Mature Cystic Teratoma: An Integrated Review
Abstract
1. Introduction
2. Epidemiology
3. Clinical Symptoms
4. Diagnosis
4.1. Image Findings
4.2. Histological Examination
4.3. Differential Diagnosis
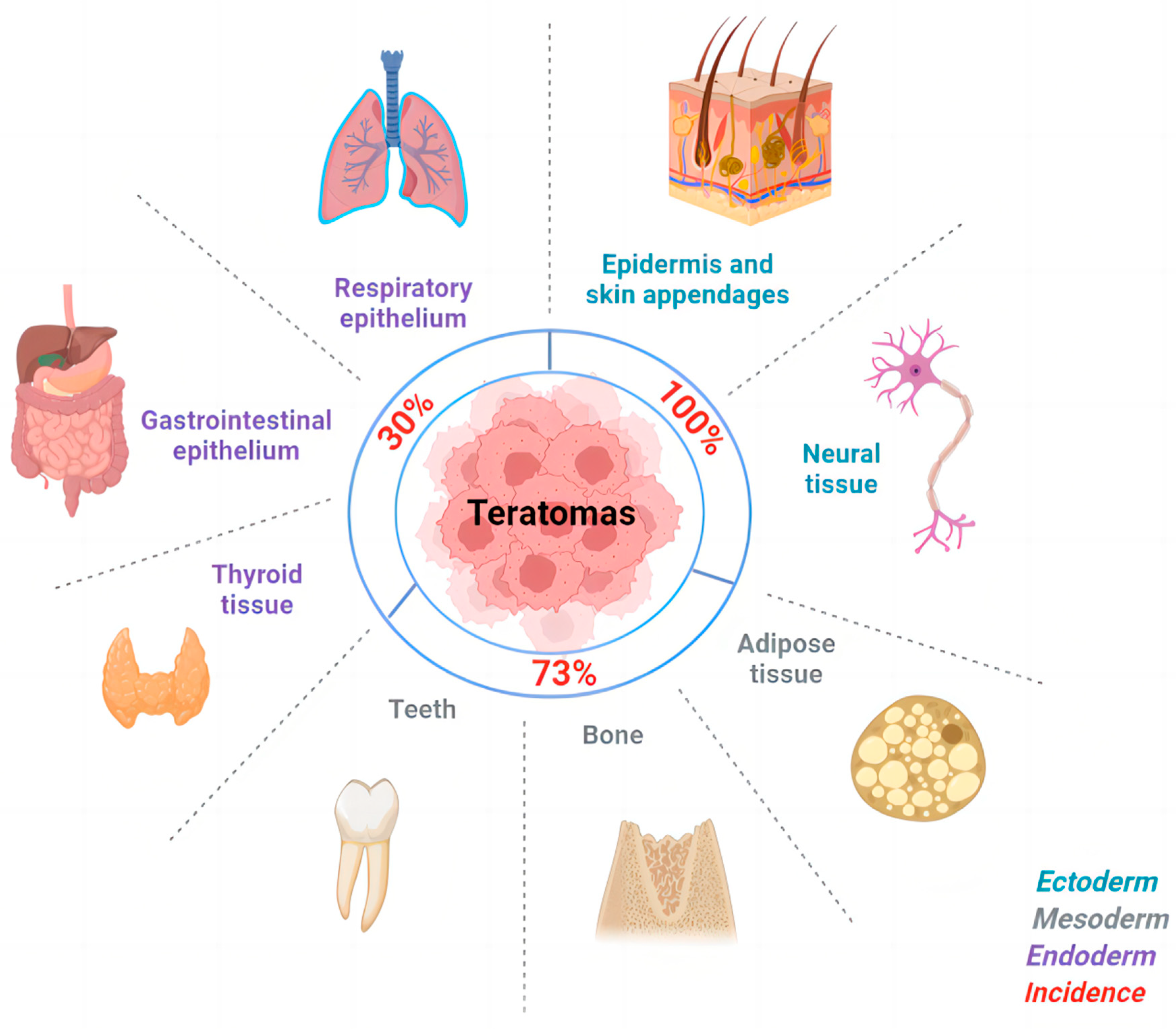
5. Cellular Origin
6. Treatment
6.1. Mature Cystic Teratomas
6.2. Malignant Transformations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Surti, U.; Hoffner, L.; Chakravarti, A.; Ferrell, R. Genetics and biology of human ovarian teratomas. I. Cytogenetic analysis and mechanism of origin. Am. J. Hum. Genet. 1990, 47, 635. [Google Scholar] [PubMed]
- Ayhan, A.; Bukulmez, O.; Genc, C.; Karamursel, B.S.; Ayhan, A. Mature cystic teratomas of the ovary: Case series from one institution over 34 years. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 88, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Bhosale, P.; Menias, C.O.; Ramalingam, P.; Jensen, C.; Iyer, R.; Ganeshan, D. Ovarian teratomas: Clinical features, imaging findings and management. Abdom. Radiol. 2021, 46, 2293–2307. [Google Scholar] [CrossRef]
- Chen, V.W.; Ruiz, B.; Killeen, J.L.; Coté, T.R.; Wu, X.C.; Correa, C.N.; Howe, H.L. Pathology and classification of ovarian tumors. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.F.; Erickson, S.; Black, W.C. Cystic ovarian teratomas: The sonographic appearance of the dermoid plug. Radiology 1985, 155, 477–478. [Google Scholar] [CrossRef]
- Saba, L.; Guerriero, S.; Sulcis, R.; Virgilio, B.; Melis, G.; Mallarini, G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur. J. Radiol. 2009, 72, 454–463. [Google Scholar] [CrossRef]
- Westhoff, C.; Pike, M.; Vessey, M. Benign ovarian teratomas: A population-based case-control study. Br. J. Cancer 1988, 58, 93–98. [Google Scholar] [CrossRef]
- Fujii, K.; Yamashita, Y.; Yamamoto, T.; Takahashi, K.; Hashimoto, K.; Miyata, T.; Kawai, K.; Kikkawa, F.; Toyokuni, S.; Nagasaka, T. Ovarian mucinous tumors arising from mature cystic teratomas—A molecular genetic approach for understanding the cellular origin. Hum. Pathol. 2014, 45, 717–724. [Google Scholar] [CrossRef]
- Templeman, C.L.; Fallat, M.E.; Lam, A.M.; Perlman, S.E.; Hertweck, S.P.; O’Connor, D.M. Managing Mature Cystic Teratomas of the Ovary. Obstet. Gynecol. Surv. 2000, 55, 738–745. [Google Scholar] [CrossRef]
- Savasi, I.; Lacy, J.A.; Gerstle, J.T.; Stephens, D.; Kives, S.; Allen, L. Management of Ovarian Dermoid Cysts in the Pediatric and Adolescent Population. J. Pediatr. Adolesc. Gynecol. 2009, 22, 360–364. [Google Scholar] [CrossRef]
- O’Neill, K.E.; Cooper, A.R. The Approach to Ovarian Dermoids in Adolescents and Young Women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Togashi, K.; Konishi, I.; Kataoka, M.L.; Koyama, T.; Ueda, H.; Fujii, S.; Konishi, J. Dermoid cysts of the ovary with malignant transformation: MR appearance. Am. J. Roentgenol. 1999, 172, 445–449. [Google Scholar] [CrossRef]
- Christopherson, W.; Councell, R. Malignant degeneration of a mature ovarian teratoma. Int. J. Gynecol. Obstet. 1989, 30, 379–384. [Google Scholar] [CrossRef]
- Hertzberg, B.S.; Kliewer, M.A. Sonography of benign cystic teratoma of the ovary: Pitfalls in diagnosis. Am. J. Roentgenol. 1996, 167, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Outwater, E.K.; Siegelman, E.S.; Hunt, J.L. Ovarian Teratomas: Tumor Types and Imaging Characteristics. Radiographics 2001, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Hackethal, A.; Brueggmann, D.; Bohlmann, M.K.; Franke, F.E.; Tinneberg, H.-R.; Münstedt, K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: Systematic review and analysis of published data. Lancet Oncol. 2008, 9, 1173–1180. [Google Scholar] [CrossRef]
- Pekar-Zlotin, M.; Rabinovich, I.; Goldrat, I.; Vaknin, Z.; Gidoni, Y.; Zur-Naaman, H.; Maymon, R.; Smorgick, N. Ovarian Dermoid Cysts Associated with Paraneoplastic Syndrome N-methyl-D-aspartic Acid Receptor Antibodies Encephalitis. J. Minim. Invasive Gynecol. 2021, 28, 1190–1193. [Google Scholar] [CrossRef]
- De Silva, K.; Kanumakala, S.; Grover, S.; Chow, C.; Warne, G. Ovarian Lesions in Children and Adolescents—An 11-year Review. J. Pediatr. Endocrinol. Metab. 2004, 17, 951–957. [Google Scholar] [CrossRef]
- Evers, M.; Rechnitzer, C.; Graem, N.; Wehner, P.S.; Schroeder, H.; Rosthoej, S.; Mosbech, C.H.; Hoei-Hansen, C.E.; Sehested, A.; Treger, T.D.; et al. Epidemiological study of paediatric germ cell tumours revealed the incidence and distribution that was expected, but a low mortality rate. Acta Paediatr. 2017, 106, 779–785. [Google Scholar] [CrossRef]
- Coleman, R.L.; Westin, S.N.; Ramirez, P.T.; Salvo, G.; Gershenson, D.M. Malignant diseases of the ovary, fallopian tube, and peritoneum. In Comprehensive Gynecology; Elsevier: Amsterdam, The Netherlands, 2021; p. 707. [Google Scholar] [CrossRef]
- Gadducci, A.; Pistolesi, S.; Guerrieri, M.E.; Cosio, S.; Carbone, F.G.; Naccarato, A.G. Malignant Transformation in Mature Cystic Teratomas of the Ovary: Case Reports and Review of the Literature. Anticancer. Res. 2018, 38, 3669–3675. [Google Scholar] [CrossRef]
- Belaid, I.; Khechine, W.; Ben Abdelkader, A.; Bedioui, A.; Ezzairi, F.; Chabchoub, I.; Boujnah, R.; Tlili, T.; Hochlaf, M.; Ben Fatma, L.; et al. Adenocarcinoma of intestinal type arising in mature cystic teratoma of ovary: A diagnostic dilemma. Clin. Case Rep. 2020, 8, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Pan, D.; Huang, B.; Weng, M.; Nie, X. KRAS mutation in adenocarcinoma of the gastrointestinal type arising from a mature cystic teratoma of the ovary. J. Ovarian Res. 2014, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Ueda, G.; Fujita, M.; Ogawa, H.; Sawada, M.; Inoue, M.; Tanizawa, O. Adenocarcinoma in a Benign Cystic Teratoma of the Ovary: Report of a Case with a Long Survival Period. Gynecol. Oncol. 1993, 48, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Ryu, S.Y.; Choi, S.C.; Lee, E.D.; Lee, K.H.; Cho, S.Y. Adenocarcinoma arising from the respiratory ciliated epithelium in a benign cystic teratoma of the ovary. Arch. Gynecol. Obstet. 2009, 280, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Edelstein, E.; Altaras, M.; Beyth, Y.; Bernheim, J. Adenocarcinoma Arising from the Gastrointestinal Epithelium in Benign Cystic Teratoma of the Ovary. Gynecol. Oncol. 1998, 70, 418–420. [Google Scholar] [CrossRef]
- Choi, W.K.; Lee, D.H.; Cho, D.H.; Jang, K.Y.; Kim, K.M. Primary malignant melanoma arising from ruptured ovarian mature cystic teratoma with elevated serum CA 19–9: A case report and review of literature. BMC Women’s Health 2019, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Q. Primary ovarian carcinoid arising in associated mature cystic teratoma. BMC Women’s Health 2022, 22, 76. [Google Scholar] [CrossRef]
- Tosuner, Z.; Sönmez, F.C.; Arici, D.S.; Dansuk, R. Carcinoid tumor arising in a mature cystic teratoma: A case report. Oncol. Lett. 2015, 9, 2236–2238. [Google Scholar] [CrossRef]
- Kim, J.-Y. A carcinoid tumor arising from a mature cystic teratoma in a 25-year-old patient: A case study. World J. Surg. Oncol. 2016, 14, 120. [Google Scholar] [CrossRef]
- Ünal, B.; Güleç, F.; Sedele, M. Oligodendroglioma Arising in Mature Cystic Teratoma. Case Rep. Oncol. Med. 2014, 2014, 745462. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Fadda, G.; Scambia, G.; Capelli, A.; Carbone, A. Oligodendroglioma arising within a mature cystic ovarian teratoma: Case report and review of the literature. Acta Obstet. Gynecol. Scand. 2002, 81, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Arévalo, M.L.; Lino-Silva, L.S.; Malagón, H.R.D. Oligodendroglial cell proliferation arising in an ovarian mature cystic teratoma. Clinicopathological, inmunohistochemical, and ultrastructural study of a case that may represent an oligodendroglioma. Ultrastruct. Pathol. 2017, 41, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ngwalle, K.; Hirakawa, T.; Tsuneyoshi, M.; Enjoji, M. Osteosarcoma arising in a benign dermoid cyst of the ovary. Gynecol. Oncol. 1990, 37, 143–147. [Google Scholar] [CrossRef]
- Kefeli, M.; Kandemir, B.; Akpolat, I.; Yildirim, A.; Kokcu, A. Rhabdomyosarcoma Arising in a Mature Cystic Teratoma With Contralateral Serous Carcinoma: Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2009, 28, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Desouki, M.M.; Fadare, O.; Chamberlain, B.K.; Shakir, N.; Kanbour-Shakir, A. Malignancy associated with ovarian teratomas: Frequency, histotypes, and diagnostic accuracy of intraoperative consultation. Ann. Diagn. Pathol. 2015, 19, 103–106. [Google Scholar] [CrossRef]
- Trabzonlu, L.; Durmaz, G.; Vural, C.; Muezzinoglu, B.; Corakci, A. Malignant tumors associated with ovarian mature teratoma: A single institution experience. Pathol. Res. Pract. 2017, 213, 518–521. [Google Scholar] [CrossRef]
- Caruso, P.A.; Marsh, M.R.; Minkowitz, S.; Karten, G. An intense clinicopathologic study of 305 teratomas of the ovary. Cancer 1971, 27, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Goel, G.; Sinha, H.H.; Anant, M.; Agarwal, M. Effects of Intraoperatively Ruptured Ovarian Dermoid Cysts. J. Gynecol. Surg. 2022, 38, 221–225. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hori, H.; Gorai, I. Chemical peritonitis caused by an iatrogenic rupture of mature cystic teratoma of the ovary during labor: A report of a case didactic to all the maternity health care workers. J. Matern. Neonatal Med. 2011, 24, 388–390. [Google Scholar] [CrossRef]
- Dalmau, J.; Gleichman, A.J.; Hughes, E.G.; Rossi, J.E.; Peng, X.; Lai, M.; Dessain, S.K.; Rosenfeld, M.R.; Balice-Gordon, R.; Lynch, D.R. Anti-NMDA-receptor encephalitis: Case series and analysis of the effects of antibodies. Lancet Neurol. 2008, 7, 1091–1098. [Google Scholar] [CrossRef]
- Zaborowski, M.P.; Spaczynski, M.; Nowak-Markwitz, E.; Michalak, S. Paraneoplastic neurological syndromes associated with ovarian tumors. J. Cancer Res. Clin. Oncol. 2015, 141, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Tüzün, E.; Wu, H.; Masjuan, J.; Rossi, J.E.; Voloschin, A.; Baehring, J.M.; Shimazaki, H.; Koide, R.; King, D. Paraneoplastic anti–N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann. Neurol. 2007, 61, 25–36. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, J.; Ren, H.; Zhou, X.; Chen, J.; Cui, L.; Lang, J.; Guan, H.; Sun, D. Surgical outcomes in patients with anti-N-methyl D-aspartate receptor encephalitis with ovarian teratoma. Am. J. Obstet. Gynecol. 2019, 221, 485.e1–485.e10. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Xu, L.; Liu, L.; Wu, X.; Zhang, Y.; Zhu, G.; Hong, Z. Anti-N-methyl-D-aspartate receptor encephalitis with accompanying ovarian teratoma in female patients from East China: Clinical features, treatment, and prognostic outcomes. Seizure 2020, 75, 55–62. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Su, Y.-C.; Huang, S.-C.; Chiang, H.-L.; Huang, P.-S. Anti-NMDAR encephalitis with ovarian teratomas: Review of the literature and two case reports. Taiwan. J. Obstet. Gynecol. 2019, 58, 313–317. [Google Scholar] [CrossRef]
- Yu, M.; Li, S.; Cheng, J.; Zhou, L.; Jiang, Z.; Di, W. Ovarian teratoma-associated anti-NMDAR encephalitis: A single-institute series of six patients from China. Arch. Gynecol. Obstet. 2021, 303, 1283–1294. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Wu, J.-D.; Chen, C.-C. The Association of Ovarian Teratoma and Anti-N-Methyl-D-Aspartate Receptor Encephalitis: An Updated Integrative Review. Int. J. Mol. Sci. 2021, 22, 10911. [Google Scholar] [CrossRef]
- Dawley, B.; Acuna, A.; Grasu, B. Ectopic production of HCG by a benign ovarian mature cystic teratoma simulating an extra-uterine pregnancy: A case report. West Va. Med. J. 2012, 108, 15–18. [Google Scholar]
- Kite, L.; Uppal, T. Ultrasound of ovarian dermoids—Sonographic findings of a dermoid cyst in a 41-year-old woman with an elevated serum hCG. Australas. J. Ultrasound Med. 2011, 14, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Takemori, M.; Nishimura, R.; Yamasaki, M.; Kawabe, Y.; Hasegawa, K. Ovarian Mixed Germ Cell Tumor Composed of Polyembryoma and Immature Teratoma. Gynecol. Oncol. 1998, 69, 260–263. [Google Scholar] [CrossRef]
- Bin Park, S.; Kim, J.K.; Kim, K.-R.; Cho, K.-S. Imaging Findings of Complications and Unusual Manifestations of Ovarian Teratomas. RadioGraphics 2008, 28, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Devaney, K.; Snyder, R.; Norris, H.J.; Tavassoli, F.A. Proliferative and histologically malignant struma ovarii: A clinicopathologic study of 54 cases. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1993, 12, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Willatt, J.; Lindsell, D. The ability of ultrasound to detect gynaecological neoplasms and their ultrasound morphological features. Australas. Radiol. 2007, 51, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Fishman, E.; Buck, J.; Hamper, U.; Sanders, R. The variable sonographic appearances of ovarian teratomas: Correlation with CT. Am. J. Roentgenol. 1988, 151, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C. Diagnostic Pathology of Ovarian Tumors; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Blackwell, W.J.; Dockerty, M.B.; Masson, J.C.; Mussey, R.D. Dermoid Cysts of the Ovary: Their Clinical and Pathologic Significance. Am. J. Obstet. Gynecol. 1946, 51, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Pyatt, R.; Hartman, D.; Downey, E.; Olson, W. CT of benign cystic teratomas. Am. J. Roentgenol. 1982, 138, 659–665. [Google Scholar] [CrossRef]
- MATZ, M.H. Benign cystic teratomas of the ovary. Obstet. Gynecol. Surv. 1961, 16, 591–605. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Manusirivithaya, S.; Sheanakul, C.; Leelahakorn, S.; Thawaramara, T.; Jesadapatarakul, S. Squamous cell carcinoma arising from dermoid cyst: Case reports and review of literature. Int. J. Gynecol. Cancer 2003, 13. [Google Scholar] [CrossRef]
- Yanai-Inbar, I.; Scully, R.E. Relation of ovarian dermoid cysts and immature teratomas: An analysis of 350 cases of immature teratoma and 10 cases of dermoid cyst with microscopic foci of immature tissue. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1987, 6, 203–212. [Google Scholar] [CrossRef]
- Atwi, D.; Kamal, M.; Quinton, M.; Hassell, L.A. Malignant transformation of mature cystic teratoma of the ovary. J. Obstet. Gynaecol. Res. 2022, 48, 3068–3076. [Google Scholar] [CrossRef]
- Corfman, P.A.; Richart, R.M. Chromosome Number and Morphology of Benign Ovarian Cystic Teratomas. N. Engl. J. Med. 1964, 271, 1241–1244. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J.; Kozak, L.P.; Eicher, E.M.; Stevens, L.C. Ovarian Teratomas in Mice are Derived from Oocytes That Have Completed the First Meiotic Division. In Genetic Mosaics and Chimeras in Mammals; Springer: Berlin/Heidelberg, Germany, 1978; pp. 33–37. [Google Scholar] [CrossRef]
- Ohama, K. Androgenesis and Parthenogenesis in Humans. Hum. Genet. 1987, 245–249. [Google Scholar] [CrossRef]
- Linder, D.; Power, J. Further evidence for post -meiotic origin of teratomas in the human female. Ann. Hum. Genet. 1970, 34, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Linder, D.; McCaw, B.K.; Hecht, F. Parthenogenic Origin of Benign Ovarian Teratomas. N. Engl. J. Med. 1975, 292, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Vortmeyer, A.O.; Devouassoux-Shisheboran, M.; Li, G.; Mohr, V.; Tavassoli, F.; Zhuang, Z. Microdissection-Based Analysis of Mature Ovarian Teratoma. Am. J. Pathol. 1999, 154, 987–991. [Google Scholar] [CrossRef]
- Patil, S.R.; EW, L. Human benign ovarian teratomas: Chromosomal and electrophoretic enzyme studies. Birth Defects Orig Artic Ser. 1978, 14, 297–301. [Google Scholar] [PubMed]
- Carritt, B.; Parrington, J.M.; Welch, H.M.; Povey, S. Diverse origins of multiple ovarian teratomas in a single individual. Proc. Natl. Acad. Sci. USA 1982, 79, 7400–7404. [Google Scholar] [CrossRef]
- Parrington, J.M.; West, L.; Povey, S. The origin of ovarian teratomas. J. Med. Genet. 1984, 21, 4–12. [Google Scholar] [CrossRef]
- Surti, U.; Deka, R.; Hoffner, L.; Majumber, P.; Chakravarti, A.; Ferrel, R. Human ovarian teratomas: Origin and gene mapping using cytogenetic and DNA markers. Am. J. Hum. Genet. 1988, 43, A34. [Google Scholar]
- Wang, W.-C.; Lai, Y.-C. Genetic analysis results of mature cystic teratomas of the ovary in Taiwan disagree with the previous origin theory of this tumor. Hum. Pathol. 2016, 52, 128–135. [Google Scholar] [CrossRef]
- Kerr, S.E.; Flotte, A.B.; McFalls, M.J.; Vrana, J.A.; Halling, K.C.; Bell, D.A. Matching Maternal Isodisomy in Mucinous Carcinomas and Associated Ovarian Teratomas Provides Evidence of Germ Cell Derivation for Some Mucinous Ovarian Tumors. Am. J. Surg. Pathol. 2013, 37, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, C.; Strambi, S.; Masoni, B.; Ghionzoli, M.; Bertocchini, A.; Sanna, B.; Morganti, R.; Messina, M.; Molinaro, F.; Tursini, S.; et al. Surgical management of ovarian teratomas in childhood: A multicentric study on 110 cases and a literature review. Gynecol. Endocrinol. 2021, 37, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Falcone, T.; Tulandi, T. Excision of ovarian dermoid cyst by laparoscopy and by laparotomy. Am. J. Obstet. Gynecol. 1995, 173, 769–771. [Google Scholar] [CrossRef]
- Zanetta, G.; Ferrari, L.; Mignini-Renzini, M.; Vignali, M.; Fadini, R. Laparoscopic excision of ovarian dermoid cysts with controlled intraoperative spillage. Safety and effectiveness. J Reprod Med. 1999, 44, 815–820. [Google Scholar]
- Childress, K.J.; Santos, X.M.; Perez-Milicua, G.; Hakim, J.; Adeyemi-Fowode, O.; Bercaw-Pratt, J.L.; Dietrich, J.E. Intraoperative rupture of ovarian dermoid cysts in the pediatric and adolescent population: Should this change your surgical management? J. Pediatr. Adolesc. Gynecol. 2017, 30, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Caspi, B.; Appelman, Z.; Rabinerson, D.; Zalel, Y.; Tulandi, T.; Shoham, Z. The growth pattern of ovarian dermoid cysts: A prospective study in premenopausal and postmenopausal women. Fertil. Steril. 1997, 68, 501–505. [Google Scholar] [CrossRef]
- Howard, F. Surgical management of benign cystic teratoma. Laparoscopy vs. laparotomy. J. Reprod. Med. 1995, 40, 495–499. [Google Scholar]
- Spinelli, C.; Strambi, S.; Liloia, C.; Bertocchini, A.; Messineo, A. Update on the surgical management of ovarian neoplasms in children and adolescents: Analysis on 32 cases. Gynecol. Endocrinol. 2016, 32, 787–791. [Google Scholar] [CrossRef]
- Mahtate, M.; Talib, S.; Slaoui, A.; Zeraidi, N.; Lakhdar, A.; Rhrab, B.; Baydada, A. Malignant Degeneration of a Mature Ovarian Teratoma. Case Rep. Obstet. Gynecol. 2021, 2021, 5527467. [Google Scholar] [CrossRef]
- Dos Santos, L.; Mok, E.; Iasonos, A.; Park, K.; Soslow, R.; Aghajanian, C.; Alektiar, K.; Barakat, R.R.; Abu-Rustum, N.R. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: A case series and review of the literature. Gynecol. Oncol. 2007, 105, 321–324. [Google Scholar] [CrossRef]
- Chen, R.-J.; Chen, K.-Y.; Chang, T.-C.; Sheu, B.-C.; Chow, S.-N.; Huang, S.-C. Prognosis and treatment of squamous cell carcinoma from a mature cystic teratoma of the ovary. J. Formos. Med. Assoc. 2008, 107, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Rim, S.-Y.; Kim, S.-M.; Choi, H.-S. Malignant transformation of ovarian mature cystic teratoma. Int. J. Gynecol. Cancer 2006, 16, 140–144. [Google Scholar] [CrossRef]
- Gadducci, A.; Guerrieri, M.E.; Cosio, S. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: A challenging question for gynecologic oncologists. Crit. Rev. Oncol. 2019, 133, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.-J.; La, V.; Peng, J.; Yu, K.-J.; Teng, N.N. Squamous Cell Carcinoma Arising From Mature Cystic Teratoma of the Ovary. Int. J. Gynecol. Cancer 2011, 21, 466–474. [Google Scholar] [CrossRef]
- Fukase, M.; Ohta, T.; Watanabe, N.; Suzuki, Y.; Seino, M.; Sudo, T.; Nagase, S. Squamous cell carcinoma arising from a mature cystic teratoma of the ovary: Successful treatment with carboplatin, paclitaxel, and bevacizumab. Gynecol. Oncol. Rep. 2020, 34, 100632. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M.H.; Beutel, G.; Grünwald, V. Targeted therapies for patients with germ cell tumors. Expert Opin. Investig. Drugs 2008, 17, 511–522. [Google Scholar] [CrossRef]
- Ribeiro, G.; Hughesdon, P.; Wiltshaw, E. Squamous carcinoma arising in dermoid cysts and associated with hypercalcemia: A clinicopathologic study of six cases. Gynecol. Oncol. 1988, 29, 222–230. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Liu, J.F.; Konstantinopoulos, P.A.; Matulonis, U.A. PARP inhibitors in ovarian cancer: Current status and future promise. Gynecol. Oncol. 2014, 133, 362–369. [Google Scholar] [CrossRef]
- O’Malley, D.M.; Matulonis, U.A.; Birrer, M.J.; Castro, C.M.; Gilbert, L.; Vergote, I.; Martin, L.P.; Mantia-Smaldone, G.M.; Martin, A.G.; Bratos, R.; et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2020, 157, 379–385. [Google Scholar] [CrossRef]
- Harbin, L.M.; Gallion, H.H.; Allison, D.B.; Kolesar, J.M. Next Generation Sequencing and Molecular Biomarkers in Ovarian Cancer—An Opportunity for Targeted Therapy. Diagnostics 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Carvajal, L.; Sanchez-Muñoz, A.; Ribelles, N.; Saez, M.; Baena, J.; Ruiz, S.; Ithurbisquy, C.; Alba, E. Targeted treatment approaches in refractory germ cell tumors. Crit. Rev. Oncol. Hematol. 2019, 143, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]

| Pathologic Equivalent | Image Findings | |
|---|---|---|
| US | Rokitansky nodule | Echogenic tubercle with cystic echo |
| Hair and watery fluid | Thin band-like echoes | |
| Presence of sebum | Dense echo pattern | |
| Sebum layered on serous fluid | Fat–fluid level | |
| Desquamative material | Floating intracystic spheres | |
| CT | Fat intralesional | Substance’s density is −20 HU or lower inside a cyst |
| Rokitansky nodule | Rounded (or bridge) structure, clearly linked to the cyst wall, projecting inside the cyst | |
| Or | ||
| Irregular mural thickening containing dense structures (calcified structures) and\or areas of fatty | ||
| MRI | Sebaceous material | T1 hyperintense and T2 hypointense |
| Squamous material | T1 hypointense and T2 hyperintense | |
| Hair | T2 hypointense | |
| Tooth | Low-signal-intensity central calcifications |
| References | Materials | Markers | Main Results | Conclusion |
|---|---|---|---|---|
| Linder D et al. [66] 1970 | 39 MCTs from 33 patients | Allelic isozymes: G6PD, PGM1, PGM3, 6PGD | 6 of 12 tumors at PGM1 locus were heterozygous in host but homozygous in tumors | MCTs are from post-meiotic origin |
| Linder D et al. [67] 1975 | 5 MCTs from 5 patients | Q-banding, G-banding, C-banding; PGM1, PGM3, 6PGD, etc. | 2 of 3 tumors at PGM1 locus, and 1 of 2 tumors at PGM3 locus were heterozygous in host but homozygous in tumors | MCTs arise from single germ cell after first meiosis |
| Eppig JJ et al. [64] 1977 | 23 teratomas from strain LT/SV mice | Gpi-1 | 21 of 23 tumors at Gpi-1 locus were heterozygous in host but homozygous in tumors | Tumors are origin from post-meiotic oocytes |
| Carritt B et al. [70] 1982 | 7 benign ovarian teratomas in one patient | C-banding, PGM1, PGD, UMPK, ME2 | 4 of 7 teratomas showed heterozygous on c-banding results | Exclude the suppression of meiosis II as amode of origin for some of these tumors. |
| Parrington JM et al. [71] 1984 | 21 benign ovarian teratomas from 14 patients | C-banding, Q-banding; 13 enzyme markers (PGD, PGM1, PGM3, etc.) | 52% of teratomas had homozygous centromeres and enzymes | Many of this group are thought to arise from duplication of a mature ovum |
| Surti U [1] 1990 | 102 benign mature teratomas from patients | Q-banding, C-banding | First reported tetraploidy and structural rearrangement in teratomas; 65% of teratomas have homozygous centromere | 65% of tumors arose from single germ cell after first meiosis or failure of second meiosis or endoreduplication of mature ovum; the rest arose by failure of first meiosis or mitotic division of premeiotic germ cell |
| Vootmeyer AO [68] 1999 | 7 mature ovarian teratomas | Microdissection; PCR using microsatellite markers | Markers showed homozygous in majority tumors | Tumors arose from post-meiotic germ cell |
| Wang WC [73] 2016 | 9 MCTs | 15 STR analysis; Methylation analysis | Most of the STR loci in tumors showed heterozygous | Exclude the parthenogenetic origin of MCTs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, L.; Wang, S.; Yeung, S.Y.; Lee, J.H.S.; Chung, J.P.W.; Chan, D.Y.L. Mature Cystic Teratoma: An Integrated Review. Int. J. Mol. Sci. 2023, 24, 6141. https://doi.org/10.3390/ijms24076141
Cong L, Wang S, Yeung SY, Lee JHS, Chung JPW, Chan DYL. Mature Cystic Teratoma: An Integrated Review. International Journal of Molecular Sciences. 2023; 24(7):6141. https://doi.org/10.3390/ijms24076141
Chicago/Turabian StyleCong, Luping, Sijia Wang, Suet Ying Yeung, Jacqueline Ho Sze Lee, Jacqueline Pui Wah Chung, and David Yiu Leung Chan. 2023. "Mature Cystic Teratoma: An Integrated Review" International Journal of Molecular Sciences 24, no. 7: 6141. https://doi.org/10.3390/ijms24076141
APA StyleCong, L., Wang, S., Yeung, S. Y., Lee, J. H. S., Chung, J. P. W., & Chan, D. Y. L. (2023). Mature Cystic Teratoma: An Integrated Review. International Journal of Molecular Sciences, 24(7), 6141. https://doi.org/10.3390/ijms24076141






