among Users of a Heat-not-burn Tobacco Product, Cigarette Smokers, and Never-Smokers in Japan: A Post-Marketing Observational Study. Nicotine Tob. Res. 2021, 23, 1143–1152. [Google Scholar] [CrossRef]
- Jensen, E.J.; Pedersen, B.; Frederiksen, R.; Dahl, R. Prospective study on the effect of smoking and nicotine substitution on leucocyte blood counts and relation between blood leucocytes and lung function. Thorax 1998, 53, 784–789. [Google Scholar] [CrossRef]
- Frans, A.; Gerin-Portier, N.; Veriter, C.; Brasseur, L. Pulmonary Gas Exchange in Asymptomatic Smokers and Nonsmokers. Scand. J. Respir Dis. 1975, 56, 233–244. [Google Scholar]
- Kullmer, T.; Kronenberger, H.; Siekmeier, R.; Clemens, M. The Value of Studies of Pulmonary Gas Exchange During Exercise for Evaluation of Reduced Arterial Oxygenation at Rest in Asymptomatic Long-Term Smokers. Pneumologie 1994, 48, 20–24. [Google Scholar]
- Ouattara, S.; Keita, M.; Tuo, N.; Dah, C.; Siransy, E.A.; Bogui, P. Effect of Smoking on Pao2 at Rest and During Moderate Exercise. Dakar Med. 2002, 47, 90–95. [Google Scholar] [PubMed]
- Lei, W.; Li, F.; Tang, X.M.; Bian, S.; Wang, J.J.; Huang, J.A. The Comparision of Two Exhaled Nitric Oxide Analyzers: Niox Vero and Sunvou-Ca2122. J. Breath Res. 2021, 15, 026007. [Google Scholar] [CrossRef] [PubMed]
| Age Cohort | ||||
|---|---|---|---|---|
| 24–34 (N = 33) | 35–60 (N = 37) | Study Overall (N = 70) | ||
| Sex n (%) | Female | 4 (12%) | 15 (41%) | 19 (27%) |
| Male | 29 (88%) | 22 (59%) | 51 (73%) | |
| Race n (%) | Black or African American | 5 (15%) | 3 (8%) | 8 (11%) |
| White | 26 (79%) | 31 (84%) | 57 (81%) | |
| Multiple | 2 (6%) | 3 (8%) | 5 (7%) | |
| Ethnicity n (%) | Hispanic or Latino | 3 (9%) | 0 (0%) | 3 (4%) |
| Not Hispanic or Latino | 30 (91%) | 37 (100%) | 67 (96%) | |
| Age a (years) | Mean (SD) | 30.0 (3.12) | 49.2 (8.10) | 40.2 (11.52) |
| FTND Score | Mean (SD) | 5.5 (1.70) | 6.0 (1.57) | 5.8 (1.63) |
| Years of Smoking b | 12.8 (4.08) | 29.3 (11.30) | 21.5 (11.94) | |
| Cigarettes Smoked per Day | 17.6 (4.48) | 17.4 (4.02) | 17.5 (4.21) | |
| Cigarette Variety n (%) | Menthol | 10 (30%) | 7 (19%) | 17 (24%) |
| Non-Menthol | 23 (70%) | 30 (81%) | 53 (76%) | |
| Urine Biomarkers | Age Cohort | |||
|---|---|---|---|---|
| Biomarker (Units) | Statistics | Time Point | 24–34 Years | 35–60 Years |
| NicEq-T (mg/24 h) | Mean ± SD (n) | Day −1 | 19.4 ± 6.6 (32) | 17.3 ± 6.4 (37) |
| Day 7 | 0.4 ± 0.2 (32) | 0.4 ± 0.1 (37) | ||
| Day 14 | 0.4 ± 0.3 (32) | 0.4 ± 0.2 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −98% * | −97% * | |
| Day 14 vs. Day −1 | −98% * | −98% * | ||
| CEMA (µg/24 h) | Mean ± SD (n) | Day −1 | 273.6 ± 115.1 (32) | 240.3 ± 91.1 (37) |
| Day 7 | 32.0 ± 12.8 (32) | 28.1 ± 14.6 (37) | ||
| Day 14 | 24.3 ± 9.3 (32) | 21.4 ± 13.1 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −88% * | −88% * | |
| Day 14 vs. Day −1 | −91% * | −91% * | ||
| NNN-T (pg/24 h) | Mean ± SD (n) | Day −1 | 16,380 ± 10,164 (32) | 20,380 ± 39,703 (37) |
| Day 7 | 240.0 ± 101.3 (32) | 251.9 ± 80.8 (37) | ||
| Day 14 | 232.9 ± 146.7 (32) | 232.7 ± 124.6 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −99% * | −99% * | |
| Day 14 vs. Day −1 | −99% * | −99% * | ||
| NAB-T (ng/24 h) | Mean ± SD (n) | Day −1 | 66.4 ± 40.4 (32) | 55.7 ± 35.3 (37) |
| Day 7 | 2.4 ± 1.0 (32) | 2.5 ± 0.8 (37) | ||
| Day 14 | 2.3 ± 1.5 (32) | 2.3 ± 1.2 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −96% * | −96% * | |
| Day 14 vs. Day −1 | −96% * | −96% * | ||
| NAT-T (ng/24 h) | Mean ± SD (n) | Day −1 | 480.4 ± 292.0 (32) | 377.0 ± 226.9 (37) |
| Day 7 | 6.0 ± 2.5 (32) | 6.2 ± 2.1 (37) | ||
| Day 14 | 5.8 ± 3.7 (32) | 5.8 ± 3.1 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −99% * | −98% * | |
| Day 14 vs. Day −1 | −99% * | −98% * | ||
| NNAL-T (ng/24 h) | Mean ± SD (n) | Day −1 | 465.3 ± 249.1 (32) | 475.3 ± 263.9 (37) |
| Day 7 | 132.7 ± 85.7 (32) | 135.8 ± 84.7 (37) | ||
| Day 14 | 84.5 ± 47.7 (32) | 81.3 ± 54.4 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −71% * | −71% * | |
| Day 14 vs. Day −1 | −82% * | −83% * | ||
| Blood Biomarkers | ||||
| Blood CoHB (%) | Mean ± SD (n) | Day −1 | 3.7 ± 1.2 (32) | 3.8 ± 1.3 (37) |
| Day 7 | 1.4 ± 0.4 (32) | 1.3 ± 0.3 (37) | ||
| Day 14 | 1.4 ± 0.3 (32) | 1.3 ± 0.3 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −62% * | −66% * | |
| Day 14 vs. Day −1 | −61% * | −65% * | ||
| Plasma Nicotine ** (ng/mL) | Mean ± SD (n) | Day −1 | 5.2 ± 6.5 (32) | 4.6 ± 5.1 (37) |
| Day 7 | 0.1 ± 0.0 (32) | 0.1 ± 0.0 (37) | ||
| Day 14 | 0.1 ± 0.0 (32) | 0.1 ± 0.0 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −98% | −98% | |
| Day 14 vs. Day −1 | −98% | −98% | ||
| Plasma Cotinine (ng/mL) | Mean ± SD (n) | Day −1 | 236.6 ± 118.9 (32) | 234.2 ± 112.7 (37) |
| Day 7 | 2.9 ± 6.7 (32) | 2.1 ± 3.0 (37) | ||
| Day 14 | 0.6 ± 0.4 (32) | 0.5 ± 0.1 (36) | ||
| Percent Change | Day 7 vs. Day −1 | −99% * | −99% * | |
| Day 14 vs. Day −1 | −100% * | −100% * | ||
| Hematological Biomarkers | Age Cohort | |||
|---|---|---|---|---|
| Biomarker (Units) | Statistics | Time Point | 24–34 Years | 35–60 Years |
| White blood cells | Mean ± SD (n) | Day −2 | 7.83 ± 1.45 (32) | 8.54 ± 2.82 (37) |
| (109/L) | Day 7 | 6.81 ± 1.38 (32) | 6.41 ± 1.74 (37) | |
| Day 14 | 6.95 ± 1.46 (32) | 6.71 ± 1.89 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −13% (<0.0014) | −25% (<0.0001) | |
| Day 14 vs. Day −2 | −11% (0.0077) | −22% (<0.0001) | ||
| Neutrophils | Mean ± SD (n) | Day −2 | 4.72 ± 1.24 (32) | 5.15 ± 2.27 (36) |
| (109/L) | Day 7 | 3.87 ± 0.98 (32) | 3.56 ± 1.33 (36) | |
| Day 14 | 3.93 ± 0.93 (32) | 3.71 ± 1.46 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −18% (<0.0001) | −31% (<0.0001) | |
| Day 14 vs. Day −2 | −17% (0.0004) | −28% (<0.0001) | ||
| Lymphocytes | Mean ± SD (n) | Day −2 | 2.23 ± 0.63 (32) | 2.49 ± 0.67 (36) |
| (109/L) | Day 7 | 2.13 ± 0.51 (32) | 2.09 ± 0.6 (36) | |
| Day 14 | 2.20 ± 0.61 (32) | 2.23 ± 0.53 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −5% (0.1201) | −16% (<0.0001) | |
| Day 14 vs. Day −2 | −1% (0.6331) | −11% (0.003) | ||
| Red blood cells | Mean ± SD (n) | Day −2 | 5.23 ± 0.37 (32) | 4.87 ± 0.43 (37) |
| (1012/L) | Day 7 | 5.11 ± 0.48 (32) | 4.75 ± 0.50 (37) | |
| Day 14 | 5.04 ± 0.41 (32) | 4.65 ± 0.52 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −2% (0.0147) | −3% (0.0038) | |
| Day 14 vs. Day −2 | −4% (<0.0001) | −5% (<0.0001) | ||
| Hematocrit (%) | Mean ± SD (n) | Day −2 | 46.91 ± 2.68 (32) | 44.02 ± 3.56 (36) |
| Day 7 | 45.75 ± 3.39 (32) | 42.79 ± 3.97 (36) | ||
| Day 14 | 45.07 ± 3.04 (32) | 42.12 ± 4.29 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −2% (0.0163) | −3% (0.0007) | |
| Day 14 vs. Day −2 | −4% (<0.0001) | −4% (<0.0001) | ||
| Hemoglobin (g/dL) | Mean ± SD (n) | Day −2 | 15.80 ± 0.96 (32) | 14.66 ± 1.41 (36) |
| Day 7 | 15.39 ± 1.11 (32) | 14.21 ± 1.48 (36) | ||
| Day 14 | 15.20± 1.06 (32) | 14.01 ± 1.57 (36) | ||
| Percent Change (p-value *) | Day 7 vs. Day −2 | −3% (0.0117) | −3% (0.0003) | |
| Day 14 vs. Day −2 | −4% (<0.0001) | −4% (<0.0001) | ||
| Physiological Biomarkers | Age Cohort | |||
|---|---|---|---|---|
| Biomarker (Units) | Statistics | Time Point | 24–34 Years | 35–60 Years |
| PAO2 (mmHg) | Mean ± SD (n) | Day −1 | 89.65 ± 9.26 (32) | 81.51 ± 11.02 (37) |
| Day 14 | 91.00 ± 10.02 (32) | 85.13 ± 8.17 (36) | ||
| Percent Change | Day 14 vs. Day −1 | 1% | 4% * | |
| PACO2 (mmHg) | Mean ± SD (n) | Day −1 | 40.13 ± 3.28 (32) | 36.95 ± 3.1 (37) |
| Day 14 | 41.41 ± 2.98 (32) | 38.91 ± 3.51 (36) | ||
| Percent Change | Day 14 vs. Day −1 | 3% * | 5% * | |
| O2 Saturation (%) | Mean ± SD (n) | Day −1 | 96.71 ± 0.85 (32) | 95.67 ± 1.68 (37) |
| Day 14 | 96.68 ± 1.3 (32) | 96.38 ± 1.10 (36) | ||
| Percent Change | Day 14 vs. Day −1 | 0% | 1% * | |
| Bicarbonate (mmol/L) | Mean ± SD (n) | Day −1 | 24.75 ± 1.87 (32) | 23.44 ± 1.76 (37) |
| Day 14 | 25.38 ± 1.75 (32) | 24.28 ± 1.94 (36) | ||
| Percent Change | Day 14 vs. Day −1 | 3% * | 4% * | |
| FeNO (ppb) | Mean ± SD (n) | Day −1 | 12.29 ± 9.07 (31) | 15.03 ± 19.56 (33) |
| Day 14 | 19.13 ± 18.42 (31) | 15.71 ± 10.78 (35) | ||
| Percent Change | Day 14 vs. Day −1 | 56% * | 5% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makena, P.; Scott, E.; Chen, P.; Liu, H.-P.; Jones, B.A.; Prasad, G.L. Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation. Int. J. Mol. Sci. 2023, 24, 6286. https://doi.org/10.3390/ijms24076286
Makena P, Scott E, Chen P, Liu H-P, Jones BA, Prasad GL. Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation. International Journal of Molecular Sciences. 2023; 24(7):6286. https://doi.org/10.3390/ijms24076286
Chicago/Turabian StyleMakena, Patrudu, Eric Scott, Peter Chen, Hsiao-Pin Liu, Bobbette A. Jones, and Gaddamanugu L. Prasad. 2023. "Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation" International Journal of Molecular Sciences 24, no. 7: 6286. https://doi.org/10.3390/ijms24076286
APA StyleMakena, P., Scott, E., Chen, P., Liu, H.-P., Jones, B. A., & Prasad, G. L. (2023). Biomarkers of Exposure and Potential Harm in Two Weeks of Smoking Abstinence: Changes in Biomarkers of Platelet Function, Oxidative Stress, and Inflammation. International Journal of Molecular Sciences, 24(7), 6286. https://doi.org/10.3390/ijms24076286





