Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps
Abstract
1. Introduction
2. Results
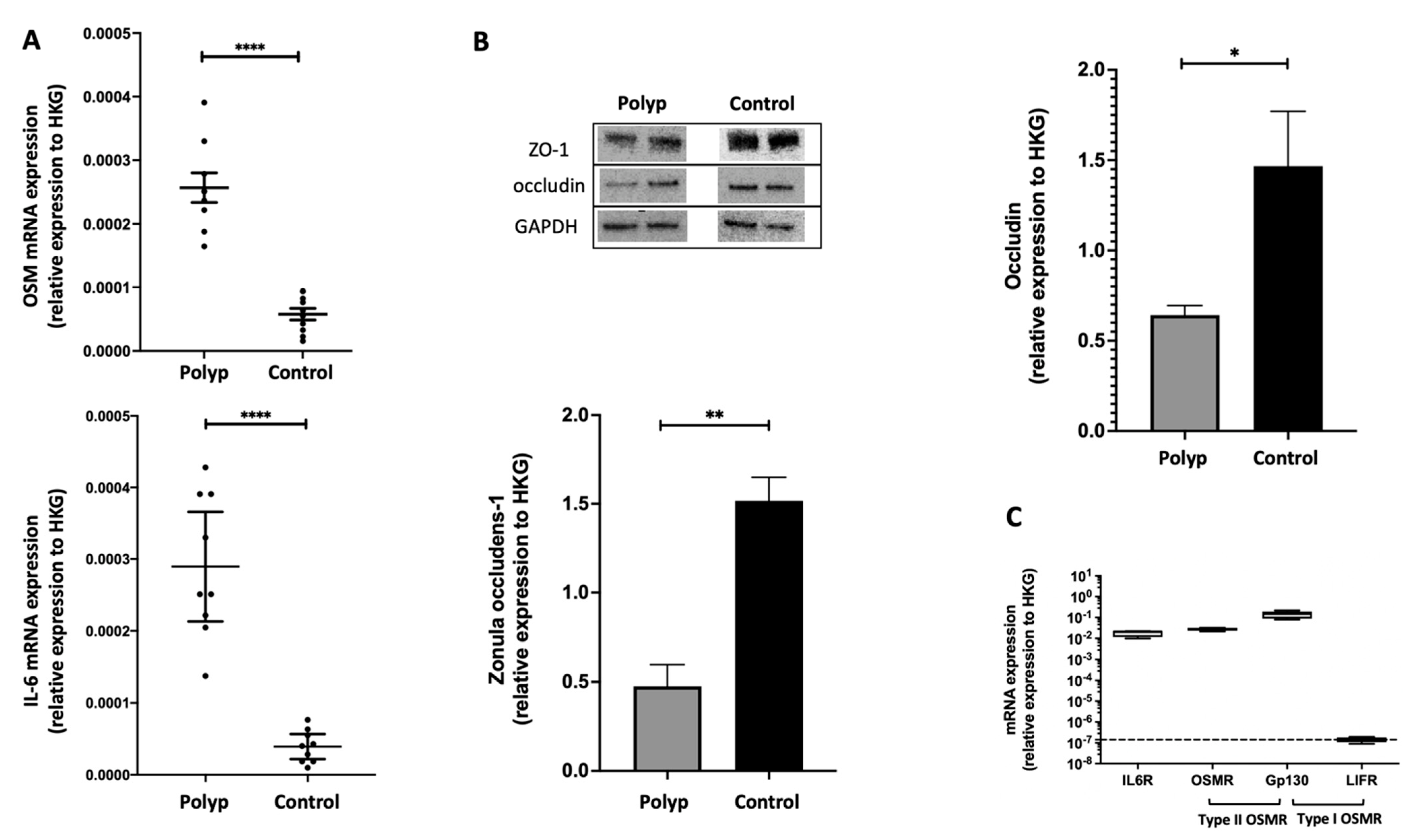
2.1. In Nasal Polyps, OSM Was Secreted and Overexpressed, and Epithelial TJs Were Altered
2.2. The IL-6 Receptor and Type II OSM Receptor Were Expressed in HNEC
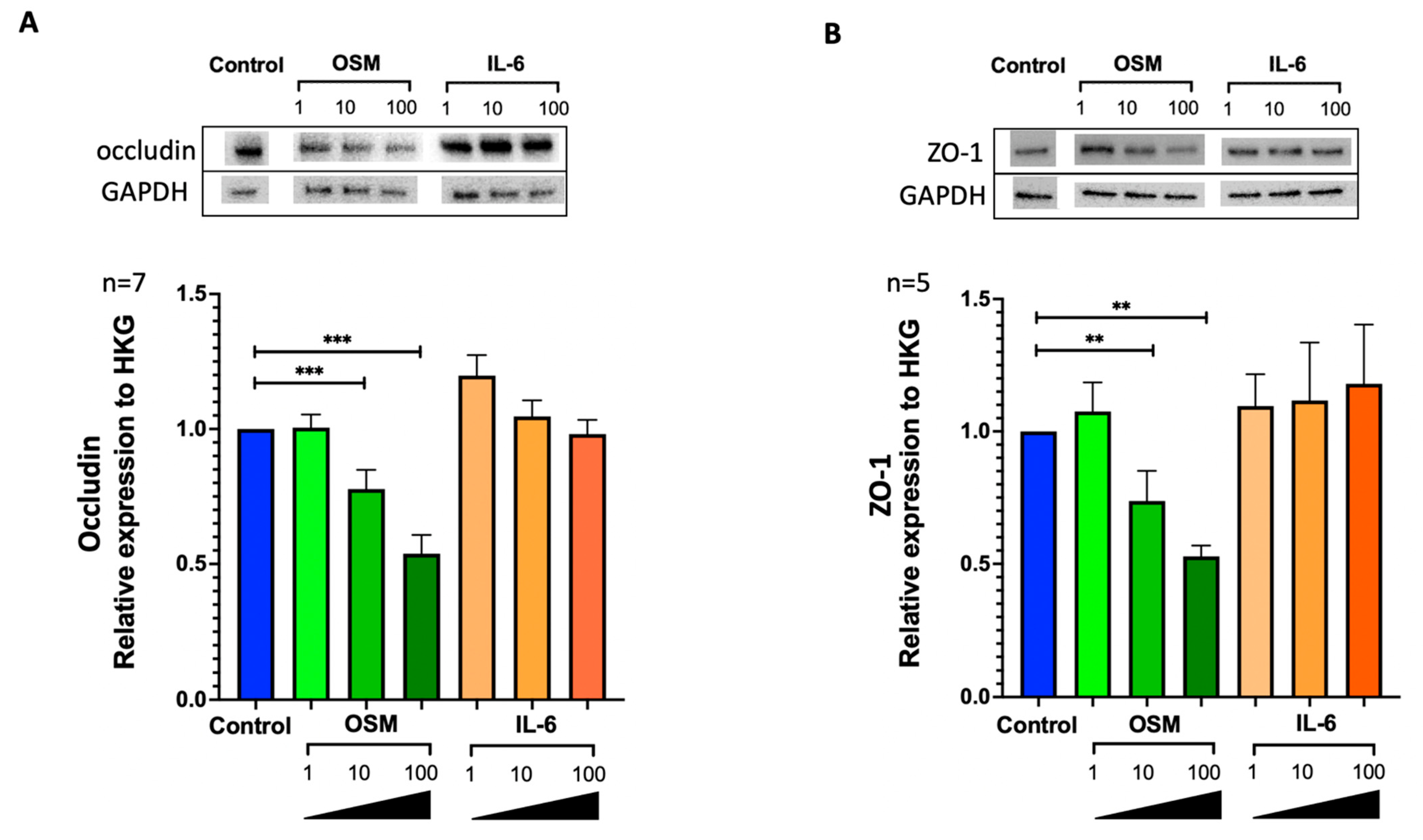
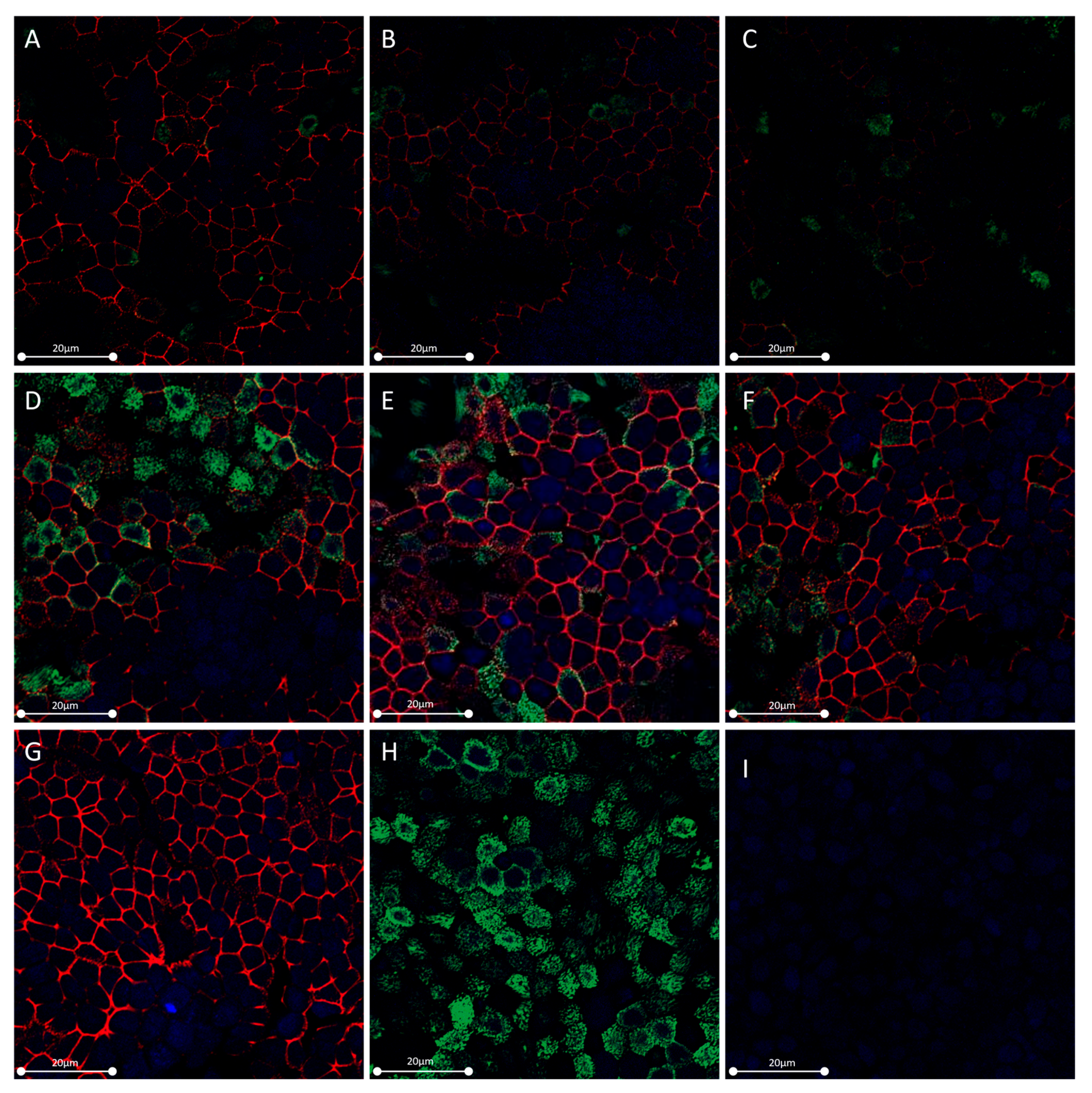
2.3. OSM but Not IL-6 Decreased Occludin and ZO-1 Expression in HNEC
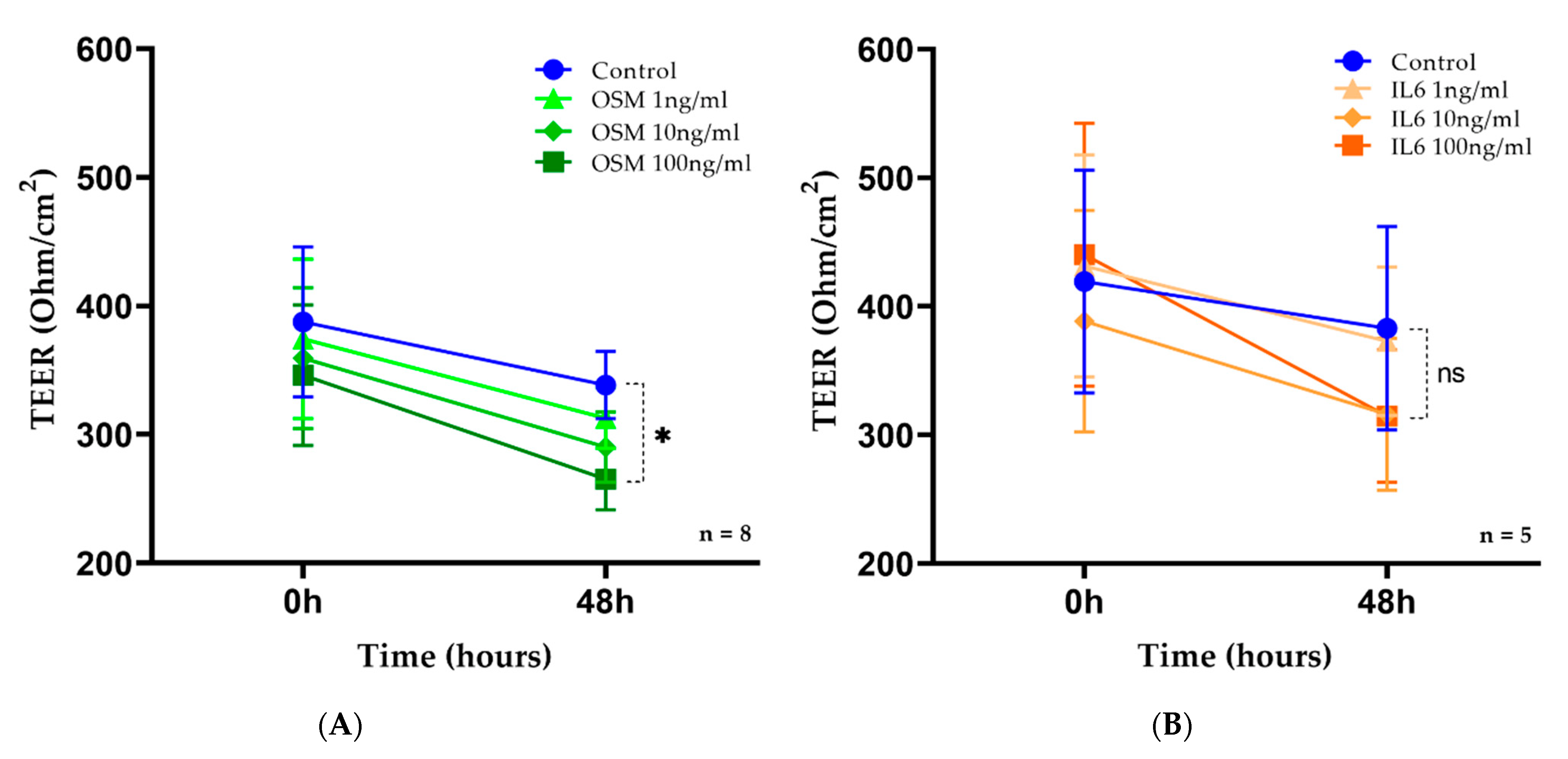
2.4. OSM but Not IL-6 Decreased TEER in HNEC
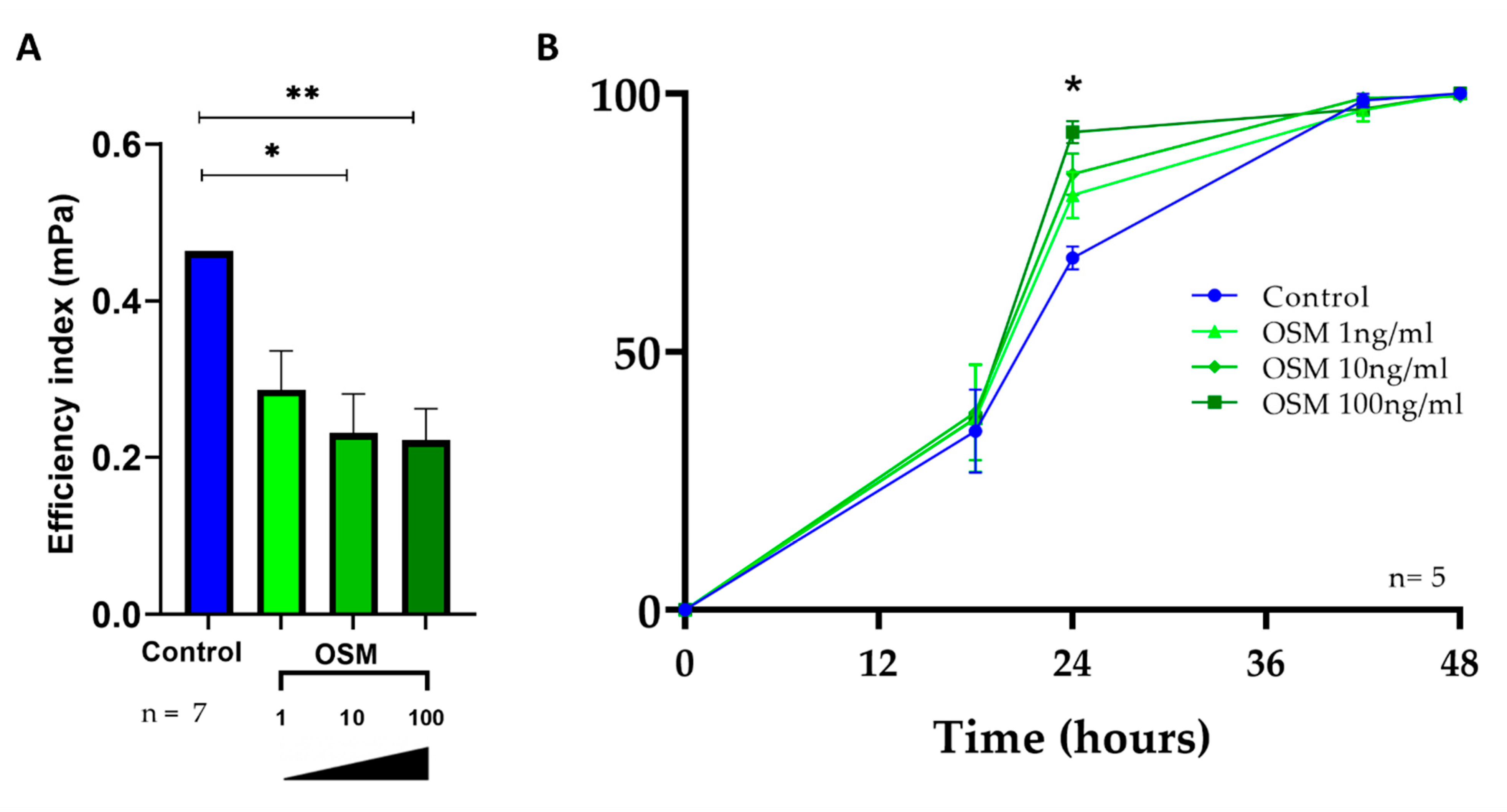
2.5. OSM Decreased Ciliary Beating Efficiency in HNEC
2.6. OSM Accelerated Wound Repair in HNEC
3. Discussion
4. Materials and Methods
4.1. Tissue, Cells Collection, and Primary Culture of HNEC
4.2. Tight Junctions Protein Expression (ZO-1 and Occludin) and IL-6 and OSM mRNA Expression in the Nasal Polyps and Control Mucosa
4.3. OSM Secretion by ELISA
4.4. IL-6 and OSM Receptors RNA Expression in HNECs (RT-qPCR)
4.5. ZO-1 and Occludin Protein Expression in HNECs (Western blot) with or without IL-6 or OSM
4.6. Actin and ZO-1 Protein Expression in HNECs (Immunolabelling) with or without IL-6 or OSM
4.7. TEER in HNECs (EVOM) with or without IL-6 or OSM
4.8. Wound Repair Rate in HNECs (Time-Lapse Images) with or without OSM
4.9. Ciliary Beating Efficiency in HNECs (High-Speed Videomicroscopy) with or without OSM
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlén, S.E.; et al. Chronic Rhinosinusitis in Europe--an Underestimated Disease. A GA2LEN Study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, P.; Vandeplas, G.; van Zele, T.; Cardell, L.-O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory Endotypes of Chronic Rhinosinusitis Based on Cluster Analysis of Biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Mullol, J.; Woessner, K.M.; Amin, N.; Mannent, L.P. Chronic Rhinosinusitis with Nasal Polyps and Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 1133–1141. [Google Scholar] [CrossRef]
- Soyka, M.B.; Wawrzyniak, P.; Eiwegger, T.; Holzmann, D.; Treis, A.; Wanke, K.; Kast, J.I.; Akdis, C.A. Defective Epithelial Barrier in Chronic Rhinosinusitis: The Regulation of Tight Junctions by IFN-γ and IL-4. J. Allergy Clin. Immunol. 2012, 130, 1087–1096.e10. [Google Scholar] [CrossRef]
- Steelant, B.; Seys, S.F.; Boeckxstaens, G.; Akdis, C.A.; Ceuppens, J.L.; Hellings, P.W. Restoring Airway Epithelial Barrier Dysfunction: A New Therapeutic Challenge in Allergic Airway Disease. Rhinology 2016, 54, 195–205. [Google Scholar] [CrossRef]
- Georas, S.N.; Rezaee, F. Epithelial Barrier Function: At the Front Line of Asthma Immunology and Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef]
- Chiba, H.; Osanai, M.; Murata, M.; Kojima, T.; Sawada, N. Transmembrane Proteins of Tight Junctions. Biochim. Biophys. Acta 2008, 1778, 588–600. [Google Scholar] [CrossRef]
- Wladislavosky-Waserman, P.; Kern, E.B.; Holley, K.E.; Eisenbrey, A.B.; Gleich, G.J. Epithelial Damage in Nasal Polyps. Clin. Allergy 1984, 14, 241–247. [Google Scholar] [CrossRef]
- Coste, A.; Wang, Q.P.; Roudot-Thoraval, F.; Chapelin, C.; Bedbeder, P.; Poron, F.; Peynègre, R.; Escudier, E. Epithelial Cell Proliferation in Nasal Polyps Could Be Up-Regulated by Platelet-Derived Growth Factor. Laryngoscope 1996, 106, 578–583. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Norton, J.E.; Hulse, K.E.; Suh, L.A.; Carter, R.G.; Rocci, E.; Harris, K.E.; Shintani-Smith, S.; Conley, D.B.; Chandra, R.K.; et al. Oncostatin M Promotes Mucosal Epithelial Barrier Dysfunction, and Its Expression Is Increased in Patients with Eosinophilic Mucosal Disease. J. Allergy Clin. Immunol. 2015, 136, 737–746.e4. [Google Scholar] [CrossRef]
- Coste, A.; Brugel, L.; Maître, B.; Boussat, S.; Papon, J.F.; Wingerstmann, L.; Peynègre, R.; Escudier, E. Inflammatory Cells as Well as Epithelial Cells in Nasal Polyps Express Vascular Endothelial Growth Factor. Eur. Respir. J. 2000, 15, 367–372. [Google Scholar] [CrossRef]
- Pawankar, R. Nasal Polyposis: An Update: Editorial Review. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 1–6. [Google Scholar] [CrossRef]
- Bartier, S.; Coste, A.; Béquignon, E. Biotherapy and Treatment of Adult Primary Chronic Rhinosinusitis with Nasal Polyps: Cellular and Molecular Bases. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 138, 355–362. [Google Scholar] [CrossRef]
- Pistochini, A.; Rossi, F.; Gallo, S.; Pirrone, C.; Preti, A.; Gornati, R.; Bernardini, G.; Castelnuovo, P. Multiple Gene Expression Profiling Suggests Epithelial Dysfunction in Polypoid Chronic Rhinosinusitis. Acta Otorhinolaryngol. Ital. 2019, 39, 169–177. [Google Scholar] [CrossRef]
- Carsuzaa, F.; Béquignon, É.; Dufour, X.; de Bonnecaze, G.; Lecron, J.-C.; Favot, L. Cytokine Signature and Involvement in Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2021, 23, 417. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 and Its Receptor: Ten Years Later. Int. Rev. Immunol. 1998, 16, 249–284. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Botelho, F.; Dubey, A.; Ayaub, E.A.; Park, R.; Yip, A.; Humbles, A.; Kolbeck, R.; Richards, C.D. IL-33 Mediates Lung Inflammation by the IL-6-Type Cytokine Oncostatin M. Mediat. Inflamm. 2020, 2020, 4087315. [Google Scholar] [CrossRef]
- Matsuda, M.; Tsurusaki, S.; Miyata, N.; Saijou, E.; Okochi, H.; Miyajima, A.; Tanaka, M. Oncostatin M Causes Liver Fibrosis by Regulating Cooperation between Hepatic Stellate Cells and Macrophages in Mice. Hepatology 2018, 67, 296–312. [Google Scholar] [CrossRef]
- Rabeony, H.; Petit-Paris, I.; Garnier, J.; Barrault, C.; Pedretti, N.; Guilloteau, K.; Jegou, J.-F.; Guillet, G.; Huguier, V.; Lecron, J.-C.; et al. Inhibition of Keratinocyte Differentiation by the Synergistic Effect of IL-17A, IL-22, IL-1α, TNFα and Oncostatin M. PLoS ONE 2014, 9, e101937. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, K.; Paris, I.; Pedretti, N.; Boniface, K.; Juchaux, F.; Huguier, V.; Guillet, G.; Bernard, F.-X.; Lecron, J.-C.; Morel, F. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J. Immunol. 2010, 184, 5263–5270. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Diveu, C.; Morel, F.; Pedretti, N.; Froger, J.; Ravon, E.; Garcia, M.; Venereau, E.; Preisser, L.; Guignouard, E.; et al. Oncostatin M Secreted by Skin Infiltrating T Lymphocytes Is a Potent Keratinocyte Activator Involved in Skin Inflammation. J. Immunol. 2007, 178, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Giot, J.-P.; Paris, I.; Levillain, P.; Huguier, V.; Charreau, S.; Delwail, A.; Garcia, M.; Garnier, J.; Bernard, F.-X.; Dagregorio, G.; et al. Involvement of IL-1 and Oncostatin M in Acanthosis Associated with Hypertensive Leg Ulcer. Am. J. Pathol. 2013, 182, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Carsuzaa, F.; Béquignon, É.; Bainaud, M.; Jégou, J.-F.; Dufour, X.; Lecron, J.-C.; Favot, L. Oncostatin M Counteracts the Fibrotic Effects of TGF-Β1 and IL-4 on Nasal-Polyp-Derived Fibroblasts: A Control of Fibrosis in Chronic Rhinosinusitis with Nasal Polyps? Int. J. Mol. Sci. 2022, 23, 6308. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 Signalling in Health and Disease. F1000Res 2020, 9, F1000 Faculty Rev-1013. [Google Scholar] [CrossRef]
- Danielsen, A.; Tynning, T.; Brokstad, K.A.; Olofsson, J.; Davidsson, A. Interleukin 5, IL6, IL12, IFN-Gamma, RANTES and Fractalkine in Human Nasal Polyps, Turbinate Mucosa and Serum. Eur. Arch. Otorhinolaryngol. 2006, 263, 282–289. [Google Scholar] [CrossRef]
- Bequignon, E.; Mangin, D.; Bécaud, J.; Pasquier, J.; Angely, C.; Bottier, M.; Escudier, E.; Isabey, D.; Filoche, M.; Louis, B.; et al. Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps: Role of IL-6 in Airway Epithelial Cell Dysfunction. J. Transl. Med. 2020, 18, 136. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-Redundant Functions of IL-6 Produced by Macrophages and Dendritic Cells in Allergic Airway Inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and Safety of Omalizumab in Nasal Polyposis: 2 Randomized Phase 3 Trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for Chronic Rhinosinusitis with Nasal Polyps (SYNAPSE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef]
- Bachert, C.; Mannent, L.; Naclerio, R.M.; Mullol, J.; Ferguson, B.J.; Gevaert, P.; Hellings, P.; Jiao, L.; Wang, L.; Evans, R.R.; et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients with Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA 2016, 315, 469–479. [Google Scholar] [CrossRef]
- Patel, G.B.; Kern, R.C.; Bernstein, J.A.; Hae-Sim, P.; Peters, A.T. Current and Future Treatments of Rhinitis and Sinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1522–1531. [Google Scholar] [CrossRef]
- Kim, D.W.; Cho, S.H. Emerging Endotypes of Chronic Rhinosinusitis and Its Application to Precision Medicine. Allergy Asthma Immunol. Res. 2017, 9, 299–306. [Google Scholar] [CrossRef]
- Cardell, L.-O.; Stjärne, P.; Jonstam, K.; Bachert, C. Endotypes of Chronic Rhinosinusitis: Impact on Management. J. Allergy Clin. Immunol. 2020, 145, 752–756. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, N.; Zhang, L.; Bachert, C. Biologics for the Treatment of Chronic Rhinosinusitis with Nasal Polyps—State of the Art. World Allergy Organ. J. 2019, 12, 100050. [Google Scholar] [CrossRef]
- Wise, S.K.; Laury, A.M.; Katz, E.H.; Den Beste, K.A.; Parkos, C.A.; Nusrat, A. Interleukin-4 and Interleukin-13 Compromise the Sinonasal Epithelial Barrier and Perturb Intercellular Junction Protein Expression. Int. Forum. Allergy Rhinol. 2014, 4, 361–370. [Google Scholar] [CrossRef]
- Babbin, B.A.; Parkos, C.A.; Mandell, K.J.; Winfree, L.M.; Laur, O.; Ivanov, A.I.; Nusrat, A. Annexin 2 Regulates Intestinal Epithelial Cell Spreading and Wound Closure through Rho-Related Signaling. Am. J. Pathol. 2007, 170, 951–966. [Google Scholar] [CrossRef]
- Kirshner, J.; Schumann, D.; Shively, J.E. CEACAM1, a Cell-Cell Adhesion Molecule, Directly Associates with Annexin II in a Three-Dimensional Model of Mammary Morphogenesis. J. Biol. Chem. 2003, 278, 50338–50345. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Bruewer, M.; Brown, G.T.; Pineda, A.A.; Ha, J.J.; Winfree, L.M.; Walsh, S.V.; Babbin, B.A.; Nusrat, A. Epithelial Cell Spreading Induced by Hepatocyte Growth Factor Influences Paxillin Protein Synthesis and Posttranslational Modification. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G886–G898. [Google Scholar] [CrossRef]
- Lazard, D.S.; Moore, A.; Hupertan, V.; Martin, C.; Escabasse, V.; Dreyfus, P.; Burgel, P.-R.; Amselem, S.; Escudier, E.; Coste, A. Muco-Ciliary Differentiation of Nasal Epithelial Cells Is Decreased after Wound Healing in Vitro. Allergy 2009, 64, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Van Zele, T.; Gevaert, P.; Watelet, J.-B.; Claeys, G.; Holtappels, G.; Claeys, C.; van Cauwenberge, P.; Bachert, C. Staphylococcus Aureus Colonization and IgE Antibody Formation to Enterotoxins Is Increased in Nasal Polyposis. J. Allergy Clin. Immunol. 2004, 114, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H. Chronic Rhinosinusitis with Nasal Polyps: Insights into Mechanisms of Disease from Emerging Biological Therapies. Expert Rev. Clin. Immunol. 2019, 15, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, A.; Hajizadeh, F.; Beigi Dargani, F.; Beyzai, B.; Aksoun, M.; Hojjat-Farsangi, M.; Zekiy, A.; Jadidi-Niaragh, F. Oncostatin M: A Mysterious Cytokine in Cancers. Int. Immunopharmacol. 2021, 90, 107158. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Oncostatin M, a Multifunctional Cytokine. Rev. Physiol. Biochem. Pharmacol. 2003, 149, 39–52. [Google Scholar]
- Stephens, J.M.; Elks, C.M. Oncostatin M: Potential Implications for Malignancy and Metabolism. Curr. Pharm. Des. 2017, 23, 3645–3657. [Google Scholar] [CrossRef]
- Elia, C.; Bucca, C.; Rolla, G.; Scappaticci, E.; Cantino, D. A Freeze-Fracture Study of Human Bronchial Epithelium in Normal, Bronchitic and Asthmatic Subjects. J. Submicrosc. Cytol. Pathol. 1988, 20, 509–517. [Google Scholar]
- Beigel, F.; Friedrich, M.; Probst, C.; Sotlar, K.; Göke, B.; Diegelmann, J.; Brand, S. Oncostatin M Mediates STAT3-Dependent Intestinal Epithelial Restitution via Increased Cell Proliferation, Decreased Apoptosis and Upregulation of SERPIN Family Members. PLoS ONE 2014, 9, e93498. [Google Scholar] [CrossRef]
- Bernard, C.; Merval, R.; Lebret, M.; Delerive, P.; Dusanter-Fourt, I.; Lehoux, S.; Créminon, C.; Staels, B.; Maclouf, J.; Tedgui, A. Oncostatin M Induces Interleukin-6 and Cyclooxygenase-2 Expression in Human Vascular Smooth Muscle Cells: Synergy with Interleukin-1beta. Circ. Res. 1999, 85, 1124–1131. [Google Scholar] [CrossRef]
- Gurluler, E.; Tumay, L.V.; Guner, O.S.; Kucukmetin, N.T.; Hizli, B.; Zorluoglu, A. Oncostatin-M as a Novel Biomarker in Colon Cancer Patients and Its Association with Clinicopathologic Variables. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2042–2047. [Google Scholar]
- Scheller, J.; Ohnesorge, N.; Rose-John, S. Interleukin-6 Trans-Signalling in Chronic Inflammation and Cancer. Scand. J. Immunol. 2006, 63, 321–329. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 Receptor System and Its Role in Physiological and Pathological Conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef]
- Wynne, M.; Atkinson, C.; Schlosser, R.J.; Mulligan, J.K. Contribution of Epithelial Cell Dysfunction to the Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps. Am. J. Rhinol. Allergy 2019, 33, 782–790. [Google Scholar] [CrossRef]
- Jiao, J.; Duan, S.; Meng, N.; Li, Y.; Fan, E.; Zhang, L. Role of IFN-γ, IL-13, and IL-17 on Mucociliary Differentiation of Nasal Epithelial Cells in Chronic Rhinosinusitis with Nasal Polyps. Clin. Exp. Allergy 2016, 46, 449–460. [Google Scholar] [CrossRef]
- Laoukili, J.; Perret, E.; Willems, T.; Minty, A.; Parthoens, E.; Houcine, O.; Coste, A.; Jorissen, M.; Marano, F.; Caput, D.; et al. IL-13 Alters Mucociliary Differentiation and Ciliary Beating of Human Respiratory Epithelial Cells. J. Clin. Investig. 2001, 108, 1817–1824. [Google Scholar] [CrossRef]
- Grosse-Onnebrink, J.; Werner, C.; Loges, N.T.; Hörmann, K.; Blum, A.; Schmidt, R.; Olbrich, H.; Omran, H. Effect of TH2 Cytokines and Interferon Gamma on Beat Frequency of Human Respiratory Cilia. Pediatr. Res. 2016, 79, 731–735. [Google Scholar] [CrossRef]
- Wanner, A. Clinical Perspectives: Role of the Airway Circulation in Drug Therapy. J. Aerosol. Med. 1996, 9, 19–23. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Yeh, C.-T.; Lin, K.-H. Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells 2020, 9, 1331. [Google Scholar] [CrossRef]
- Lecron, J.-C.; Charreau, S.; Jégou, J.-F.; Salhi, N.; Petit-Paris, I.; Guignouard, E.; Burucoa, C.; Favot-Laforge, L.; Bodet, C.; Barra, A.; et al. IL-17 and IL-22 Are Pivotal Cytokines to Delay Wound Healing of S. Aureus and P. Aeruginosa Infected Skin. Front. Immunol. 2022, 13, 984016. [Google Scholar] [CrossRef]
- Wise, S.K.; Den Beste, K.A.; Hoddeson, E.K.; Parkos, C.A.; Nusrat, A. Sinonasal Epithelial Wound Resealing in an in Vitro Model: Inhibition of Wound Closure with IL-4 Exposure. Int. Forum. Allergy Rhinol. 2013, 3, 439–449. [Google Scholar] [CrossRef]
- Lee, S.-N.; Ahn, J.-S.; Lee, S.G.; Lee, H.-S.; Choi, A.M.K.; Yoon, J.-H. Integrins Avβ5 and Avβ6 Mediate IL-4-Induced Collective Migration in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2019, 60, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gitto, S.; Lori, G.; Marra, F.; Parola, M.; Cannito, S.; Gentilini, A. Oncostatin M: From Intracellular Signaling to Therapeutic Targets in Liver Cancer. Cancers 2022, 14, 4211. [Google Scholar] [CrossRef] [PubMed]
- Fritz, D.K.; Kerr, C.; Tong, L.; Smyth, D.; Richards, C.D. Oncostatin-M up-Regulates VCAM-1 and Synergizes with IL-4 in Eotaxin Expression: Involvement of STAT6. J. Immunol. 2006, 176, 4352–4360. [Google Scholar] [CrossRef] [PubMed]
- Papon, J.-F.; Coste, A.; Gendron, M.-C.; Cordonnier, C.; Wingerstmann, L.; Peynègre, R.; Escudier, E. HLA-DR and ICAM-1 Expression and Modulation in Epithelial Cells from Nasal Polyps. Laryngoscope 2002, 112, 2067–2075. [Google Scholar] [CrossRef]
- Forero, A.; Fenstermacher, K.; Wohlgemuth, N.; Nishida, A.; Carter, V.; Smith, E.A.; Peng, X.; Hayes, M.; Francis, D.; Treanor, J.; et al. Evaluation of the Innate Immune Responses to Influenza and Live-Attenuated Influenza Vaccine Infection in Primary Differentiated Human Nasal Epithelial Cells. Vaccine 2017, 35, 6112–6121. [Google Scholar] [CrossRef]
- Ramanathan, M.; Lee, W.-K.; Dubin, M.G.; Lin, S.; Spannhake, E.W.; Lane, A.P. Sinonasal Epithelial Cell Expression of Toll-like Receptor 9 Is Decreased in Chronic Rhinosinusitis with Polyps. Am. J. Rhinol. 2007, 21, 110–116. [Google Scholar] [CrossRef]
- Bequignon, E.; Dhommée, C.; Angely, C.; Thomas, L.; Bottier, M.; Escudier, E.; Isabey, D.; Coste, A.; Louis, B.; Papon, J.-F.; et al. FcRn-Dependent Transcytosis of Monoclonal Antibody in Human Nasal Epithelial Cells In Vitro: A Prerequisite for a New Delivery Route for Therapy? Int. J. Mol. Sci. 2019, 20, 1379. [Google Scholar] [CrossRef]
- Jeanson, L.; Guerrera, I.C.; Papon, J.-F.; Chhuon, C.; Zadigue, P.; Prulière-Escabasse, V.; Amselem, S.; Escudier, E.; Coste, A.; Edelman, A. Proteomic Analysis of Nasal Epithelial Cells from Cystic Fibrosis Patients. PLoS ONE 2014, 9, e108671. [Google Scholar] [CrossRef]
- Botterel, F.; Gross, K.; Ibrahim-Granet, O.; Khoufache, K.; Escabasse, V.; Coste, A.; Cordonnier, C.; Escudier, E.; Bretagne, S. Phagocytosis of Aspergillus Fumigatus Conidia by Primary Nasal Epithelial Cells in Vitro. BMC Microbiol. 2008, 8, 97. [Google Scholar] [CrossRef]
- Botterel, F.; Cordonnier, C.; Barbier, V.; Wingerstmann, L.; Liance, M.; Coste, A.; Escudier, E.; Bretagne, S. Aspergillus Fumigatus Causes in Vitro Electrophysiological and Morphological Modifications in Human Nasal Epithelial Cells. Histol. Histopathol. 2002, 17, 1095–1101. [Google Scholar]
- Khoufache, K.; Puel, O.; Loiseau, N.; Delaforge, M.; Rivollet, D.; Coste, A.; Cordonnier, C.; Escudier, E.; Botterel, F.; Bretagne, S. Verruculogen Associated with Aspergillus Fumigatus Hyphae and Conidia Modifies the Electrophysiological Properties of Human Nasal Epithelial Cells. BMC Microbiol. 2007, 7, 5. [Google Scholar] [CrossRef]
- Cao, H.; Ouyang, H.; Grasemann, H.; Bartlett, C.; Du, K.; Duan, R.; Shi, F.; Estrada, M.; Seigel, K.E.; Coates, A.L.; et al. Transducing Airway Basal Cells with a Helper-Dependent Adenoviral Vector for Lung Gene Therapy. Hum. Gene Ther. 2018, 29, 643–652. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, D.W.; Lee, S.H.; Kolliputi, N.; Hong, S.J.; Suh, L.; Norton, J.; Hulse, K.E.; Seshadri, S.; Conley, D.B.; et al. Age-Related Increased Prevalence of Asthma and Nasal Polyps in Chronic Rhinosinusitis and Its Association with Altered IL-6 Trans-Signaling. Am. J. Respir. Cell Mol. Biol. 2015, 53, 601–606. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Pedersen, M.H.; Cencic, A.; Budde, B.B. Application of Measurements of Transepithelial Electrical Resistance of Intestinal Epithelial Cell Monolayers to Evaluate Probiotic Activity. Appl. Environ. Microbiol. 2005, 71, 7528–7530. [Google Scholar] [CrossRef]
- Yeo, N.-K.; Jang, Y.J. Rhinovirus Infection-Induced Alteration of Tight Junction and Adherens Junction Components in Human Nasal Epithelial Cells. Laryngoscope 2010, 120, 346–352. [Google Scholar] [CrossRef]
- Lechapt-Zalcman, E.; Prulière-Escabasse, V.; Advenier, D.; Galiacy, S.; Charrière-Bertrand, C.; Coste, A.; Harf, A.; D’Ortho, M.-P.; Escudier, E. Transforming Growth Factor-Beta1 Increases Airway Wound Repair via MMP-2 Upregulation: A New Pathway for Epithelial Wound Repair? Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L1277–L1282. [Google Scholar] [CrossRef]
- Bottier, M.; Blanchon, S.; Pelle, G.; Bequignon, E.; Isabey, D.; Coste, A.; Escudier, E.; Grotberg, J.B.; Papon, J.-F.; Filoche, M.; et al. A New Index for Characterizing Micro-Bead Motion in a Flow Induced by Ciliary Beating: Part I, Experimental Analysis. PLoS Comput. Biol. 2017, 13, e1005605. [Google Scholar] [CrossRef]
- Bottier, M.; Peña Fernández, M.; Pelle, G.; Isabey, D.; Louis, B.; Grotberg, J.B.; Filoche, M. A New Index for Characterizing Micro-Bead Motion in a Flow Induced by Ciliary Beating: Part II, Modeling. PLoS Comput. Biol. 2017, 13, e1005552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carsuzaa, F.; Bequignon, E.; Bartier, S.; Coste, A.; Dufour, X.; Bainaud, M.; Lecron, J.C.; Louis, B.; Tringali, S.; Favot, L.; et al. Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2023, 24, 6094. https://doi.org/10.3390/ijms24076094
Carsuzaa F, Bequignon E, Bartier S, Coste A, Dufour X, Bainaud M, Lecron JC, Louis B, Tringali S, Favot L, et al. Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps. International Journal of Molecular Sciences. 2023; 24(7):6094. https://doi.org/10.3390/ijms24076094
Chicago/Turabian StyleCarsuzaa, Florent, Emilie Bequignon, Sophie Bartier, André Coste, Xavier Dufour, Matthieu Bainaud, Jean Claude Lecron, Bruno Louis, Stéphane Tringali, Laure Favot, and et al. 2023. "Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps" International Journal of Molecular Sciences 24, no. 7: 6094. https://doi.org/10.3390/ijms24076094
APA StyleCarsuzaa, F., Bequignon, E., Bartier, S., Coste, A., Dufour, X., Bainaud, M., Lecron, J. C., Louis, B., Tringali, S., Favot, L., & Fieux, M. (2023). Oncostatin M Contributes to Airway Epithelial Cell Dysfunction in Chronic Rhinosinusitis with Nasal Polyps. International Journal of Molecular Sciences, 24(7), 6094. https://doi.org/10.3390/ijms24076094




