Muscle Mechanics and Thick Filament Activation: An Emerging Two-Way Interaction for the Vertebrate Striated Muscle Fine Regulation
Abstract
1. Introduction
2. Detached Myosin Stable States
2.1. X-ray
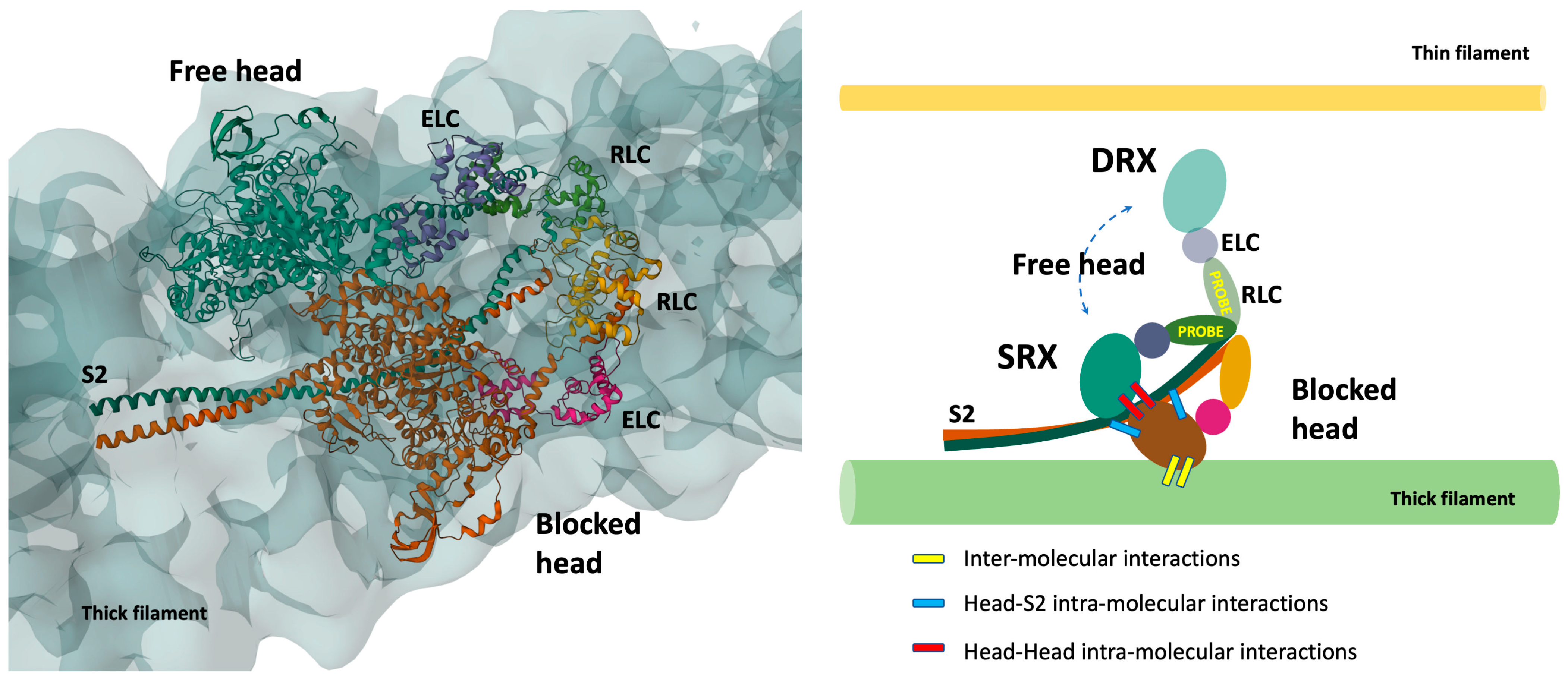
2.2. CryoEM
2.3. Mant-ATP
2.4. RLC-Probes
3. Non-Mechanical Modulators of the Detached Myosin Stable States and Reciprocal Relationships
3.1. Temperature and Ionic Strength
3.2. Small Molecules Directly Targeting the Myosin Motors
3.3. RLC and MyBP-C Phosphorylation
3.4. Genetic Modifications or Alterations
4. Thick Filament Activation and Contractile Performance
5. From the Activation of the Thick Filament to Muscle Mechanical Response
6. From the Mechanical Perturbation to the Thick Filament Activation
7. Influence of Mechanical Modulation of the Thick Filament Activation on Contractile Performance
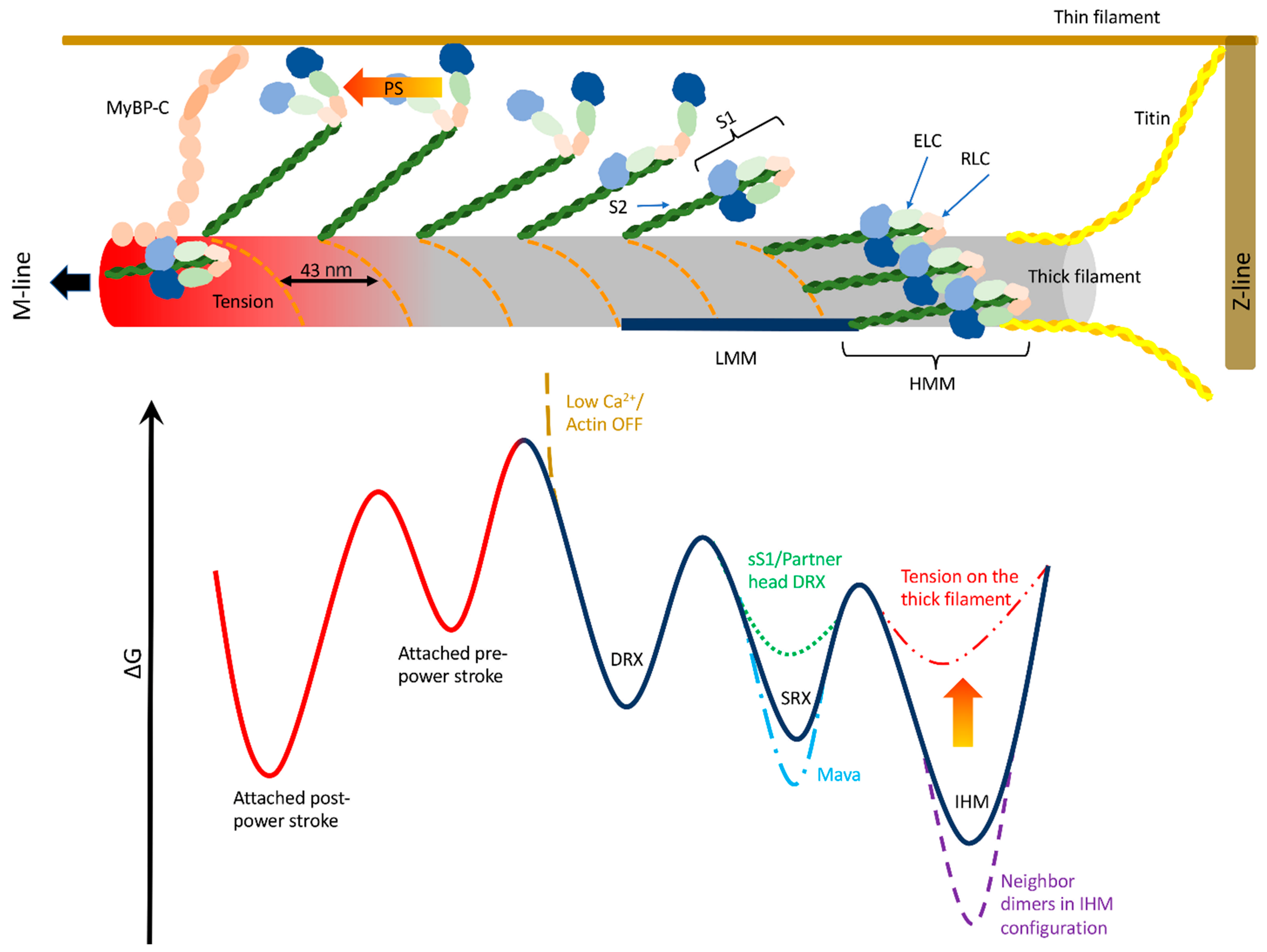
8. Proposed Working Model
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Squire, J. The Structural Basis of Muscular Contraction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4613-3183-4. [Google Scholar]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of Contraction in Striated Muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Huxley, H.E. Structural Changes in the Actin- and Myosin-Eontaining Filaments during Contraction. Cold Spring Harb. Symp. Quant. Biol. 1973, 37, 361–376. [Google Scholar] [CrossRef]
- Shaffer, J.F.; Kensler, R.W.; Harris, S.P. The Myosin-Binding Protein C Motif Binds to F-Actin in a Phosphorylation-Sensitive Manner. J. Biol. Chem. 2009, 284, 12318–12327. [Google Scholar] [CrossRef]
- Previs, M.J.; Previs, S.B.; Gulick, J.; Robbins, J.; Warshaw, D.M. Molecular Mechanics of Cardiac Myosin-Binding Protein C in Native Thick Filaments. Science 2012, 337, 1215–1218. [Google Scholar] [CrossRef]
- Kampourakis, T.; Yan, Z.; Gautel, M.; Sun, Y.-B.; Irving, M. Myosin Binding Protein-C Activates Thin Filaments and Inhibits Thick Filaments in Heart Muscle Cells. Proc. Natl. Acad. Sci. USA 2014, 111, 18763–18768. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.W.; Li, A.; dos Remedios, C.G.; Cooke, R. The Role of Super-Relaxed Myosin in Skeletal and Cardiac Muscle. Biophys. Rev. 2014, 7, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.V.; Adhikari, A.S.; Sarkar, S.S.; Ruppel, K.M.; Spudich, J.A. Hypertrophic Cardiomyopathy and the Myosin Mesa: Viewing an Old Disease in a New Light. Biophys. Rev. 2017, 10, 27–48. [Google Scholar] [CrossRef]
- Piazzesi, G.; Caremani, M.; Linari, M.; Reconditi, M.; Lombardi, V. Thick Filament Mechano-Sensing in Skeletal and Cardiac Muscles: A Common Mechanism Able to Adapt the Energetic Cost of the Contraction to the Task. Front. Physiol. 2018, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Spudich, J.A. Three Perspectives on the Molecular Basis of Hypercontractility Caused by Hypertrophic Cardiomyopathy Mutations. Pflügers Arch.-Eur. J. Physiol. 2019, 471, 701–717. [Google Scholar] [CrossRef]
- Craig, R.; Padrón, R. Structural Basis of the Super- and Hyper-Relaxed States of Myosin II. J. Gen. Physiol. 2022, 154, e202113012. [Google Scholar] [CrossRef]
- Irving, M. Regulation of Contraction by the Thick Filaments in Skeletal Muscle. Biophys. J. 2017, 113, 2579–2594. [Google Scholar] [CrossRef]
- Day, S.M.; Tardiff, J.C.; Ostap, E.M. Myosin Modulators: Emerging Approaches for the Treatment of Cardiomyopathies and Heart Failure. J. Clin. Investig. 2022, 132, e148557. [Google Scholar] [CrossRef]
- Lehman, S.J.; Crocini, C.; Leinwand, L.A. Targeting the Sarcomere in Inherited Cardiomyopathies. Nat. Rev. Cardiol. 2022, 19, 353–363. [Google Scholar] [CrossRef]
- Barrick, S.K.; Greenberg, M.J. Cardiac Myosin Contraction and Mechanotransduction in Health and Disease. J. Biol. Chem. 2021, 297, 101297. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, L.; Washio, T.; Yanagida, T. Including Thermal Fluctuations in Actomyosin Stable States Increases the Predicted Force per Motor and Macroscopic Efficiency in Muscle Modelling. PLoS Comput. Biol. 2016, 12, e1005083. [Google Scholar] [CrossRef] [PubMed]
- Huxley, H.E.; Faruqi, A.R.; Kress, M.; Bordas, J.; Koch, M.H.J. Time-Resolved X-Ray Diffraction Studies of the Myosin Layer-Line Reflections during Muscle Contraction. J. Mol. Biol. 1982, 158, 637–684. [Google Scholar] [CrossRef]
- Linari, M.; Brunello, E.; Reconditi, M.; Fusi, L.; Caremani, M.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. Force Generation by Skeletal Muscle Is Controlled by Mechanosensing in Myosin Filaments. Nature 2015, 528, 276–279. [Google Scholar] [CrossRef]
- Brunello, E.; Fusi, L.; Ghisleni, A.; Park-Holohan, S.-J.; Ovejero, J.G.; Narayanan, T.; Irving, M. Myosin Filament-Based Regulation of the Dynamics of Contraction in Heart Muscle. Proc. Natl. Acad. Sci. USA 2020, 117, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Linari, M.; Piazzesi, G.; Dobbie, I.; Koubassova, N.; Reconditi, M.; Narayanan, T.; Diat, O.; Irving, M.; Lombardi, V. Interference Fine Structure and Sarcomere Length Dependence of the Axial X-Ray Pattern from Active Single Muscle Fibers. Proc. Natl. Acad. Sci. USA 2000, 97, 7226–7231. [Google Scholar] [CrossRef]
- Huxley, H.E.; Brown, W. The Low-Angle X-Ray Diagram of Vertebrate Striated Muscle and Its Behaviour during Contraction and Rigor. J. Mol. Biol. 1967, 30, 383–434. [Google Scholar] [CrossRef]
- Haselgrove, J.C.; Huxley, H.E. X-Ray Evidence for Radial Cross-Bridge Movement and for the Sliding Filament Model in Actively Contracting Skeletal Muscle. J. Mol. Biol. 1973, 77, 549–568. [Google Scholar] [CrossRef]
- Haselgrove, J.C. X-Ray Evidence for Conformational Changes in the Myosin Filaments of Vertebrate Striated Muscle. J. Mol. Biol. 1975, 92, 113–143. [Google Scholar] [CrossRef] [PubMed]
- Alamo, L.; Koubassova, N.; Pinto, A.; Gillilan, R.; Tsaturyan, A.; Padrón, R. Lessons from a Tarantula: New Insights into Muscle Thick Filament and Myosin Interacting-Heads Motif Structure and Function. Biophys. Rev. 2017, 9, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, J.L.; Zhao, F.-Q.; Craig, R.; Egelman, E.H.; Alamo, L.; Padrón, R. Atomic Model of a Myosin Filament in the Relaxed State. Nature 2005, 436, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.; Taylor, D.; Trybus, K.M.; Taylor, K. Three-Dimensional Image Reconstruction of Dephosphorylated Smooth Muscle Heavy Meromyosin Reveals Asymmetry in the Interaction between Myosin Heads and Placement of Subfragment 2. Proc. Natl. Acad. Sci. USA 2001, 98, 4361–4366. [Google Scholar] [CrossRef]
- Alamo, L.; Ware, J.S.; Pinto, A.; Gillilan, R.E.; Seidman, J.G.; Seidman, C.E.; Padrón, R. Effects of Myosin Variants on Interacting-Heads Motif Explain Distinct Hypertrophic and Dilated Cardiomyopathy Phenotypes. eLife 2017, 6, e24634. [Google Scholar] [CrossRef]
- Zoghbi, M.E.; Woodhead, J.L.; Moss, R.L.; Craig, R. Three-Dimensional Structure of Vertebrate Cardiac Muscle Myosin Filaments. Proc. Natl. Acad. Sci. USA 2008, 105, 2386–2390. [Google Scholar] [CrossRef]
- AL-Khayat, H.A.; Kensler, R.W.; Squire, J.M.; Marston, S.B.; Morris, E.P. Atomic Model of the Human Cardiac Muscle Myosin Filament. Proc. Natl. Acad. Sci. USA 2013, 110, 318–323. [Google Scholar] [CrossRef]
- Knupp, C.; Morris, E.; Squire, J.M. The Interacting Head Motif Structure Does Not Explain the X-Ray Diffraction Patterns in Relaxed Vertebrate (Bony Fish) Skeletal Muscle and Insect (Lethocerus) Flight Muscle. Biology 2019, 8, 67. [Google Scholar] [CrossRef]
- Koubassova, N.A.; Tsaturyan, A.K.; Bershitsky, S.Y.; Ferenczi, M.A.; Padrón, R.; Craig, R. Interacting-Heads Motif Explains the X-Ray Diffraction Pattern of Relaxed Vertebrate Skeletal Muscle. Biophys. J. 2022, 121, 1354–1366. [Google Scholar] [CrossRef]
- Padrón, R.; Ma, W.; Duno-Miranda, S.; Koubassova, N.; Lee, K.H.; Pinto, A.; Alamo, L.; Bolaños, P.; Tsaturyan, A.; Irving, T.; et al. The Myosin Interacting-Heads Motif Present in Live Tarantula Muscle Explains Tetanic and Posttetanic Phosphorylation Mechanisms. Proc. Natl. Acad. Sci. USA 2020, 117, 11865–11874. [Google Scholar] [CrossRef]
- Jung, H.S.; Burgess, S.A.; Billington, N.; Colegrave, M.; Patel, H.; Chalovich, J.M.; Chantler, P.D.; Knight, P.J. Conservation of the Regulated Structure of Folded Myosin 2 in Species Separated by at Least 600 Million Years of Independent Evolution. Proc. Natl. Acad. Sci. USA 2008, 105, 6022–6026. [Google Scholar] [CrossRef]
- Spudich, J.A. The Myosin Mesa and a Possible Unifying Hypothesis for the Molecular Basis of Human Hypertrophic Cardiomyopathy. Biochem. Soc. Trans. 2015, 43, 64–72. [Google Scholar] [CrossRef]
- Robert-Paganin, J.; Auguin, D.; Houdusse, A. Hypertrophic Cardiomyopathy Disease Results from Disparate Impairments of Cardiac Myosin Function and Auto-Inhibition. Nat. Commun. 2018, 9, 4019. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin ATP Turnover Rate Is a Mechanism Involved in Thermogenesis in Resting Skeletal Muscle Fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, P.E.; Beishuizen, A.; Witt, C.C.; de Waard, M.C.; Girbes, A.R.J.; Spoelstra-de Man, A.M.E.; Niessen, H.W.M.; Manders, E.; van Hees, H.W.H.; van den Brom, C.E.; et al. Diaphragm Muscle Fiber Weakness and Ubiquitin–Proteasome Activation in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2015, 191, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Phung, L.A.; Foster, A.D.; Miller, M.S.; Lowe, D.A.; Thomas, D.D. Super-Relaxed State of Myosin in Human Skeletal Muscle Is Fiber-Type Dependent. Am. J. Physiol.-Cell Physiol. 2020, 319, C1158–C1162. [Google Scholar] [CrossRef] [PubMed]
- Walklate, J.; Kao, K.; Regnier, M.; Geeves, M.A. Exploring the Super-Relaxed State of Myosin in Myofibrils from Fast-Twitch, Slow-Twitch, and Cardiac Muscle. J. Biol. Chem. 2022, 298, 101640. [Google Scholar] [CrossRef]
- Rohde, J.A.; Roopnarine, O.; Thomas, D.D.; Muretta, J.M. Mavacamten Stabilizes an Autoinhibited State of Two-Headed Cardiac Myosin. Proc. Natl. Acad. Sci. USA 2018, 115, E7486–E7494. [Google Scholar] [CrossRef]
- Anderson, R.L.; Trivedi, D.V.; Sarkar, S.S.; Henze, M.; Ma, W.; Gong, H.; Rogers, C.S.; Gorham, J.M.; Wong, F.L.; Morck, M.M.; et al. Deciphering the Super Relaxed State of Human β-Cardiac Myosin and the Mode of Action of Mavacamten from Myosin Molecules to Muscle Fibers. Proc. Natl. Acad. Sci. USA 2018, 115, E8143–E8152. [Google Scholar] [CrossRef]
- Fusi, L.; Huang, Z.; Irving, M. The Conformation of Myosin Heads in Relaxed Skeletal Muscle: Implications for Myosin-Based Regulation. Biophys. J. 2015, 109, 783–792. [Google Scholar] [CrossRef]
- Fusi, L.; Brunello, E.; Yan, Z.; Irving, M. Thick Filament Mechano-Sensing Is a Calcium-Independent Regulatory Mechanism in Skeletal Muscle. Nat. Commun. 2016, 7, 13281. [Google Scholar] [CrossRef]
- Reconditi, M.; Brunello, E.; Fusi, L.; Linari, M.; Martinez, M.F.; Lombardi, V.; Irving, M.; Piazzesi, G. Sarcomere-Length Dependence of Myosin Filament Structure in Skeletal Muscle Fibres of the Frog. J. Physiol. 2014, 592, 1119–1137. [Google Scholar] [CrossRef] [PubMed]
- Wray, J.S. Structure of Relaxed Myosin Filaments in Relation to Nucleotide State in Vertebrate Skeletal Muscle. J. Muscle Res. Cell Motil. 1987, 8, 62. [Google Scholar]
- Lowy, J.; Popp, D.; Stewart, A.A. X-Ray Studies of Order-Disorder Transitions in the Myosin Heads of Skinned Rabbit Psoas Muscles. Biophys. J. 1991, 60, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Malinchik, S.; Xu, S.; Yu, L.C. Temperature-Induced Structural Changes in the Myosin Thick Filament of Skinned Rabbit Psoas Muscle. Biophys. J. 1997, 73, 2304–2312. [Google Scholar] [CrossRef] [PubMed]
- Park-Holohan, S.-J.; Brunello, E.; Kampourakis, T.; Rees, M.; Irving, M.; Fusi, L. Stress-Dependent Activation of Myosin in the Heart Requires Thin Filament Activation and Thick Filament Mechanosensing. Proc. Natl. Acad. Sci. USA 2021, 118, e2023706118. [Google Scholar] [CrossRef]
- Caremani, M.; Fusi, L.; Linari, M.; Reconditi, M.; Piazzesi, G.; Irving, T.C.; Narayanan, T.; Irving, M.; Lombardi, V.; Brunello, E. Dependence of Thick Filament Structure in Relaxed Mammalian Skeletal Muscle on Temperature and Interfilament Spacing. J. Gen. Physiol. 2021, 153, e202012713. [Google Scholar] [CrossRef]
- Nag, S.; Trivedi, D.V. To Lie or Not to Lie: Super-Relaxing with Myosins. eLife 2021, 10, e63703. [Google Scholar] [CrossRef]
- Caremani, M.; Brunello, E.; Linari, M.; Fusi, L.; Irving, T.C.; Gore, D.; Piazzesi, G.; Irving, M.; Lombardi, V.; Reconditi, M. Low Temperature Traps Myosin Motors of Mammalian Muscle in a Refractory State That Prevents Activation. J. Gen. Physiol. 2019, 151, 1272–1286. [Google Scholar] [CrossRef]
- Gollapudi, S.K.; Yu, M.; Gan, Q.-F.; Nag, S. Synthetic Thick Filaments: A New Avenue for Better Understanding the Myosin Super-Relaxed State in Healthy, Diseased, and Mavacamten-Treated Cardiac Systems. J. Biol. Chem. 2021, 296, 100114. [Google Scholar] [CrossRef] [PubMed]
- Nogara, L.; Naber, N.; Pate, E.; Canton, M.; Reggiani, C.; Cooke, R. Piperine’s Mitigation of Obesity and Diabetes Can Be Explained by Its up-Regulation of the Metabolic Rate of Resting Muscle. Proc. Natl. Acad. Sci. USA 2016, 113, 13009–13014. [Google Scholar] [CrossRef]
- Green, E.M.; Wakimoto, H.; Anderson, R.L.; Evanchik, M.J.; Gorham, J.M.; Harrison, B.C.; Henze, M.; Kawas, R.; Oslob, J.D.; Rodriguez, H.M.; et al. A Small-Molecule Inhibitor of Sarcomere Contractility Suppresses Hypertrophic Cardiomyopathy in Mice. Science 2016, 351, 617–621. [Google Scholar] [CrossRef]
- Chu, S.; Muretta, J.M.; Thomas, D.D. Direct Detection of the Myosin Super-Relaxed State and Interacting-Heads Motif in Solution. J. Biol. Chem. 2021, 297, 101157. [Google Scholar] [CrossRef]
- Kawas, R.F.; Anderson, R.L.; Ingle, S.R.B.; Song, Y.; Sran, A.S.; Rodriguez, H.M. A Small-Molecule Modulator of Cardiac Myosin Acts on Multiple Stages of the Myosin Chemomechanical Cycle. J. Biol. Chem. 2017, 292, 16571–16577. [Google Scholar] [CrossRef]
- Awinda, P.O.; Bishaw, Y.; Watanabe, M.; Guglin, M.A.; Campbell, K.S.; Tanner, B.C.W. Effects of Mavacamten on Ca2+ Sensitivity of Contraction as Sarcomere Length Varied in Human Myocardium. Br. J. Pharmacol. 2020, 177, 5609–5621. [Google Scholar] [CrossRef]
- Kampourakis, T.; Sun, Y.-B.; Irving, M. Orientation of the N- and C-Terminal Lobes of the Myosin Regulatory Light Chain in Cardiac Muscle. Biophys. J. 2015, 108, 304–314. [Google Scholar] [CrossRef]
- Kampourakis, T.; Sun, Y.-B.; Irving, M. Myosin Light Chain Phosphorylation Enhances Contraction of Heart Muscle via Structural Changes in Both Thick and Thin Filaments. Proc. Natl. Acad. Sci. USA 2016, 113, E3039–E3047. [Google Scholar] [CrossRef] [PubMed]
- Colson, B.A.; Locher, M.R.; Bekyarova, T.; Patel, J.R.; Fitzsimons, D.P.; Irving, T.C.; Moss, R.L. Differential Roles of Regulatory Light Chain and Myosin Binding Protein-C Phosphorylations in the Modulation of Cardiac Force Development. J. Physiol. 2010, 588, 981–993. [Google Scholar] [CrossRef]
- Nag, S.; Trivedi, D.V.; Sarkar, S.S.; Adhikari, A.S.; Sunitha, M.S.; Sutton, S.; Ruppel, K.M.; Spudich, J.A. The Myosin Mesa and the Basis of Hypercontractility Caused by Hypertrophic Cardiomyopathy Mutations. Nat. Struct. Mol. Biol. 2017, 24, 525–533. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Bowman, B.F.; Stull, J.T. Myosin Light Chain Phosphorylation in Vertebrate Striated Muscle: Regulation and Function. Am. J. Physiol. Cell Physiol. 1993, 264, C1085–C1095. [Google Scholar] [CrossRef]
- Ponnam, S.; Sevrieva, I.; Sun, Y.-B.; Irving, M.; Kampourakis, T. Site-Specific Phosphorylation of Myosin Binding Protein-C Coordinates Thin and Thick Filament Activation in Cardiac Muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 15485–15494. [Google Scholar] [CrossRef] [PubMed]
- Ponnam, S.; Kampourakis, T. Microscale Thermophoresis Suggests a New Model of Regulation of Cardiac Myosin Function via Interaction with Cardiac Myosin-Binding Protein C. J. Biol. Chem. 2022, 298, 101485. [Google Scholar] [CrossRef]
- McNamara, J.W.; Singh, R.R.; Sadayappan, S. Cardiac Myosin Binding Protein-C Phosphorylation Regulates the Super-Relaxed State of Myosin. Proc. Natl. Acad. Sci. USA 2019, 116, 11731–11736. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.R.; Li, A.; Beck-Previs, S.; Kennedy, G.G.; Warshaw, D.M. Imaging ATP Consumption in Resting Skeletal Muscle: One Molecule at a Time. Biophys. J. 2020, 119, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.S.; Trivedi, D.V.; Sarkar, S.S.; Song, D.; Kooiker, K.B.; Bernstein, D.; Spudich, J.A.; Ruppel, K.M. β-Cardiac Myosin Hypertrophic Cardiomyopathy Mutations Release Sequestered Heads and Increase Enzymatic Activity. Nat. Commun. 2019, 10, 2685. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, C.N.; Garfinkel, A.C.; Venturini, G.; Wakimoto, H.; Repetti, G.; Alamo, L.; Sharma, A.; Agarwal, R.; Ewoldt, J.F.; Cloonan, P.; et al. Myosin Sequestration Regulates Sarcomere Function, Cardiomyocyte Energetics, and Metabolism, Informing the Pathogenesis of Hypertrophic Cardiomyopathy. Circulation 2020, 141, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.S.; Trivedi, D.V.; Morck, M.M.; Adhikari, A.S.; Pasha, S.N.; Ruppel, K.M.; Spudich, J.A. The Hypertrophic Cardiomyopathy Mutations R403Q and R663H Increase the Number of Myosin Heads Available to Interact with Actin. Sci. Adv. 2020, 6, eaax0069. [Google Scholar] [CrossRef]
- Yuan, C.-C.; Kazmierczak, K.; Liang, J.; Ma, W.; Irving, T.C.; Szczesna-Cordary, D. Molecular Basis of Force-PCa Relation in MYL2 Cardiomyopathy Mice: Role of the Super-Relaxed State of Myosin. Proc. Natl. Acad. Sci. USA 2022, 119, e2110328119. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, C.N.; Wakimoto, H.; Garfinkel, A.C.; McDonough, B.; Liao, D.; Jiang, J.; Tai, A.C.; Gorham, J.M.; Lunde, I.G.; Lun, M.; et al. Hypertrophic Cardiomyopathy Mutations in MYBPC3 Dysregulate Myosin. Sci. Transl. Med. 2019, 11, eaat1199. [Google Scholar] [CrossRef]
- Huxley, A.F. Muscle Structure and Theories of Contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef]
- Caremani, M.; Marcello, M.; Morotti, I.; Pertici, I.; Squarci, C.; Reconditi, M.; Bianco, P.; Piazzesi, G.; Lombardi, V.; Linari, M. The Force of the Myosin Motor Sets Cooperativity in Thin Filament Activation of Skeletal Muscles. Commun. Biol. 2022, 5, 1266. [Google Scholar] [CrossRef] [PubMed]
- Hooijman, P.; Stewart, M.A.; Cooke, R. A New State of Cardiac Myosin with Very Slow ATP Turnover: A Potential Cardioprotective Mechanism in the Heart. Biophys. J. 2011, 100, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Kampourakis, T.; Irving, M. The Regulatory Light Chain Mediates Inactivation of Myosin Motors during Active Shortening of Cardiac Muscle. Nat. Commun. 2021, 12, 5272. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Ma, W.; Hu, Z.; Daneshparvar, N.; Taylor, D.W.; McCammon, J.A.; Irving, T.C.; Edwards, R.J.; Taylor, K.A. The Myosin II Coiled-Coil Domain Atomic Structure in Its Native Environment. Proc. Natl. Acad. Sci. USA 2021, 118, e2024151118. [Google Scholar] [CrossRef] [PubMed]
- Herron, T.J.; Korte, F.S.; McDonald, K.S. Power Output Is Increased after Phosphorylation of Myofibrillar Proteins in Rat Skinned Cardiac Myocytes. Circ. Res. 2001, 89, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, R.; Gresham, K.S.; Verma, S.; Stelzer, J.E. Cardiac Myosin Binding Protein-C Phosphorylation Modulates Myofilament Length-Dependent Activation. Front. Physiol. 2016, 7, 38. [Google Scholar] [CrossRef]
- Caremani, M.; Pinzauti, F.; Powers, J.D.; Governali, S.; Narayanan, T.; Stienen, G.J.M.; Reconditi, M.; Linari, M.; Lombardi, V.; Piazzesi, G. Inotropic Interventions Do Not Change the Resting State of Myosin Motors during Cardiac Diastole. J. Gen. Physiol. 2019, 151, 53–65. [Google Scholar] [CrossRef]
- Robinett, J.C.; Hanft, L.M.; Geist, J.; Kontrogianni-Konstantopoulos, A.; McDonald, K.S. Regulation of Myofilament Force and Loaded Shortening by Skeletal Myosin Binding Protein C. J. Gen. Physiol. 2019, 151, 645–659. [Google Scholar] [CrossRef]
- Sewanan, L.R.; Shen, S.; Campbell, S.G. Mavacamten Preserves Length-Dependent Contractility and Improves Diastolic Function in Human Engineered Heart Tissue. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1112–H1123. [Google Scholar] [CrossRef]
- Ma, W.; Henze, M.; Anderson, R.L.; Gong, H.; Wong, F.L.; del Rio, C.L.; Irving, T. The Super-Relaxed State and Length Dependent Activation in Porcine Myocardium. Circ. Res. 2021, 129, 617–630. [Google Scholar] [CrossRef]
- Scellini, B.; Piroddi, N.; Dente, M.; Vitale, G.; Pioner, J.M.; Coppini, R.; Ferrantini, C.; Poggesi, C.; Tesi, C. Mavacamten Has a Differential Impact on Force Generation in Myofibrils from Rabbit Psoas and Human Cardiac Muscle. J. Gen. Physiol. 2021, 153, e202012789. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, R.; Li, J.; Doh, C.Y.; Verma, S.; Stelzer, J.E. Impact of the Myosin Modulator Mavacamten on Force Generation and Cross-Bridge Behavior in a Murine Model of Hypercontractility. J. Am. Heart Assoc. 2018, 7, e009627. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for Treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy (EXPLORER-HCM): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2020, 396, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Heitner, S.B.; Jacoby, D.; Lester, S.J.; Owens, A.; Wang, A.; Zhang, D.; Lambing, J.; Lee, J.; Semigran, M.; Sehnert, A.J. Mavacamten Treatment for Obstructive Hypertrophic Cardiomyopathy. Ann. Intern. Med. 2019, 170, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Reconditi, M.; Brunello, E.; Linari, M.; Bianco, P.; Narayanan, T.; Panine, P.; Piazzesi, G.; Lombardi, V.; Irving, M. Motion of Myosin Head Domains during Activation and Force Development in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2011, 108, 7236–7240. [Google Scholar] [CrossRef] [PubMed]
- Fusi, L.; Percario, V.; Brunello, E.; Caremani, M.; Bianco, P.; Powers, J.D.; Reconditi, M.; Lombardi, V.; Piazzesi, G. Minimum Number of Myosin Motors Accounting for Shortening Velocity under Zero Load in Skeletal Muscle. J. Physiol. 2016, 595, 1127–1142. [Google Scholar] [CrossRef]
- Hu, Z.; Taylor, D.W.; Edwards, R.J.; Taylor, K.A. Coupling between Myosin Head Conformation and the Thick Filament Backbone Structure. J. Struct. Biol. 2017, 200, 334–342. [Google Scholar] [CrossRef]
- Reconditi, M.; Brunello, E.; Fusi, L.; Linari, M.; Lombardi, V.; Irving, M.; Piazzesi, G. Myosin Motors That Cannot Bind Actin Leave Their Folded OFF State on Activation of Skeletal Muscle. J. Gen. Physiol. 2021, 153, e202112896. [Google Scholar] [CrossRef]
- Hill, C.; Brunello, E.; Fusi, L.; Ovejero, J.G.; Irving, M. Myosin-Based Regulation of Twitch and Tetanic Contractions in Mammalian Skeletal Muscle. eLife 2021, 10, e68211. [Google Scholar] [CrossRef]
- Hill, C.; Brunello, E.; Fusi, L.; Ovejero, J.G.; Irving, M. Activation of the Myosin Motors in Fast-twitch Muscle of the Mouse Is Controlled by Mechano-sensing in the Myosin Filaments. J. Physiol. 2022, 600, 3983–4000. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Gong, H.; Irving, T. Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2643. [Google Scholar] [CrossRef] [PubMed]
- Reconditi, M.; Caremani, M.; Pinzauti, F.; Powers, J.D.; Narayanan, T.; Stienen, G.J.M.; Linari, M.; Lombardi, V.; Piazzesi, G. Myosin Filament Activation in the Heart Is Tuned to the Mechanical Task. Proc. Natl. Acad. Sci. USA 2017, 114, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Pinzauti, F.; Pertici, I.; Reconditi, M.; Narayanan, T.; Stienen, G.J.M.; Piazzesi, G.; Lombardi, V.; Linari, M.; Caremani, M. The Force and Stiffness of Myosin Motors in the Isometric Twitch of a Cardiac Trabecula and the Effect of the Extracellular Calcium Concentration: Mechanical Properties of Cardiac Myosin. J. Physiol. 2018, 596, 2581–2596. [Google Scholar] [CrossRef]
- Marcucci, L.; Washio, T.; Yanagida, T. Proposed Mechanism for the Length Dependence of the Force Developed in Maximally Activated Muscles. Sci. Rep. 2019, 9, 1317. [Google Scholar] [CrossRef]
- Mun, J.Y.; Previs, M.J.; Yu, H.Y.; Gulick, J.; Tobacman, L.S.; Beck Previs, S.; Robbins, J.; Warshaw, D.M.; Craig, R. Myosin-Binding Protein C Displaces Tropomyosin to Activate Cardiac Thin Filaments and Governs Their Speed by an Independent Mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 2170–2175. [Google Scholar] [CrossRef]
- Ma, W.; Childers, M.; Murray, J.; Moussavi-Harami, F.; Gong, H.; Weiss, R.; Daggett, V.; Irving, T.; Regnier, M. Myosin Dynamics during Relaxation in Mouse Soleus Muscle and Modulation by 2′-deoxy-ATP. J. Physiol. 2020, 598, 5165–5182. [Google Scholar] [CrossRef]
- Nogara, L.; Naber, N.; Pate, E.; Canton, M.; Reggiani, C.; Cooke, R. Spectroscopic Studies of the Super Relaxed State of Skeletal Muscle. PLoS ONE 2016, 11, e0160100. [Google Scholar] [CrossRef]
- Kuhn, E.R.; Naik, A.R.; Lewis, B.E.; Kokotovich, K.M.; Li, M.; Stemmler, T.L.; Larsson, L.; Jena, B.P. Nanothermometry Reveals Calcium-Induced Remodeling of Myosin. Nano Lett. 2018, 18, 7021–7029. [Google Scholar] [CrossRef]
- Ma, W.; Nag, S.; Gong, H.; Qi, L.; Irving, T.C. Cardiac Myosin Filaments Are Directly Regulated by Calcium. J. Gen. Physiol. 2022, 154, e202213213. [Google Scholar] [CrossRef]
- Marcucci, L.; Reggiani, C. Mechanosensing in Myosin Filament Solves a 60 Years Old Conflict in Skeletal Muscle Modeling between High Power Output and Slow Rise in Tension. Front. Physiol. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Kampourakis, T.; Ponnam, S.; Irving, M. Hypertrophic Cardiomyopathy Mutation R58Q in the Myosin Regulatory Light Chain Perturbs Thick Filament-Based Regulation in Cardiac Muscle. J. Mol. Cell. Cardiol. 2018, 117, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, V.; van der Velden, J. The Frank-Starling Law: A Jigsaw of Titin Proportions. Biophys. Rev. 2017, 9, 259–267. [Google Scholar] [CrossRef]
- Mateja, R.D.; Greaser, M.L.; de Tombe, P.P. Impact of Titin Isoform on Length Dependent Activation and Cross-Bridge Cycling Kinetics in Rat Skeletal Muscle. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 804–811. [Google Scholar] [CrossRef]
- Ait-Mou, Y.; Hsu, K.; Farman, G.P.; Kumar, M.; Greaser, M.L.; Irving, T.C.; de Tombe, P.P. Titin Strain Contributes to the Frank–Starling Law of the Heart by Structural Rearrangements of Both Thin- and Thick-Filament Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Hessel, A.L.; Ma, W.; Mazara, N.; Rice, P.E.; Nissen, D.; Gong, H.; Kuehn, M.; Irving, T.; Linke, W.A. Titin Force in Muscle Cells Alters Lattice Order, Thick and Thin Filament Protein Formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2209441119. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, L.; Washio, T.; Yanagida, T. Titin-Mediated Thick Filament Activation, through a Mechanosensing Mechanism, Introduces Sarcomere-Length Dependencies in Mathematical Models of Rat Trabecula and Whole Ventricle. Sci. Rep. 2017, 7, 5546. [Google Scholar] [CrossRef]
- Sequeira, V.; Najafi, A.; McConnell, M.; Fowler, E.D.; Bollen, I.A.E.; Wüst, R.C.I.; dos Remedios, C.; Helmes, M.; White, E.; Stienen, G.J.M.; et al. Synergistic Role of ADP and Ca2+ in Diastolic Myocardial Stiffness. J. Physiol. 2015, 593, 3899–3916. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; de Locht, M.; Schuldt, M.; Schönleitner, P.; Willigenburg, M.; Bollen, I.; Goebel, M.; Ottenheijm, C.A.C.; der Velden, J.; Helmes, M.; et al. End-diastolic Force Pre-activates Cardiomyocytes and Determines Contractile Force: Role of Titin and Calcium. J. Physiol. 2019, 597, 4521–4531. [Google Scholar] [CrossRef]
- Zhang, X.; Kampourakis, T.; Yan, Z.; Sevrieva, I.; Irving, M.; Sun, Y.-B. Distinct Contributions of the Thin and Thick Filaments to Length-Dependent Activation in Heart Muscle. eLife 2017, 6, e24081. [Google Scholar] [CrossRef]
- Campbell, K.S.; Janssen, P.M.L.; Campbell, S.G. Force-Dependent Recruitment from the Myosin Off State Contributes to Length-Dependent Activation. Biophys. J. 2018, 115, 543–553. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcucci, L. Muscle Mechanics and Thick Filament Activation: An Emerging Two-Way Interaction for the Vertebrate Striated Muscle Fine Regulation. Int. J. Mol. Sci. 2023, 24, 6265. https://doi.org/10.3390/ijms24076265
Marcucci L. Muscle Mechanics and Thick Filament Activation: An Emerging Two-Way Interaction for the Vertebrate Striated Muscle Fine Regulation. International Journal of Molecular Sciences. 2023; 24(7):6265. https://doi.org/10.3390/ijms24076265
Chicago/Turabian StyleMarcucci, Lorenzo. 2023. "Muscle Mechanics and Thick Filament Activation: An Emerging Two-Way Interaction for the Vertebrate Striated Muscle Fine Regulation" International Journal of Molecular Sciences 24, no. 7: 6265. https://doi.org/10.3390/ijms24076265
APA StyleMarcucci, L. (2023). Muscle Mechanics and Thick Filament Activation: An Emerging Two-Way Interaction for the Vertebrate Striated Muscle Fine Regulation. International Journal of Molecular Sciences, 24(7), 6265. https://doi.org/10.3390/ijms24076265






